Abstract
Caffeine improves short-to-moderate distance running performance, but the effect of caffeine on repeated sprints are equivocal. This research determined if caffeine improved exercise tolerance during repeated-sprint exercise. iCV is a running velocity that distinguishes intermittent running velocities (velocities ≤ iCV) that are sustainable from those resulting in a predictable time to exhaustion (velocities > iCV). Seven physically active men (age = 21.6 ± 1.5 years, body mass = 72.8 ± 5.1 kg, VO2max = 56.9 ± 9.8 mL/kg/min) ingested caffeine (5 mg/kg) or placebo (crossover design) 60 min prior to an intermittent critical velocity (iCV) test. The treadmill grade and velocity at VO2max (vVO2max) were used for iCV testing, and consisted of 3 bouts (10 sec running and 10 sec passive rest) at 130, 110 and 120% vVO2max. Each bout continued until volitional exhaustion and was separated by 20 min of passive rest. Total distance and duration were recorded to determine exercise tolerance using the iCV model. Caffeine ingestion increased running duration at 110% vVO2max (p = 0.02), but not at 120 (p = 0.93) and 130% vVO2max (p = 0.14). Caffeine did not improve iCV model parameters. A single dose of caffeine consumed 60 min before repeated-sprints can improve performance at 110% vVO2max, but not at higher velocities.
Keywords: repeated-sprint ability, dietary supplements, ergogenic aids, intermittent exercise, running
INTRODUCTION
Many team-sport athletes perform repeated maximal or near-maximal effort sprints interspersed with recovery over 1–4 h, known as repeated sprint exercise (RSE) (14). Resistance to fatigue during RSE is known as repeated-sprint ability (RSA) (14), and characterized by a reduction in velocity/power output from a series of short (< 10 sec) sprints with brief recovery intervals (> 30 sec) (7, 11). RSA provides evidence for the presence of fatigue, but it fails to define a threshold where fatigue increases during RSE (11). A well-known feature of steady-state high-intensity exercise is the curvilinear relationship between running velocity and time to exhaustion, termed critical velocity or critical speed (5). This has been modeled to RSE, and identifies a novel fatigue threshold known as the intermittent critical velocity (iCV) (12, 13). iCV is a running velocity that distinguishes intermittent running velocities (velocities ≤ iCV) that are sustainable from those resulting in a predictable time to exhaustion (velocities > iCV). iCV, therefore, represents the boundary of exercise tolerance during intermittent high-intensity running (12, 13). The iCV model also describes an intermittent anaerobic running capacity (iARC) and critical rest interval (CRI). The iARC represents a finite distance that can be achieved before the accumulation of metabolites associated with fatigue, while CRI is a theoretical rest period that will enable RSE to continue without the onset of fatigue (12, 13). These measures are determined from a series of maximal effort bouts interspersed with short rest intervals. This approach may have utility since athletes engage in similar intensity efforts during high-quality intermittent exercise training sessions (31).
Caffeine is widely used by athletes before competition to improve endurance performance, muscular strength and power, and augment exercise training adaptations (8, 9, 15, 17, 34). Up to 89% of competitive athletes consume caffeine, and trained participants report daily consumption > 300 mg day (8, 27). There are several mechanisms that may be responsible for caffeine’s ergogenic properties. As an adenosine-receptor antagonist, caffeine reduces perception of pain and exertion (15, 22). Caffeine has also been reported to augment blood flow and muscle oxygenation by activation of endothelial nitric oxide synthase (32, 38). In addition, caffeine improves muscle function by modifying K+ and Ca2+ kinetics (1, 23). The dose that has typically been tested on running performance is 3–10 mg/kg body mass consumed 60 min before the activity (9, 15, 27). While this dosing strategy appears to be effective when running for 20–45 min, the benefits of caffeine may not extend to longer duration running events (9). This may be due to the pharmacokinetics of caffeine, i.e. peak plasma concentrations are achieved within 45 min of oral ingestion, and the half-life is 3–4 h (19).
A single dose of caffeine (300 mg) ingested by recreationally active males before iCV testing did not improve RSE (36). Limited by the study design, the caffeine supplement contained other compounds and this may have interfered with caffeine’s metabolism (24, 36). Therefore, it remains unknown if caffeine, alone, improves iCV model parameters. The objective of this research was to establish if a moderate dose of caffeine consumed 60 min before iCV testing improves RSE performance. Our hypothesis was that caffeine would extend running time at VO2max velocities, improve iCV parameters and decrease ratings of perceived exertion (RPE).
