Abstract
Resistance training (RT) with blood flow restriction (BFR) appears to accelerate muscle hypertrophy and strength gains in older populations. However, the training-related effects of RT with BFR upon blood pressure (BP) and cardiac autonomic modulation in the elderly remains unclear. The objective of this study is to compare the chronic effects of low-intensity RT performed with soft BFR (BFR) vs. high-intensity (HI) and low-intensity RT (CON) without BFR on BP and heart rate variability (HRV) indices in older adults. Thirty-two physically inactive participants (72 ± 7 yrs) performed RT for upper and lower limbs (50-min sessions, 3 times/week) for 12 weeks, being assigned into three groups: a) BFR; 30% of 1 repetition maximum (RM) with BFR corresponding to 50% of arterial occlusion pressure; b) HI; 70% of 1RM without BFR; c) CON; 30% of 1 RM without BFR. Resting BP and HRV were assessed at rest in the supine position, before and after exercise interventions. Systolic BP (Δ = −7.9 ± 8.0 mmHg; p = 0.002; effect size = 0.62), diastolic BP (Δ = trace length by the duration of the test 5.0 ± 6.0 mmHg; p = 0.007; effect size = 0.67) and mean arterial pressure (Δ = −6.3 ± 6.5 mmHg; p = 0.003/effect size = 0.77) reduced after BFR, remaining unaltered in HI and CON. HRV indices of sympathetic and vagal modulation did not change in all groups (p ≥ 0.07 for all comparisons). 12-wk RT with low intensity and relatively soft BFR substantially reduced BP at rest in older adults vs. traditional RT performed with either low or high intensity. Those reductions were not parallel to changes in autonomic modulation.
Keywords: Elderly, heart rate variability, strength training, cardiovascular physiology, exercise
INTRODUCTION
The aging process is associated to metabolic and cardiovascular disorders (3, 25), and hypertension is acknowledged as a major risk factor of cardiovascular disease and premature mortality (8, 21). Despite the advances in treatment approaches (21), the prevalence of hypertension is still high (45), particularly in old age (17). On the other hand, regular physical exercise is accepted as a non-pharmacological strategy to prevent and treat patients with hypertension (5). Although high quality evidence demonstrates that both aerobic and resistance training may reduce the cardiovascular risk among adults with hypertension (1, 27), new modalities of training to optimize the beneficial effects and safety of physical training in the elderly are always warranted (18, 31).
In the last few years, different research groups have investigated the clinical benefits of exercises performed with blood flow restriction (BFR) (18, 22, 28). It is currently accepted that BFR training provokes substantial muscle gains in different populations, such as patients with musculoskeletal disorders or the elderly (7, 20). On the other hand, BFR has been also suggested to elicit unfavorable hemodynamic responses (33, 36, 43), due to an over activation of exercise pressor reflex in response to external mechanical compression on arterial walls (38). The consequent sympathetic hyperactivity tends to increase heart rate (HR), blood pressure (BP), and systemic vascular resistance (SVR) (38). However, few studies have concomitantly investigated hemodynamic and autonomic acute responses to exercise performed with BFR (15, 39), all of them with healthy and physically active young participants.
In addition, trials comparing hemodynamic responses to acute RT with BFR vs. traditional RT produced mixed results (12). The limited studies with older participants reported that adding BFR to light-weight RT increased BP, HR, and SVR to greater levels than traditional RT performed with high intensity (60- to 70% of 1RM) (33, 36). These findings are suggestive that RT with BFR might increase the hemodynamic stress, which would be harmful for older patients with hypertension. On the other hand, a prior systematic review indicated that BP reduction during postexercise recovery would be greater after RT with vs. without BFR (12). Considering that chronic changes in BP may relate to acute postexercise reductions (24), this raises interesting possibilities in regards to clinical applications of this modality of training in patients with hypertension. Unfortunately, data on hemodynamic effects of BFR training are scarce (13, 23), particularly in older populations (30). Actually, we could not find trials investigating these outcomes after chronic RT performed with BFR in the elderly. This would be nonetheless important, given its applicability to frail individuals unable to perform RT with high loads and the great prevalence of hypertension in the elderly.
