Abstract
PURPOSE
This study aims to identify chest computed tomography (CT) characteristics of coronavirus disease 2019 (COVID-19), investigate the association between CT findings and laboratory or demographic findings, and compare the accuracy of chest CT with reverse transcription-polymerase chain reaction (RT-PCR).
METHODS
Overall, 120 of 159 consecutive cases isolated due to suspected COVID-19 at our hospital between 17 and 25 March 2020 were included in this retrospective study. All patients underwent both chest CT and RT-PCR at first admission. The patients were divided into two groups: laboratory-confirmed COVID-19 and clinically diagnosed COVID-19. Clinical findings, laboratory findings, radiologic features and CT severity index (CT-SI) of the patients were noted. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of chest CT were calculated for the diagnosis of COVID-19, using RT-PCR as reference.
RESULTS
The laboratory-confirmed and clinically diagnosed COVID-19 groups consisted of 69 (M/F 43/26, mean age 50.9±14.0 years) and 51 patients (M/F 24/27, mean age 50.9±18.8 years), respectively. Dry cough (62.3% vs. 52.9%), fever (30.4% vs. 25.5%) and dyspnea (23.2% vs. 27.5%) were the most common admission symptoms in the laboratory-confirmed and clinically diagnosed COVID-19 groups, respectively. Bilateral multilobe involvement (83.1% vs. 57.5%), peripheral distribution (96.9% vs. 97.5%), patchy shape (75.4% vs. 70.0%), ground-glass opacities (GGO) (96.9% vs. 100.0%), vascular enlargement (56.9% vs. 50.0%), intralobular reticular density (40.0% vs. 40.0%) and bronchial wall thickening (27.7% vs. 45.0%) were the most common CT findings in the laboratory-confirmed and clinically diagnosed COVID-19 subgroups, respectively. Except for the bilateral involvement and white blood cell (WBC) count, no difference was found between the clinical, laboratory, and parenchymal findings of the two groups. Positive correlation was found between CT-SI and, lactate dehydrogenase (LDH) and C-reactive protein (CRP) values in the laboratory-confirmed COVID-19 subgroup. Chest CT and RT-PCR positivity rates among patients with suspected COVID-19 were 87.5% (105/120) and 57.5% (69/120), respectively. The sensitivity, specificity, PPV, NPV and accuracy rates of chest CT were determined as 94.2% (95% confidence interval [CI], 85.8–98.4), 21.57% (95% CI, 11.3–35.3), 61.90% (95% CI, 58.2–65.5), 73.3% (95% CI, 48.2–89.1) and 63.3% (95% CI, 54.1–71.9), respectively.
CONCLUSION
Chest CT has high sensitivity and low specificity in the diagnosis of COVID-19. The clinical, laboratory, and CT findings of laboratory-confirmed and clinically diagnosed COVID-19 patients are similar.
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first appeared in Wuhan, China in December 2019, spread to the whole world in a short time, and was declared as a pandemic by the World Health Organization (WHO) on March 11, 2020. According to the WHO data, as of April 11, 2020, the number of confirmed COVID-19 cases has exceeded 1.5 million and the total loss of life caused by the virus has exceeded 100 000, worldwide. In Turkey, in the last one month, since the first case was declared on March 10th, the total number of confirmed cases has gone over 50 000, and loss of life exceeded 1000 (1–3).
The incubation period after exposure to the virus is between 1 and 14 days, and lasts an average of 5 days, until the occurrence of the most common symptoms such as dry cough, fever, dyspnea, and fatigue (4, 5). Early diagnosis of COVID-19 cases is necessary for timely isolation and treatment of the patients. In the diagnosis of COVID-19, the demonstration of viral nucleic acid by RT-PCR is considered to be the reference standard. However, because of delays in obtaining the test results, the need for experienced personnel, inaccessibility and high false negativity of the RT-PCR test, methods that will obtain faster results are required. In recent studies, the importance of chest CT in the diagnosis of COVID-19 has been emphasized and CT sensitivity has been reported as above 90% (6–10). Due to the relatively easy accessibility and high accuracy of CT, it can be used to quickly confirm patients with COVID-19 and to have detailed information about disease involvement.
The aim of this study is to identify the chest CT characteristics of COVID-19, investigate the association between CT-SI and laboratory or demographic findings, and compare the accuracy of chest CT with RT-PCR at first admission.
Methods
Patient population and study design
This retrospective study was approved by our hospital board (protocol no: 2020.3/08-303) and written informed consent was obtained from all participants. This study included all 159 consecutive adult cases isolated due to suspected COVID-19 at our hospital between the dates of 17–25 March, when the first COVID-19 cases were appearing in Turkey. Among these, 36 patients with less than 2 RT-PCR tests and 3 patients with nondiagnostic chest CT were excluded. In total, 120 of 159 patients were included in the study. Of these 120 patients, those with RT-PCR test positivity were considered laboratory-confirmed COVID-19, and those with a negative result were accepted as clinically diagnosed COVID-19.