METHODS
Participants
Seven physically active men volunteered for the study (Table 1). Participants completed a health-history questionnaire, and were disqualified from study participation if they had cardiovascular, pulmonary, muscular, or metabolic disease; acute or chronic muscle pain or injury; suffered from seizures; were not between 18–25 years old; had a pacemaker or other internal device; followed a specialized or restricted diet; had unexplained weight loss in the past 6 months; or experienced adverse events after caffeine consumption. The Department of Defense Health-related behaviors survey was also used to verify that participants met aerobic physical activity guidelines (Table 1). All participants completed a self-reported 7 d caffeine recall to determine daily caffeine consumption (Table 1). On average, the participants in this study were regular caffeine consumers, but consumed less caffeine than described in trained participants (≥ 300 mg/d) (26, 28). Dietary intake prior to iCV testing was reported with a 24-h dietary recall using the Automated Self-Administered 24-h Dietary Assessment Tool (ASA24) developed by the National Cancer Institute (Bethesda, MD). Total energy and macronutrient intake were not significantly different between the caffeine (total energy 3036 ± 753 kcal; carbohydrate 297 ± 84 g; protein 143.0 ± 17.8 g; fat 143.0 ± 27.2 g) and placebo sessions (total energy 3217 ± 899 kcal, p = 0.51; carbohydrate 424 ± 104 g, p = 0.11; protein 154.4 ± 32.6, p = 0.54; fat 110.9 ± 18.7 g, p = 0.18). Each participant was briefed on the procedures and risks associated with study participation before providing written informed consent. The study was approved by the Kansas State University Institutional Review Board (#9607).
Table 1.
Participant Characteristics (N = 7)
| Age (years) | Height (cm) | Body mass (kg) | Body Mass Index (kg/m2) | Body Fat (%) | VO2max (mL/kg/min) | Caffeine consumption (mg/day) | Moderate physical activity (min/week) | Vigorous physical activity (min/week) |
|---|---|---|---|---|---|---|---|---|
| 21.6±1.5 | 179.7±6.8 | 72.8±5.1 | 22.5±0.9 | 14.0±2.2 | 56.9±9.8 | 98.3±95.4 | 283.8±124.9 | 124.0±116.3 |
Data are presented as mean ± standard deviation.
Protocol
A double-blind, counterbalanced, crossover design was employed to determine the effects of acute caffeine supplementation on exercise tolerance during RSE using the iCV model. The design was chosen to minimize participant recruitment needs while achieving adequate statistical power. Eight participants were needed based on a large effect size of caffeine supplementation (0.8), at α = 0.05 with 80% power (39). Caffeine and placebo (biotin) pills were used based on recommendations from a registered dietitian, and a moderate dose of caffeine (5 mg/kg body mass) was selected based on previous investigations (37). The iCV model was used to evaluate exercise tolerance during RSE because it is reliable and has been previously used in other ergogenic evaluations (13, 36). Participants visited the laboratory three times over two weeks. All testing was scheduled between 8:00 AM and 12:00 PM. Participants were encouraged to continue their normal exercise and dietary habits during the study. Participants were instructed to refrain from caffeine and alcohol for 12 h, and vigorous physical activity for 24 h before testing. Additionally, participants refrained from eating 2–3 h prior to VO2max testing. Participants were provided a standardized meal (Boost™ meal replacement shake) 3 h prior to each iCV test that comprised ~20% of their estimated total energy expenditure (18). Multiple servings of the meal replacement shake (kcal = 240, carbohydrate = 41 g, protein = 10 g, fat = 4 g) were used to satisfy the estimated energy expenditure when necessary. A list of the meal replacement shake ingredients is provided in Supplement A. All procedures and measurements performed were compliant with the International Journal of Exercise Science guidelines (25).
Anthropometric Measurements
Height was measured using a stadiometer. Body mass, body mass index, basal metabolic rate, and percent fat were determined using bioelectrical impedance analysis in standard mode (TBF-300A; Tanita, Japan).
VO2max protocol
On their first laboratory visit, participants performed a graded exercise test (GXT) to volitional exhaustion on a treadmill (Woodway Pro, Waukesha, WI) to determine VO2max and velocity at VO2max (vVO2max). The GXT consisted of two 3-min warm-up stages at 4- and 5 km/h. Treadmill velocity was set to 6 – 10 km/h, based on participant’s reported level of fitness, and increased by 0.5 km/h every min until 95% of the predicted maximal heart rate (220- age) was achieved. The velocity was then decreased by 1.0 km/h and the grade increased by 1.0% every min until volitional exhaustion. VO2max was confirmed using a validation protocol after 15–20 min of passive recovery (29). Briefly, participants lowered themselves onto the treadmill set at the highest grade, and 110% of vVO2max achieved during the GXT. VO2 was recorded using open circuit spirometry (TrueOne 2400, Parvo-Medics, Waukesha, WI) and measured continuously throughout the GXT and the VO2max validation tests. The three highest 15-s VO2 values were averaged and considered the VO2max values for the GXT and VO2max validation protocols. VO2max was considered valid if the VO2 obtained on the VO2max validation test was less than 0.2 L/min higher than that achieved during the GXT. All participants performed the validation test and achieved their VO2max.