In short, older adults often exhibit muscle atrophy and strength deficits and RT is widely indicated to improve their muscle function, functional independence, and overall health. More recently, low-intensity RT performed with BFR emerged as a promising strategy to increase muscle mass and strength of frail older individuals. However, there is a lack of research with this age group in which concerns the effects of BFR training on hemodynamic outcomes, particularly BP. Given this gap in the literature, this randomized trial compared the effects of low-intensity RT performed with BFR (BFR) vs. high-intensity RT (HI) without BFR on resting BP in older adults. Additionally, autonomic modulation was assessed by means of HRV, as a potential mechanism underlying BP changes. It has been hypothesized that BFR would be more effective than HI to reduce BP in the elderly, and that this effect would be related to improved cardiac autonomic modulation.
METHODS
Participants
Thirty-two physically inactive adults (22 females) aged ≥ 65-yrs-old (72 ± 7 years; 64.0 ± 10.6 kg; 25.4 ± 2.7 kg/m2; 133.0 ± 13.2/73.9 ± 7.5 mmHg) participated in the study. The initial screening included clinical history, physical examination, treadmill exercise testing, and vascular Doppler assessment. Exclusion criteria were defined based on their influence on RT performance: severe visual and audition limitations, neurological, musculoskeletal or orthopedic disabilities, or cognitive deficit. Additional exclusion criteria considered their potential influence on outcomes or aggravation of clinical conditions resulting from hemodynamic effects of BFR: kidney, liver or autoimmune diseases; cancer; severe anemia; obesity; chronic obstructive pulmonary disease; uncontrolled hypertension; unstable angina; recent myocardial infarction, stroke or peripheral vascular disease (< 6 months); chronic heart failure; use of medications influencing the autonomic nervous system; smoking habit.
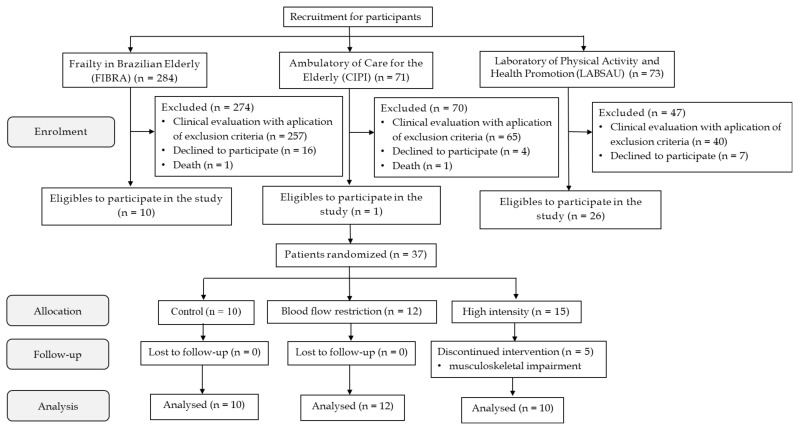
Volunteers were recruited from the Rio de Janeiro section of the ‘Frailty Study in Brazilian Elderly’ (FIBRA-RJ; n = 284), from the ‘Elderly Care Clinic of the Piquet Carneiro Polyclinic’ (PPC; n = 71), and from the ‘Laboratory of Physical Activity and Health Promotion’ (LABSAU, n = 73) at the University of Rio de Janeiro State (UERJ). Out of 37 individuals considered eligible after the application of exclusion criteria, five presented musculoskeletal impairments during HI (about 19%), and 32 concluded the experimental protocol. Figure 1 presents the flow chart of the enrollment, allocation and follow up of participants.
Figure 1.