Clinical data
All patients were evaluated in pandemic outpatient clinics established in the emergency department. Clinical symptoms of cases such as fever (≥37.3 °C), cough, dyspnea, sore throat, fatigue, and the international travel history of the patient or their relatives were noted. Subsequently, all patients adhering to the possible case definition were subjected to laboratory-blood tests and non-contrast chest CT, at the time of initial admission. To verify SARS-CoV-2 positive patients, RT-PCR test was performed in the Republic of Turkey Ministry of Health, General Directorate of Public Health Microbiology Reference Laboratory. For the test, a combined swab sample was taken from the oropharynx and nasopharynx. The first laboratory tests and chest CT examinations of the cases were done 1–3 days before the RT-PCR test. Patients with negative first RT-PCR were examined for the second time after an interval of 3–5 days. At least 2 RT-PCR tests were performed on each patient, and cases that were negative two times in a row were considered negative. The demographic characteristics, clinical signs and symptoms, and laboratory results of the patients were obtained from the patient files.
CT scanning
Chest CT was performed for all patients, by using a 16-slice CT scanner (Optima 520 CT, General Electric (GE) company). CT images were obtained with the patient in the supine position at full inspiration and without contrast medium by using standard clinical protocols. The following parameters were used: tube voltage, 120 kV; automatic tube current modulation, 100–250 mAs; slice thickness, 1.25 mm without interslice gap. Images were reconstructed with a slice thickness of 5 mm. The reconstructed images were transmitted to the workstation and picture archiving and communication systems (PACS, Extreme PACS co.) for multiplanar reconstruction post-processing.
CT image analysis
CT images were evaluated blinded to the RT-PCR results, by two radiologists. In case of conflict, a result was reached by consensus. CT findings were evaluated according to the Thoracic Imaging in COVID-19 Infection version 2 of the British Society of Thoracic Imaging (BSTI) (11). According to BSTI, non-COVID-19 cases were categorized as CT negative; indeterminate COVID-19, probable COVID-19 and classic COVID-19 cases were categorized as CT positive. Lung involvement, number of involved lobes, shape (patchy, nodular or patchy, and nodular), location (peripheral, central, or peripheral and central), appearance (ground-glass opacity [GGO], GGO and consolidation, or consolidation alone) and the size of the largest lesion were recorded. In addition, parenchymal findings such as vascular enlargement, intralobular reticular density, air bronchogram, subpleural curvilinear lines, bronchial wall thickening, fibrous bands, halo sign, reversed halo sign, presence of cavitation, and extrapulmonary findings such as pleural effusion, pleural thickening, and enlarged lymph nodes (defined as a lymph node with short axis >10 mm) were recorded. Also, as Xie et al. (6) described, we calculated the CT-SI according to the degree of lesion distribution. For calculation, the lungs were divided into three zones: upper (above the carina), middle (below the carina up to the inferior pulmonary vein), and lower (below the pulmonary vein). Separate scoring was done for each zone. They were scored as follows: 0 for 0% involvement, 1 for <25% involvement, 2 for 25%–50% involvement, 3 for 50%–75% involvement and 4 for >75% involvement. Total score of 1–5 was considered to indicate mild involvement, 6–11 moderate involvement, and ≥12 severe involvement.
Statistical analysis
Descriptive statistics were used to summarize the characteristics of the patients. Categorical data were presented as n (%) and, normal distributions were shown as mean ± standard deviation (SD). Independent t-test was used in the comparison of normally distributed variables between the groups and chi-square test or Fisher’s exact test were used to compare categorical variables. When the contingency table is larger than 2×2 with more than 20% of expected values lower than 5 lattice points, the Fisher–Freeman–Halton test was performed. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of chest CT were calculated for the diagnosis of COVID-19, using RT-PCR as reference. The relations between clinical variables and CT variables were evaluated by the Spearman’s correlation coefficient. p values were calculated for two-tailed comparisons and p < 0.05 values were accepted as statistically significant. SPSS 17.0 was used for the statistical analyses.
Results
While the RT-PCR test of 69 of the 120 patients included in the study was positive (laboratory-confirmed COVID-19), the RT-PCR test of 51 patients was negative (clinically diagnosed COVID-19). The laboratory-confirmed COVID-19 group consisted of 69 patients between the ages of 22 and 88 years (mean ± SD, 50.9±14.0 years), and the clinically diagnosed COVID-19 group consisted of 51 adult patients between the ages of 19 and 89 years (50.9±18.8 years) (p = 0.997). There were 43 male and 26 female patients in the laboratory-confirmed COVID-19, and 24 male and 27 female patients in the clinically diagnosed COVID-19 group (p = 0.139) (Table 1).
Table 1.
