Abstract
Athletes with cervical level spinal cord injuries (SCI) have an impaired ability to thermoregulate during exercise, leading to an increased core temperature (Tcore) due to a decrease in sweat response. Elevated Tcore may result in premature onset of fatigue and decreased athletic performance. Therefore, precooling techniques that decrease Tcore before exercise may increase the storage capacity for metabolic heat production, thereby delaying the time before reaching a critically high Tcore. The purpose of this study was to investigate the effects of pre-exercise ice slurry ingestion as a precooling method in elite athletes with SCI during a wheelchair rugby match simulation. Employing a field-based, counterbalanced-design, participants were administered 6.8 g/kg of room temperature (PLB) or ice slurry (IS) beverage during a 20-minute precooling period, before engaging in a 50 and 60 minute on-court training session on day 1 and 2, respectively. Physiological measures, including Tcore and heart rate, and perceptual measures including gastrointestinal and thermal comfort, and rating of perceived exertion, were monitored throughout precooling (minutes 10, 20) and exercise (minutes 10–60). IS had a large effect on Tcore at the midpoint of exercise on day 1 (minute 30) (ES=0.73) and 2 (minute 40) (ES=1.17). Independent samples T-tests revealed significant differences in the perception of thermal comfort between IS and PLB at the midpoint of exercise on day 1 (minute 30) (p=0.04), but not day 2 (minute 40) (p=0.05), indicating that IS may help participants to feel cooler during exercise. Although further research is warranted, pre-exercise ice slurry ingestion may provide an effective means for delaying an increase in Tcore in some athletes with SCI during a wheelchair rugby match.
Keywords: Thermoregulation, cooling, tetraplegia
INTRODUCTION
Athletes with spinal cord injuries (SCI) face challenges unlike those of able-bodied (AB) persons, including altered sweat rates, delayed gastric emptying, smaller working muscle mass, and altered blood flow redistribution, due to physiologic alterations and adaptations as a result of injury (7, 20). Reduction in the afferent and efferent signals of the sympathetic nervous system due to the loss of the supraspinal control, regulated by the hypothalamus results in greater increases in core temperature in athletes with complete and incomplete SCI compared to their AB counterparts (9). Individuals with the highest level and completeness of SCI may experience greater degrees and severity of physiologic changes as compared to individuals with lower level SCI. Research has indicated that individuals with tetraplegia with a complete lesion at C 5/6 exhibit more than a two-fold difference in thermometrically-estimated heat storage as compared to individuals with lesions at T1 and L1 (4). Furthermore, athletes with SCI have altered autonomic nervous system control, resulting in a reduced sweat response below the level of injury (14). Blood-flow redistribution and sweating are two major thermoregulatory effectors, suggesting that many athletes with SCI have impaired ability to thermoregulate during exercise as the body cannot dissipate heat at the rate which it is internally produced (9, 5, 15). As increased core temperature is commonly cited as one of the main reasons for decreased athletic performance, athletes with SCI, and especially cervical SCI, may experience premature onset of fatigue and decreased performance, especially when exercising in the heat (5, 6).
Precooling has been investigated as a means of reducing core temperature before exercise in order to increase the available temperature margin and delay the time before reaching a critical level (9). Webborn et al. (22) compared the use of cooling vests before and during exercise in the heat (32°C, 50% RH) during an intermittent sprint protocol with wheelchair rugby and wheelchair tennis athletes. Compared to the control (no cooling), participants wearing a cooling vest were able to sprint longer (p<0.05) and perform 6–8 more sprints (p<0.05). Furthermore, increases in core temperature were delayed for the precooling trial (p<0.01), as compared to both the during exercise and control conditions. The efficacy of precooling techniques like cold water immersion and cooling garments is established in the literature in both AB athletes and athletes with SCI; however, these precooling strategies may not prove practical for competition settings (5, 22, 8).