Flow chart of participants enrolment, allocation, follow-up and data analysis. FIBRA-RJ – Rio de Janeiro Section of the Study Frailty in Brazilian Elderly; CIPI – Care for the Elderly; LABSAU – Laboratory of Physical Activity and Health Promotion
Protocol
Participants were randomized in a counter-balanced order into three groups: a) Blood flow restriction (BFR) – loads corresponding to 30% of 1RM with BFR applied by a cuff inflated to approximately 50% of arterial occlusion pressure at rest (rAOP); b) High intensity (HI) – loads corresponding to 70% of 1RM without BFR; c) Control (CON) – loads corresponding to 30% of 1RM without BFR. Interventions lasted 12 weeks (or 36 sessions), and participants attending < 85% of RT sessions were excluded from the study.
Data collection pre and post intervention included two visits to the laboratory, interspersed with 24- to 48 h intervals. On the first visit, participants underwent anthropometry, BP, and HRV assessments. On the second visit, 1 repetition maximum (1-RM) tests were performed. The participants were instructed to not engage in any other regular physical activity during the experiment, and all evaluations were performed by trained researchers blinded for the purposes of the study and group allocation.
This randomized controlled clinical trial was approved by local Ethics Committee (CAAE 17782513.0.0000.5282) and registered at a WHO credited office (identification TCTR20170131001). All participants signed written informed consents prior to enrolling in the study, as recommended by the Declaration of Helsinki. This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (29).
Body mass and height were measured by means of calibrated electronic scale (FilizolaTM, Sao Paulo, SP, Brazil) and wall stadiometer (SannyTM, Sao Paulo, SP, Brazil), respectively. BMI was calculated as the ratio between body mass and height squared (kg/m2). Loads corresponding to 1-RM were determined for all exercises. Prior to 1-RM tests, participants underwent specific warm up with intensity of approximately 50% of maximum estimated load, followed by 5 attempts to reach 1-RM interspersed with 3–5 min intervals. Familiarization sessions were performed before tests to improve the technique of movements, therefore increasing the validity of tests and reducing the risk of injury. The tests were performed in the same order applied in RT sessions.
BFR was performed using a nylon cuff size 11 x 85 cm (Model SC10TM, D. E. Hokanson Inc, Bellevue, WA, USA) connected to a pneumatic cuff inflator Hokanson TD312 (D. E. Hokanson Inc, Bellevue, WA, USA) placed over the arms and legs. Pressure was applied by a cuff inflated to approximately 50% of rAOP (19) in the proximal portion of upper and lower limbs. Exercises of upper and lower limbs were alternate, and cuff pressure was maintained during all sets of a given exercise, including resting intervals (continuous BFR). The cuff was deflated during the transition between exercises for upper and lower limbs and vice versa (about 60 s). The duration of BFR in each exercise was of 3 min on average.
Resting BP and HRV were measured at baseline and after interventions, at the same time of day (7–11 a.m.) and in a controlled temperature room (22–25°C). Before measurements, participants were instructed to avoid any type of physical exercise, and not to ingest alcoholic and caffeinated beverages in the prior 24 h. A semi-automated oscillometric device (LifeWindowTM LW6000, Digicare Biomedical Technology, West Palm Beach, FL, USA) was used to measure BP in the supine position, according to standard recommendations (32).