Comparison of demographic, clinical, and laboratory characteristics of laboratory-confirmed and clinically diagnosed COVID-19 patients
| Laboratory-confirmed COVID-19 (n=69) | Clinically diagnosed COVID-19 (n=51) | P | ||
|---|---|---|---|---|
| Patient demographics | ||||
| Age (years), mean±SD | 50.9±14.0 | 50.9±18.8 | 0.997 | |
| Gender (M/F) | 43/26 | 24/27 | 0.139 | |
|
| ||||
| Clinical findings | ||||
| Fever (≥37.3°C ) | 21/69 (30.4) | 13/51 (25.5) | 0.697 | |
| Dry cough | 43/69 (62.3) | 27/51 (52.9) | 0.399 | |
| Sore throat | 23/69 (33.3) | 18/51 (35.3) | 0.977 | |
| Fatigue | 20/69 (29.0) | 17/51 (33.3) | 0.757 | |
| Dyspnea | 16/69 (23.2) | 14/51 (27.5) | 0.749 | |
|
| ||||
| History of suspicious contact | 18/69 (26.1) | 11/51 (21.6) | 0.722 | |
|
| ||||
| History of travel abroad | 2/69 (2.9) | 2/51 (3.9) | 1.000 | |
|
| ||||
| Laboratory tests | ||||
|
| ||||
| WBC count (normal range, 4–10 ×103/uL) | Low | 12/68 (17.6) | 4/50 (8.0) | < 0.001 |
| Normal | 52/68 (76.5) | 29/50 (58.0) | ||
| High | 4/68 (5.9) | 17/50 (34.0) | ||
|
| ||||
| Lymphocyte count (normal range, 1–4 ×103/mm3) | Low | 19/68 (27.9) | 12/50 (24.0) | 0.882 |
| Normal | 47/68 (69.1) | 36/50 (72.0) | ||
| High | 2/68 (2.9) | 2/50 (4.0) | ||
|
| ||||
| Neutrophil to lymphocyte ratio, mean±SD | 2.69±1.84 | 4.74±4.44 | 0.112 | |
|
| ||||
| CRP (normal range, 0.00–0.50 mg/L) | Normal | 16/69 (23.2) | 17/48 (35.4) | 0.216 |
| High | 53/69 (76.8) | 31/48 (64.6) | ||
|
| ||||
| LDH (normal range, 125–220 U/L) | Normal | 16/54 (29.6) | 15/44 (34.1) | 0.799 |
| High | 38/54 (70.4) | 29/44 (65.9) | ||
Data are presented as n/N (%) unless otherwise noted.
M/F, Male/Female; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase.
During their first admission, symptoms of laboratory-confirmed COVID-19 patients were fever (30.4%), dry cough (62.3%), dyspnea (23.2%), sore throat (33.3%), and fatigue (29.0%). Symptoms of clinically diagnosed COVID-19 patients were fever (25.5%), dry cough (52.9%), dyspnea (27.5%), sore throat (35.3%), and fatigue (33.3%). There was no difference between the symptoms of the laboratory-confirmed COVID-19 and clinically diagnosed COVID-19 patients during their first admission (Table 1). Two (2.9%) of the laboratory-confirmed COVID-19 patients had a history of being abroad in the last 14 days, and 18 (26.1%) had a suspicious contact history. Of the clinically diagnosed COVID-19 patients, 2 (3.9%) had international travel history and 11 (21.6%) had suspicious contact history.
Laboratory findings of the cases are shown in Table 1. WBC count was low in 17.6% and high in 5.9% of laboratory-confirmed COVID-19 patients, while it was low in 8.0% and high in 34.0% of clinically diagnosed COVID-19 patients (p < 0.001). Lymphocyte count and mean neutrophil to lymphocyte ratio (NLR) were not statistically different between the laboratory-confirmed and clinically diagnosed groups. CRP (76.8% vs. 64.6%) and LDH (70.4% vs. 65.9%) values were found to be relatively higher in the laboratory-confirmed COVID-19 group compared with the clinically diagnosed COVID-19 group, but the difference was not statistically significant (p = 0.216 and p = 0.799, respectively).
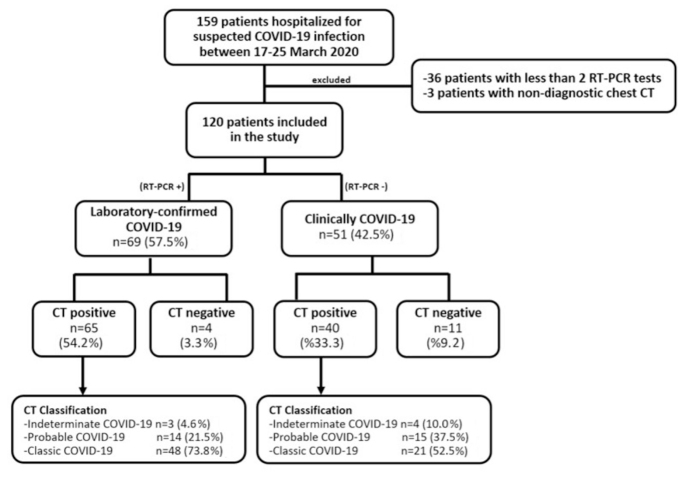
In our study, chest CT and RT-PCR test positivity rates were 87.5% (105/120) and 57.5% (69/120), respectively, among patients suspected of having COVID-19. In the evaluation of CT findings, 4 of 69 patients with RT-PCR positivity (5.8%) was evaluated as non-COVID-19, 3 (4.4%) as indeterminate COVID-19, 14 (20.3%) as probable COVID-19 and 48 (69.6%) as classic COVID-19. In the clinically diagnosed COVID-19 group, 11 of 51 patients (21.6%) were evaluated as non-COVID-19, 4 (7.8%) as indeterminate COVID-19, 15 (29.4%) as probable COVID-19, and 21 (41.2%) as classic COVID-19 (Fig. 1). Accordingly, there was a statistically significant difference between the laboratory-confirmed COVID-19 and clinically diagnosed COVID-19 groups in terms of CT positivity (p = 0.021).
