Abstract
Understanding the neurobiological underpinnings of abstinence from drugs of abuse is critical to allow better recovery and ensure relapse prevention in addicted subjects. By comparing the long-term transcriptional consequences of morphine and cocaine exposure, we identified the metabotropic glutamate receptor subtype 4 (mGluR4) as a promising pharmacological target in morphine abstinence. We evaluated the behavioral and molecular effects of facilitating mGluR4 activity in abstinent mice. Transcriptional regulation of marker genes of medium spiny neurons (MSNs) allowed best discriminating between 4-week morphine and cocaine abstinence in the nucleus accumbens (NAc). Among these markers, Grm4, encoding mGluR4, displayed down-regulated expression in the caudate putamen and NAc of morphine, but not cocaine, abstinent mice. Chronic administration of the mGluR4 positive allosteric modulator (PAM) VU0155041 (2.5 and 5 mg/kg) rescued social behavior, normalized stereotypies and anxiety and blunted locomotor sensitization in morphine abstinent mice. This treatment improved social preference but increased stereotypies in cocaine abstinent mice. Finally, the beneficial behavioral effects of VU0155041 treatment in morphine abstinent mice were correlated with restored expression of key MSN and neural activity marker genes in the NAc. This study reports that chronic administration of the mGluR4 PAM VU0155041 relieves long-term deleterious consequences of morphine exposure. It illustrates the neurobiological differences between opiate and psychostimulant abstinence and points to pharmacological repression of excessive activity of D2-MSNs in the NAc as a promising therapeutic lever in drug addiction.
Subject terms: Reward, Addiction
Introduction
Drug addiction is a chronic psychiatric disorder characterized by loss of control over consumption despite negative consequences [1]. Addicted subjects wanting to quit drug use face a true challenge, as protracted abstinence leads to negative affect, anxiety disorders, and social withdrawal [2–4] that increase the propensity for relapse [5]. A better understanding of the long-lasting neurobiological underpinnings of the abstinent state thus appears invaluable to improve relapse prevention [6–8]. Although addiction to most drugs of abuse share similar clinical features, a unitary view of addiction is challenged by different drug-seeking behaviors and brain adaptations across drug classes, particularly for narcotics versus psychostimulants [9–12]. The neurobiological mechanisms underlying abstinence and vulnerability to relapse are thus also likely to diverge between drugs.
In previous reports, we have evidenced common long-term transcriptional adaptations that develop within the central extended amygdala (central EA: central amygdala and bed nucleus of the stria terminalis, CeA and BNST, respectively) upon protracted abstinence to morphine, nicotine, Δ9-tetrahydrocannabinol (THC) and alcohol [13, 14] but not cocaine [14]. These adaptations mostly involved genes with an enriched expression in medium spiny GABAergic neurons (MSNs), a key brain substrate for drug reward and addiction [15–17], and notably 14 genes belonging to a huntingtin (HTT)-related gene network [13, 14]. Consistent with shared molecular alterations, protracted abstinence from the aforementioned four drugs induced similar behavioral deficits, including impaired social abilities, motor stereotypies and exacerbated anxiety-like behavior [14, 18–20] that were not observed following cocaine abstinence, except for blunted social preference [14]. Remarkably, behavioral deficits evidenced in mice abstinent from morphine, nicotine, alcohol or THC were similar to those detected in mice lacking the mu opioid receptor (Oprm1-/-), a mouse model of autism [21, 22], and, more generally, in most mouse models of this neurodevelopmental disorder [23, 24]. Such similarities may reflect common striatal dysfunction and altered reward processing [25, 26], possibly involving compromised opioid function [27–29]. Shared neurobiological mechanisms between drug addiction and autism spectrum disorders interestingly argue for the development of novel therapeutic interventions that would benefit to both pathologies, with the caveat that addiction to cocaine/psychostimulants may diverge from narcotic drugs in their responsiveness to such approaches.
In the present study, we extended our comparison of morphine versus cocaine abstinence-induced transcriptional changes to the caudate putamen (CPu) and nucleus accumbens (NAc), two more brain regions where MSNs represent the main cell type, and analyzed separately the transcriptomes of the CeA and BNST. Not only we confirmed that morphine and cocaine abstinence produce contrasting regulations in gene expression, especially genes with MSN-enriched expression, but we detected down-regulated levels of Grm4 (encoding the presynaptic mGlu4 glutamate receptor - mGluR4) transcripts in the CPu and NAc of morphine, and not cocaine, abstinent mice. Decreased Grm4 expression retained our attention as another common feature between morphine abstinent and Oprm1-/- mice [21, 30], as we evidenced in the latter a rescue of sociability, stereotypic behavior and anxiety-like behavior under chronic facilitation of mGluR4 activity [21]. Activation of mGluR4 was shown to inhibit GABA release by indirect, D2 dopamine receptor-expressing MSNs (D2-MSNs), with therapeutic benefit in Parkinson’s disease [31, 32]. Interestingly, negative affect in opioid addiction and dependence may involve D2-MSNs rather than direct, D1 dopamine receptor-expressing MSNs (D1-MSNs) [33–35]. Here we tested whether chronic administration of VU0155041, a positive allosteric modulator (PAM) and partial agonist of mGluR4, can alleviate the deleterious behavioral and molecular consequences of abstinence to morphine, and presumably not to cocaine. This study suggests that mGluR4 is a promising pharmacological target to treat the long-term behavioral consequences of opioid use disorder (OUD) and further argues for the involvement of distinct neurobiological processes in opiate versus psychostimulant addiction.
Methods and materials
Subjects
In all experiments, we used C57BL6/J male mice aged 8–12 weeks (Charles River, Lyon, France). Mice were group housed and maintained on a 12 h light/dark cycle (lights on at 7:00 AM) at controlled temperature (21 ± 1 °C); food and water were available ad libitum, except otherwise stated. Mice in the same cage received the same treatment. All experimental procedures were conducted in accordance with the European Communities Council Directive 2010/63/EU and approved by the Comité d’Ethique pour l’Expérimentation Animale de l’ICS et de l’IGBMC (Com’Eth, 2012-033) and Comité d’Ethique en Expérimentation animale Val de Loire (C2EA-19).
Drugs
Chronic morphine and cocaine treatments were performed as described previously [14] (details in Supplementary 1). Mice were injected twice daily with vehicle (i.p., NaCl 0.9%) or morphine (i.p., escalating doses from 20 mg/kg to 100 mg/kg over 5 days and one injection on day 6 at 100 mg/kg) or cocaine (s.c., 25 mg/kg during 5.5 days). They were left drug-free for 3 weeks before chronic daily treatment with vehicle (NaCl 0.9%) or VU0155041 (Tocris, Bristol, UK; 2.5 or 5 mg/kg, i.p.) started. Behavioral testing started after 8 days of VU0155041 administration, to evaluate chronic effects of treatment. This treatment was maintained for 8–15 consecutive days, allowing comprehensive behavioral phenotyping (see time lines in Fig. S1). On testing days, VU0155041 (or vehicle) was administered 30 min before behavioral assays. All compounds were administered in a volume of 10 ml/kg.
Antibodies
In Western blot experiments, primary antibodies targeting GAPDH (Rabbit mAb, ref. 2118; RRID: AB_561053) (1:2000) from Cell Signaling Technology and synaptophysin (mouse SYP antibody [D-4], ref. sc-17750; RRID: AB_628311) (1:2000) from Santa Cruz Biotechnology were used at the indicated dilutions. They were combined with the following secondary antibodies: goat anti rabbit IRDye®800CW (LI-COR®, USA, 926-3221; RRID: AB_621843) (1:15000) and goat anti mouse IRDye®680RD (LI-COR®, USA, 926-68070; RRID: AB_10956588) (1:15000).
Behavioral experiments
As described previously [14, 30], social behavior was explored using the direct social interaction and three-chamber tests, stereotyped/perseverative behavior was assessed by scoring spontaneous motor stereotypies and buried marbles in the marble burying test and by analyzing alternation patterns in the Y-maze, and anxiety-like behavior was evaluated in the novelty-suppressed feeding test. Locomotor activity was recorded using video-tracking (Viewpoint, Lyon, France). Behavioral assays started on week 4 after cessation of morphine or cocaine exposure, on day 8 of VU0155041/vehicle treatment. On testing days, mice received pharmacological treatment 30 min prior to testing. Detailed protocols can be found in Supplementary1; experimental cohorts are detailed in Fig. S1.
Real-time quantitative polymerase chain reaction analysis
Real-time quantitative Polymerase Chain Reaction (qRT-PCR) analysis was performed on brain samples as described previously [14, 21, 36] (see dissection in Fig. S2, supplementary experimental procedures in Supplementary1 and list of probes in Table S1).
Western blot experiments
Mouse brains were dissected 45 min after social interaction and brain regions of interest were punched out [21, 37]. Tissues were processed and protein expression was measured as described previously [37] (see supplementary experimental procedures in Supplementary 1).
Statistics
Statistical analyses were performed using Statistica 9.0 software (StatSoft, Maisons-Alfort, France). For all comparisons, values of p < 0.05 were considered as significant. Statistical significance in behavioral experiments was assessed using one or three-way analysis of variance (drug, stimulus, treatment, and time effects) followed by Newman–Keuls post-hoc test. Significance of quantitative real-time PCR (qRT-PCR) results was assessed after transformation (if x < 1, y = 1-1/x; if x > 1, y = x-1, x: qRT-PCR data) using a two-tailed t-test, and p value was adjusted using Benjamin–Hochberg correction for multiple testing. Unsupervised clustering analysis was performed on transformed qRT-PCR data [14, 21, 36] using complete linkage with correlation distance (Pearson correlation) for drug, treatment, and brain region (Cluster 3.0 and Treeview software). When used for clustering analysis (Fig. 5A), behavioral data were normalized to vehicle-vehicle condition and transformed using the same formula as qRT-PCR data.