Cardiac autonomic modulation was assessed through HRV during 30 min by means of a cardiotachometer Polar RS800cx (PolarTM, Kempele, Finland), after 15 min at rest in the supine position (41). Data were extracted and downloaded for analysis by a specific software (PolarTM Precision Performance, Polar). HRV indices were analyzed using KubiosTM HRV software (Biomedical Signal Analysis Group, Department of Applied Physics, University of Kuopio, Kuopio, Finland), considering the last 5 min of recordings. Sampling frequency was 1,000 Hz and signal artifacts were filtered by excluding R-R intervals with differences of more than 20 % compared to the preceding R-R interval. Filtering was performed in less than 1 % of R-R sequences for each subject in the study. In the time domain, the following indices were obtained: average of all normal R-R intervals (RR interval), standard deviation of all normal NN intervals (SDNN), root-mean of square successive NN interval difference (rMSSD), and percentage of normal-normal NN intervals whose difference exceeds 50 ms (pNN50). These markers are acknowledged to reflect vagal modulation within short periods (41). In the frequency domain, the power spectrum was obtained with the Fast Fourier transform method (Welch’s periodogram: 1,024 points, 50% overlap and Hamming window). In this study, we have analyzed bands corresponding to low-frequency (LF: 0.04–0.15 Hz), high-frequency (HF: 0.15–0.40 Hz), and total power (TP; meaning LF plus HF). While TP and LF are influenced by both sympathetic and parasympathetic outflows, HF is acknowledged to reflect vagal modulation. The LF:HF ratio was used as a marker of sympathovagal balance, as previously described (41). The duration of exercise sessions was of approximately 50 min and included: a) 15 min warm-up on treadmill with intensity corresponding to 30% of heart rate reserve; b) 30-min of exercises for upper and lower limbs in the following order: elbow flexion with halter, leg press, elbow extension with pulley and leg extension. Participants performed 3 sets of 10 reps with loads corresponding to 30% (HI and CON) or 70% (BFR) of 1RM, and 1 min of rest between sets and exercises; c) 5-min recovery with light stretching exercises. All training sessions occurred from 7- to 10 am, 3 times a week for 12 weeks (totalizing 36 sessions) and were ministered by experienced trainers blinded for the purposes of the study.
Statistical Analysis
The statistical power was calculated a priori using the software G*Power 3.1.9.4 4 (Universitat Kiel, Germany), considering a power of 0.8, probabilistic error of 5 % and effect size of 0.5, resulting in a sample size of 32 subjects. Data normality was tested by the Shapiro Wilk test and results were expressed as mean ± standard deviation (SD) or median [percentiles 25–75]), whenever appropriate. Baseline demographic and clinical characteristics among groups were compared by 1-way ANOVA, and categorical variables were compared by chi-square tests. Within group differences were determined by paired Student t-tests or Wilcoxon tests. To verify differences among groups, a 1-way ANOVA was applied to compare absolute deltas (post minus pre-intervention), followed by Bonferroni post hoc verifications in the event of significant F ratios. Cohen’s d effect-sizes were calculated for statistically significant differences (14). All calculations were performed using the NCSSTM software (Kaysville, UT, USA) and significance level was set at p ≤ 0.05.
RESULTS
No clinical complications were observed during the experiment, and participants who performed the exercise with BFR did not report discomfort or pain during the training sessions. On the other hand, five individuals from HI were excluded due to orthopedic or muscular problems. Table 1 presents demographic characteristics, medication, and clinical history of each group. No differences across groups were detected at baseline.
Table 1.
Demographic characteristics, medications and clinical history of the study groups (mean ± SD and %).
| Variable | CON (n = 10) | HI (n = 10) | BFR (n = 12) | p Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (yrs) | 72 ± 8 | 73 ± 7 | 71 ± 6 | 0.70 |
| Weight (kg) | 60.9 ± 9.6 | 64.8 ± 11.9 | 65.9 ± 10.5 | 0.54 |
| Height (cm) | 160.3 ± 9.2 | 157.9 ± 9.7 | 158.2 ± 10.5 | 0.84 |
| Body mass index (kg/m2) | 23.8 ± 2.4 | 25.8 ± 2.9 | 26.3 ± 2.4 | 0.08 |
| Female (%) | 6 (60) | 8 (80) | 8 (66.7) | 0.61 |
| Medications – n (%) | ||||
| Diuretic | 1 (10) | 2 (20) | 2 (16.7) | 0.82 |
| Beta-blocker | 2 (20) | 0 (0) | 1 (8.3) | 0.30 |
| Angiotensin converting enzyme inhibitor | 4 (40) | 4 (40) | 4 (33.3) | 0.93 |
| Anticoagulant | 1 (10) | 1 (10) | 1 (8.3) | 0.98 |
| Anti-arrhtymic | 0 (0) | 1 (10) | 0 (0) | 0.32 |
| Antidiabetic | 2 (20) | 2 (20) | 2 (16.7) | 0.97 |
| Statins | 2 (20) | 2 (20) | 1 (8.3) | 0.67 |
| Clinical history – n (%) | ||||
| Diabetes Mellitus | 2 (20) | 2 (20) | 2 (16.7) | 0.97 |
| Hypertension | 5 (50) | 5 (50) | 6 (50) | 1 |
| Dyslipidemia | 2 (20) | 2 (20) | 2 (16.7) | 0.97 |
| Venous insufficiency | 1 (10) | 1 (10) | 1 (8.3) | 0.98 |
CON: control; HI: high intensity; BFR: blood flow restriction; p-value: p values of the ANOVA.