Figure 1.
The flowchart of patients according to RT-PCR and chest CT classification.
When RT-PCR is accepted as the reference standard, the sensitivity, specificity, PPV, NPV and accuracy rates of chest CT were determined to be 94.2% (95% CI, 85.8%–98.4%), 21.6% (11.3%–35.3%), 61.9% (58.2%–65.5%), 73.3% (48.2%–89.1%) and 63.3% (54.1%–71.9%), respectively (Table 2).
Table 2.
Diagnostic performance of chest CT for COVID-19 disease, with RT-PCR as the reference standard
| TP | TN | FP | FN | |
|---|---|---|---|---|
| n | 65 | 11 | 40 | 4 |
| Percentage (95% CI) | ||||
| Sensitivity (65/69) | 94.2 (85.8–98.4) | |||
| Specificity (11/51) | 21.6 (11.3–35.3) | |||
| Positive predictive value (65/105) | 61.9 (58.2–65.5) | |||
| Negative predictive value (11/15) | 73.3 (42.8–89.1) | |||
| Accuracy (76/120) | 63.3 (54.1–71.9) | |||
CT, computed tomography; COVID-19, coronavirus 2019; RT-PCR, reverse transcription polymerase chain reaction; CI, confidence interval; TP, true positive; TN, true negative; FP, false positive; FN, false negative.
We performed a subgroup analysis of 65 patients with laboratory-confirmed COVID-19 and CT positivity, and 40 patients with clinically diagnosed COVID-19 and CT positivity (Table 3). In the laboratory-confirmed COVID-19 subgroup (n=65), there was bilateral lung involvement in 83.1% of the cases, and unilateral involvement in 16.9% (9.2% right, 7.7% left). While single lobe was involved in 10.8% of cases, multiple lobe involvement was present in 89.2%. Maximum lesion size was less than 1 cm in one case, and more than 3 cm in more than half of the cases. In more than half of the cases, the lesions were located both centrally and peripherally, and approximately half of them were only located peripherally, while only 2 (3.1%) were located only centrally. In 75.4% of the cases, lesions were observed as patchy, and in 15.4% they were observed in nodular form. In the evaluation of CT parenchymal findings, the following features were observed: vascular enlargement (56.9%), intralobular reticular density (40.0%), bronchial wall thickening (27.7%), subpleural curvilinear lines (24.6%), air bronchogram (27.7%), fibrous band (26.2%), subpleural sparing (21.5%), halo sign (20.0%), and reversed halo sign (6.2%). Cavitation and tree-in-bud were not observed in any cases. Pleural thickening in 11 cases (16.9%), lymphadenopathy in 5 cases (7.7%) and pleural effusion in one case (1.5%) were observed in the evaluation of extrapulmonary findings. Examples of chest CT findings are shown in Figs. 2 and 3.
Table 3.
Chest CT features of patients with laboratory-confirmed and clinically diagnosed COVID-19
| CT feature analysis | Laboratory-confirmed and CT positive subgroup (n=65) | Clinically diagnosed and CT positive subgroup (n=40) | p |
|---|---|---|---|
| Lung involvement | |||
| Bilateral | 54 (83.1) | 23 (57.5) | 0.010 |
| Right | 6 (9.2) | 6 (15.0) | |
| Left | 5 (7.7) | 11 (27.5) | |
|
| |||
| Number of lobes involved | |||
| 1 | 7 (10.8) | 10 (25.0) | 0.420 |
| 2 | 6 (9.2) | 4 (10.0) | |
| 3 | 11 (16.9) | 6 (15.0) | |
| 4 | 6 (9.2) | 3 (7.5) | |
| 5 | 35 (53.8) | 7 (17.5) | |
|
| |||
