Abstract
PURPOSE
Neck ultrasonography (US), computed tomography (CT), and 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) are all known to be useful imaging modalities for detecting supraclavicular lymph node (SCN) metastasis in breast cancer. The authors compared the diagnostic values of neck US, CT, and PET/CT in the detection of SCN metastasis in breast cancer.
METHODS
SCN metastases identified in neck US, CT, or PET/CT during follow-up visits of patients with breast cancer were pathologically confirmed with the use of US-guided fine-needle aspiration cytology. The clinicopathological factors of the patients were analyzed, and the statistical parameters including sensitivity, specificity, positive and negative predictive values, false-positive and false-negative rates, and accuracy of neck US, CT, and PET/CT were compared.
RESULTS
Among 32 cases of suspicious SCNs, 24 were pathologically confirmed as metastasis of breast cancer. The sensitivity of US + CT was 91.7%, which was the same as that of PET/CT, while the sensitivity rates of US alone and CT alone were 87.5% and 83.3%, respectively. Accuracy was 99.8% in PET/CT alone and 98.1% in US + CT. The false-negative rate was 0.1% in US + PET/CT, while it was 0.2% in PET/CT and US + CT, 0.3% in US alone and 0.4% in CT alone.
CONCLUSION
PET/CT can be the first choice for detecting SCN metastases in breast cancer. However, if PET/CT is unavailable for any reason, US + CT could be a good second option to avoid false-negative results.
The overall survival and mortality rates of patients with cancer have improved with early diagnosis and the development of new treatment modalities (1–4). However, paradoxically, the incidence of recurrence or metastasis has increased with the prolonged overall survival period.
Supraclavicular lymph nodes (SCNs) are one of the common sites of regional recurrence of breast cancer. The breast cancer recurrence in the axillary lymph nodes is generally surgically removable and shows better prognosis than the recurrence in supraclavicular lymph nodes (SCNs), which does not show good prognosis even if it has been removed because of concurrent or subsequent distant metastases (5). However, early detection of SCN metastasis may improve the operability and expand the opportunities for curative therapy (6–8).
Neck ultrasonography (US), computed tomography (CT), and 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) have been used for the detection of SCN metastasis in breast cancer (9–11). According to the National Comprehensive Cancer Network (NCCN) guidelines, although diagnostic contrast-enhanced chest CT is only recommended for clinical stages I–IIB with pulmonary symptoms, suspicious SCNs can be incidentally detected on neck areas shown in chest CT images (12).
Based on several studies, the sensitivity and specificity were 75%–100% and 55%–99% for US, 25%–98% and 65%–99% for CT, and 74%–92% and 61%–79% for PET/CT, respectively (13–16). However, to the authors’ knowledge, there is no study that compares various imaging modalities for the detection of SCN metastasis in breast cancer. Herein, the authors compared the statistical parameters of neck US, CT, and PET/CT in detecting SCN metastasis in breast cancer.
Methods
All the procedures in this study were performed in accordance with the ethical standards of the institutional review board of the Kyungpook National University Chilgok Hospital. Informed consent was obtained from all patients and the protocol used in this study was approved by the Institutional Review Board Committee of the Kyungpook National University Chilgok Hospital (KNUCH 2015-05-205), and all the experiments were performed in accordance with relevant guidelines and regulations.
A total of 1148 patients with breast cancer who had undergone surgery between 2008 and 2013 were included in this study. The treatment strategy for patients with operable breast cancer was determined through multidisciplinary team discussions, which comprised breast and plastic surgeons, oncologists, radiologists, pathologists, radiation oncologists, and a rehabilitation physician. After completing adjuvant treatments, each patient was followed up one or two times annually for at least five years for surveillance. Locoregional recurrence (including recurrence in the breast, axilla, or supra/infraclavicular area), distant metastasis, and death were evaluated through blood tests, tumor markers, mammography, breast and neck US, chest X-ray, bone scan, chest (including neck area) or abdominal CT, and 18F-FDG PET/CT. In addition, the clinicopathological factors were analyzed retrospectively.