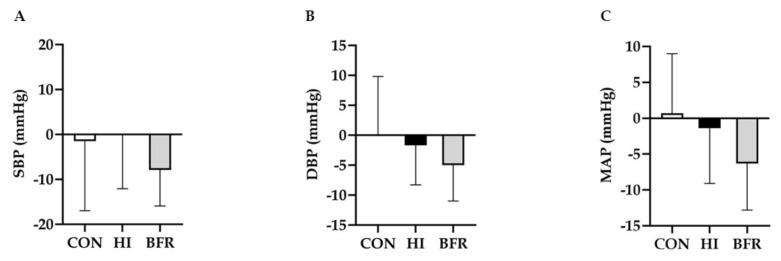
Figure 2 exhibits post-intervention differences between groups for resting BP. Systolic BP (p = 0.002/effect size = 0.62), diastolic BP (p = 0.007/effect size = 0.67) and mean arterial pressure (p = 0.003/effect size = 0.77) reduced in BFR and remained unaltered in HI and CON (p ≥ 0.16, for both conditions).
Figure 2.
Post-intervention differences for resting blood pressure assessment of the study groups. CON – control; HI – high intensity; BFR – blood flow restriction; SBP – systolic blood pressure; DBP – diastolic blood pressure; MAP – mean arterial pressure; * Significantly different within group comparison – pre and post intervention (SBP, p = 0.002/effect size = 0.62; DBP, p = 0.007/effect size = 0.67; MAP, p = 0.003/effect size = 0.77).
Table 2 presents results for autonomic modulation as reflected by HRV indices, before and after interventions. No significant change was found for HR at rest, or any of indices in time and frequency domains (p ≥ 0.07 for all comparisons).
Table 2.
Results of heart rate variability assessment of the study groups, before and after intervention with strength training
| Variable | CON (n = 10) | p-value | HI (n = 10) | p-value | BFR (n = 12) | p-value | p-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Before | After | Before | After | Before | After | Δ Comparisons | ||||
| Heart rate variability | ||||||||||
| HR (bpm) | 62 ± 10 | 63 ± 8 | 0.40 | 71 ± 11 | 68 ± 14 | 0.28 | 65 ± 10 | 65 ± 10 | 0.47 | 0.76 |
| RR interval (ms) | 987.5 ± 160.8 | 964.7 ± 130.4 | 0.28 | 861.3 ± 141.5 | 907.7 ± 163.4 | 0.23 | 938.8 ± 142.4 | 933.8 ± 138.7 | 0.45 | 0.61 |
| SDNN (ms) | 23.1 [15.7–50.5] | 18.3 [11.0–40.6] | 0.08 | 23.1 [14.4–33.3] | 19.5 [ 14.0–23.2] | 0.28 | 18.7 [15.5–25.9] | 21.9 [13.5–28.1] | 0.41 | 0.36 |
| rMSSD (ms) | 25.2 [14.9–35.8] | 23.7 [10.3–48.1] | 0.19 | 23.7 [13.3–40.0] | 23.1 [13.0–28.8] | 0.43 | 20.9 [13.0–33.8] | 22.7 [14.2–32.0] | 0.47 | 0.95 |
| pNN50 (%) | 4.1 [0–14.3] | 3.5 [0–22.0] | 0.26 | 5.6 [0.3–19.8] | 2.1 [0.2–5.5] | 0.30 | 0.8 [0.1–9.3] | 1.5 [0.1–10.6] | 0.25 | 0.63 |
| LF (n.u.) | 51.6 ± 24.3 | 49.2 ± 29.4 | 0.35 | 58.5 ± 22.5 | 43.9 ± 22.5 | 0.07 | 50.3 ± 18.9 | 49.0 ± 18.6 | 0.37 | 0.29 |
| HF (n.u.) | 48.3 ± 24.3 | 50.7 ± 29.4 | 0.35 | 41.5 ± 22.5 | 56.1 ± 22.4 | 0.07 | 49.6 ± 18.8 | 50.9 ± 18.5 | 0.38 | 0.29 |
| LF:HF ratio | 0.77 [0.46–3.42] | 0.88 [0.28–3.54] | 0.25 | 1.63 [0.99–3.31] | 0.59 [0.35–2.33] | 0.12 | 1.24 [0.56–1.87] | 0.91 [0.61–1.71] | 0.43 | 0.30 |