| Max diameter of lesion (cm) | |||
| <1 cm | 1 (1.5) | 1 (2.5) | 0.782 |
| 1–3 cm | 29 (44.6) | 15 (37.5) | |
| >3 cm | 35 (53.8) | 24 (60.0) | |
|
| |||
| CT severity index | |||
| Mild (≤5) | 31 (47.7) | 19 (47.5) | 1.000 |
| Moderate (6–11) | 26 (40.0) | 16 (40.0) | |
| Severe (≥12) | 8 (12.3) | 5 (12.5) | |
|
| |||
| Distribution of lesions | |||
| Peripheral and central | 33 (50.8) | 26 (65.0) | 0.327 |
| Peripheral | 30 (46.2) | 13 (32.5) | |
| Central | 2 (3.1) | 1 (2.5) | |
|
| |||
| Shape of lesions | |||
| Patchy | 49 (75.4) | 28 (70.0) | 0.663 |
| Nodular | 10 (15.4) | 6 (15.0) | |
| Patch and nodular | 6 (9.2) | 6 (15.0) | |
|
| |||
| Appearance of lesions | |||
| GGO | 42 (64.6) | 20 (50.0) | 0.128 |
| GGO and consolidation | 21 (32.3) | 20 (50.0) | |
| Consolidation | 2 (3.1) | 0 (0.0) | |
|
| |||
| CT parenchymal findings | |||
| Vascular enlargement | 37 (56.9) | 20 (50.0) | 0.624 |
| Intralobular reticular density | 26 (40.0) | 16 (40.0) | 1.000 |
| Bronchial wall thickening | 18 (27.7) | 15 (45.0) | 0.293 |
| Subpleural curvilinear lines | 16 (24.6) | 4 (10.0) | 0.110 |
| Air bronchogram | 18 (27.7) | 9 (22.5) | 0.718 |
| Fibrous band | 17 (26.2) | 8 (20.0) | 0.629 |
| Subpleural sparing | 14 (21.5) | 7 (17.5) | 0.802 |
| Halo sign | 13 (20.0) | 5 (12.5) | 0.469 |
| Reversed halo sign | 4 (6.2) | 0 (0.0) | 0.295 |
|
| |||
| Extrapulmonary manifestation | |||
| Pleural thickening | 11 (16.9) | 5 (12.5) | 0.739 |
| Lymphadenopathy | 5 (7.7) | 5 (12.5) | 0.500 |
| Pleural effusion | 1 (1.5) | 2 (5.0) | 0.556 |
CT, computed tomography; COVID-19, coronavirus disease 2019; GGO, ground-glass opacity.
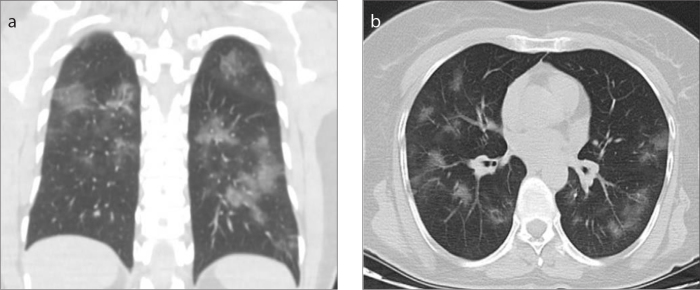
Figure 2. a, b.
Non-contrast chest CT images in a 52-year-old male COVID-19 patient presenting with dry cough. Coronal CT image (a) shows bilateral patchy ground-glass opacities in multiple lung segments. Axial image (b) shows central and peripheral distribution.
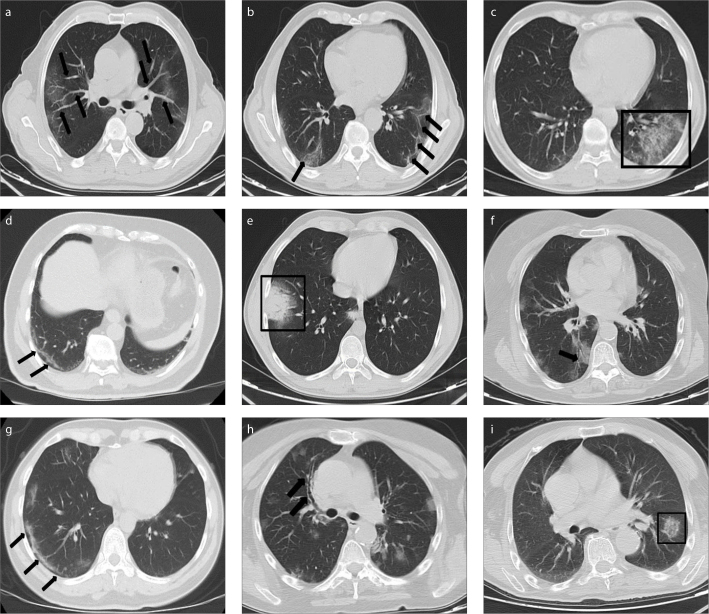
Figure 3. a–i.
CT features of COVID-19 disease. Axial unenhanced chest CT images show: (a), pure peripheral ground-glass opacities (GGOs) with vascular enlargements (black arrows); (b), bilateral GGOs and fibrous bands in the lower lobes (black arrows); (c), GGO with intralobular reticular densities in the left lower lobe (black frame); (d), subpleural curvilinear lines in the right lower lobe (black arrows); (e), consolidation with air bronchograms surrounded by a ground-glass halo representing halo sign in the anterobasal segment of the right lower lobe (black frame), with another focal consolidation also seen in the medial part of the posterobasal segment of the right lower lobe; (f), bilateral (dominantly in the right lung) GGOs in the lower lobes and air bronchogram in the right subpleural area (black arrow); (g), relative sparing of the lung periphery indicating subpleural sparing sign (black arrows); (h), bilateral patchy GGOs and bronchial wall thickening in the anterior segment of the right upper lobe (black arrows); and (i), reversed halo sign defined as central GGO surrounded by denser consolidation in the left lower lobe (black frame).