Ice slurries are icy mixtures consumed as a beverage that may be a practical and affordable precooling method to decrease core temperature before exercise, in turn increasing time to exhaustion (18, 23). Ross et al. (16) conducted a study in which trained cyclists completed a time trial in hot conditions (32°C–35°C at 50%–60% RH), and found that participants who precooled using both ice slurry (14 g/kg) and iced towels for 30 minutes before exercise demonstrated a 3.0% increase in power output (p=0.04) and a 1.3% performance improvement (p=0.08) compared to the control condition. Similarly, Siegel and colleagues (18) examined the difference between 7.5 g/kg of ice slurry (−1°C) and cold water (4°C) consumed during a 30-minute precooling period on run performance in the heat in AB participants. Following ice slurry ingestion, running time was longer (p=0.001) and rectal temperature remained lower for the first 30 minutes of exercise (p=0.001) versus cold water. However, other studies examining the effectiveness of an ice slurry in AB participants have found no significant difference in performance (17).
To date, Forsyth and colleagues (5) were the first to examine the efficacy of ice slurry ingestion during 20 minutes of precooling in participants with SCI. Comparing the effects of cold water immersion, ice slurry ingestion, and ice slurry ingestion plus the application of iced towels in wheelchair basketball athletes during 60 minutes of passive rest, researchers found cold water immersion to have the greatest cooling effect. While researchers failed to observe significant changes in core temperature after ice slurry consumption, it was suggested that the volume of ice slurry administered could prove beneficial if exercise began immediately after ingestion. However, Forsyth et al. (5) did not employ an exercise protocol to raise core temperature after precooling.
Therefore, the purpose of this study was to investigate the effects of pre-exercise ice slurry ingestion as a practical and affordable precooling strategy on physiological and perceptual measures during two simulated on-court wheelchair rugby session in elite athletes with SCI.
METHODS
Participants
Elite wheelchair rugby athletes (n=13) were recruited from the U.S. National Wheelchair Rugby Team. Self-reported descriptive and SCI characteristics are presented in Table 1. Twelve participants completed data collection on both days, with one participant excluded as the telemetric pill was passed after the completion of day 1, and a second pill was not ingested. All participants were tetraplegic, with cervical level SCI. Remaining autonomic function among participants was not controlled for; therefore, it is possible that some participants were able to thermoregulate better than others.
Table 1.
Participant descriptive characteristics (n=13).
| Participant | Age (Years) | Body Mass (kg) | Injury | Injury Level | Years Since Injury | AIS | IWRF Classification |
|---|---|---|---|---|---|---|---|
| 1 | 26 | 46.8 | Congenital | - | - | Unknown | 2.0 |
| 2 | 25 | 73.6 | I | C6 | 3 | B | 2.5 |
| 3 | 32 | 90.9 | I | C6 | 13 | Unknown | 2.5 |
| 4 | 33 | 54.5 | I | C6 | 19 | Unknown | 1.0 |
| 5 | 38 | 95.4 | I | C7 | 15 | B | 2.0 |
| 6 | 34 | 53.6 | I | C5 | 15 | B | 1.0 |
| 7 | 45 | 84.1 | I | C6 | 24 | Unknown | 2.5 |
| 8 | 27 | 56.4 | I | C5 | 14 | B | 0.5 |
| 9 | 29 | 74.1 | I | C5 | 13 | B | 1.5 |
| 10 | 37 | 72.7 | I | C2 | 11 | Unknown | 2.5 |
| 11 | 29 | 63.6 | I | C5 | 9 | B | 1.0 |
| 12 | 35 | 70.4 | I | C7 | 20 | B | 2.0 |
| 13 | 27 | 50 | I | C6 | 12 | B | 1.0 |
|
| |||||||
| Mean (SD) | 32.1 (5.8) | 68.2 (15.7) | 14 (5.4) | ||||
Note: I = incomplete lesion; C = complete lesion; AIS = American Spinal Injury Association Impairment Scale; IWRF = International Wheelchair Rugby Federation
All participants provided informed consent before participating, and ethical approval was granted by the Central Washington University Human Subjects Review Committee. This investigation adhered to the ethical guideline of the International Journal of exercise Science (13).
Protocol
A field-based design was employed to mimic the conditions typical of a wheelchair rugby match, and to assess the practicalities of ice slurry ingestion as a precooling strategy considering the requirements, circumstances, and constraints of competition settings (12). Data was collected over two consecutive days in an environmentally-controlled, thermoneutral gymnasium (21°C). Using a randomized, counterbalanced design, the same procedures were repeated on each day of collection. After refraining from exercise for 12 hours prior to the trial, participants began precooling at the same time each day.