Supplementation protocol
Participants returned to the laboratory for the second visit after a minimum of 48 h. Participants consumed caffeine (5 mg/kg) or a biotin placebo (Solgar, 300 μg) 60 min before testing. The placebo was indistinguishable from the caffeine pill. After ingesting the supplement and prior to warming-up for the iCV test, participants completed the dietary recall. Participants returned to the laboratory after at least a 7 d washout period to repeat exercise testing with the other supplement.
iCV protocol
The iCV test protocol followed the same procedures used in the reliability study conducted by Fukuda and colleagues (13). In order to minimize learning effects and ensure safety, participants were familiarized with brief intermittent running bouts, and then completed a 5 min warm-up at a self-selected pace. The treadmill (Woodway Pro, Waukesha, WI) grade was set to the highest level obtained during the GXT for iCV testing. Participants completed 3 sets at 130-, 110- and 120% vVO2max interspersed by 20 min of passive recovery, each to volitional fatigue. As specified by Fukuda and colleagues (13), the order of iCV test running bouts were not randomized across participants. Each set consisted of 10 sec of running followed by 10 sec of passive recovery. Water was available ad libitum during passive rest periods. Participants mounted and dismounted the treadmill (Woodway Pro, Waukesha, WI, USA) by lowering themselves onto the treadmill belt during running bouts and straddling the treadmill during passive rest.
Participants repeated 10 sec running bouts during each set until they could no longer maintain the selected velocity or complete the full 10 sec running bout. The total duration (running + rest), running duration only, the number of running bouts (determined by lap counter), and total distance covered were determined for each set. The linear, total distance model (L-TD) (13) was used to determine iCV and iARC with the following equation, where total distance was (time × velocity) and time was the exercise duration:
The iCV represents the slope of the regression line of exercise distance and duration, while iARC represents the y-intercept.
CRI was determined with the following equation, where TD, ∑INT and iCVCRI represented the total distance of all sets, the total number of running bouts completed in all sets, and the slope of the linear regression between total distance and the total duration of the exercise bout (exercise + rest), respectively:
The L-TD model was also used to establish an alternative iCV to determine the CRI (iCVCRI):
RPE and Adverse Event Measurements
Participants reported their ratings of perceived exertion (RPE) using the 15-point Borg scale after each set (4). RPE was averaged for each iCV test. Participants completed a brief questionnaire immediately after the last exercise bout and indicated, “yes” or “no”, if they perceived an effect from the supplement that was provided. An online survey was used to determine if any adverse events occurred in the immediate hours after testing and the following day. Participants were asked to complete surveys prior to bed and upon awakening.
Statistical Analysis
Data were analyzed with SPSS version 25.0 (IBM, Armonk, NY, USA). The level of significance was set at α = 0.05. Three volunteers did not complete the study due to scheduling conflicts (n = 1) or failure to follow-up after testing (n = 2), leaving a final sample of seven participants. Normality was determined using a Kolmogorov-Smirnov test. Exercise duration (110, 120, and 130% of vVO2max), RPE, iCV, iARC, and CRI were normally distributed and analyzed using a paired t-test, and reported as means ± SD and 95% CI. iCV and iARC display the highest reliability (ICC = 0.89, SEM = 0.2 m/s, CV = 12.4–15.4%; ICC = 0.80, SEM = 28.6 m, CV = 45.2–49.9%) and CRI displays moderate reliability (ICC = 0.59, SEM = 1.5 s, CV = 8.2–10.7%) (13). Hedge’s g was used to indicate effect size (ES) and was classified as “trivial” (< 0.19), “small” (0.20–0.49), “moderate” (0.50–0.79), and “large” (> 0.80) (10). Adverse events measures were reported as percentage of individuals reporting an event in each condition.