In the clinically diagnosed COVID-19 subgroup (n=40), there was bilateral lung involvement in 57.5% of the cases, and unilateral involvement in 42.5% (15.0% right, 27.5% left) of the cases. While single lobe was involved in 25.0% of cases, multiple lobe involvement was present in 75.0%. Maximum lesion size was less than 1 cm in one case, and more than 3 cm in more than half of the cases. In approximately two-thirds of the cases, the lesions were located both centrally and peripherally, and in approximately one-third of the cases they were only located peripherally, while in only one case (2.5%), they were located only centrally. Lesions were observed as patchy in 70.0% of the cases, and in nodular form in 15.0%. In the evaluation of CT parenchymal findings, the following features were observed: vascular enlargement (50.0%), intralobular reticular density (40.0%), bronchial wall thickening (45.0%), subpleural curvilinear lines (10.0%), air bronchogram (22.5%), fibrous band (20.0%), subpleural sparing (17.5%), and halo sign (12.5%). Reversed halo sign, cavitation and tree-in-bud were not observed in any cases. Pleural thickening and lymphadenopathy were observed in 5 cases each (12.5%) and pleural effusion was observed in two cases (5.0%) in the evaluation of extrapulmonary findings. In the comparison of CT parenchymal findings, no statistically significant difference was found between the two subgroups, except for bilateral lung involvement (Table 3).
According to CT-SI, 47.7% of laboratory-confirmed COVID-19 subgroup was classified as mild, 40.0% as moderate and 12.3% as severe; 47.5% of clinically diagnosed COVID-19 subgroup was classified as mild, 40.0% as moderate, and 12.5% as severe. There was no significant difference between the mean CT-SI of subgroups (p = 0.893). The mean CT-SI was 6.2±3.6 in patients with laboratory-confirmed COVID-19 subgroup; it was significantly higher in patients over 40 years of age (mean±SD, 6.6±3.7) compared with those under 40 years of age (4.6±2.3) (p = 0.019). Similarly, the average of CT-SI was 6.3±4.9 in the clinically diagnosed COVID-19 subgroup, and it was significantly higher in patients over 40 years of age (7.9±5.2) compared with those under 40 years of age (3.4±2.8) (p = 0.001).
In the laboratory-confirmed COVID-19 subgroup, no difference was detected between the mean CT-SI of male (6.4±3.8) and female (5.7±3.2) patients (p = 0.425). Similarly, no difference was detected between the mean CT-SI of male (5.2±4) and female (7.5±5.7) patients in the clinically diagnosed COVID-19 subgroup (p = 0.157). While CT-SI correlated with maximum lesion diameter, LDH, CRP, neutrophil/lymphocyte ratio in laboratory-confirmed COVID-19 subgroup, no correlation was detected in clinically diagnosed COVID-19 subgroup except age and maximum lesion diameter (Table 4).
Table 4.
Correlation of clinical and laboratory variables with CT severity index in laboratory-confirmed and clinically diagnosed COVID-19 subgroups
| Variables | CT-SI of laboratory-confirmed COVID-19 patients (n=65) | CT-SI of clinically diagnosed COVID-19 patients (n=40) |
|---|---|---|
| Age (years) | ||
| r | 0.251 | 0.662 |
| p | 0.144 | 0.001 |
|
| ||
| Max diameter of lesion (cm) | ||
| r | 0.627 | 0.380 |
| p | 0.001 | 0.019 |
|
| ||
| WBC count | ||
| r | −0.035 | 0.058 |
| p | 0.781 | 0.720 |
|
| ||
| Neutrophil count | ||
| r | 0.098 | −0.311 |
| p | 0.480 | 0.084 |
|
| ||
| Lymphocyte count | ||
| r | −0.187 | −0.012 |
| p | 0.139 | 0.940 |
|
| ||
| Neutrophil to lymphocyte ratio | ||
| r | 0.336 | −0.197 |
| p | 0.013 | 0.280 |
|
| ||
| CRP | ||
| r | 0.438 | 0.270 |
| p | 0.001 | 0.101 |
|
| ||
| LDH | ||
| r | 0.318 | −0.049 |
| p | 0.023 | 0.782 |
CT, computed tomography; COVID-19, coronavirus disease 2019; CT-SI, computed tomography severity index; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase.
Discussion
In our study, the most common complaints of patients were dry cough, sore throat and fatigue, while dyspnea and fever were observed less commonly. In the literature, the most common symptoms in COVID-19 patients were fever, cough, and dyspnea (12–14). Although the clinical symptoms in our study are similar to those in the literature, fever and dyspnea symptoms were found less frequently, which may be because of the early admission of patients due to the pandemic.
In the literature, there are studies reporting lymphocytopenia in up to 60% of COVID-19 patients, and in our study, this rate was 27.9% (10, 15). In our study, the lymphocyte counts of the laboratory-confirmed COVID-19 and clinically diagnosed COVID-19 groups were similar. Similar to the other studies, WBC count was normal or low in a high percentage of both groups. In this study, LDH and CRP values were found to be high in both groups, similar to the literature (14, 16). We found no significant difference between the admission symptoms and laboratory findings of laboratory-confirmed COVID-19 and clinically diagnosed COVID-19 patients. Considering the similarity of the clinical and laboratory findings, as well as the limitations of the molecular test, there may have been false-negative cases among the clinically diagnosed COVID-19 group despite two negative RT-PCR tests. In subgroup analysis, a positive correlation was found only between CT-SI and, LDH and CRP values in the laboratory-confirmed COVID-19 subgroup. Therefore, high LDH and CRP values could be used as a parameter to inform the prevalence of lung involvement.