Among the 1148 patients with breast cancer, the patients who had suspicious SCNs in neck US, chest CT, or PET/CT underwent fine-needle aspiration cytology (FNAC) to confirm true metastasis. The statistical parameters including sensitivity, specificity, positive and negative predictive value, false-positive and false-negative rates, and accuracy were calculated and compared between neck US, CT, 18F-FDG PET/CT, and combination methods.
Neck US
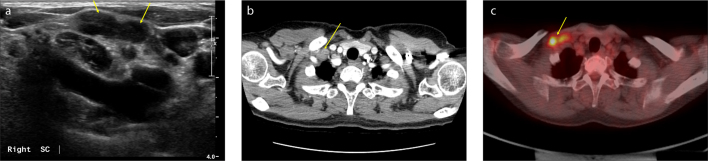
For SCN examination during surveillance, all the patients were monitored with neck and breast US, which were performed by two breast radiologists. For the evaluation of SCNs, a linear array transducer was used with frequencies of 12–14 MHz. When the radiologists suspected SCN metastasis during examination, they performed US-guided FNAC immediately for pathological confirmation (Fig. 1a).
Figure 1. a–c.
During the follow-up of a 55-year-old female patient with breast cancer, two suspicious supraclavicular lymph nodes (SCNs) on the right side of the neck were identified in US, chest CT, and 18F-FDG PET/CT. These lymph nodes were diagnosed as metastatic lymph nodes through fine-needle aspiration cytology. During the neck US shown in (a), two abutting suspicious SCNs were found in the lateral portion of the right carotid artery. The enlarged lymph nodes are hypoechoic with cortical thickening and loss of fatty hilum (arrows). Chest CT image (b) shows the presence of suspicious SCNs. Two abutted, enlarged SCNs on the right side of the neck show heterogeneous enhancement (arrow). In 18F-FDG PET/CT shown in (c), metastatic SCNs are seen on the right side of the neck (arrow) with a SUVmax 10.9.
Chest CT (expanded to the neck area)
The patients underwent chest CT that covered the area from the jaw to the upper abdomen to evaluate distant metastasis in the thorax and regional lymph nodes. Although conventional chest CT generally includes the lung and mediastinal area, which is chest level, the protocol of chest CT in the authors’ institution was modified to cover the lower neck level including the supraclavicular and infraclavicular areas.
Both traditional CT and contrast-enhanced CT were utilized in this study, and all the scans were acquired in both axial and coronal planes. The slice spacing was 2.5–3 mm, and the CT images were reviewed by experienced radiologists (Fig. 1b).
18F-FDG PET/CT
Before the administration of 18F-FDG PET/CT, at least 6 hours of fasting time was required and blood glucose levels of all the patients were checked. The patients’ blood glucose concentrations were managed to maintain levels of <150 mg/dL, and their examinations were rescheduled if their glucose levels were elevated. A dose of approximately 3.7–5.55 MBq/kg of 18F-FDG was injected intravenously, and the patients were asked to rest for one hour before image acquisition.
Before PET scan, a low-dose CT scan without contrast enhancement was conducted from the skull vertex to the knee level under supine position with quiet respiration for attenuation correction. Consequently, PET/CT scans were performed using a 16-slice or 64-slice CT Discovery® PET/CT 600 or 690 apparatus (General Electric Healthcare). PET scans with maximum spatial resolutions of 5.1 mm (Discovery PET/CT 600) and 4.9 mm (Discovery PET/CT 690) were also performed from the skull vertex to the knees at 1.5 minutes per bed position. The PET images, obtained by the Discovery PET/CT scanners, were reconstructed with a 192×192 matrix, an ordered subset expectation maximum iterative reconstruction algorithm (4 iterations, 16 subsets), a Gaussian filter of 6.4 mm, and a slice thickness of 3.27 mm (Discovery PET/CT 600 or 690).
The obtained PET/CT images were interpreted by nuclear medicine physicians. A visually identifiable 18F-FDG activity, which showed higher intensity than surrounding tissue and was not associated with benign or physiological 18F-FDG uptake, was considered a positive finding (Fig. 1c).