RESULTS
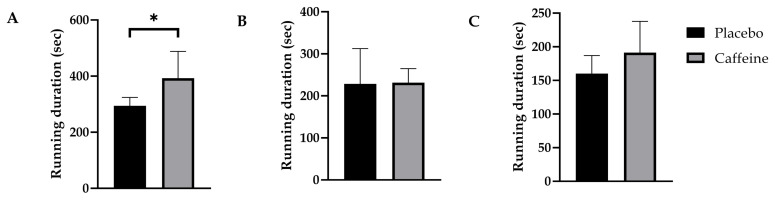
Participant characteristics are shown in Table 1. Of the running velocities tested, caffeine extended mean running time by 99 sec at 110 vVO2max (placebo 294 ± 29 sec, 95%CI [267, 322]; caffeine 393 ± 95 sec, 95%CI [304, 481]; p = 0.020, g = 1.4) (Figure 1). While there were no significant differences between placebo and caffeine at 120% (placebo 229 ± 84 sec, 95%CI [151, 306]; caffeine 231 ± 33.4 sec, 95%CI [201, 262]; p = 0.93, g = 0.045) or 130% vVO2max (placebo 160 ± 10 sec, 95%CI [135, 185]; caffeine 191 ± 46 sec, 95%CI [149, 234]; p = 0.14, g = 0.83), 6 of the 7 participants increased their running duration at 130% vVO2max.
Figure 1.
Differences in exercise duration during the iCV test between placebo and caffeine conditions (N = 7). (A) Exercise duration during 110% of PV bout. (B) Exercise duration during 120% of PV bout. (C) Exercise duration during 130% of PV bout. Data are presented as means ± SD. * Denotes significant differences (p < 0.05) between the caffeine and placebo condition.
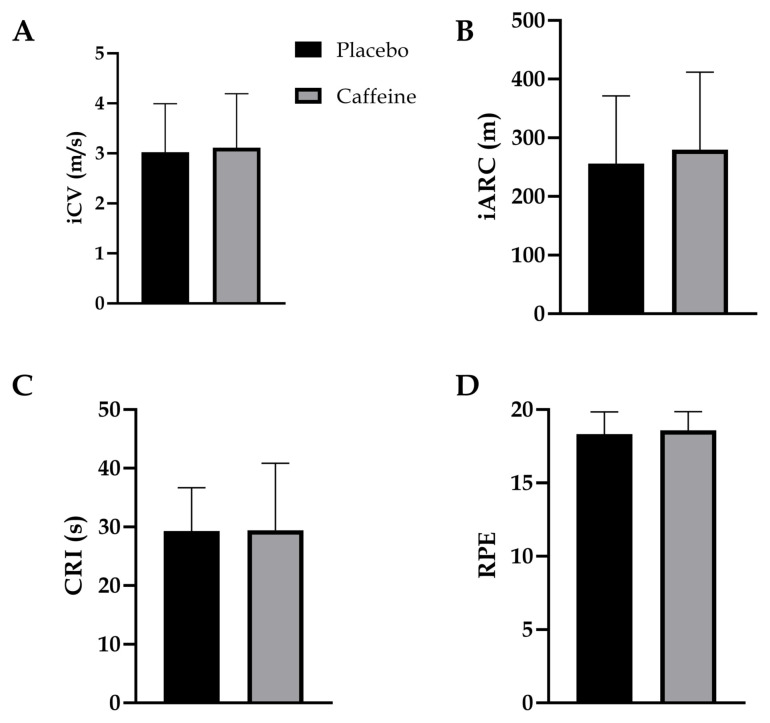
Figure 2 illustrates iCV, iARC, CRI or RPE between conditions. iCV was not significant different between the caffeine (3.11 ± 1.1 m/s, 95%CI [2.1, 4.1]) and placebo condition (3.02 ± 0.97, 95%CI [2.1, 3.9], p = 0.81, g = 0.08). iARC was not significantly different between the caffeine (280.0 ± 132.1 m, 95%CI [157.8, 402]) and placebo condition (256.1 ± 115.2 m, 95%CI [149.5, 362.6], p = 0.63, g = 0.180). CRI was not significantly different between the caffeine (29.4 ± 11.4 s, 95%CI [18.8, 40.0]) and placebo condition (29.3 ± 7.4 s, 95%CI [22.5, 36.1], p = 0.97, g = 0.009). RPE was not significantly different between the caffeine (18.3 ± 1.5, 95%CI [16.9, 19.7]) and the placebo condition (18.6 ± 1.3, 95%CI [17.4, 19.8], p = 0.16, g = 0.20). Participants reported a perceived effect of the supplement (85.7% vs. 28.6%), increased activeness (42.9% vs. 14.3%) and gastrointestinal problems (28.2% vs. 0%) with caffeine compared to placebo. Additionally, one participant reported indigestion (14.3%) after consuming the placebo.