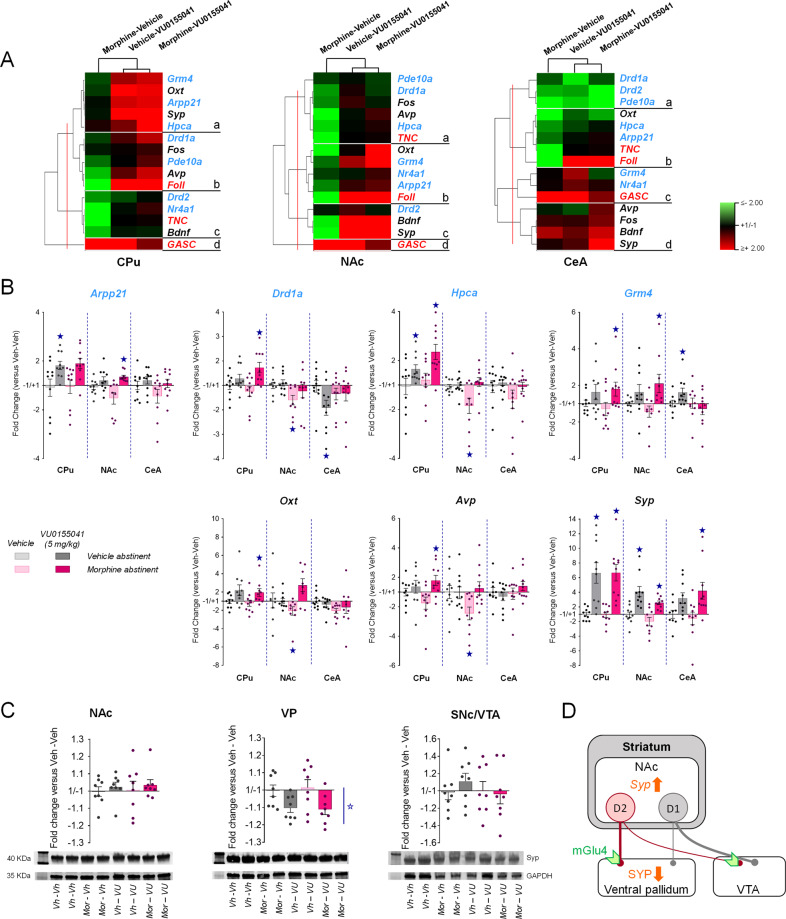
Fig. 5. Transcriptional regulations induced in the striatum and amygdala of morphine abstinent mice by chronic facilitation of mGluR4 signaling.
We evaluated the effects of chronic VU0155041 treatment (5 mg/kg) on transcriptional changes induced by morphine versus vehicle abstinence in the CPu, NAC, and CeA. We focused on the seven MSN marker genes (blue characters) highlighted in Fig. 1, Oxt and Avp coding for the social neuropeptides oxytocin and vasopressin, Fos as a marker of neuronal activity, and Bdnf and Syp as markers of synaptic plasticity. A Clustering analysis organized gene expression in 4 clusters per region. In the CPu and NAc, chronic VU0155041 restored (CPu: clusters b and c; NAc: clusters a, b,c) or increased (CPu: cluster a) the expression of most genes tested, which clustered with social parameters. In the CeA, rescued expression under VU0155041 treatment was observed in a single cluster (cluster b). B Among MSN marker genes highlighted in Fig. 1B, Drd1a and Hpca displayed significant down-regulation in the NAc, but not Arpp21, Gmr4, or Pde10a. VU0155041 rescued the expression of Drd1a and Hpca and increased the expression of Arpp21 and Grm4 in this region. Similarly, down-regulated expression of Oxt and Avp in the NAc of morphine-vehicle mice was rescued by VU0155041 treatment. Syp expression was increased upon VU0155041 exposure in the three regions tested. C Western blot analysis failed to detect modifications of synaptophysin (SYP) protein levels in the NAc, CPu and VTA but revealed a significant decrease in SYP immunoreactivity in the VP, (D) which represents the main projection area of NAc D2-MSNs. Gene (n = 9–10 per group) and protein (n = 8 per group) expression data are expressed as fold change versus vehicle-vehicle group for each condition (scatter plots and mean ± SEM). Comparison to vehicle-vehicle group: star p < 0.05 (two-tailed t-test, p value adjusted using Benjamin–Hochberg correction for multiple testing). Foll: number of following episodes, GASC: grooming after social contact, TNC: time in nose contact. qRT-PCR data used for clustering are displayed in Table S3.
Results
Deregulated expression of MSN marker genes discriminated between morphine and cocaine abstinence
We first compared the long-term consequences of a history of morphine or cocaine exposure at transcriptional level across the CPu, NAc, BNST and CeA, by assessing the expression of a set of 30 candidate genes, including the 14 HTT-related genes regulated by morphine abstinence (Adora2, Arpp21, Bcl11b, Gcnt2, Cnr1, Drd1a, Fam107a, Foxp1, Gpr88, Hpca, Nr4a1, Pde10a, St8sia3, Strip2) [13], 13 additional marker genes of MSNs identified from the literature (see Table S1) [38–40] and the Oxt gene, coding the neuropeptide oxytocin, as a marker of social behavior [30]. We used hierarchical clustering analysis of qRT-PCR data obtained for each drug in each brain region studied (Fig. 1, Table S2) to compare gene expression profiles.
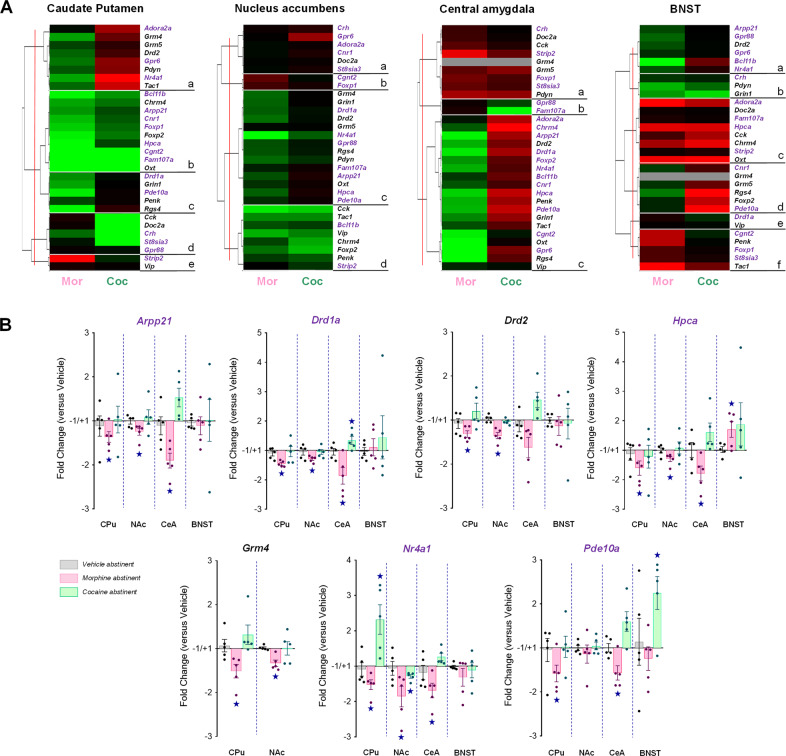
Fig. 1. Deregulated expression of MSN marker genes in the CPu, NAc and CeA allows to best discriminate between morphine and cocaine abstinence.
A We used qRT-PCR to compare the expression of 30 candidate genes, among which 14 previously identified HTT-related genes (purple characters), 13 additional MSN marker genes and Oxt as a marker transcript of social behavior, in the CPu, NAc, CeA and BNST under morphine and cocaine abstinence conditions compared to their saline counterparts. Hierarchical clustering organized gene expression data in three to six clusters per region. In a majority of these clusters, HTT-related and MSN marker genes displayed down-regulated expression under morphine conditions contrasting with up-regulated or unchanged expression under cocaine conditions. This pattern of gene expression was observed in the CPu (clusters a and c), NAc (cluster c), CeA (cluster c) and, to a lesser extent, in the BNST (cluster a). B Seven MSN marker genes displayed consistent down-regulated expression in the CPu, NAc and CeA under morphine conditions contrasting with no regulation or upregulation under cocaine abstinence, among which Grm4, coding for the mGlu4 receptor. Gene expression data are expressed as fold change versus vehicle abstinent for each condition (mean ± SEM, n = 5 per group). Comparison to vehicle group: p < 0.05 (two-tailed t-test, p value adjusted using Benjamin–Hochberg correction for multiple testing). qRT-PCR data used for clustering are displayed in Table S2.
In the four regions tested, clustering analysis organized gene expression in three to six clusters (Fig. 1A). Convergent effects of morphine and cocaine abstinence were observed in discrete clusters in the CPu (cluster b), NAc (cluster d) and CeA (cluster a) and in three out of six clusters in the BNST (clusters b,c,e). The remaining clusters revealed divergent, when not opposed, influence on gene expression. Interestingly, in a majority of these clusters, HTT-related and MSN marker genes displayed down-regulated expression under morphine conditions contrasting with up-regulated or unchanged expression under cocaine conditions. This pattern of gene expression was observed in the CPu (clusters a and c, 13 genes), NAc (cluster c, 14 genes), CeA (cluster c, 19 genes) and, to a lesser extent, in the BNST (cluster a, 6 genes). Thus, morphine abstinence was associated with down-regulated expression of numerous MSN-enriched genes in the CPu, NAc and CeA, an effect that was not observed under cocaine abstinence. Notably, we noticed seven genes with consistent down-regulated expression in the CPu, NAc and CeA under morphine conditions contrasting with no regulation or upregulation under cocaine abstinence: Arpp21, Drd1a, Drd2, Hpca, Grm4, Nr4a1 and Pde10a (Fig. 1B). Among them, Grm4 retained our attention for coding mGluR4, which we had previously identified as a promising target to alleviate deficient social behavior.
Chronic VU0155041 administration relieved social behavior deficits in morphine and cocaine abstinent mice
We then tested whether facilitating mGluR4 activity by chronically administering a PAM of mGluR4, VU0155041 (2.5 or 5 mg/kg), in morphine abstinent mice would rescue their behavioral deficits. For comparison purpose, we administered the same treatment (VU0155041, 5 mg/kg) to cocaine abstinent mice.