US-guided FNAC
When a suspicious SCN was detected in the neck US, chest CT, or PET/CT images, FNAC was performed for pathological confirmation. In case of multiple suspicious lymph nodes, US-guided FNAC was performed on the most suspicious lymph node.
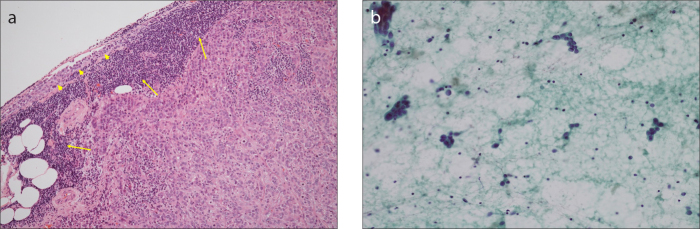
FNAC was performed under US; each lesion was aspirated with a 21-gauge needle using the to-and-fro technique. The aspirated sample was smeared on two pairs of glass slides and was immediately fixed with 95% alcohol. All the FNAC slides were prepared for Papanicolaou staining based on the standard method and assessed for diagnostic adequacy, which was confirmed by experienced cytopathologists (Fig. 2).
Figure 2. a, b.
Histopathologic findings of a metastatic SCN in breast cancer. Panel (a) shows histologic section of a lymph node demonstrating tumor cell deposits replacing the normal cells (arrows). In addition, normal lymphoid tissues are observed on the periphery (arrowheads) (×40, hematoxylin and eosin staining). In the smear of fine-needle aspiration cytology method (b), the lymph node shows clusters of pleomorphic malignant cells (×200, Papanicolaou stain).
Final diagnosis
The final diagnosis was decided based on the cytopathological findings. A true-negative finding was defined when the pathological result did not show any suspicious malignant component in the SCN examined and no interval change within 1 year after the procedure. However, the cases with changing patterns in the SCNs in the images were excluded if their final diagnoses were not determined with pathological results.
A false-positive finding was defined as a suspicious SCN indicated in any of the images, with no evidence of metastasis in subsequent cytological examinations. A false-negative finding was defined as no evidence of a suspicious SCN in any of the images, but during the follow-up period, a suspicious lymph node is detected and confirmed as metastasis on cytological examinations. The follow-up interval ranged from 6 months to 1 year. A true-negative finding was defined as negative findings on all the images and on the follow-up period of at least 1 year (13).
Statistical analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) or number and percentage. To assess the statistical parameters for the detection of SCN metastasis in breast cancer, the sensitivity, specificity, positive and negative predictive values, false-positive and false-negative rates, and accuracy of neck US, CT, and 18F-FDG PET/CT were calculated and compared.
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) Statistics version 25.0 (IBM Corp.). Categorical variables were analyzed using the chi-square test in a univariate analysis, and oncological outcomes were assessed using the Kaplan–Meier analysis to identify factors affecting locoregional recurrence, distant metastasis, or death. p < 0.05 was considered statistically significant.
Results
The mean age of the patients was 50.1±10.4 years, and 24 patients (2.1%) had been diagnosed with bilateral breast cancer. The mean clinical and pathological tumor sizes were 2.3±1.5 cm and 1.8±1.1 cm, respectively. Most of the breast cancers were invasive ductal carcinomas (n=1053, 91.7%), and their pathological stage was IA (n=565, 49.2%). Neoadjuvant and adjuvant chemotherapies were performed in 104 (9.1%) and 695 patients (60.5%), respectively. Adjuvant radiotherapy and hormonal treatment were applied to 676 (58.9%) and 810 (70.6%) patients, respectively (Table 1).
Table 1.