Figure 2.
iCV parameters and ratings of perceived exertion between placebo and caffeine conditions (N = 7). (A) Intermittent critical velocity. (B) Intermittent Anaerobic Running Capacity. (C) Critical rest interval. (D) Ratings of perceived exertion. Data are presented as means ± SD. No significant differences were found between conditions.
DISCUSSION
The purpose of our investigation was to determine the effect of acute caffeine supplementation on exercise tolerance during RSE in physically active males utilizing the iCV model. The ability to resist fatigue during RSE is a key attribute to many sporting disciplines, where athletes are reported to use ergogenic aids (8, 14). Improvements in iCV and CRI reflect resilience to fatigue and rapid recovery during RSE, respectively (12). In this study, we determined that ingesting 5 mg/kg of caffeine 60 min before iCV testing increased running duration at 110% vVO2max, but not at 120- or 130%, or in any parameter of exercise tolerance, or RPE during RSE.
Our findings, similar to other reports, suggest that caffeine supplementation may improve some, but not all sprints during RSE (4, 8, 22, 23). Extending the duration of high-intensity running, increases the distance covered by an athlete. Interestingly, Del Coso et al. (2012) reported that caffeine improved the distance covered at the end of the first half of a simulated soccer match, and at some running intensities but not all. Our investigation found that caffeine improved the second set of sprints; thus, caffeine may improve RSE in a time and intensity dependent manner. The utility of improving the exercise tolerance at 110% of vVO2max may have implications for middle distance athletes since 110%vVO2max is a similar running velocity attained during high-intensity intermittent training sessions (31).
In the previous study by Spradley and colleagues (36), the highest running velocity used during iCV testing was at 110% of vVO2max, yet they did not find an ergogenic effect like we did at the same velocity. Caffeine extends exercise duration between 75–85% of vVO2max during continuous exercise; however, it is unknown if caffeine improved RSE at exercise intensities below 110% of vVO2max (9). The investigation by Spradley and colleagues (2012) reported that a multi-ingredient pre-workout supplement containing an absolute dose of 300 mg of caffeine (< 4 mg/kg of body weight) did not improve iCV or iARC. It is possible that the dose of caffeine used by Spradley and the current investigation was insufficient to cause an increase in exercise tolerance to RSE. Investigators have reported that ergogenic doses of caffeine range from 3–10 mg/kg body mass (9, 15, 28), but for RSA a dose found to be ergogenic was 6 mg/kg body mass (20, 21). Thus, higher doses of caffeine may be necessary to offset the perturbations to the muscle milieu during RSE by increasing calcium release from the sarcoplasmic reticulum and retaining potassium ions (1, 14, 23).
Moderate-doses of caffeine act on multiple target tissues to antagonize adenosine receptors (A1 and A2A receptors), which decrease RPE (22). Our study found no significant difference in RPE after acute caffeine supplementation, similar to other reports (2, 37). In some investigations, caffeine results in an increase in performance with no changes in RPE (2, 35). This suggests that caffeine is ergogenic by maintaining RPE at higher workloads, which is reported to enhance exercise training adaptations when caffeine-containing products are consumed prior to training sessions (34).
The current investigation employed a study design with participants serving as their own controls, provided standardized meals prior to iCV testing, and was the first study to determine the effects of acute caffeine supplementation on exercise tolerance during RSE using the iCV model; all of which, contributed to the study’s strengths. However, by only recruiting young, physically active men, our results cannot be generalized to women, middle-age adults, or participants with lower physical activity levels. Caffeine metabolism is prolonged by estrogen and oral contraceptives, however, improvements in performance have been reported to be similar between sexes (35). Additionally, a large effect size was used to determine sample size and a larger number of participants would have allowed for the determination of a small effect during RSE. By failing to recruit enough participants to achieve adequate statistical power, these findings may be prone to increased type 2 error (i.e., false negative) and merits further investigation.
Although our study lacked invasive measures to determine plasma caffeine concentration and caffeine metabolism, the likelihood that caffeine supplementation from caffeinated pills increases plasma caffeine concentration has substantial support given that it is used as a standard when comparing caffeinated products (19). Interestingly, caffeine is likely ergogenic in the absence of elevated plasma caffeine concentrations, as suggested by caffeine’s ergogenic properties when mouth rinsing caffeinated fluids (3). However, caffeine may not be ergogenic for all athletes, which may be in part associated with genetic influences from the CYP1A2 and ADORA2A polymorphisms (30, 40). The CYP1A2 gene encodes cytochrome P450 1A2, an enzyme responsible for 95% of caffeine metabolism and determines the pharmacokinetic response of caffeine metabolism (16). Carriers of the C allele (slow metabolizers) in the CYP1A2 gene have been characterized as “non-responders” after caffeine supplementation, although these findings are preliminary with conflicting reports (16, 30). We were not able to characterize the genetic differences among our participants, which presents an avenue for future research.