Participants were randomly assigned by the team High Performance Manager to a placebo (PLB) or ice slurry (IS) beverage on day 1 and 2. Previous research has reported than 6.8 g/kg ice slurry is a well-tolerated volume in similar populations (5). Therefore, the IS group received 6.8 g/kg of ice slurry (−2°C) administered in two 3.4 g/kg boluses provided 10 minutes apart to ensure consistent ingestion (5). Participants were given 10 minutes per bolus to consume IS, for a total of two bolus doses within the 20-minute precooling period. Cups were weighed with IS to ensure accuracy of each dose. IS was made using a 6% carbohydrate (CHO)-electrolyte containing sports drink (Gatorade, PepsiCo, USA) (80 kcal, 22 g CHO, and 0 grams protein per 12 fluid ounces) using a commercial slushie machine (Bunn-O-Matic Corporation, Illinois, USA). Participants were given both a straw and spoon to maximize consumption of IS. The PLB group received an isocaloric 6% CHO-electrolyte (Gatorade, PepsiCo, USA) containing beverage at room temperature (20°C–25°C) at a matched volume and administration.
Other food and fluid intake were controlled during the precooling period, and only consumption of IS and PLB beverages was permitted. During the exercise period, other fluid intake was permitted ad libitum and participants had access to spray bottles and fans to mimic the conditions typical of a match-play setting. However, additional consumption of IS was not permitted.
A field-based, self-paced protocol allowed for a direct assessment of the physiologic response to exercise, which directly correlates with how athletes fare in competition. Therefore, while the work performed was similar for the two days, it is difficult to determine whether total work was identical.
After participants completed precooling, a structured warmup was completed under the direction of the strength coach. The warmup was similar in structure and duration on both days, and progressed in intensity, beginning with low-intensity stretches using an elastic resistance band and building to wheelchair propulsions and sprints (push/pulls) and “tows” in which players propelled themselves down the court while pushing or pulling another teammate and their chair.
Participants completed a 50-minute wheelchair rugby match simulation with play divided into 6-minute periods on day 1, while day 2 consisted of a 60-minute game simulation with play divided into 4-minute periods. The exercise pattern of a standard wheelchair rugby match was followed, with intermittent sprints of wheelchair propulsion on a regulation-size indoor basketball court. Normal stoppages occurred for breaks between quarters, substitutions, timeouts, and penalty calls. Coaches and referees were present to ensure the conditions mimicked that of a standard wheelchair rugby match.
Data was collected over two consecutive days during a wheelchair rugby training camp. All participants were competing to be named for a spot on the international traveling team; therefore, participants had a vested interest in playing at a high caliber which would be expected during regular competition.
Baseline measures (core temperature, heart rate, gastrointestinal comfort, thermal comfort) were collected before beverage ingestion and 10 and 20 minutes into the precooling period.
Core temperature (Tcore) was recorded every 10 minutes throughout each trial using CorTemp® Ingestible Core Body Temperature Sensors and data was wirelessly transmitted to a CorTemp Data Recorder worn outside the body (22, 11, 9). Telemetric sensors were ingested 11 hours before the first trial. Participants ingested a second sensor approximately 11 hours before the start of the second trial, if the first sensor passed via bowel movement.
Heart rate (HR) was measured every 10 minutes throughout the duration of precooling and exercise with a wearable HR monitor (Polar 3200, Polar, USA).
Gastrointestinal (GI) and thermal comfort were measured less frequently than Tcore and HR due to time constraints during match play. GI and thermal comfort were recorded at 10 and 20 minutes during the precooling period, 30 and 40 minutes into the exercise periods, as well as at the conclusion of the 50- and 60-minute exercise periods on day 1 and 2, respectively. A five-point Likert scale, ranging very uncomfortable to very comfortable, was used to assess GI comfort (5). Thermal comfort was evaluated using a zero (unbearably cold) to eight-point (unbearably hot) scale (5). Rating of perceived exertion (RPE) was recorded every 10 minutes during the exercise session using the Borg Scale (6–20) (18).
Statistical Analysis
Data was analyzed by making probabilistic magnitude based inferences about the true values of the effect of intervention on outcomes. Effect Size (ES) magnitudes were calculated as the difference in means/standard deviation (SD) within groups and were qualified as follows, based on observations of the literature on various precooling methods: small, <0.3; moderate, 0.3–0.6; large, 0.6–0.8; very large, >0.8 (16).