Clinicopathological characteristics of breast cancer patients who underwent surgery and additional treatment
| Variables | n=1148 | |
|---|---|---|
| Age (years), mean±SD | 50.1±10.4 | |
|
| ||
| Body mass index (kg/m2), mean±SD | 23.6±3.3 | |
|
| ||
| History of bilateral breast cancer, n (%) | 24 (2.1) | |
|
| ||
| Hospital stay (days), mean±SD | 12.4±4.6 | |
|
| ||
| Clinical tumor size (cm), mean±SD | 2.3±1.5 | |
|
| ||
| Pathological tumor size (cm), mean±SD | 1.8±1.1 | |
|
| ||
| Types of tumor, n (%) | Invasive ductal carcinoma | 1053 (91.7) |
| Invasive lobular carcinoma | 38 (3.3) | |
| Mucinous carcinoma | 27 (2.4) | |
| Others | 30 (2.6) | |
|
| ||
| Histological grade, n (%) | 1 | 269 (23.4) |
| 2 | 597 (52.0) | |
| 3 | 282 (24.6) | |
|
| ||
| Pathological stage, n (%) | IA | 565 (49.2) |
| IB | 2 (0.2) | |
| IIA | 348 (30.3) | |
| IIB | 132 (11.5) | |
| IIIA | 69 (6.0) | |
| IIIB | 3 (0.3) | |
| IIIC | 29 (2.5) | |
|
| ||
| Estrogen receptor positive, n (%) | 798 (69.5) | |
|
| ||
| Progesterone receptor positive, n (%) | 690 (60.1) | |
|
| ||
| c-erbB2 gene positive, n (%) | 221 (19.3) | |
|
| ||
| Triple-negative breast cancer, n (%) | 86 (7.5) | |
|
| ||
| Type of breast surgery, n (%) | Breast conserving surgery | 636 (55.4) |
| Mastectomy | 512 (44.6) | |
|
| ||
| Type of axillary surgery, n (%) | Sentinel lymph node biopsy | 807 (70.3) |
| Axillary lymph node dissection | 341 (29.7) | |
|
| ||
| Neoadjuvant chemotherapy, n (%) | 104 (9.1) | |
|
| ||
| Adjuvant chemotherapy, n (%) | 695 (60.5) | |
|
| ||
| Adjuvant radiotherapy, n (%) | 676 (58.9) | |
|
| ||
| Adjuvant hormonal therapy, n (%) | 810 (70.6) | |
SD, standard deviation.
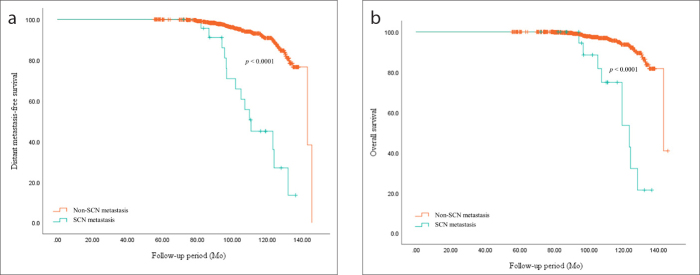
During the mean follow-up period of 100.54±18.5 months, there were 64 cases (5.6%) of locoregional recurrences, 85 cases (7.4%) of distant metastases, and 52 cases (4.5%) of death (Table 2). Among the locoregional recurrence cases, there were 32 cases of suspicious SCNs and 24 of these (75.0%) were pathologically confirmed as true SCN metastases (Table 3). In addition, the patients who were diagnosed with SCN metastasis showed significantly worse oncological outcomes in distant metastasis-free survival and overall survival (p < 0.001) (Fig. 3).
Table 2.
Oncological outcomes of breast cancer patients who underwent surgery and additional treatments
| Follow-up period (months), mean±SD | 100.54±18.5 |
|
| |
| Disease-free survival, n (%) | 1026 (89.4) |
|
| |
| Locoregional recurrencea, n (%) | 64 (5.6) |
| Ipsilateral breast | 23 (2.0) |
| Ipsilateral axillary lymph node | 25 (2.2) |
| Ipsilateral supraclavicular lymph node | 24 (2.1) |
| Ipsilateral internal mammary lymph node | 8 (0.7) |
|
| |
| Distant metastasisa, n (%) | 85 (7.4) |
| Lung | 48 (4.2) |
| Bone | 38 (3.3) |
| Liver | 34 (3.0) |
| Brain | 14 (1.2) |
| Mediastinal lymph node | 9 (0.8) |
| Others | 6 (0.5) |
|
| |
| Death, n (%) | 52 (4.5) |
SD, standard deviation.