In the direct social interaction test (Fig. 2A), morphine abstinent mice displayed a marked deficit in social interaction that was dose-dependently normalized by VU0155041 treatment (partially at 2.5 mg/kg, completely at 5 mg/kg), as evidenced by restored time (abstinence × dose interaction: F2,57 = 56.2, p < 0.0001), number (abstinence x dose: F2,57 = 28.5, p < 0.0001) and duration of nose contacts (abstinence × dose: F2,57 = 70.1, p < 0.0001), as well as number of following (abstinence × dose: F2,57 = 13.3, p < 0.0001) and grooming episodes (abstinence × dose: F2,57 = 20.7, p < 0.0001), especially after a social contact (abstinence × dose: F2,57 = 35.3, p < 0.0001). In contrast, cocaine abstinent mice behaved similarly as vehicle abstinent mice in this test, and VU0155041 treatment had no significant impact on their social behavior, except for an increase in their number of following episodes (abstinence × treatment: F1,28 = 8.9, p < 0.01).
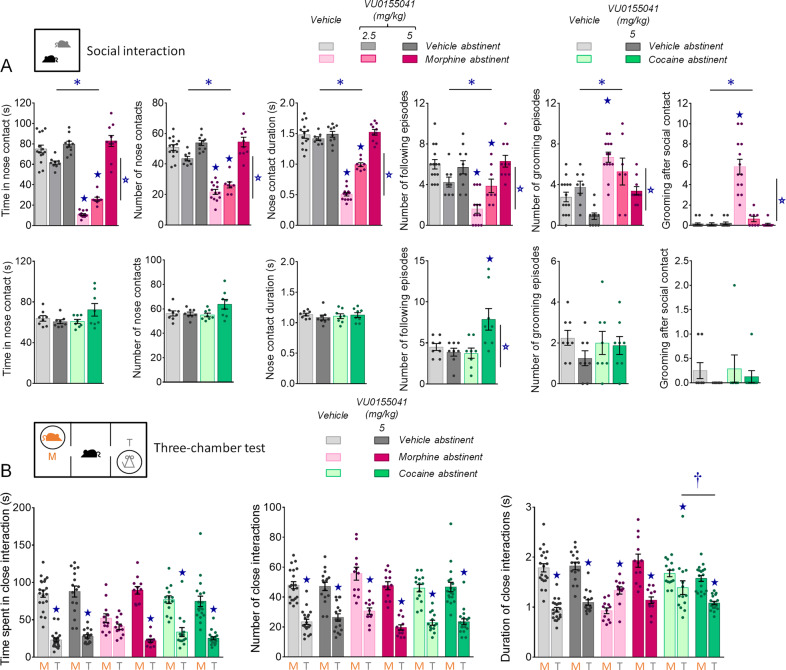
Fig. 2. Effects of chronic VU0155041 administration on social behaviors in morphine and cocaine abstinent mice.
A In the direct social interaction test, facilitation of mGluR4 activity in morphine abstinent mice (top row, n = 8–14 per group) dose-dependently normalized social interaction parameters. In cocaine abstinent mice (bottom row, n = 8–9 per group), VU0155041 at the dose of 5 mg/kg had no significant effect on social behavior, except for an increase in the number of following episodes. B In the three-chamber test, chronic administration of VU0155041 at 5 mg/kg restored social preference in morphine abstinent mice. In cocaine abstinent mice, the difference between close contact duration with the toy and close contact duration with the mouse was significantly increased by mGluR4 PAM treatment. Results are shown as scatter plots and mean ± sem. Asterisk: abstinence effect (morphine/cocaine versus vehicle), open star: treatment effect (VU0155041 versus vehicle), solid star: abstinence × treatment interaction, dagger: stimulus (toy versus mouse) × abstinence × treatment, comparison to close contact duration with the toy in vehicle abstinent mice (two-way ANOVA or three-way ANOVA with stimulus as repeated measure, followed by Newman–Keuls post-hoc test); p < 0.05.
In the three-chamber test (Fig. 2B), morphine abstinent mice failed to show preference for interacting with the mouse rather than the toy, as evidenced by similar time spent interacting with both stimuli, and longer duration of their close interactions with the toy than with the mouse. Chronic administration of VU0155041 at 5 mg/kg restored social preference in morphine abstinent mice, rescuing longer time (stimulus × abstinence × treatment: F2,86 = 7.4, p < 0.01) and duration of close contacts (stimulus × abstinence × treatment: F2,86 = 23.3, p < 0.0001) with the mouse versus the toy. In cocaine abstinent mice, altered social preference was evidenced by a reduction in the difference between close contact duration with the toy versus the mouse. Chronic VU0155041 significantly shortened the duration of interactions with the toy in these mice. Thus, chronic facilitation of mGluR4 activity relieved social deficits in both morphine and cocaine abstinent mice.
Chronic VU0155041 treatment normalized stereotypic behavior and anxiety-like behavior in morphine abstinent mice
We further evaluated the effects of chronic VU0155041 treatment by assessing its influence on other, non-social, long-term behavioral adaptations detected in morphine abstinent mice. We first assessed stereotyped/perseverative behavior in drug abstinent mice. Chronic VU0155041 administration (2.5 and 5 mg/kg) decreased rearing in morphine-exposed mice (abstinence × dose: F2,56 = 7.8, p < 0.01), dose-dependently reduced grooming in both morphine and vehicle groups (abstinence: F1,56 = 242.1, p < 0.0001; dose: F2,56 = 12.3, p < 0.0001), increased burying behavior in vehicle abstinent mice (abstinence × dose: F2,56 = 3.3, p < 0.05) and finally suppressed circling and head shakes in morphine abstinent mice while increasing the occurrence of these behaviors in vehicle controls (Circling - abstinence × dose: F2,56 = 29.1, p < 0.0001; abstinence × dose: F2,56 = 29.1, p < 0.0001; Head shakes - abstinence × dose: F2,56 = 25.9, p < 0.0001) (Fig. 3A). In vehicle and cocaine abstinent mice, chronic VU0155041 treatment (5 mg/kg) had no effect on cocaine abstinence-induced increase in rearing frequency (abstinence: F1,30 = 7.4, p < 0.05) but increased the frequency of circling episodes (treatment: F1,30 = 7.2, p < 0.05).
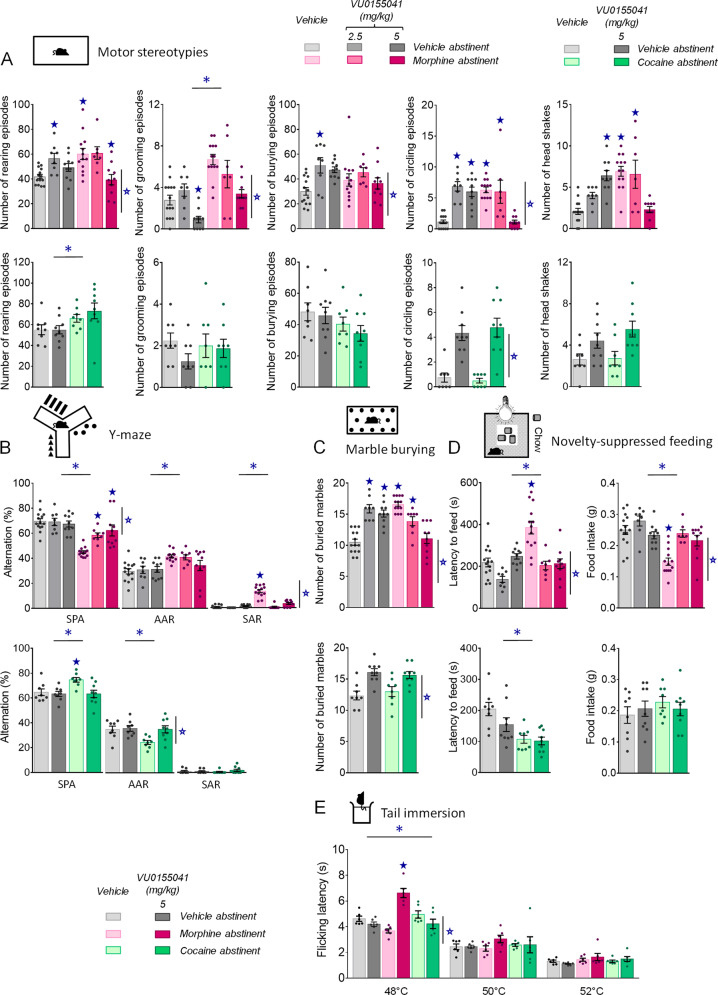
Fig. 3. Chronic facilitation of mGluR4 signaling relieved motor stereotypies, perseverative behavior, elevated anxiety and lowered nociceptive thresholds in morphine abstinent mice.
A Under morphine versus vehicle conditions (upper row, n = 8–14 per group), morphine abstinent mice displayed spontaneous stereotyped grooming, circling and head shakes that were dose-dependently suppressed by chronic VU0155041 administration. Under cocaine versus vehicle conditions (lower row, n = 8–9 per group), this treatment increased the frequency of circling episodes. B In the Y-maze test, chronic VU0155041 administration in morphine abstinent mice (upper panel) normalized their pattern of exploration by suppressing same arm returns. Under cocaine versus vehicle conditions (lower panel), mGluR4 PAM suppressed cocaine abstinence-induced increase in spontaneous alternation by restoring the number of alternate arm returns to vehicle values. C In the marble burying test, chronic VU0155041 dose-dependently normalized the number of marbles buried by morphine abstinent mice while increasing this number in vehicle abstinent controls; similarly this treatment increased marble burying in vehicle and cocaine abstinent mice. D In the novelty-suppressed feeding test, VU0155041 normalized the latency to eat in the arena and food intake of morphine abstinent mice in the home cage. Latency to feed was reduced in cocaine abstinent mice. E In the tail immersion test, at 48 °C, nociceptive thresholds tended to be decreased in morphine abstinent mice treated with vehicle; VU0155041 was analgesic at this temperature and in morphine abstinent mice only. No effect was detected at 50 and 52 °C. Asterisk: abstinence effect (morphine/cocaine versus vehicle); open star: treatment effect (VU0155041 versus vehicle); solid star: abstinence × treatment interaction (two-way ANOVA followed by Newman–Keuls post-hoc test); p < 0.05. AAR: alternate arm returns, SAR: same arm returns, SPA: spontaneous alternation.