Bilateral multilobe involvement, peripheral (including peripheral and central) distribution, patchy shape and GGO appearance were the most common chest CT findings of COVID-19 in the entire study population. These findings are very similar to the CT findings of COVID-19 reported in the literature (10, 12, 17–20).
The incidence of certain parenchymal findings described in chest CT in COVID-19 pneumonia was evaluated in the performed studies. In this study, when the laboratory-confirmed COVID-19 group was evaluated, vascular enlargement (56.9%) was the most common parenchymal finding. Similarly, high rates (71.3%–82.9%) were found in many studies (15, 21, 22). Intralobular reticular density (40.0%) is the second most common finding and has been reported in the literature ranging from 14% to 89% (9, 19, 23). Bronchial wall thickening was observed in 27.7% of our cases and this rate is slightly higher compared to the other studies (11%–23%) (18, 24, 25). Subpleural curvilinear lines were 24.6% in our study, consistent with the literature (18, 25). The incidence of air bronchogram sign varies widely in the literature from 21% to 80%, in our study this rate was 27.7% (12, 23, 24). Although pleural thickening has mostly not been reported in the literature, it was found to be 16.9% in our study. Mediastinal-hilar lymph node enlargement has been reported in the literature ranging from 4% to 8% and was observed in 7.7% (5/65) of our laboratory-confirmed COVID-19 cases (12, 18, 23, 26). In our study, the clinically diagnosed COVID-19 group exhibits similar parenchymal findings as the laboratory-confirmed COVID-19 group. This may be related to the low specificity of chest CT or false negativity of the RT-PCR test.
Identification of parenchymal findings of COVID-19 pneumonia will help increase the diagnostic performance of chest CT. Particularly in false-negative RT-PCR cases, chest CT will make an important contribution to diagnosis when evaluated together with history, clinical findings, and laboratory results. In addition, recognizing these findings is valuable in distinguishing other viral pneumonia types, other infectious processes such as organizing pneumonia and noninfectious processes (such as hypersensitivity pneumonia, vasculitis or connective tissue disease) from COVID-19 pneumonia.
Early diagnosis and timely isolation of COVID-19 is important because it is highly contagious. In the diagnosis of COVID-19, it is essential to show the virus by molecular methods, but RT-PCR test has its limitations. Chest CT has an important place in the evaluation of COVID-19 patients since it is easily accessible compared with RT-PCR, gives faster results, and provides information about the severity of disease involvement (13, 27). Furthermore, there are studies showing that chest CT is more sensitive than RT-PCR test in the diagnosis of COVID-19 (27). As in the systematic review of 1014 patients by Ai et al. (28), in our study, positive chest CT (87.5% vs. 88%) ratio was higher than positive RT-PCR (57.5% vs. 59%) for the diagnosis of COVID-19. Chest CT sensitivity for COVID-19 detection ranges from 47% to 100%, and pooled sensitivity was reported as 94% in a meta-analysis (27). Similarly, chest CT sensitivity was found as 94.2% in our study.
In the same meta-analysis including 5 studies, chest CT specificity ranged from 25% to 56% in COVID-19 detection and pooled specificity was found as 37% (8–10, 27, 28). In our study, the specificity was found to be 21.6%, similar to studies by Ai et al. (25%) and Cheng et al. (26%) (28, 29). The reason for the low specificity in our study may be that the cases with two consecutive RT-PCR negativity were considered negative; it has been shown that RT-PCR repeated 3 or 4 times increased the COVID-19 positivity rate by 20%–25%, due to the relatively low sensitivity of the RT-PCR test (10, 28). This shows that two consecutive negative RT-PCR tests are not sufficient to exclude COVID-19. If COVID-19 is suspected clinically or radiologically, the RT-PCR test should be repeated.
In studies conducted in countries with different COVID-19 prevalence before April 2020, PPV of chest CT in the diagnosis of COVID-19 varied between 1.5% and 30.7%, and NPV varied between 95.4% and 99.8% (27). In our study, the high PPV (61.9%) can be explained by not using CT as a screening method and the patients included in the study being isolated in the hospital with a high suspicion of clinically diagnosed COVID-19. The low rate of NPV (11/15; 73.3%) in our study can be explained by the number of patients included in the study and the difference in disease prevalence between countries. Additionally, three out of four RT-PCR positive CT negative cases had follow-up CT, and no finding compatible with COVID-19 was detected in follow-up imaging. This may be due to the fact that some cases experience this disease without lung involvement or due to early initiation of treatment.