Almost 90% of participants indicated a perceived effect from the supplement during the caffeine condition which may indicate poor blinding to the treatment conditions. Despite reports of increased effect from participants correctly identifying a supplement with an active ingredient, we failed to confirm these findings via their RSE performance (33). Lastly, while our iCV protocol replicated Fukuda and colleagues (13), the authors did not provide rationale for the 130%, 110%, and 120% PV testing order; results may differ if the running set intensities were randomized between participants.
RSA is a key attribute among many team sport athletes and remains a priority for strength and conditioning specialists and coaches. Our findings suggest that an acute dose of caffeine provides significant improvements in tolerance to RSE in physically active men at 110% of vVO2max, but not at higher velocities, nor did caffeine have any effect on RPE. Our RSA protocol utilized exhaustive RSE, which may replicate prolonged gameplay (i.e., overtime) or some highintensity interval training sessions.
Supplement A - Meal replacement shake ingredients
Water, Glucose Syrup, Sugar, Milk Protein Concentrate, And Less Than 2% Of Vegetable Oil (Canola, High Oleic Sunflower, Corn) Vitamins And Minerals‡, Soy Protein Isolate, Gum Acacia, Fructooligosaccharides, Inulin (From Chicory), Cellulose Gel And Gum, Salt, Soy Lecithin, Natural And Artificial Flavor, Carrageenan, Stevia Leaf Extract
‡Vitamins And Minerals: Magnesium Phosphate, Potassium Citrate, Potassium Chloride, Calcium Carbonate, Sodium Ascorbate, Choline Bitartrate, Calcium Phosphate, Ferrous Sulfate, Dl-Alpha Tocopheryl Acetate, Zinc Sulfate, Niacinamide, Calcium Pantothenate, Manganese Sulfate, Pyridoxine Hydrochloride, Riboflavin, Thiamine Hydrochloride, Vitamin A Palmitate, Copper Sulfate, Folic Acid, Potassium Iodide, Vitamin K1, Chromium Chloride, Sodium Selenite, Biotin, Sodium Molybdate, Vitamin D3, Vitamin B12
REFERENCES
- 1.Allen DG, Westerblad H. The effects of caffeine on intracellular calcium, force and the rate of relaxation of mouse skeletal muscle. J Physiol. 1995;487:331–342. doi: 10.1113/jphysiol.1995.sp020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astorino TA, Cottrell T, Talhami Lozano A, Aburto-Pratt K, Duhon J. Effect of caffeine on RPE and perceptions of pain, arousal, and pleasure/displeasure during a cycling time trial in endurance trained and active men. Physiol Behav. 2012;106:211–217. doi: 10.1016/j.physbeh.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Beaven CM, Maulder P, Pooley A, Kilduff L, Cook C. Effects of caffeine and carbohydrate mouth rinses on repeated sprint performance. Appl Physiol Nutr Metab. 2013;38:633–637. doi: 10.1139/apnm-2012-0333. [DOI] [PubMed] [Google Scholar]
- 4.Borg G. Psychophysical bases of perceived exertion. Med Sci Sport Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 5.Broxterman RM, Ade CJ, Poole DC, Harms CA, Barstow TJ. A single test for the determination of parameters of the speed-time relationship for running. Respir Physiol Neurobiol. 2013;185:380–385. doi: 10.1016/j.resp.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Del Coso J, Muñoz-Fernández VE, Muñoz G, Fernández-Elías VE, Ortega JF, Hamouti N, et al. Effects of a Caffeine-Containing Energy Drink on Simulated Soccer Performance. PLoS One. 2012;7:e31380. doi: 10.1371/journal.pone.0031380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Coso J, Portillo J, Muñoz G, Abián-Vicén J, Gonzalez-Millán C, Muñoz-Guerra J. Caffeine-containing energy drink improves sprint performance during an international rugby sevens competition. Amino Acids. 2013;44:1511–1519. doi: 10.1007/s00726-013-1473-5. [DOI] [PubMed] [Google Scholar]
- 8.Desbrow B, Leveritt M. Awareness and use of caffeine by athletes competing at the 2005 Ironman Triathlon World Championships. Int J Sport Nutr Exerc Metab. 2006;16:545–558. doi: 10.1123/ijsnem.16.5.545. [DOI] [PubMed] [Google Scholar]