Additional analyses were conducted using IBM SPSS statistics (version 23). The effect of each treatment (i.e. IS, PLB) on Tcore over the precooling and exercise periods were compared using a repeated measures ANOVA (treatment x time). Separate analyses examined the effects of precooling on thermal comfort, GI comfort, RPE, and HR. Independent t-tests were used to identify differences between treatment groups (i.e. IS, PLB) at each time point for the thermal comfort, GI comfort, RPE, and HR. Data are reported as mean +/− standard deviation (SD) and results were considered significant at p≤0.05.
RESULTS
While the amount of work performed by the participants on day 1 and 2 was similar, methods for measuring total work in this population are not yet established; therefore, it is difficult to determine workload and directly compare days 1 and 2. Therefore, results were analyzed separately for day 1 and 2.
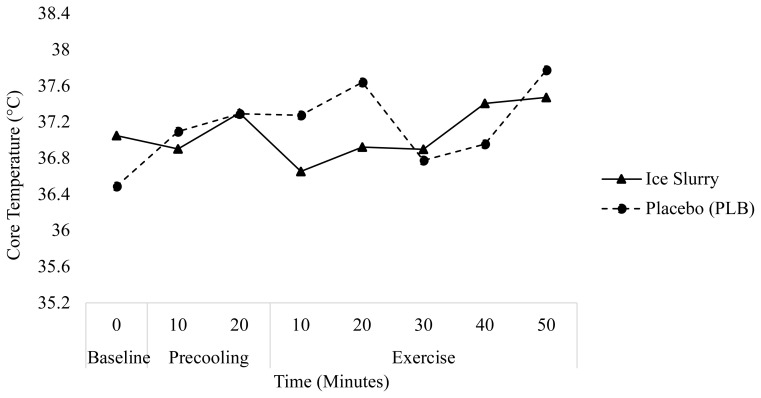
The changes in Tcore over time for days 1 and 2 are presented in Figures 1 and 2, respectively. Tcore for each condition and day are presented in Table 2. Analysis of Tcore over time revealed IS had a large effect on Tcore at the midpoint of exercise (minute 30) (ES=0.73) and a moderate effect at the end of exercise (minute 50) (ES=0.51) on day 1. On day 1, a repeated measures ANOVA displayed no main effect on Tcore over time for IS (p=0.13), but there was a main effect of time on Tcore for PLB (p=0.02). Pairwise comparison revealed interactions between baseline and minute 50 in the PLB group (p=0.04) on day 1, indicating Tcore was 1.30°C ± 0.24°C higher at minute 50 as compared to baseline.
Figure 1.
Change in core temperature (°C) over time during the precooling and exercise periods on trial day 1.
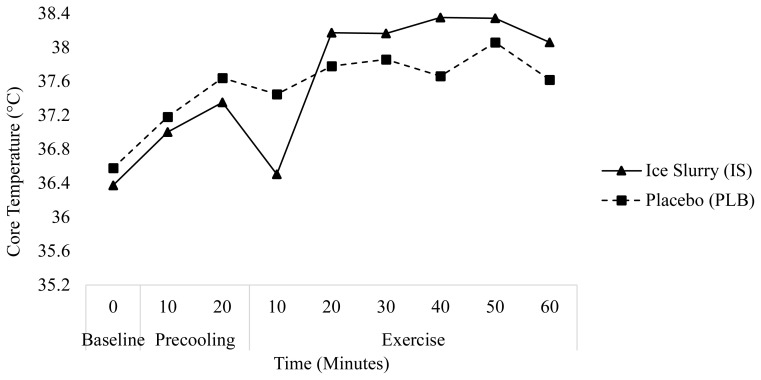
Figure 2.
Change in core temperature (°C) over time during the precooling and exercise periods on trial day 2.
Table 2.