When exploring a Y-maze, morphine abstinent mice displayed impaired spontaneous alternation, mostly due to increased number of perseverative same arm returns. Chronic mGluR4 PAM administration dose-dependently restored spontaneous alternation (abstinence × dose: F2,57 = 8.6, p < 0.001) by suppressing same arm returns (abstinence × dose: F2,57 = 39.5, p < 0.0001); no effect of the treatment was detected in vehicle abstinent mice (Fig. 3B). In this test, cocaine-exposed mice showed an increase in spontaneous alternation that was suppressed by chronic VU0155041 (abstinence × dose: F1,30 = 4.3, p < 0.05) by normalizing the number of alternate arm returns (abstinence × dose: F1,30 = 4.2, p = 0.05).
In the marble burying test (Fig. 3C), chronic VU0155041 reduced the number of marbles buried by morphine abstinent mice to the level of non-treated vehicle abstinent mice but increased this number when administered to vehicle abstinent mice (abstinence × dose: F2,57 = 7.8, p < 0.01). Similarly, VU0155041 increased marble burying in cocaine and vehicle abstinent mice (treatment: F1,30 = 86.8, p < 0.0001).
We assessed anxiety-like behavior in abstinent mice using the novelty-suppressed feeding test (Fig. 3D). Chronic mGluR4 PAM administration normalized latency to feed and food intake in morphine abstinent mice since the lower dose (abstinence × dose: F2,57 = 10.5, p < 0.001), with no effect in vehicle or cocaine abstinent mice. Latency to feed was reduced in cocaine abstinent mice (abstinence: F1,30 = 17.5, p = 0.001).
Finally, we evaluated nociceptive thresholds in morphine and cocaine abstinent mice under vehicle or VU0155041 treatment (5 mg/kg). Morphine abstinent mice showed a tendency for decreased nociceptive thresholds at 48 °C, a temperature at which chronic mGluR4 PAM demonstrated analgesic effects in morphine-exposed mice only (abstinence × treatment: F2,30 = 28.5, p < 0.0001) (Fig. 3E).
In conclusion, chronic mGluR4 PAM administration relieved motor stereotypies, perseverative behavior and excessive anxiety-like behavior and produced analgesia in morphine abstinent mice, with little or no effect in vehicle or cocaine abstinent mice.
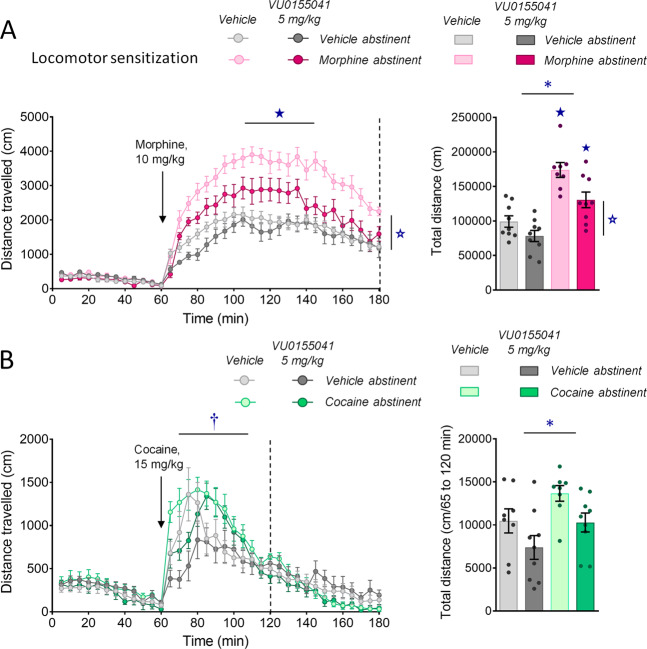
Chronic mGluR4 facilitation inhibited locomotor sensitization to morphine exposure
Then, we assessed morphine and cocaine locomotor sensitization in mice made abstinent from morphine or cocaine and chronically treated with VU0155041 (5 mg/kg) or vehicle. In morphine abstinent mice treated with vehicle, we observed that a single injection of morphine (10 mg/kg) 4 weeks after cessation of chronic morphine exposure induced a greater locomotor response than in vehicle abstinent mice, demonstrating locomotor sensitization. Chronic VU0155041 administration significantly blunted, although not completely suppressed, this conditioned response (Fig. 4A, left panel), as shown by diminished total distance travelled after morphine injection compared to vehicle-treated mice (right panel) (65–180 min; abstinence × treatment: F1,31 = 4.8, p < 0.05; time × abstinence: F23,713 = 4.4, p < 0.0001). In cocaine abstinent mice, an acute injection of cocaine (15 mg/kg) induced detectable locomotor sensitization over the first 60 min of the test; chronic VU0155041 treatment delayed the peak of cocaine-induced locomotor activity but did not significantly decreased cocaine-induced locomotion (65–120 min; time × abstinence: F11,352 = 3.0, p < 0.001; time x treatment: F11,352 = 5.4, p < 0.0001) (Fig. 4B). In conclusion, VU0155041 administration was able to dampen morphine-induced locomotor sensitization in morphine abstinent mice.
Fig. 4. Chronic VU0155041 treatment inhibits morphine-induced locomotor sensitization.
A Morphine abstinent mice (n = 8–9 per group) displayed greater locomotor response to acute morphine (10 mg/kg) than vehicle abstinent mice and this sensitization was significantly decreased upon chronic VU0155041 (5 mg/kg, left panel) as shown by diminished total distance travelled after morphine injection compared to vehicle-treated mice (right panel). B In cocaine abstinent mice (n = 8–9 per group), acute cocaine (15 mg/kg) induced detectable locomotor sensitization during the first 60 min of the test; chronic VU0155041 (5 mg/kg) delayed the peak of locomotor activity (left panel) without decreasing cocaine-induced locomotion (right panel). Results are shown as scatter plots and/or mean ± sem. Asterisk: abstinence effect (morphine/cocaine versus vehicle); open star: treatment effect (VU0155041 versus vehicle); dagger: time × treatment interaction; solid star: abstinence × treatment interaction (two- or three-way ANOVA followed by Newman–Keuls post-hoc test); p < 0.05.
VU0155041 treatment rescued the expression of MSN marker genes prominently in the NAc
To gain insight into the molecular mechanisms involved in beneficial effects of VU0155041 treatment, we evaluated its effects on transcriptional regulations induced by morphine abstinence in the CPu, NAc and CeA, where gene expression was the most contrasted between morphine and cocaine abstinence. This experiment was performed following a session of social interaction (Fig. S3A), to allow correlations between gene expression and social behavior. We focused on the seven MSN marker genes highlighted in Fig. 1 (blue characters in Fig. 5A), in addition to Oxt and Avp coding for the social neuropeptides oxytocin and vasopressin, Fos as a marker of neuronal activity, and Bdnf (coding for brain derived Neurotrophic factor - BDF) and Syp (coding for the vesicular protein synaptophysin) as markers of synaptic plasticity.
We performed hierarchical clustering analysis of qRT-PCR data for each brain region to visualize the influence of VU0155041 treatment on gene expression (Fig. 5A, Table S3). Overall, transcriptional profiles in morphine abstinent mice treated with VU0155041 (morphine-VU0155041) were closer to those of VU0155041-treated vehicle abstinent (vehicle-VU0155041) mice than vehicle-treated morphine abstinent (morphine-vehicle) mice. This was particularly true in the CPu and NAc, where gene expression under morphine-VU0155041 and vehicle-VU0155041 conditions were the most similar. In the CPu, this pattern was mostly driven by an increase of gene expression under VU0155041 treatment (clusters a and b). In the NAc, the expression profile was dominated by a down-regulation under morphine-vehicle conditions (clusters a, b, c) that was rescued by chronic VU0155041. The modulation of social parameters (time in nose contact and following episodes) across treatment conditions followed a similar pattern and clustered with the expression of MSN marker genes (all but Drd2), Fos, Avp and Oxt in the NAc (clusters a and b). In the CeA, rescued expression under VU0155041 treatment was observed in a single cluster (cluster b), also gathering social parameters. Together, these results indicate that VU0155041 treatment rescued morphine-induced down-regulation of MSN marker gene expression prominently in the NAc, an effect that was tightly correlated with the behavioral improvements observed in morphine-VU0155041 mice.
Focusing on MSN marker genes, down-regulated expression under morphine-vehicle conditions was confirmed in the NAc (but not the CPu) for Drd1a and Hpca, while it failed to reach significance for Arpp21, Gmr4, and Pde10a. VU0155041 rescued (Drd1a, Hpca) or increased (Arpp21, Grm4) the expression of these genes. Interestingly, the expression of Oxt and Avp was also decreased in the NAc of morphine-vehicle mice, and chronic mGluR4 PAM treatment similarly rescued their expression. Finally, VU0155041 induced Syp expression in the three regions tested, in both vehicle and morphine abstinent mice.
Intrigued by Syp expression data in the striatum, especially in the NAc, we assessed synaptophysin (SYP) protein levels using western blotting. We dissected out the NAc, as the main substrate of long-term opiate-induced behavioral deficits, its projection sites: ventral pallidum (VP) and ventral tegmental area (VTA), and the neighboring CPu. At behavioral level, we verified again that chronic VU0155041 rescued morphine abstinence-induced deficit in social interaction (Fig. S3B). At molecular level, we failed to detect modifications of SYP protein levels in the NAc, CPu and SNc/VTA (Figs. 5D, S3B, S4 and S5). In the VP, however, we measured a significant decrease in SYP immunoreactivity. Thus beneficial effects of chronic VU0155041 were associated with a decrease in SYP levels in the VP, the main projection site of NAc D2-MSNs (Fig. 5D).