- 9.Doherty M, Smith PM. Effects of caffeine ingestion on exercise testing: A meta-analysis. Int J Sport Nutr Exerc Metab. 2004;14:626–646. doi: 10.1123/ijsnem.14.6.626. [DOI] [PubMed] [Google Scholar]
- 10.Durlak JA. How to Select, Calculate, and Interpret Effect Sizes. J Pediatr Psychol. 2009;34:917–928. doi: 10.1093/jpepsy/jsp004. [DOI] [PubMed] [Google Scholar]
- 11.Evans M, Tierney P, Gray N, Hawe G, Macken M, Egan B. Acute ingestion of caffeinated chewing gum improves repeated sprint performance of team sport athletes with low habitual caffeine consumption. Int J Sport Nutr Exerc Metab. 2018;28:221–227. doi: 10.1123/ijsnem.2017-0217. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda D, Smith A, Kendall K, Cramer J, Stout J. The Determination of Critical Rest Interval from the intermittent Critical Velocity Test in Club-Level Collegiate Hockey and Rugby Players. J Strength Cond Res. 2011;25:889–895. doi: 10.1519/JSC.0b013e31820f5036. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda D, Smith AE, Kendall KL, Hetrick RP, Hames RL, Cramer JT, et al. The reliability of the intermittent critical velocity test and assessment of critical rest interval in men and women. Eur J Appl Physiol. 2012;112:1197–1205. doi: 10.1007/s00421-011-2076-z. [DOI] [PubMed] [Google Scholar]
- 14.Girard O, Mendez-villanueva A, Bishop DJ. Repeated-Sprint Ability Part I : Factors Contributing to Fatigue Repeated-Sprint Ability – Part I Factors Contributing to Fatigue. 2011 doi: 10.2165/11590550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Grgic J, Mikulic P, Schoenfeld BJ, David ·, Bishop J, Pedisic Z. The Influence of Caffeine Supplementation on Resistance Exercise: A Review Key Points. 2019;49:17–30. doi: 10.1007/s40279-018-0997-y. [DOI] [PubMed] [Google Scholar]
- 16.Grgic J, Pickering C, Bishop DJ, Schoenfeld BJ, Mikulic P, Pedisic Z. CYP1A2 genotype and acute effects of caffeine on resistance exercise, jumping, and sprinting performance. J Int Soc Sports Nutr. 2020;17:1–11. doi: 10.1186/s12970-020-00349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grgic J, Trexler ET, Lazinica B, Pedisic Z. Effects of caffeine intake on muscle strength and power: A systematic review and meta-analysis. J Int Soc Sports Nutr. 2018;15:1–10. doi: 10.1186/s12970-018-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James WPT, Schofield EC. Human energy requirements. A manual for planners and nutritionists. Hum energy Requir A Man planners Nutr. 1990 [Google Scholar]
- 19.Kamimori GH, Karyekar CS, Otterstetter R, Cox DS, Balkin TJ, Belenky GL, et al. The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. 2002 doi: 10.1016/s0378-5173(01)00958-9. [DOI] [PubMed] [Google Scholar]
- 20.Lee CL, Cheng CF, Lin JC, Huang HW. Caffeine’s effect on intermittent sprint cycling performance with different rest intervals. Eur J Appl Physiol. 2012;112:2107–2116. doi: 10.1007/s00421-011-2181-z. [DOI] [PubMed] [Google Scholar]
- 21.Lee CL, Lin JC, Cheng CF. Effect of caffeine ingestion after creatine supplementation on intermittent high-intensity sprint performance. Eur J Appl Physiol. 2011;111:1669–1677. doi: 10.1007/s00421-010-1792-0. [DOI] [PubMed] [Google Scholar]
- 22.McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. 2016;71:294–312. doi: 10.1016/j.neubiorev.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Mohr M, Nielsen JJ, Bangsbo J. Caffeine intake improves intense intermittent exercise performance and reduces muscle interstitial potassium accumulation. J Appl Physiol. 2011;111:1372–1379. doi: 10.1152/japplphysiol.01028.2010. [DOI] [PubMed] [Google Scholar]
- 24.Naderi A, Earnest CP, Lowery RP, Wilson JM, Willems MET. Co-ingestion of Nutritional Ergogenic Aids and High-Intensity Exercise Performance. Sport Med. 2016;46:1407–1418. doi: 10.1007/s40279-016-0525-x. [DOI] [PubMed] [Google Scholar]