| Total power (ms2) | 802.0 ± 869.7 | 751.8 ± 811.5 | 0.28 | 682.5 ± 714.2 | 675.5 ± 1223.1 | 0.25 | 570.3 ± 718.4 | 514.8 ± 415.8 | 0.78 | 0.78 |
Mean ± SD; median: [percentiles 25–75]); CON – control; HI – high intensity; BFR – blood flow restriction; HR – heart rate; BPM – beats per minute; RR interval – average of all normal R-R intervals; SDNN – standard deviation of all normal NN interval; rMSSD – root- mean of square sucessive NN interval difference; pNN50 – percent of normal-normal NN intervals whose difference exceeds 50 ms; LF – low frequency band; HF – high frequency band; LF:HF ratio – sympathovagal balance; Δ comparisons tested by one-way ANOVA.
DISCUSSION
The main finding of this study was that 12 weeks of RT with loads corresponding to 30% of 1-RM combined with relatively soft BFR (50% rAOP) lowered BP in older adults vs. RT performed traditionally with either 70% or 30% of 1-RM. On the other hand, no training-related changes in HRV indices reflecting autonomic modulation occurred. This suggests that RT with BFR might be a complementary strategy within exercise programs to manage hypertension, particularly in older individuals unable to tolerate high mechanical stress (RT intensity > 70% of 1RM). The reductions of approximately 7 mmHg in systolic BP and 5 mmHg in diastolic BP are clinically meaningful, since decreases of 5–6 mmHg in BP have been associated to substantially lower the risk of stroke and coronary heart disease in patients with hypertension (9).
However, our data differ from prior chronic studies with young and elderly individuals (13, 30), in which 6–10 weeks of either resistance or aerobic training performed with high BFR (~200 mmHg) did not produce changes in BP. Different methodological designs, as training characteristics (frequency, intensity, duration, or mode) (27), BFR level (high vs. low occlusion pressures), and application mode (continuous vs. intermittent BFR) (31) may help to explain the discrepant findings in those trials and our study. It is worthy to notice that BP changes after RT performed traditionally were not detected, irrespective of their intensity (30% or 70% of 1-RM). The chronic effects of isolate RT on BP are not consensual. While some studies showed reductions (6, 35), others failed to confirm these changes (2, 44), which can be also due to differences in methodological designs or sample characteristics (1).