The organs could be duplicated.
Table 3.
Pathological results of FNAC in 32 cases of suspicious SCNs in breast cancer
| Pathological results | n=32 |
|---|---|
| Metastatic carcinoma, n (%) | 24 (75.0) |
| Benign or reactive hyperplasia, n (%) | 6 (18.8) |
| Inadequate sample, n (%) | 2 (6.3) |
FNAC, fine needle aspiration cytology; SCNs, supraclavicular lymph node.
Figure 3. a, b.
Comparison of oncologic outcomes between metastatic supraclavicular lymph node (SCN) and non-metastatic SCN in breast cancer. In panel (a), distant metastasis-free survival was significantly superior in patients with breast cancer who were not diagnosed with SCN metastasis (p < 0.0001). In panel (b), overall survival was also significantly superior in patients with breast cancer who did not have SCN metastasis (p < 0.0001).
The sensitivities of neck US, CT, and PET/CT in detecting SCN metastasis were 87.5%, 83.3%, and 91.7%, respectively. Furthermore, the sensitivities of US + CT and US + PET/CT were 91.7% and 95.8%, respectively. The specificity of PET/CT was 100%, and those of other modalities ranged from 98.3% to 99.4%. The positive predictive value of PET/CT was also 100%, and those of other modalities ranged from 53.7% to 76.7%. Although PET/CT had no false-positive findings, the false-positive rate of US + CT was 1.7%. However, the lowest false-negative rate of 0.1% was achieved when US and PET/CT was combined, and the false-negative rates of PET/CT and US + CT were similar (0.2%). The accuracy was calculated as 99.8% for PET/CT and ranged from 98.1% to 99.3% for other modalities (Table 4).
Table 4.
Statistical parameters of neck US, CT, PET/CT, and combination methods for the detection of SCN metastases in breast cancer
| US | CT | PET/CT | US+CT | US+PET/CT | |
|---|---|---|---|---|---|
| Sensitivity (%) | 21/24 (87.5) | 20/24 (83.3) | 22/24 (91.7) | 22/24 (91.7) | 23/24 (95.8) |
| Specificity (%) | 1117/1124 (99.4) | 1111/1124 (98.8) | 1124/1124 (100.0) | 1105/1124 (98.3) | 1117/1124 (99.4) |
| Positive predictive value (%) | 21/28 (75.0) | 20/33 (60.6) | 22/22 (100.0) | 22/41 (53.7) | 23/30 (76.7) |
| Negative predictive value (%) | 1117/1120 (99.7) | 1111/1115 (99.6) | 1124/1126 (99.8) | 1105/1107 (99.8) | 1117/1118 (99.9) |
| False positive rate (%) | 7/1124 (0.6) | 13/1124 (1.2) | 0 | 19/1124 (1.7) | 7/1124 (0.6) |
| False negative rate (%) | 3/1120 (0.3) | 4/1115 (0.4) | 2/1126 (0.2) | 2/1107 (0.2) | 1/1118 (0.1) |
| Accuracy (%) | 1138/1148 (99.1) | 1131/1148 (98.5) | 1146/1148 (99.8) | 1127/1148 (98.1) | 1140/1148 (99.3) |
US, ultrasonography; CT, computed tomography; PET/CT, positron emission tomography/computed tomography; SCN, supraclavicular lymph node.
Discussion
The axillary lymph nodes and SCNs are the most common sites of regional recurrence in breast cancer (17, 18). Although the recurrence in axillary lymph nodes is generally curable with surgical removal, the recurrence in SCNs should be treated with a combination of local control and systemic treatment (5). However, the early detection of regional lymph node metastasis in breast cancer may improve progression-free survival and overall survival (19, 20).