Discussion
Transcriptional regulation of MSN marker genes in the NAc allows best discriminating between morphine and cocaine abstinence
We previously evidenced persistent and contrasted transcriptional regulations in the central EA of 4-week morphine versus cocaine abstinent mice for a set of HTT-related genes known for their enriched expression in MSNs [13, 14]. In the present study, we extended our investigation to a wider collection of MSN marker genes and to Oxt, coding for the social neuropeptide oxytocin. First, we revealed that gene expression patterns in the CeA were more discriminative than those in the BNST to distinguish between morphine and cocaine abstinence. Then, we found enduring transcriptional modifications in two more brain regions where MSNs represent the main cell type, CPu and NAc. Overall, modified expression of MSN maker genes, belonging or not to the HTT-centered network, allowed to discriminate between morphine and cocaine abstinence conditions in the CPu, NAc, and CeA. This result indicates that abstinence from both drugs significantly impacted MSN population, but in different, if not opposite, directions. A majority of MSN marker genes displayed common down-regulated expression under morphine condition; they were indifferently genes with enriched expression in D1- (Drd1a, Pdyn, Tac1) or D2-MSNs (Drd2, Foxp1, Penk, Grm4, Rgs4) or in both MSN populations (Arpp21, Hpca, Nr4a1, Pde10a, Rgs4) [38, 39]. Thus, prolonged abstinence from morphine, and not cocaine, inhibited the expression of a set of genes in D1- and D2-MSNs. These data substantiate the notion of differential neurobiological substrates involved in opiate versus psychostimulant addiction [9, 14, 41, 42].
The seven MSN-enriched genes Arpp21, Drd1a, Drd2, Hpca, Grm4, Nr4a1, and Pde10a [38, 43–45], captured our interest for displaying highly discriminative patterns of expression between morphine and cocaine conditions and sharing consistent down-regulation in the CPu, NAc and CeA. Arpp21 codes for Cyclic AMP regulated phosphoprotein 21 (ARPP-21) so called regulator of calmodulin signaling that modulates striatal calcium and dopamine signaling [46, 47]. When phosphorylated, ARPP-21 amplifies D1 but attenuates D2 dopamine receptor signaling in striatal MSNs [47]. Drd1a and Drd2 genes code for the dopamine receptors D1 and D2, respectively. D1 receptor couples Gs proteins and stimulates the activity of Drd1a expressing MSNs; conversely, D2 receptor is coupled to Gi/o proteins, inhibiting the activity of Drd2 expressing MSNs [48]. Hpca encodes the neural calcium sensor protein hippocalcin, a main contributor of the slow afterhyperpolarization (sAHP) [49, 50]. In morphine abstinent rats, sAHP is attenuated in the NAc MSNs, increasing the excitability of a subset of MSNs [51], possibly D2-MSNs [34]. Grm4, preponderantly expressed in D2-MSNs [52], encodes the presynaptic mGlu4 glutamate receptor, negatively coupled to adenylyl cyclase, which decreases excitability and reduces neurotransmitter release at axon terminals [53]. Nr4a1 is a nuclear receptor/immediate early gene and marker of striosomes, gathering most D1 MSNs in the CPu. Altered Nr4a1 expression compromises transcription of multiple members of the D1 receptor signaling cascade [54]. Pde10a encodes the cyclic nucleotide phosphodiesterase 10A (PDE10A) that regulates cGMP and cAMP signaling cascades and striatal function [55, 56]. Inhibition of PDE10A stimulates the activity of MSNs, with D2-MSNs being more sensitive to this effect [57, 58]. Therefore, down-regulated expression of the seven above genes in morphine abstinent mice potentially favored the activity of D2 over D1 MSNs, tilting the striatofugal balance toward excessive D2 MSN tone. Altered striatofugal balance, notably in the NAc, represents a key cellular mechanism in drug addiction [17, 59, 60]. Recent work has elegantly evidenced, by measuring dopamine-induced cAMP signaling in the NAc, that, along repeated opioid exposure, this balance progressively shifts toward preponderant D2-MSN activity, maintained during abstinence [61], as previously observed in morphine withdrawal [34]. Exacerbated D2-MSN activity could drive the negative affect associated to morphine dependence and withdrawal, increasing the risk of relapse [33–35]. Here, we hypothesized that pharmacological intervention allowing to dampen D2-MSN activity, as by facilitating mGluR4 activity, should relieve long-term behavioral alterations in morphine abstinent mice.
Chronic facilitation of mGluR4 activity relieves behavioral deficits and dampens locomotor sensitization in morphine abstinent mice
Morphine abstinent mice display a dramatic alteration of their social behavior [14, 18, 20, 62–64]. Chronic administration of the mGluR4 PAM VU0155041 dose-dependently normalized social interaction to vehicle-abstinent levels and restored the preference for making more and longer contacts with the mouse versus the toy in the three-chamber test. Chronic VU0155041 similarly restored social behavior in the Oprm1-/- mouse model of autism, together with the associated activation of the reward circuit [21]. Social interactions are pleasurable events in humans and rodents and therefore activate the brain reward circuit [27, 65, 66]. Exacerbated/prolonged D2-MSN tone, notably in the NAc, as observed in morphine abstinent mice [34, 61], was shown to induce aversion [67–69]. Hence, excessive D2-MSN activity in morphine abstinent mice may compromise social interactions by hampering their rewarding properties, and chronic VU0155041 treatment would restore social behavior by normalizing this activity. Prior studies have evidenced relieving effects of the serotonin reuptake inhibitor fluoxetine [18] and oxytocin analog carbetocin [20] on social deficit in morphine abstinent mice. Remarkably, oxytocin promotes social reward by triggering long-term depression at NAc D1 and D2-MSN neurons, an effect that requires serotonin release [70]. Shared beneficial effects of VU0155041, fluoxetine and carbetocin on social behavior in morphine abstinent mice may thus involve common inhibition of D2-MSNs.
Besides social impairments, morphine abstinent mice display motor stereotypies, stereotypic marble burying and perseverative behavior in the Y-maze, as previously reported [14]. Here we show that chronic VU0155041 administration, at the highest dose, suppressed these behaviors. Similarly, VU0155041 alleviated stereotypies in Oprm1-/- [21] and relieved stereotypies in mice lacking the Elfn2 gene (Elfn2-/-), which also display autistic-like symptoms [71]. Interestingly, stereotypic behavior was shown to involve NAc D2-MSNs in another mouse model of autism, Neuroligin-3 knockout mice [72]. Hence, VU0155041 may reduce stereotypies in morphine abstinent mice by repressing D2-MSN activity.
We next verified that conflict anxiety-like behavior was exacerbated in morphine abstinent mice, as evidenced by increased latency to eat in the novelty-suppressed feeding test [14]. From the lowest dose we tested, chronic VU0155041 normalized anxiety-like behavior, as previously observed in Oprm1-/- and Elfn2-/- mice [21, 71]. This result is consistent with previous reports of facilitating mGluR4 activity having anxiolytic effects in mice [73–75] and rats [76]. In addition, VU0155041 treatment produced analgesia only in morphine abstinent mice whose tendency for lowered nociceptive thresholds is reminiscent of withdrawal symptoms [77]. Stimulation of mGluR4 produces analgesia under chronic pain or inflammatory conditions only [78, 79]. Interestingly, opioid-induced hyperalgesia was shown to involve an activation of the glutamatergic system [80] that may represent the neurobiological substrate for analgesic effects of VU0155041 in morphine abstinent mice. Finally, morphine-exposed mice displayed significant locomotor sensitization to morphine after prolonged abstinence, consistent with previous findings [14, 81], which was dampened by VU0155041. Morphine-induced sensitization involves D1 receptor activation in striatal D1-MSNs [81–83], whose activity may be impacted by mGluR4 either directly in D1-MSNs [84, 85] or through corticostriatal terminals forming synapses at D1-MSNs [86]. However, an effect of VU0155041 at D2-MSNs cannot be ruled out, as they also contribute to drug-induced sensitization [87, 88].
Collectively, blunted social behavior, stereotyped behavior, elevated anxiety-like behavior and low nociceptive thresholds in morphine abstinent mice fit with clinical reports of social withdrawal, impaired cognitive flexibility and high comorbidity with anxiety disorder and depression in patients with a history of chronic opioid exposure [89–96]. Importantly, such negative affect, combined with conditioned effects of opioids, are considered as the two major causes of relapse in OUD [97, 98]; they were both significantly alleviated in morphine abstinent mice under VU0155041 treatment. However, this was observed at distinct, single time points for each test, social interaction being the exception (D8 and D15, Fig. S3A and B). The kinetics of chronic VU0155041 effects over days of administration and after cessation of treatment will deserve further investigation.
In contrast with morphine, cocaine abstinence had little impact on sociability, as previously shown [14], and VU0155041 treatment either preserved or even improved this behavior. Chronic VU0155041, however, induced motor stereotypies (circling, head shakes, marble burying) in these mice as it did in vehicle abstinent mice, possibly by tilting the D1/D2-MSN balance toward D1-MSN activity, which triggers stereotypic behaviors [99]. Contrary to morphine abstinence, cocaine abstinence was associated with decreased anxiety in the novelty-suppressed feeding test [14]; chronic facilitation of mGlu4 activity had no influence on this response. Finally, VU0155041 failed to significantly reduce cocaine-induced locomotor sensitization; however, this failure may be due to the weak amplitude of cocaine- compared to morphine-induced response. These results further illustrate the neurobiological differences between opiate and cocaine abstinence.