This is probably the first study investigating the chronic effect of RT performed with BFR upon autonomic activity. Previous research investigated only autonomic responses after acute aerobic or resistance exercises combined with BFR (15, 40). For instance, increased sympathovagal balance and longer parasympathetic recovery have been reported after walking with intensity corresponding to 70% of maximal aerobic capacity vs. 40% of maximal aerobic capacity with 50% BFR in older adults (15). Another trial observed a 30-min reduction in vagal modulation of healthy young individuals, after traditional high-intensity RT vs. low-intensity RT with BFR (40). It is currently accepted that physical exercise may decrease the sympathetic neural influence (1), by improving the baroreflex function (34) and thereby lowering SVR and BP (16). This overall premise was not confirmed in the present study. Our results showed that RT with BFR was not effective to promote changes in sympathovagal balance, so that our hypothesis was rejected. The present BFR protocol included exercises performed with relatively light loads (30% of 1 RM), soft BFR pressure (50% rAOP (differently from other methods where the cuff pressure are set to the point of arterial blood flow occlusion as 100% of rAOP), and low volume (3 sets of 10 TM in 4 exercises for upper and lower limbs). In consequence, the stimulation of exercise pressor reflex was probably limited and sympatho-excitatory activity through muscle efferent stimulation tempered, which could be a concern, considering possible abnormal cardiovascular responses during exercise, leading to an increased the risk of cardiovascular events in clinical populations (10). Changes in HRV indices were not observed after traditional RT as well, regardless of its intensity (30% or 70% of 1-RM). The impact of RT alone upon cardiac autonomic outflow is not a pacified issue. Data from current studies in older populations are mixed (4), and further research is necessary for a better understanding of autonomic adaptations due to RT in different populations.
A direct consequence of our findings is that autonomic changes are hardly to account for as a potential mechanism of exercise-related hypotension due to RT performed with BFR. The BP reduction probably resulted from vascular mechanisms, as improved endothelial function (increased vasodilation) (37), or structural adaptations (larger lumen diameter, greater vasculature distensibility, and angiogenesis). This premise is reinforced by a recent study (37) demonstrating that 4 weeks of RT with BFR improved endothelial function in elderly subjects, when compared to controls that underwent RT without BFR. Moreover, a case report of a nonagenarian sarcopenic patient published by our group, showed that 8 weeks of RT with low BFR (about 50% rAOP) improved his endothelium-dependent vasodilation during reactive hyperemia (26). It could be speculated that the blood reperfusion after cuff deflation increased the shear stress mediated by nitric oxide production (42), resulting in improved endothelial function (11). Evidently, further research is needed to test this hypothesis and ratify our results. The main limitation of the present study was the small sample size (type II error), which may have limited the statistical power of comparisons between groups, particularly in regard to HRV. Additionally, the peripheral vascular resistance was not measured, which would allow to associate this mechanism to BP reduction following BFR. The lack of methodological standardization of RT with BFR also limits the generalization of the present findings. Additional studies with RT performed with BFR, applying different levels of blood restriction (or vascular occlusion) and loads, as well as including populations with distinct clinical characteristics are therefore warranted.
In conclusion, our data suggest that 12 weeks (36 sessions) of RT performed with 30% of 1-RM and combined with relatively soft BFR (~65 mmHg) was capable to reduce the resting BP in older adults vs. traditional RT performed with either 30% or 70% 1-RM. These findings indicate a potential hypotensive effect of RT performed with BFR. The antihypertensive effects of BFR training seemed not to be centrally mediated by changes in cardiac autonomic outflow, since HRV indices remained unaltered. Future studies investigating the potential effects of RT performed with BFR upon BP in older adults should include larger groups with distinct clinical characteristics, and apply different loads and BFR pressures. Moreover, the effects of BFR training upon hemodynamic determinants of BP, as SVR and cardiac output should be quantified to ascertain the mechanisms underlying hypotensive responses, therefore helping to clarify the potential application of BFR training as a clinical tool in the management of hypertension in the elderly.