Changes in Tcore (°C) over time for IS and PLB on day 1 and 2.
| Day | Condition | Baseline (0 min) | End of Precooling (20 min) | Delta (0→20 min) | End of Exercise (70/80 min) | Delta (20→70/80 min) | Delta (0→70/80 min) |
|---|---|---|---|---|---|---|---|
| 1 | IS | 36.38 (1.65) | 37.33 (0.92) | +0.97 (1.28) | 38.34 (0.75) | +0.99 (0.83) | +1.97 (1.20) |
| PLB | 36.46 (0.62) | 37.64 (0.48) | +1.18 (0.55) | 38.06 (0.62) | +0.42 (0.55) | +1.59 (0.62) | |
| 2 | IS | 37.05 (0.44) | 37.3 (0.62) | +0.25 (0.53) | 38.06 (0.39) | +0.76 (0.51) | +1.01 (0.42) |
| PLB | 36.49 (0.72) | 37.29 (0.45) | +0.8 (0.58) | 37.61 (0.91) | +0.33 (0.68) | +1.13 (0.81) |
Values are Mean (SD).
On day 2, IS ingestion had a very large effect on Tcore at the midpoint of exercise (minute 40) (ES=1.17) and a large effect on Tcore at the end of exercise (minute 60) (ES=0.63). On day 2, a repeated measures ANOVA revealed a significant main effect of time for IS (p=0.01), with pairwise comparisons showing interactions between minutes 10 and 60 of exercise. No significant main effects were observed in the PLB group on day 2.
The increase in Tcore from baseline to the end of precooling was greater on both days 1 (+1.18 ± 0.55°C) and 2 (+0.8 ± 0.58°C) for PLB as compared to IS (+0.97 ± 1.28°C and +0.25 ± 0.53°C for day 1 and 2, respectively). Conversely, from the end of precooling until the end of exercise, the increase in Tcore was greater for IS (+0.99 ± 0.83°C, and +0.72 ± 0.51°C for day 1 and 2, respectively) compared to PLB (+0.42 ± 0.55°C, and +0.33 ± 0.68°C).
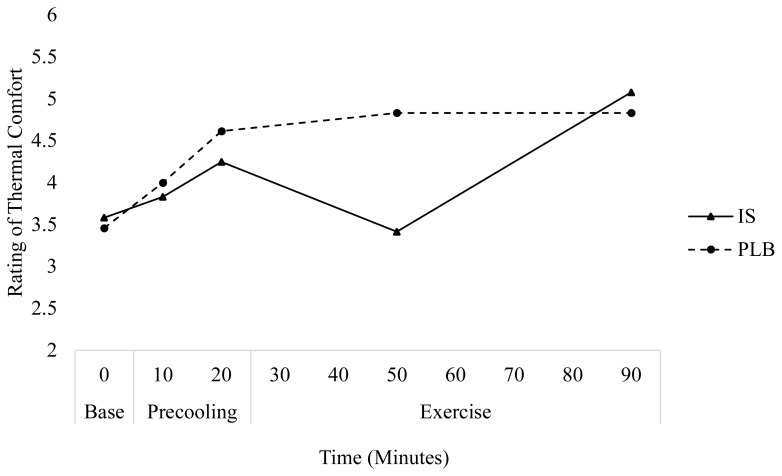
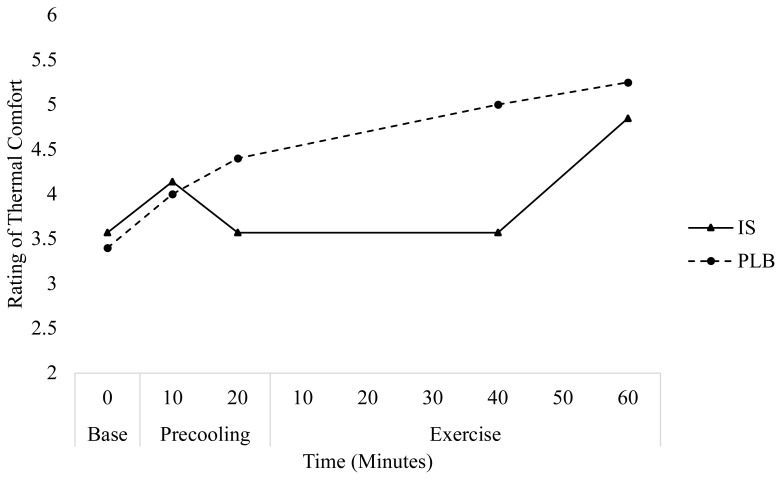
IS had a very large effect on the rating of thermal comfort at the midpoint of exercise (minutes 30 and 40, respectively) on days 1 (ES=1.30) and 2 (ES=1.47), indicating a beneficial effect of IS on perceived thermal comfort. Independent samples T-tests revealed significant differences between IS and PLB at the midpoint of exercise on day 1 (p=0.04), but not day 2 (p=0.05). Further, following IS ingestion, participants rated thermal comfort 1.3±0.8 and 1.5±0.2 points lower for day 1 and 2, respectively, at the mid-point of exercise as compared to PLB. At the end of exercise, IS ingestion had a very large effect thermal comfort on day 1 (ES=0.79) and a moderate effect on day 2 (ES=0.41). An independent samples T-tests revealed no significant differences between treatments (PLB, IS) for rating of thermal comfort on either day at the end of exercise. No significant effects were observed in the subjective rating of GI comfort between time and treatment for either condition.