According to the NCCN guidelines, mammography performed every 12 months is sufficient for surveillance of breast cancer if there are no clinical signs and symptoms suggestive of recurrent disease (12). However, for Asian women with breast cancer, mammography alone is not enough because of their high incidence rates of dense breasts (21). Furthermore, for the early detection of asymptomatic regional lymph node metastasis, additional US and CT may be helpful and PET/CT can also be a complementary tool (9, 10, 13, 15, 22).
Several studies have reported that PET/CT scanning is a very useful modality to identify not only SCN metastases but also tumor cell viability with maximum standard uptake values (SUVmax) (13, 15, 23, 24). Although there were several reports about the disadvantages of PET/CT (13, 25), its statistical parameters in detecting SCN metastases in breast cancer were excellent in this study.
Neck CT can also be a useful imaging modality to identify SCN metastases. Uematsu et al. (26) reported that lymph nodes ranging from 0.5 mm to 26.5 mm in size can be detected using high-resolution helical CT and that the findings are well correlated with pathological results. However, neck CT for breast cancer surveillance is not recommended due to its less information and lower sensitivity, specificity, and accuracy in detecting suspicious SCN than neck US (9, 10, 22). If chest CT is planned to evaluate the chest, the scanning range can be expanded to the neck area for further evaluation of SCN metastases in breast cancer.
Neck US is very easy and familiar to surgeons, and it has the following advantages: it has a high sensitivity and positive predictive value, it is radiation-free, it is compatible with additional simultaneous procedures, and it can identify detailed morphological features (27, 28). However, the US quality is operator-dependent, which means a novice is more likely to miss or misinterpret suspicious lesions or not see every regional area closely (29).
In this study, the authors intended to determine the superiority of various statistical parameters of US alone, CT alone, PET/CT alone, US + CT, and US + PET/CT. The authors found that most parameters were similar in various imaging modalities, but slightly higher with PET/CT. Moreover, the sensitivity was slightly increased and the false-negative rate was decreased when neck US was combined with PET/CT. The false-negative rate is considered an important factor due to its impact on the patient’s survival. Consequently, the false-negative rate of PET/CT was the lowest among all the imaging modalities, and the false-negative rate of US + CT was lower than that of US alone or CT alone in detecting SCN metastases in breast cancer. However, the accuracy rate was highest when PET/CT was performed alone. This is due to the lower specificity of US + PET/CT (99.4%) than that of PET/CT alone (100%).
Although the NCCN guidelines do not include neck US, CT, and PET/CT for breast cancer surveillance, asymptomatic regional recurrence or distant metastasis cannot be detected without these imaging modalities. Because the tumor burden is highly correlated with the disease prognosis, additional cancer monitoring is necessary for patients at high risk of metastatic breast cancer. Although PET/CT alone would be enough for monitoring of SCN metastases in breast cancer, eventually US-guided FNAC or biopsy is necessary to confirm true metastasis. In addition, US + CT may be considered rather than US alone or CT alone, if PET/CT is not available.
The limitation of this study was that the images were not obtained on the same day. In several cases, CT or PET/CT was conducted after pathological results were confirmed, which may affect the interpretation of the CT or PET/CT images. In addition, FNAC was only selectively performed in patients with suspicious SCNs. Even on cases with suspicious SCNs identified on CT or PET/CT, US-guided FNAC could not be performed if the suspicious lymph nodes were not seen in US. For those cases, the physicians had to carefully follow up to determine any change in the lymph nodes within a 6-month or 1-year period.
In conclusion, the early detection of asymptomatic SCN metastasis in breast cancer is important to predict and improve the disease prognosis. Among the aforementioned imaging modalities, which are all good modalities in evaluating regional lymph node recurrence, PET/CT can be the first choice for detecting SCN metastasis in breast cancer. However, if PET/CT is unavailable for any reason, a combination of neck US and CT can be a good second option, instead of neck US or CT alone, in patients with breast cancer who are at high risk of metastasis or recurrent disease.
Main points.
Neck ultrasound, CT and 18F-FDG PET/CT are all useful imaging modalities for detection of metastatic supraclavicular lymph node in breast during surveillance.