- 25.Navalta J, Stone W, Lyons S. Ethical Issues Relating to Scientific Discovery in Exercise Science. Int J Exerc Sci. 2019;12:1. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor P, Motl R, Broglio S, Ely M. Dose-dependent effect of caffeine on reducing leg muscle pain during cycling exercise is unrelated to systolic blood pressure. Pain. 2004;109:291–298. doi: 10.1016/j.pain.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Pickering C, Kiely J. Are the Current Guidelines on Caffeine Use in Sport Optimal for Everyone? Interindividual Variation in Caffeine Ergogenicity, and a Move Towards Personalised Sports Nutrition. Sport Med. 2017;48 doi: 10.1007/s40279-017-0776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickering C, Kiely J. What Should We Do About Habitual Caffeine Use in Athletes ? Sport Med. 2018:1–10. doi: 10.1007/s40279-018-0980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poole D, Jones AM, Jones AM. Validity of criteria for establishing maximal O2 uptake during ramp exercise test during ramp exercise tests. Eurpean J Appl Physiol. 2008;102:403–410. doi: 10.1007/s00421-007-0596-3. [DOI] [PubMed] [Google Scholar]
- 30.Puente C, Abián-Vicén J, Coso J, Del Lara B, Salinero JJ. The CYP1A2 -163C>A polymorphism does not alter the effects of caffeine on basketball performance. PLoS One. 2018;13:1–14. doi: 10.1371/journal.pone.0195943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson GP, Killer SC, Stoyanov Z, Stephens H, Read L, James LJ, Bailey SJ. Influence of dietary nitrate supplementation on high-intensity intermittent running performance at different doses of normobaric hypoxia in endurance-trained males. International Journal of Sport Nutrition and Exercise Metabolism. 2020;31(1):1–8. doi: 10.1123/ijsnem.2020-0198. [DOI] [PubMed] [Google Scholar]
- 32.Ruíz-Moreno C, Lara B, Brito de Souza D, Gutiérrez-Hellín J, Romero-Moraleda B, Cuéllar-Rayo Á, et al. Acute caffeine intake increases muscle oxygen saturation during a maximal incremental exercise test. Br J Clin Pharmacol. 2019:1–7. doi: 10.1111/bcp.14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders B, de Oliveira LF, da Silva RP, de Salles Painelli V, Gonçalves LS, Yamaguchi G, et al. Placebo in sports nutrition: a proof-of-principle study involving caffeine supplementation. Scand J Med Sci Sport. 2017;27:1240–1247. doi: 10.1111/sms.12793. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz NA, McKinley-Barnard SK, Blahnik ZJ. Effect of Bang® Pre-Workout Master Blaster® combined with four weeks of resistance training on lean body mass, maximal strength, mircoRNA expression, and serum IGF-1 in men: A randomized, double-blind, placebo-controlled trial. J Int Soc Sports Nutr. 2019;16:1–15. doi: 10.1186/s12970-019-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skinner TL, Desbrow BEN, Arapova J, Schaumberg MA, Osborne J, Grant GD, et al. Women Experience the Same Ergogenic Response to Caffeine as Men. 2019 doi: 10.1249/MSS.0000000000001885. [DOI] [PubMed] [Google Scholar]
- 36.Spradley BD, Crowley KR, Tai C-Y, Kendall KL, Fukuda DH, Esposito EN, et al. Ingesting a pre-workout supplement containing caffeine, B-vitamins, amino acids, creatine, and beta-alanine before exercise delays fatigue while improving reaction time and muscular endurance. 2012 doi: 10.1186/1743-7075-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein JA, Ramirez M, Heinrich KM. Acute caffeine supplementation does not improve performance in trained crossfit® athletes. Sports. 2020;8 doi: 10.3390/sports8040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umemura T, Ueda K, Nishioka K, Hidaka T, Takemoto H, Nakamura S3, et al. Effects of Acute Administration of Caffeine on Vascular Function. Am J Cardiol. 2006;98:1538–1541. doi: 10.1016/j.amjcard.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 39.Warren G, Park N, Maresca R, Mckibans K, Millard-Stafford M. Effect of Caffeine Ingestion on Muscular Strength and Endurance: A Meta-Analysis. Med Sci Sport Exerc. 2010;42:1375–1387. doi: 10.1249/MSS.0b013e3181cabbd8. [DOI] [PubMed] [Google Scholar]
- 40.Womack CJ, Saunders MJ, Bechtel MK, Bolton DJ, Martin M, Luden ND, et al. The influence of a CYP1A2 polymorphism on the ergogenic effects of caffeine. J Int Soc Sport Nutr. 2012;9:1–6. doi: 10.1186/1550-2783-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]