Figure 3.
Change in rating of thermal comfort over time for each treatment on day 1.
Figure 4.
Change in rating of thermal comfort over time for each treatment during day 2.
No effects were observed in RPE between time and treatment for either condition during day 1 or 2. IS had a small effect on RPE at the midpoint of exercise for both days 1 and 2 (IS: 7.8±1.3, PLB: 13.8±2.9, ES=0.26 and IS: 11.0±3.0, PLB: 11.6±3.1, ES=0.43, respectively) as well as at the endpoint of exercise (IS: 13.8±1.9, PLB: 12.0±4.4, ES=0.39 and IS: 10.9±4.1, PLB: 13.0±2.0, ES=0.59, respectively). PLB had a small effect on RPE at the midpoint of exercise on day 1 (13.8±2.9, ES=0.42) and a moderate effect at the midpoint of exercise on day 2 (11.6±3.1, ES=0.63). Conversely, PLB had a moderate effect on RPE at the end of exercise on day 1 (12.0±4.4, ES=0.63) and a small effect at the end of exercise on day 2 (13.0±2.0, ES=0.40).
DISCUSSION
The purpose of this study was to investigate the effects of pre-exercise ice slurry ingestion as a practical and affordable precooling strategy on physiological and perceptual measures during two similar on-court match simulation in elite wheelchair rugby players. The primary findings suggest that ice slurry ingestion before exercise may delay an increase in Tcore from the end of precooling and through the first ~30 minutes of exercise. Therefore, although more research is needed, the current study suggests that ice slurry ingestion may be an effective precooling method to delay increases in Tcore in elite wheelchair rugby players during the initial 30 minutes of match simulation held in a thermoneutral environment.
These findings are consistent with previous research in AB populations where Byrne et al. (3) noted that following cold fluid infusion, intragastric temperature was not similar to the control condition until minute 15 of exercise, signifying a possible long-lasting reduction in intragastric temperature following cold fluid, including ice, intake. This is further supported by Stevens et al. (19) who reported that following ice slurry ingestion, rectal temperature was lower at the onset of exercise and remained lower for the first 2 km of a 5 km run trial compared to a room temperature control beverage. Similar to our findings where Tcore remained lower following IS ingestion than PLB until minutes 20 and 30 of exercise on day 1 and 2, respectively, Forsyth et al. (5) reported that in athletes with SCI, Tcore remained lower at 40 and 50 minutes into a period of passive rest after participants precooled with ice slurry and iced towels as compared to no cooling. Therefore, the findings of the current study suggest that ice slurry ingestion generates a small decrease in Tcore during precooling and the first 30 minutes of game.
Conversely, the IS treatment demonstrated a greater increase in Tcore from the onset to the end of the exercise period compared to PLB. Yeo et al. (23) reported similar findings in AB populations, wherein despite similar increases in gastrointestinal temperature (Tgi) throughout precooling and warm-up, participants who ingested IS 30 minutes before engaging in a 10 km run trial finished the exercise period with a higher Tgi compared to participants who consumed a room temperature control beverage. This may be due to a number of factors. Siegel et al. (17) proposed that a higher Tcore following ice slurry ingestion could be due to the stimulation of internal thermoreceptors, which may have the potential to convey a “false” sense of the body’s thermal state, and in turn elicit the perception of exercise as being easier at a given thermal load. For athletic performance, this sensation may be advantageous, as players perceive less thermal stress, and play harder and longer. However, without a true sense of the body’s thermal state, hyperthermic conditions may be masked, putting the athlete in potential danger. This should be noted in athletes with SCI as tetraplegic individuals possess a lesser degree of afferent input regarding their thermal state (9). Perhaps the most notable limitation in the present study is that total work was not measured; therefore, it is not possible to conclude whether the IS group actually performed more work. Although HR data was collected, due to the high-level injuries of the participants and the consequent likelihood of cardiovascular dysfunction, HR was an unreliable indicator of work performed. However, approximately half of participants (58.3% on day 1 and 50% on day 2) in both conditions, experienced exercise-induced hyperthermia (EIH), defined by the American College of Sports Medicine (ACSM) as Tcore > 37.8°C–38.3°C (21). Despite this, no participant terminated participation early or demonstrated symptoms of heat exhaustion (Tcore < 40°C) or heat stroke (Tcore > 40°C) (22). Future research should examine and determine a valid method (ie GPS or acceleromter) for capturing the total work performed during wheelchair-based activity in athletes with SCI in order better assess the effectiveness of ice slurry as a precooling intervention during match-play.