Compared with pathologic results, combination of neck ultrasound and CT showed excellent results in terms of false negativity, even if the accuracy was calculated as 99.3%–99.5% in all three methods.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Rue M, Vilaprinyo E, Lee S, et al. Effectiveness of early detection on breast cancer mortality reduction in catalonia (spain) BMC Cancer. 2009;9:326. doi: 10.1186/1471-2407-9-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor R, Gregory M, Sexton K, et al. Breast cancer mortality and screening mammography in new zealand: Incidence-based and aggregate analyses. J Med Screen. 2019;26:35–43. doi: 10.1177/0969141318776039. [DOI] [PubMed] [Google Scholar]
- 3.McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in england. Brit J Cancer. 2015;112(Suppl 1):S108–115. doi: 10.1038/bjc.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narod SAIJ, Miller AB. Why have breast cancer mortality rates declined? J Cancer Policy. 2015;5:8–17. doi: 10.1016/j.jcpo.2015.03.002. [DOI] [Google Scholar]
- 5.Pedersen AN, Moller S, Steffensen KD, et al. Supraclavicular recurrence after early breast cancer: A curable condition? Breast Cancer Res Treat. 2011;125:815–822. doi: 10.1007/s10549-010-0918-8. [DOI] [PubMed] [Google Scholar]
- 6.Dellapasqua S, Bagnardi V, Balduzzi A, et al. Outcomes of patients with breast cancer who present with ipsilateral supraclavicular or internal mammary lymph node metastases. Clin Breast Cancer. 2014;14:53–60. doi: 10.1016/j.clbc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Lu WL, Jansen L, Post WJ, Bonnema J, Van de Velde JC, De Bock GH. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: A meta-analysis. Breast Cancer Res Treat. 2009;114:403–412. doi: 10.1007/s10549-008-0023-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen SC, Chang HK, Lin YC, et al. Prognosis of breast cancer after supraclavicular lymph node metastasis: Not a distant metastasis. Ann Surg Oncol. 2006;13:1457–1465. doi: 10.1245/s10434-006-9012-1. [DOI] [PubMed] [Google Scholar]
- 9.Moon HJ, Kim MJ, Kim EK, et al. US surveillance of regional lymph node recurrence after breast cancer surgery. Radiology. 2009;252:673–681. doi: 10.1148/radiol.2523081977. [DOI] [PubMed] [Google Scholar]
- 10.van Vliet EP, van der Lugt A, Kuipers EJ, et al. Ultrasound, computed tomography, or the combination for the detection of supraclavicular lymph nodes in patients with esophageal or gastric cardia cancer: A comparative study. J Surg Oncol. 2007;96:200–206. doi: 10.1002/jso.20819. [DOI] [PubMed] [Google Scholar]
- 11.Mao YHS, Harisinghani M. Radiologic assessment of lymph nodes in oncologic patients. Curr Radiol Rep. 2014;2:36. doi: 10.1007/s40134-013-0036-6. [DOI] [Google Scholar]
- 12.Goetz MP, Gradishar WJ, Anderson BO, et al. NCCN guidelines insights: Breast cancer, version 3.2018. J Natl Compr Canc Netw. 2019;17:118–126. doi: 10.6004/jnccn.2019.0009. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kim J, Moon HJ, et al. Supraclavicular lymph nodes detected by 18f-fdg pet/CT in cancer patients: Assessment with 18f-fdg pet/CT and sonography. AJR Am J Roentgenol. 2012;198:187–193. doi: 10.2214/AJR.11.6999. [DOI] [PubMed] [Google Scholar]
- 14.van Vliet EP, Steyerberg EW, Eijkemans MJ, Kuipers EJ, Siersema PD. Detection of distant metastases in patients with oesophageal or gastric cardia cancer: A diagnostic decision analysis. Brit J Cancer. 2007;97:868–876. doi: 10.1038/sj.bjc.6603960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sung YM, Lee KS, Kim BT, et al. Nonpalpable supraclavicular lymph nodes in lung cancer patients: Preoperative characterization with 18f-fdg pet/CT. AJR Am J Roentgenol. 2008;190:246–252. doi: 10.2214/AJR.07.2508. [DOI] [PubMed] [Google Scholar]