VU0155041-induced transcriptional changes in the NAc are coherent with a repression of D2-MSN activity and restauration of social reward
We proposed above that mGluR4 PAM treatment relieved social behavior in morphine abstinent mice by repressing D2-MSN and boosting D1-MSN activity in the NAc. Our qPCR results in morphine abstinent mice treated or not with VU0155041 were consistent with this hypothesis. Indeed, VU0155041 restored or increased expression of Arpp21, Drd1a, Hpca and Grm4 in the NAc, where their respective encoded proteins can either dampen D2-MSN activity or facilitate D1-MSN activity. Interestingly, these transcriptional regulations were tightly correlated with pro-social behaviors. NAc Bdnf expression tended to be decreased in our experiments, as previously shown under prolonged morphine abstinence [100], while VU0155041 tended to increase this expression. Reduced BDNF signaling in the NAc would decrease GABAergic activity specifically in D1-MSNs [101], favoring excessive D2 tone. Furthermore, Bdnf and Syp displayed very similar expression patterns in the NAc and, in a lesser extent, in the CPu, consistent with the demonstrated ability of the former in triggering expression of the second [102, 103]. Globally, VU0155041 treatment induced an increase in Syp mRNA levels, most notably in the striatum. As regards the NAc, this phenomenon appears to be a compensatory mechanism, as SYP protein levels were decreased in the VP, the main projection area of NAc D2-MSNs. The mGlu4 receptor is located presynaptically at axon terminals where its activation represses neurotransmitter release. Thus, reduced expression of SYP in the VP could have resulted from the inhibitory action of mGluR4 PAM treatment on GABA release from indirect MSNs in this region. These data point to repression of D2-MSN activity in the NAc as a key substrate supporting social effects of mGluR4 facilitation in morphine abstinent mice.
The NAc is a hub region for the integration of reward processes [104–106], a key brain substrate in drug addiction [107, 108] and finally a critical substrate for social behavior. Consistent with their pleasurable properties, social stimuli activate the NAc in humans [109, 110] and this activation is blunted in patients with ASD [27], depression [111], schizophrenia [112] or OUD [113] who display impaired social behavior. In rodents, activating neuromodulator or neuropeptide receptors in the NAc stimulates or drives social behavior [70, 114–116] and models of ASD, schizophrenia or depression show abnormal volume, connectivity and/or function of the NAc [26, 117–121]. In our study, Fos expression induced in the NAc by social exposure was dampened in morphine abstinent mice, suggesting blunted social reward, and rescued upon VU0155041 treatment, as we previously observed in Oprm1-/- mice [21]. Moreover, NAc expression of Oxt and Avp transcripts, plausibly transported from the hypothalamus [122], was restored by mGluR4 PAM treatment. The former gene codes for oxytocin, critically involved in mediating social reward in the NAc [70, 123]; the role of vasopressin (encoded by Avp) in the NAc remains poorly understood, but vasopressin dysregulation has been highlighted in autism [124]. Together, these results argue for a restoration of social reward under VU0155041 treatment, likely through a resetting the D1/D2-MSN balance in the NAc. Further investigations will be required, however, to confirm this hypothesis and also to explore the role of NAc and extra-NAc mGluR4 populations in regulating social behavior. Of note, Grm4 expression was not down-regulated upon VU0155041 administration, suggesting limited tolerance to pharmacological treatment.
One ought to point out some limitations to our study. The low sample number of mice allocated to each experimental condition in transcriptome analyses resulted in lower statistical power that may lie behind the lack of significance of some regulations of gene expression, despite consistent expression patterns between independent experiments (Figs. 1 and 5). Moreover, this study was performed in male mice only, to allow direct comparisons with previous reports [13, 125] and one should thus be cautious regarding transposition to females. In Oprm1-/- mice, however, VU0155041 was as efficient to relieve deficient social behavior, stereotypies and anxiety in females as in males [21], which bodes well for beneficial effects in female morphine abstinent mice and for potential clinical applications.
Conclusion
The present study reports that facilitating glutamate mGluR4 activity can relieve the deleterious long-term behavioral and molecular consequences of opiate, but not cocaine, exposure. These results highlight the neurobiological differences between opiate and psychostimulant abstinence, arguing against a unitary view of drug addiction [9, 41]. They also substantiate the view of negative affect in opiate abstinence as resulting from excessive activity of striatal D2-MSNs, more specifically in the NAc [33–35, 61]. In this context, pharmacological compounds repressing striatopallidal activity appear as promising candidates for the treatment of OUD, possibly by restoring “liking” [126] for non-drug, notably social, stimuli. More broadly, opiate addiction sharing striking phenotypic and neurobiological features with other diseases considered as “reward deficiency syndromes”, such as autism or depression [27, 120, 127, 128], our work opens promising avenues toward the development of common therapeutic strategies for these pathologies.
Funding and disclosure
This work was supported by the Institut National de Recherche pour l’Agriculture, l’alimentation et l’Environnement (INRAE), Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (Inserm), and Université de Tours. We thank Région Centre (ARD2020 Biomédicament – GPCRAb) and LabEx MabImprove for financial support. JLM acknowledges postdoctoral fellowship from the Fondation Université de Strasbourg, generously granted by Pierre Fabre Laboratories. LPP acknowledges postdoctoral support from the Marie-Curie/AgreenSkills Program. The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary information
Acknowledgements
We are grateful to Dr B.L. Kieffer for fruitful discussions and support at an early stage of this project. We thank G. Duval and D. Memedov for animal care and technical assistance (Institut de Génétique et de Biologie Moléculaire et Cellulaire). We thank the Experimental Unit PAO-1297 (EU0028, Animal Physiology Experimental Facility, DOI: 10.15454/1.5573896321728955E12) from the INRAE-Val de Loire Centre for animal breeding and care.
Author contributions
JAJB, JG, LPP and JLM designed the experiments. JAJB, LPP, JG, TL, AL and JLM performed behavioral experiments. JAJB, YC and LPP performed qRT-PCR experiments. YC performed western blot experiments. JAJB, LPP, JG, YC and JLM analyzed the data. JAJB and JLM wrote the paper. All authors discussed the results and commented on the paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jerome A. J. Becker, Email: jerome.becker@inserm.fr
Julie Le Merrer, Email: julie.le-merrer@inserm.fr.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00927-x).
References
- 1.APA. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013.
- 2.Goodwin RD, Stayner DA, Chinman MJ, Wu P, Tebes JK, Davidson L. The relationship between anxiety and substance use disorders among individuals with severe affective disorders. Compr Psychiatry. 2002;43:245–52.. doi: 10.1053/comp.2002.33500. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–62. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–68.. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–31.. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 6.Becker JA, Le Merrer J. Long-term transcriptional consequences of drug exposure as cues to understand vulnerability to relapse: advances and perspectives. J Stud Alcohol Drugs. 2016;77:692–5. doi: 10.15288/jsad.2016.77.692. [DOI] [PubMed] [Google Scholar]
- 7.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–84.. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:545–51.. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–20.. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Pirro S, Galati G, Pizzamiglio L, Badiani A. The Affective and Neural Correlates of Heroin versus Cocaine Use in Addiction Are Influenced by Environmental Setting But in Opposite Directions. J Neurosci. 2018;38:5182–95. doi: 10.1523/JNEUROSCI.0019-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albertson DN, Schmidt CJ, Kapatos G, Bannon MJ. Distinctive profiles of gene expression in the human nucleus accumbens associated with cocaine and heroin abuse. Neuropsychopharmacology. 2006;31:2304–12.. doi: 10.1038/sj.npp.1301089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, et al. Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol. 2012;17:1–12. doi: 10.1111/j.1369-1600.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 14.Becker JAJ, Kieffer BL, Le, Merrer J. Differential behavioral and molecular alterations upon protracted abstinence from cocaine versus morphine, nicotine, THC and alcohol. Addict Biol. 2017;22:1205–17.. doi: 10.1111/adb.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–25.. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Dobbs LK, Lemos JC, Alvarez VA. Restructuring of basal ganglia circuitry and associated behaviors triggered by low striatal D2 receptor expression: implications for substance use disorders. Genes Brain Behav. 2017;16:56–70. doi: 10.1111/gbb.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, et al. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–44.. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia W, Liu R, Shi J, Wu B, Dang W, Du Y, et al. Differential Regulation of MAPK Phosphorylation in the Dorsal Hippocampus in Response to Prolonged Morphine Withdrawal-Induced Depressive-Like Symptoms in Mice. PLoS ONE. 2013;8:e66111. doi: 10.1371/journal.pone.0066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, et al. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology. 2014;39:855–65.. doi: 10.1038/npp.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker JA, Clesse D, Spiegelhalter C, Schwab Y, Le Merrer J, Kieffer BL. Autistic-like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacology. 2014;39:2049–60.. doi: 10.1038/npp.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oddi D, Crusio WE, D’Amato FR, Pietropaolo S. Monogenic mouse models of social dysfunction: implications for autism. Behav Brain Res. 2013;251:75–84. doi: 10.1016/j.bbr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homberg JR, Kyzar EJ, Nguyen M, Norton WH, Pittman J, Poudel MK, et al. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci Biobehav Rev. 2016;65:292–312. doi: 10.1016/j.neubiorev.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Rothwell PE. Autism spectrum disorders and drug addiction: common pathways, common molecules, distinct disorders. Front Neurosci. 2016;10:20. doi: 10.3389/fnins.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuccillo MV. Striatal Circuits as a Common Node for Autism Pathophysiology. Front Neurosci. 2016;10:27. doi: 10.3389/fnins.