COVID-19 has a higher mortality in male and elderly patients (30). Clinically severe COVID-19 cases were reported to have higher chest burden on CT imaging (31). In another study, a significant correlation was observed between the chest X-ray score for COVID-19 and age. There is an increased risk of developing severe COVID-19 in men over 50 and women over 80 years of age (32). In our study, when the CT-SI scores were evaluated, severe involvement was not observed in any of the patients under 40 years of age. At the time of first admission, CT-SI score was higher in patients ≥40 years old compared with those <40 years old in both laboratory-confirmed and clinically diagnosed COVID-19 subgroups. However, there was no difference between sex and CT-SI in either group. In line with this information, as stated in the literature, it can be thought that the disease progresses more seriously in elderly patients than young people (17, 18). Similarly, it is thought that CT-SI and disease severity may be related, and more detailed studies are needed in this regard.
The limitations of our study are the acceptance of two consecutive RT-PCR tests as negative and not performing further repeat tests, the absence of other viral panel tests in RT-PCR negative patients, and the relatively low number of patients.
In conclusion, chest CT has high sensitivity and low specificity in the diagnosis of COVID-19. The clinical, laboratory and CT findings of the laboratory-confirmed and clinically diagnosed COVID-19 patients are similar. Laboratory findings correlate with CT-SI in COVID-19.
Main points.
For the diagnosis of COVID-19, the sensitivity, specificity, PPV, NPV and accuracy rates of chest CT are 94.2%, 21.6%, 61.9%, 73.3%, and 63.3% respectively, using RT-PCR as reference.
Bilateral multilobe involvement, peripheral (including peripheral and central) distribution, patchy shape, and GGO appearance are the most common CT findings of COVID-19.
CT severity index correlated with maximum lesion diameter, LDH and CRP values in the laboratory-confirmed and CT positive COVID-19 patient subgroup.
The clinical, laboratory, and CT findings of laboratory-confirmed and clinically diagnosed COVID-19 patients are similar.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 2.Turkish Ministry of Health. COVID-19 (SARS-CoV-2 INFECTION) GUIDE-Study of Scientific Board. Turkish Ministry of Health; Ankara, Turkey: https://covid19.saglik.gov.tr/ [Google Scholar]
- 3.World Health Organization. Coronavirus disease 2019 (COVID-19) situation report–51. World Health Organization; Geneva: 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10. [Google Scholar]
- 4.Park M, Cook AR, Lim JT, Sun Y, Dickens BL. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020;9:967. doi: 10.3390/jcm9040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80:394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;296:E41–45. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sverzellati N, Milone F, Balbi M. How imaging should properly be used in COVID-19 outbreak: an Italian experience. Diagn Interv Radiol. 2020;26:204–206. doi: 10.5152/dir.2020.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432. 200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caruso D, Zerunian M, Polici M, et al. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020:201237. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen Z, Chi Y, Zhang L, et al. Coronavirus disease 2019: ınitial detection on chest CT in a retrospective multicenter study of 103 Chinese subjects. Radiol Cardiothorac Imaging. 2020;2:e200092. doi: 10.1148/ryct.2020200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.British Society of Thoracic Imaging. Thoracic Imaging in COVID 19 Infection Guidance for the Reporting Radiologist Version 2. https://www.bsti.org.uk/standards-clinical-guidelines/clinical-guidelines/bsti-covid-19-guidance-for-the-reporting-radiologist/
- 12.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020. Apr 15, [Epub Ahead of Print] [DOI] [PMC free article] [PubMed]
- 15.Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. Am J Roentgenol. 2020;215:338–343. doi: 10.2214/AJR.20.22961. [DOI] [PubMed] [Google Scholar]
- 16.Luo N, Zhang H, Zhou Y, et al. Utility of chest CT in diagnosis of COVID-19 pneumonia. Diagn Interv Radiol. 2020 May 29; doi: 10.5152/dir.2020.20144. [Epub Ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z, Guo D, Li C, et al. Coronavirus disease 2019: initial chest CT findings. Eur Radiol. 2020;2:1–9. doi: 10.1007/s00330-020-06816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Güneyli S, Atçeken Z, Doğan H, Altınmakas E, Atasoy KÇ. Radiological approach to COVID-19 pneumonia with an emphasis on chest CT. Diagn Interv Radiol. 2020;26:323–332. doi: 10.5152/dir.2020.20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (covid-19) pneumonia: A multicenter study. Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214:1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 23.Song F, Shi N, Shan F, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon SH, Lee KH, Kim JY, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): Analysis of nine patients treated in Korea. Korean J Radiol. 2020;21:498–504. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J, Wu X, Zeng W, et al. Chest CT findings in patients with coronavirus disease 2019 and ıts relationship with clinical features. Invest Radiol. 2020;55:257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30:3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. 2020:201343. doi: 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng Z, Lu Y, Cao Q, et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22959. [DOI] [PubMed] [Google Scholar]
- 30.Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Heal. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang R, Li X, Liu H, et al. Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiol Cardiothorac Imaging. 2020;2:e200047. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borghesi A, Zigliani A, Masciullo R, et al. Radiographic severity index in COVID-19 pneumonia: relationship to age and sex in 783 Italian patients. Radiol Medica. 2020;125:461–464. doi: 10.1007/s11547-020-01202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]