- 16.Ryu EBOK, Jeong KS. Supraclavicular lymph node metastasis from various malignancies: Assessment with 18f-fluorodeoxyglucose positron emission tomography/CT, contrast-enhanced CT and ultrasound. J Korean Soc Radiol. 2012;66:83–92. doi: 10.3348/jksr.2012.66.1.83. [DOI] [Google Scholar]
- 17.Lopez F, Rodrigo JP, Silver CE, et al. Cervical lymph node metastases from remote primary tumor sites. Head Neck. 2016;38(Suppl 1):E2374–2385. doi: 10.1002/hed.24344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SH, Lee J, Lee JE, et al. Local and regional recurrence following mastectomy in breast cancer patients with 1–3 positive nodes: Implications for postmastectomy radiotherapy volume. Radiat Oncol J. 2018;36:285–294. doi: 10.3857/roj.2018.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Gioia D, Stieber P, Schmidt GP, Nagel D, Heinemann V, Baur-Melnyk A. Early detection of metastatic disease in asymptomatic breast cancer patients with whole-body imaging and defined tumour marker increase. Brit J Cancer. 2015;112:809–818. doi: 10.1038/bjc.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Glas NA, Bastiaannet E, Engels CC, et al. Validity of the online predict tool in older patients with breast cancer: A population-based study. Brit J Cancer. 2016;114:395–400. doi: 10.1038/bjc.2015.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo HM, Lee EH, Ko K, et al. Prevalence of women with dense breasts in korea: Results from a nationwide cross-sectional study. Cancer Res Treat. 2019;51:1295–1301. doi: 10.4143/crt.2018.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Kwak JY, Choi JW, et al. Impact of US surveillance on detection of clinically occult locoregional recurrence after mastectomy for breast cancer. Ann Surg Oncol. 2010;17:2670–2676. doi: 10.1245/s10434-010-1087-z. [DOI] [PubMed] [Google Scholar]
- 23.Riegger C, Koeninger A, Hartung V, et al. Comparison of the diagnostic value of FDG-PET/CT and axillary ultrasound for the detection of lymph node metastases in breast cancer patients. Acta Radiol. 2012;53:1092–1098. doi: 10.1258/ar.2012.110635. [DOI] [PubMed] [Google Scholar]
- 24.Li XF, Du Y, Ma Y, Postel GC, Civelek AC. (18)F-fluorodeoxyglucose uptake and tumor hypoxia: Revisit (18)F-fluorodeoxyglucose in oncology application. Transl Oncol. 2014;7:240–247. doi: 10.1016/j.tranon.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprinz C, Altmayer S, Zanon M, et al. Effects of blood glucose level on 18F-FDG uptake for pet/CT in normal organs: A systematic review. PLoS One. 2018;13:e0193140. doi: 10.1371/journal.pone.0193140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uematsu T, Sano M, Homma K. In vitro high-resolution helical CT of small axillary lymph nodes in patients with breast cancer: Correlation of CT and histology. AJR Am J Roentgenol. 2001;176:1069–1074. doi: 10.2214/ajr.176.4.1761069. [DOI] [PubMed] [Google Scholar]
- 27.Guo R, Lu G, Qin B, Fei B. Ultrasound imaging technologies for breast cancer detection and management: A review. Ultrasound Med Bio. 2018;44:37–70. doi: 10.1016/j.ultrasmedbio.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright T, McGechan A. Breast cancer: New technologies for risk assessment and diagnosis. Mol Diagn. 2003;7:49–55. doi: 10.2165/00066982-200307010-00009. [DOI] [PubMed] [Google Scholar]
- 29.Henderson RE, Walker BF, Young KJ. The accuracy of diagnostic ultrasound imaging for musculoskeletal soft tissue pathology of the extremities: A comprehensive review of the literature. Chiropractic Manual Ther. 2015;23:31. doi: 10.1186/s12998-015-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]