Stevens et al. (19) noted that even slight 0.4°C to 0.5°C decreases in Tcore following IS ingestion are associated with a transient decrease in the perception of thermal comfort at the beginning of exercise. In support of this, despite the greater increase in Tcore observed in the IS group throughout exercise in the present study, participants in the IS group reporting feeling cooler at the end of exercise on both days compared to the PLB group. Recent research has demonstrated that perception of thermal sensation is an important contributor to self-selected exercise intensity, suggesting that if players are feeling cooler, exercise intensity increases (19). In the current study, no differences in HR or RPE were observed under either condition at any time point. However, given the intermittent nature of wheelchair rugby, the field-based setting of this study, and constraints of training camp, researchers cannot conclude whether participants in one condition were playing harder than the other.
Finally, the volume and timing of ice slurry ingestion were well-tolerated. Previous research has indicated that 6.8 g/kg ice slurry is a well-tolerated volume in similar populations (5). While other studies have used higher volumes of IS in AB populations, due to the risk of autonomic dysreflexia in individuals with SCI, the present study used a conservative volume of IS. Participants noted no GI distress related to IS ingestion, as GI comfort from baseline throughout precooling and the entirety of exercise, was rated within a “comfortable” range (9,4,14) by all participants on both days. Future research should examine the timing of ingestion, including the viability and effectiveness of ad libitum IS ingestion during exercise and rest periods, such as half-time during a match. Furthermore, most studies examining the effectiveness of precooling methods in SCI populations fail to employ a standard measure of performance, making it difficult to assess whether there is a clear performance benefit associated with any precooling method (9).
While the primary limitation of this study is that the amount of assigned work performed on day 1 and 2 were similar, we have no way of assessing total work. However, the results have high external validity given the field-based design and application. Our results may be more practical in mimicking the conditions that athletes experience during competition, where intensity and duration of work varies each day.
In conclusion, pre-exercise ice slurry ingestion may be an effective method to delay the time before reaching a critically high Tcore during exercise in athletes with SCI. Despite this, ingesting ice slurry prior to exercise may result in a greater overall increase in Tcore throughout the duration of exercise. Accurate methods for assessing total work during wheelchair-based activity are warranted to better determine the efficacy of IS on performance outcomes in this population. Furthermore, ice slurry is a practical and well-tolerated precooling method as it did not compromise athlete comfort or interrupt the sequence of pre-event or half-time routines.