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellissier LP, Gandia J, Laboute T, Becker JAJ, Le, Merrer J. mu opioid receptor, social behaviour and autism spectrum disorder: reward matters. Br J Pharmacol. 2018;175:2750–69.. doi: 10.1111/bph.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkisyan D, Hussain MZ, Watanabe H, Kononenko O, Bazov I, Zhou X, et al. Downregulation of the endogenous opioid peptides in the dorsal striatum of human alcoholics. Front Cell Neurosci. 2015;9:187. doi: 10.3389/fncel.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackwood CA, Hoerle R, Leary M, Schroeder J, Job MO, McCoy MT, et al. Molecular Adaptations in the Rat Dorsal Striatum and Hippocampus Following Abstinence-Induced Incubation of Drug Seeking After Escalated Oxycodone Self-Administration. Mol Neurobiol. 2019;56:3603–15. doi: 10.1007/s12035-018-1318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pujol CN, Pellissier LP, Clément C, Becker JAJ, Le Merrer J. Back-translating behavioral intervention for autism spectrum disorders to mice with blunted reward restores social abilities. Transl Psychiatry. 2018;8:197. doi: 10.1038/s41398-018-0247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amalric M, Lopez S, Goudet C, Fisone G, Battaglia G, Nicoletti F, et al. Group III and subtype 4 metabotropic glutamate receptor agonists: discovery and pathophysiological applications in Parkinson’s disease. Neuropharmacology. 2013;66:53–64. doi: 10.1016/j.neuropharm.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, et al. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23:7218–26.. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y, Wienecke CF, Nachtrab G, Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530:219–22.. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enoksson T, Bertran-Gonzalez J, Christie MJ. Nucleus accumbens D2- and D1-receptor expressing medium spiny neurons are selectively activated by morphine withdrawal and acute morphine, respectively. Neuropharmacology. 2012;62:2463–71.. doi: 10.1016/j.neuropharm.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 35.Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, et al. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci USA. 2016;113:757–62.. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pujol CN, Pellissier LP, Clement C, Becker JAJ, Le Merrer J. Back-translating behavioral intervention for autism spectrum disorders to mice with blunted reward restores social abilities. Transl Psychiatry. 2018;8:197–212.. doi: 10.1038/s41398-018-0247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laboute T, Gandia J, Pellissier LP, Corde Y, Rebeillard F, Gallo M, et al. The orphan receptor GPR88 blunts the signaling of opioid receptors and multiple striatal GPCRs. eLife. 2020;9:e50519. doi: 10.7554/eLife.50519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–62.. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho H, Both M, Siniard A, Sharma S, Notwell JH, Wallace M, et al. A guide to single-cell transcriptomics in adult rodent brain: the medium spiny neuron transcriptome revisited. Front Cell Neurosci. 2018;12:159. doi: 10.3389/fncel.2018.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puighermanal E, Castell L, Esteve-Codina A, Melser S, Kaganovsky K, Zussy C, et al. Functional and molecular heterogeneity of D2R neurons along dorsal ventral axis in the striatum. Nat Commun. 2020;11:1957. [DOI] [PMC free article] [PubMed]
- 41.Vassilev P, Avvisati R, Koya E, Badiani A. Distinct Populations of Neurons Activated by Heroin and Cocaine in the Striatum as Assessed by catFISH. eNeuro. 2020;7:ENEURO.0394-19.2019. doi: 10.1523/ENEURO.0394-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozburn AR, Janowsky AJ, Crabbe JC. Commonalities and Distinctions Among Mechanisms of Addiction to Alcohol and Other Drugs. Alcohol Clin Exp Res. 2015;39:1863–77.. doi: 10.1111/acer.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL, et al. Immunohistochemical localization of PDE10A in the rat brain. Brain Res. 2003;985:113–26.. doi: 10.1016/s0006-8993(03)02754-9. [DOI] [PubMed] [Google Scholar]
- 44.Paterlini M, Revilla V, Grant AL, Wisden W. Expression of the neuronal calcium sensor protein family in the rat brain. Neuroscience. 2000;99:205–16.. doi: 10.1016/s0306-4522(00)00201-3. [DOI] [PubMed] [Google Scholar]
- 45.Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–18.. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nair AG, Bhalla US, Hellgren Kotaleski J. Role of DARPP-32 and ARPP-21 in the Emergence of Temporal Constraints on Striatal Calcium and Dopamine Integration. PLoS Comput Biol. 2016;12:e1005080. doi: 10.1371/journal.pcbi.1005080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rakhilin SV, Olson PA, Nishi A, Starkova NN, Fienberg AA, Nairn AC, et al. A network of control mediated by regulator of calcium/calmodulin-dependent signaling. Science. 2004;306:698–701. doi: 10.1126/science.1099961. [DOI] [PubMed] [Google Scholar]
- 48.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–66.. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. Hippocalcin gates the calcium activation of the slow afterhyperpolarization in hippocampal pyramidal cells. Neuron. 2007;53:487–93.. doi: 10.1016/j.neuron.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim KS, Kobayashi M, Takamatsu K, Tzingounis AV. Hippocalcin and KCNQ channels contribute to the kinetics of the slow afterhyperpolarization. Biophysical J. 2012;103:2446–54.. doi: 10.1016/j.bpj.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Shi M, Ling H, Wei C, Liu Y, Liu Z, et al. Effects of morphine withdrawal on the membrane properties of medium spiny neurons in the nucleus accumbens shell. Brain Res Bull. 2013;90:92–9. doi: 10.1016/j.brainresbull.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, et al. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999;407:33–46. [PubMed] [Google Scholar]
- 53.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cirnaru MD, Melis C, Fanutza T, Naphade S, Tshilenge KT, Muntean BS, et al. Nuclear Receptor Nr4a1 Regulates Striatal Striosome Development and Dopamine D1 Receptor Signaling. eNeuro. 2019;6:ENEURO.0305-19.2019. doi: 10.1523/ENEURO.0305-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siuciak JA, McCarthy SA, Chapin DS, Fujiwara RA, James LC, Williams RD, et al. Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: evidence for altered striatal function. Neuropharmacology. 2006;51:374–85.. doi: 10.1016/j.neuropharm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Nishi A, Kuroiwa M, Miller DB, O’Callaghan JP, Bateup HS, Shuto T, et al. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–71.. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki K, Kimura H. TAK-063, a novel PDE10A inhibitor with balanced activation of direct and indirect pathways, provides a unique opportunity for the treatment of schizophrenia. CNS Neurosci Ther. 2018;24:604–14.. doi: 10.1111/cns.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Threlfell S, Sammut S, Menniti FS, Schmidt CJ, West AR. Inhibition of Phosphodiesterase 10A Increases the Responsiveness of Striatal Projection Neurons to Cortical Stimulation. J Pharmacol Exp Ther. 2009;328:785–95.. doi: 10.1124/jpet.108.146332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, et al. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–95.. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kruyer A, Chioma VC, Kalivas PW. The Opioid-Addicted Tetrapartite Synapse. Biol Psychiatry. 2020;87:34–43. doi: 10.1016/j.biopsych.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muntean BS, Dao MT, Martemyanov KA. Allostatic Changes in the cAMP System Drive Opioid-Induced Adaptation in Striatal Dopamine Signaling. Cell Rep. 2019;29:946–60 e2. doi: 10.1016/j.celrep.2019.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lutz PE, Ayranci G, Chu-Sin-Chung P, Matifas A, Koebel P, Filliol D, et al. Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive-like behaviors during heroin abstinence. Neuropsychopharmacology. 2014;39:2694–705.. doi: 10.1038/npp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lalanne L, Ayranci G, Filliol D, Gaveriaux-Ruff C, Befort K, Kieffer BL, et al. Kappa opioid receptor antagonism and chronic antidepressant treatment have beneficial activities on social interactions and grooming deficits during heroin abstinence. Addict Biol. 2017;22:1010–21.. doi: 10.1111/adb.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma H, Wang N, Wang X, Jia M, Li Y, Cui C. Wnt7a in Mouse Insular Cortex Contributes to Anxiety-like Behavior During Protracted Abstinence from Morphine. Neuroscience. 2018;394:164–76.. doi: 10.1016/j.neuroscience.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 65.Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn Sci. 2007;11:419–27.. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Trezza V, Baarendse PJ, Vanderschuren LJ. On the interaction between drugs of abuse and adolescent social behavior. Psychopharmacol (Berl) 2014;231:1715–29.. doi: 10.1007/s00213-014-3471-z. [DOI] [PubMed] [Google Scholar]
- 67.Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, et al. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry. 2019;25:3241–55. doi: 10.1038/s41380-019-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–84.. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunn HA, Zucca S, Dao M, Orlandi C, Martemyanov KA. ELFN2 is a postsynaptic cell adhesion molecule with essential roles in controlling group III mGluRs in the brain and neuropsychiatric behavior. Mol Psychiatry. 2019;24:1902–19.. doi: 10.1038/s41380-019-0512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK, et al. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duvoisin RM, Villasana L, Davis MJ, Winder DG, Raber J. Opposing roles of mGluR8 in measures of anxiety involving non-social and social challenges. Behav Brain Res. 2011;221:50–4. doi: 10.1016/j.bbr.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalinichev M, Le Poul E, Bolea C, Girard F, Campo B, Fonsi M, et al. Characterization of the novel positive allosteric modulator of the metabotropic glutamate receptor 4 ADX88178 in rodent models of neuropsychiatric disorders. J Pharmacol Exp Ther. 2014;350:495–505. doi: 10.1124/jpet.114.214437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slawinska A, Wieronska JM, Stachowicz K, Palucha-Poniewiera A, Uberti MA, Bacolod MA, et al. Anxiolytic- but not antidepressant-like activity of Lu AF21934, a novel, selective positive allosteric modulator of the mGlu(4) receptor. Neuropharmacology. 2013;66:225–35.. doi: 10.1016/j.neuropharm.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 76.Stachowicz K, Klak K, Klodzinska A, Chojnacka-Wojcik E, Pilc A. Anxiolytic-like effects of PHCCC, an allosteric modulator of mGlu4 receptors, in rats. Eur J Pharm. 2004;498:153–6. doi: 10.1016/j.ejphar.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Tilson HA, Rech RH, Stolman S. Hyperalgesia during withdrawal as a means of measuring the degree of dependence in morphine dependent rats. Psychopharmacologia. 1973;28:287–300. doi: 10.1007/BF00429309. [DOI] [PubMed] [Google Scholar]