REFERENCES
- 1.Armstrong LE, Casa DJ, Millard-Stafford M, Moran DS, Pyne SW, Roberts WO American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sport Exerc. 2007;39(3):556–72. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- 2.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 3.Byrne C, Owen C, Cosnefroy A, Lee JKW. Self-paced exercise performance in the heat after pre-exercise cold-fluid ingestion. J Athl Train. 2011;46(6):592–9. doi: 10.4085/1062-6050-46.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsyth P, Miller J, Pumpa K, Thompson K, Jay O. Independent influence of spinal cord injury level on thermoregulation during exercise. Med Sci Sport Exerc. 2019;51(8):1710–9. doi: 10.1249/MSS.0000000000001978. [DOI] [PubMed] [Google Scholar]
- 5.Forsyth P, Pumpa K, Knight E, Miller J. Physiological and perceptual effects of precooling in wheelchair basketball athletes. J Spinal Cord Med. 2016;39(6):671–8. doi: 10.1080/10790268.2016.1180098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999;86(3):1032–9. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- 7.Goosey-Tolfrey V, Kremlin J, Price M. Spinal cord injuries. In: Broad E, editor. Sports Nutrition for Paralympic Athletes. 1st ed. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 8.Goosey-Tolfrey V, Swainson M, Boyd C, Atkinson G, Tolfrey K. The effectiveness of hand cooling at reducing exercise-induced hyperthermia and improving distance-race performance in wheelchair and able-bodied athletes. J Appl Physiol. 2008;105(1):37–43. doi: 10.1152/japplphysiol.01084.2007. [DOI] [PubMed] [Google Scholar]
- 9.Griggs KE, Leicht CA, Price MJ, Goosey-Tolfrey VL. Thermoregulation during intermittent exercise in athletes with a spinal-cord injury. Int J Sports Physiol Perform. 2015;10(4):469–75. doi: 10.1123/ijspp.2014-0361. [DOI] [PubMed] [Google Scholar]
- 10.Griggs KE, Price MJ, Goosey-Tolfrey VL. Cooling athletes with a spinal cord injury. Sports Med. 2015;45(1):9–21. doi: 10.1007/s40279-014-0241-3. [DOI] [PubMed] [Google Scholar]
- 11.HQ, Inc. CorTemp Sensor. 2019. Retrieved from: http://www.hqinc.net/cortemp-sensor-2/
- 12.Lee JKW, Kenefick RW, Cheuvront SN. Novel cooling strategies for military training and operations. J Strength Cond Res. 2015;29(Suppl 11):S77–S81. doi: 10.1519/JSC.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 13.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritchett RC, Al-Nawaiseh AM, Pritchett KK, Nethery V, Bishop PA, Green JM. Sweat gland density and response during high-intensity exercise in athletes with spinal cord injuries. Biol Sport. 2015;32(3):249–54. doi: 10.5604/20831862.1163370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price MJ. Thermoregulation during exercise in individuals with spinal cord injuries. Sports Med. 2006;36(10):863–79. doi: 10.2165/00007256-200636100-00005. [DOI] [PubMed] [Google Scholar]
- 16.Ross MLR, Garvican LA, Jeacocke NA, Laursen PB, Abbiss CR, Martin DT, Burke LM. Novel precooling strategy enhances time trial cycling in the heat. Med Sci Sports Exerc. 2011;43(1):123–33. doi: 10.1249/MSS.0b013e3181e93210. [DOI] [PubMed] [Google Scholar]
- 17.Siegel R, Laursen PB. Keeping your cool: possible mechanisms for enhanced exercise performance in the heat with internal cooling methods. Sports Med. 2012;42(2):89–98. doi: 10.2165/11596870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Siegel R, Maté J, Brearley MB, Watson G, Nosaka K, Laursen PB. Ice slurry ingestion increases core temperature capacity and running time in the heat. Med Sci Sports Exerc. 2010;42(4):717–25. doi: 10.1249/MSS.0b013e3181bf257a. [DOI] [PubMed] [Google Scholar]
- 19.Stevens CJ, Thoseby B, Sculley DV, Callister R, Taylor L, Dascombe BJ. Running performance and thermal sensation in the heat are improved with menthol mouth rinse but not ice slurry ingestion. Scand J Med Sci Sports. 2016;26(10):1209–16. doi: 10.1111/sms.12555. [DOI] [PubMed] [Google Scholar]
- 20.Thijssen DHJ, Steendijk S, Hopman MTE. Blood redistribution during exercise in subjects with spinal cord injury and controls. Med Sci Sports Exerc. 2009;41(6):1249–54. doi: 10.1249/MSS.0b013e318196c902. [DOI] [PubMed] [Google Scholar]
- 21.Trbovich M, Ortega C, Schroeder J, Fredrickson M. Effect of a cooling vest on core temperature in athletes with and without spinal cord injury. Top Spinal Cord Inj Rehabil. 2014;20(1):70–80. doi: 10.1310/sci2001-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webborn N, Price MJ, Castle PC, Goosey-Tolfrey VL. Effects of two cooling strategies on thermoregulatory responses of tetraplegic athletes during repeated intermittent exercise in the heat. J Appl Physiol. 2005;98(6):2101–7. doi: 10.1152/japplphysiol.00784.2004. [DOI] [PubMed] [Google Scholar]