- 78.Vilar B, Busserolles J, Ling B, Laffray S, Ulmann L, Malhaire F, et al. Alleviating pain hypersensitivity through activation of type 4 metabotropic glutamate receptor. J Neurosci. 2013;33:18951–65.. doi: 10.1523/JNEUROSCI.1221-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zussy C, Gomez-Santacana X, Rovira X, De Bundel D, Ferrazzo S, Bosch D, et al. Dynamic modulation of inflammatory pain-related affective and sensory symptoms by optical control of amygdala metabotropic glutamate receptor 4. Mol Psychiatry. 2018;23:509–20.. doi: 10.1038/mp.2016.223. [DOI] [PubMed] [Google Scholar]
- 80.Laulin JP, Larcher A, Celerier E, Le Moal M, Simonnet G. Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. Eur J Neurosci. 1998;10:782–5. doi: 10.1046/j.1460-9568.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- 81.Borgkvist A, Valjent E, Santini E, Herve D, Girault JA, Fisone G. Delayed, context- and dopamine D1 receptor-dependent activation of ERK in morphine-sensitized mice. Neuropharmacology. 2008;55:230–7. doi: 10.1016/j.neuropharm.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 82.Tao YM, Yu C, Wang WS, Hou YY, Xu XJ, Chi ZQ, et al. Heteromers of mu opioid and dopamine D1 receptors modulate opioid-induced locomotor sensitization in a dopamine-independent manner. Br J Pharmacol. 2017;174:2842–61.. doi: 10.1111/bph.13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent beta-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology. 2011;36:551–8. doi: 10.1038/npp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuramoto E, Fujiyama F, Unzai T, Nakamura K, Hioki H, Furuta T, et al. Metabotropic glutamate receptor 4-immunopositive terminals of medium-sized spiny neurons selectively form synapses with cholinergic interneurons in the rat neostriatum. J Comp Neurol. 2007;500:908–22.. doi: 10.1002/cne.21216. [DOI] [PubMed] [Google Scholar]
- 85.Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–20.. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- 86.Iskhakova L, Smith Y. mGluR4-containing corticostriatal terminals: synaptic interactions with direct and indirect pathway neurons in mice. Brain Struct Funct. 2016;221:4589–99.. doi: 10.1007/s00429-016-1187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Porter-Stransky KA, Petko AK, Karne SL, Liles LC, Urs NM, Caron MG, et al. Loss of beta-arrestin2 in D2 cells alters neuronal excitability in the nucleus accumbens and behavioral responses to psychostimulants and opioids. Addict Biol. 2020;25:e12823. doi: 10.1111/adb.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song SS, Kang BJ, Wen L, Lee HJ, Sim HR, Kim TH, et al. Optogenetics reveals a role for accumbal medium spiny neurons expressing dopamine D2 receptors in cocaine-induced behavioral sensitization. Front Behav Neurosci. 2014;8:336. doi: 10.3389/fnbeh.2014.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grella CE, Karno MP, Warda US, Niv N, Moore AA. Gender and comorbidity among individuals with opioid use disorders in the NESARC study. Addictive Behav. 2009;34:498–504. doi: 10.1016/j.addbeh.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson B, Ulberg S, Shivale S, Donaldson J, Milczarski B, Faraone SV. Fibromyalgiaautism, and opioid addiction as natural and induced disorders of the endogenous opioid hormonal system. Disco Med. 2014;18:209–20. [PubMed] [Google Scholar]
- 91.McDonald S, Darke S, Kaye S, Torok M. Deficits in social perception in opioid maintenance patients, abstinent opioid users and non-opioid users. Addiction. 2013;108:566–74.. doi: 10.1111/add.12040. [DOI] [PubMed] [Google Scholar]
- 92.Fatseas M, Denis C, Lavie E, Auriacombe M. Relationship between anxiety disorders and opiate dependence-a systematic review of the literature: implications for diagnosis and treatment. J Subst Abus Treat. 2010;38:220–30.. doi: 10.1016/j.jsat.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 93.von Hippel C, Henry JD, Terrett G, Mercuri K, McAlear K, Rendell PG. Stereotype threat and social function in opioid substitution therapy patients. Br J Clin Psychol. 2017;56:160–71.. doi: 10.1111/bjc.12128. [DOI] [PubMed] [Google Scholar]
- 94.Schiltenwolf M, Akbar M, Hug A, Pfuller U, Gantz S, Neubauer E, et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician. 2014;17:9–20. [PubMed] [Google Scholar]
- 95.Fischer B, Murphy Y, Kurdyak P, Goldner EM. Depression - A major but neglected consequence contributing to the health toll from prescription opioids? Psychiatry Res. 2016;243:331–4. doi: 10.1016/j.psychres.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 96.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–87.. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 97.Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, et al. Opioid use disorder. Nat Rev Dis Prim. 2020;6:3. doi: 10.1038/s41572-019-0137-5. [DOI] [PubMed] [Google Scholar]
- 98.Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44–53. doi: 10.1016/j.biopsych.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 99.Bouchekioua Y, Tsutsui-Kimura I, Sano H, Koizumi M, Tanaka KF, Yoshida K, et al. Striatonigral direct pathway activation is sufficient to induce repetitive behaviors. Neurosci Res. 2018;132:53–57. doi: 10.1016/j.neures.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 100.Khalil-Khalili M, Rashidy-Pour A, Bandegi AR, Yousefi B, Jorjani H, Miladi-Gorji H. Effects of BDNF receptor antagonist on the severity of physical and psychological dependence, morphine-induced locomotor sensitization and the ventral tegmental area-nucleus accumbens BDNF levels in morphine- dependent and withdrawn rats. Neurosci Lett. 2018;668:7–12. doi: 10.1016/j.neulet.2017.12.061. [DOI] [PubMed] [Google Scholar]
- 101.Koo JW, Lobo MK, Chaudhury D, Labonte B, Friedman A, Heller E, et al. Loss of BDNF signaling in D1R-expressing NAc neurons enhances morphine reward by reducing GABA inhibition. Neuropsychopharmacology. 2014;39:2646–53.. doi: 10.1038/npp.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coffey ET, Akerman KE, Courtney MJ. Brain derived neurotrophic factor induces a rapid upregulation of synaptophysin and tau proteins via the neurotrophin receptor TrkB in rat cerebellar granule cells. Neurosci Lett. 1997;227:177–80.. doi: 10.1016/s0304-3940(97)00335-2. [DOI] [PubMed] [Google Scholar]
- 103.Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem. 2001;276:37585–93.. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- 104.Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 105.Mannella F, Gurney K, Baldassarre G. The nucleus accumbens as a nexus between values and goals in goal-directed behavior: a review and a new hypothesis. Front Behav Neurosci. 2013;7:135. doi: 10.3389/fnbeh.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- 107.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58.. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 108.Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–76.. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ, Troiani V, et al. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia. 2013;51:2062–9. doi: 10.1016/j.neuropsychologia.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmidt SNL, Fenske SC, Kirsch P, Mier D. Nucleus accumbens activation is linked to salience in social decision making. Eur Arch Psychiatry Clin Neurosci. 2018;269:701–12. doi: 10.1007/s00406-018-0947-6. [DOI] [PubMed] [Google Scholar]
- 111.Lee J, Jimenez AM, Reavis EA, Horan WP, Wynn JK, Green MF. Reduced Neural Sensitivity to Social vs Nonsocial Reward in Schizophrenia. Schizophrenia Bull. 2019;45:620–28.. doi: 10.1093/schbul/sby109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharma A, Satterthwaite TD, Vandekar L, Katchmar N, Daldal A, Ruparel K, et al. Divergent relationship of depression severity to social reward responses among patients with bipolar versus unipolar depression. Psychiatry Res Neuroimaging. 2016;254:18–25. doi: 10.1016/j.pscychresns.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Strathearn L, Mertens CE, Mayes L, Rutherford H, Rajhans P, Xu G, et al. Pathways Relating the Neurobiology of Attachment to Drug Addiction. Front Psychiatry. 2019;10:737. doi: 10.3389/fpsyt.2019.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manduca A, Servadio M, Damsteegt R, Campolongo P, Vanderschuren LJ, Trezza V. Dopaminergic Neurotransmission in the Nucleus Accumbens Modulates Social Play Behavior in Rats. Neuropsychopharmacology. 2016;41:2215–23.. doi: 10.1038/npp.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ. Nucleus accumbens mu-opioid receptors mediate social reward. J Neurosci. 2011;31:6362–70.. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci. 2015;10:561–70.. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Francis TC, Lobo MK. Emerging Role for Nucleus Accumbens Medium Spiny Neuron Subtypes in Depression. Biol Psychiatry. 2017;81:645–53.. doi: 10.1016/j.biopsych.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Platt RJ, Zhou Y, Slaymaker IM, Shetty AS, Weisbach NR, Kim JA, et al. Chd8 Mutation Leads to Autistic-like Behaviors and Impaired Striatal Circuits. Cell Rep. 2017;19:335–50.. doi: 10.1016/j.celrep.2017.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Asif-Malik A, Dautan D, Young AMJ, Gerdjikov TV. Altered cortico-striatal crosstalk underlies object recognition memory deficits in the sub-chronic phencyclidine model of schizophrenia. Brain Struct Funct. 2017;222:3179–90.. doi: 10.1007/s00429-017-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry. 2015;77:212–22.. doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lipina TV, Fletcher PJ, Lee FH, Wong AH, Roder JC. Disrupted-in-schizophrenia-1 Gln31Leu polymorphism results in social anhedonia associated with monoaminergic imbalance and reduction of CREB and beta-arrestin-1,2 in the nucleus accumbens in a mouse model of depression. Neuropsychopharmacology. 2013;38:423–36.. doi: 10.1038/npp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bigler RL, Kamande JW, Dumitru R, Niedringhaus M, Taylor AM. Messenger RNAs localized to distal projections of human stem cell derived neurons. Sci Rep. 2017;7:611. doi: 10.1038/s41598-017-00676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–8. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oztan O, Garner JP, Constantino JN, Parker KJ. Neonatal CSF vasopressin concentration predicts later medical record diagnoses of autism spectrum disorder. Proc Natl Acad Sci USA. 2020;117:10609–13.. doi: 10.1073/pnas.1919050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Becker JA, Kieffer BL, Le, Merrer J. Differential behavioral and molecular alterations upon protracted abstinence from cocaine versus morphine, nicotine, THC and alcohol. Addict Biol. 2017;22:1205–17.. doi: 10.1111/adb.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychologist. 2016;71:670–79.. doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koob GF, Le, Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 128.Ortega LA, Solano JL, Torres C, Papini MR. Reward loss and addiction: Opportunities for cross-pollination. Pharm Biochem Behav. 2017;154:39–52. doi: 10.1016/j.pbb.2017.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.