REFERENCES
- 1.American College of Sports medicine Position Stand. American College of Sports Medicine Position Stand Med Sience Sport Exerc. 1998;30:975–91. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 2.Anton MM, Cortez-Cooper MY, DeVan AE, Neidre DB, Cook JN, Tanaka H. Resistance training increases basal limb blood flow and vascular conductance in aging humans. J Appl Physiol. 2006;101(5):1351–5. doi: 10.1152/japplphysiol.00497.2006. [DOI] [PubMed] [Google Scholar]
- 3.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: A report of the american college of cardiology foundation task force on clinical expert consensus documents. Circulation. 2011;123(21):2434–506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- 4.Bhati P, Moiz JA, Menon GR, Hussain ME. Does resistance training modulate cardiac autonomic control? A systematic review and meta-analysis. Clin Auton Res. 2019;29(1):75–103. doi: 10.1007/s10286-018-0558-3. [DOI] [PubMed] [Google Scholar]
- 5.Buchner DM. Physical Activity and Prevention of Cardiovascular Disease in Older Adults. Clin Geriatr Med. 2009;25(4):661–75. doi: 10.1016/j.cger.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Carter JR, Ray CA, Downs EM, Cooke WH. Strength training reduces arterial blood pressure but not sympathetic neural activity in young normotensive subjects. J Appl Physiol. 2003;94(6):2212–6. doi: 10.1152/japplphysiol.01109.2002. [DOI] [PubMed] [Google Scholar]
- 7.Centner C, Wiegel P, Gollhofer A, König D. Effects of Blood Flow Restriction Training on Muscular Strength and Hypertrophy in Older Individuals: A Systematic Review and Meta-Analysis. Sport Med. 2019;49(1) doi: 10.1007/s40279-018-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 9.Collins RRP. Blood pressure, stroke, and coronary heart disease Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 10.Cristina-Oliveira M, Meireles K, Spranger MD, O’Leary DS, Roschel HPT. Clinical safety of blood flow-restricted training? A comprehensive review of altered muscle metaboreflex in cardiovascular disease during ischemic exercise. Am J Physiol Hear Circ Physiol. 2020;318(1):H90–109. doi: 10.1152/ajpheart.00468.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies KJA. Cardiovascular adaptive homeostasis in exercise. Front Physiol. 2018;9(MAY):1–11. doi: 10.3389/fphys.2018.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingos E, Polito MD. Blood pressure response between resistance exercise with and without blood flow restriction: A systematic review and meta-analysis. Life Sci. 2018;209(August):122–31. doi: 10.1016/j.lfs.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Fahs CA, Rossow LM, Loenneke JP, Thiebaud RS, Kim D, Bemben DA, et al. Effect of different types of lower body resistance training on arterial compliance and calf blood flow. Clin Physiol Funct Imaging. 2012;32(1):45–51. doi: 10.1111/j.1475-097X.2011.01053.x. [DOI] [PubMed] [Google Scholar]
- 14.Farinatti PTV, Brandão C, Soares PPS, Duarte A. Acute effects of stretching exercise on the heart rate variability in subjects with low flexibility levels. J Strength Cond Res. 2011;25(6):1579–85. doi: 10.1519/JSC.0b013e3181e06ce1. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira MLV, Sardeli AV, De Souza GV, Bonganha V, Santos LDC, Castro A, et al. Cardiac autonomic and haemodynamic recovery after a single session of aerobic exercise with and without blood flow restriction in older adults. J Sports Sci. 2017;35(24):2412–20. doi: 10.1080/02640414.2016.1271139. [DOI] [PubMed] [Google Scholar]
- 16.de Freitas Brito A, de Oliveira CVC, do Socorro Brasileiro-Santos M, da Cruz Santos A. Resistance exercise with different volumes: Blood pressure response and forearm blood flow in the hypertensive elderly. Clin Interv Aging. 2014;9:2151–8. doi: 10.2147/CIA.S53441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fryar Cheryl D, Ostchega Yechiam, Hales Craig, Zhang Guangyu, Kruszon-Moran D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. 2019;(289):2015–6. [PubMed] [Google Scholar]
- 18.Grutter Lopes K, Alexandre Bottino D, Farinatti P, Coelho De Souza M, das G, Alves Maranhão P, De Araujo CMS, et al. Strength training with blood flow restriction – a novel therapeutic approach for older adults with sarcopenia? A case report. Clin Interv Aging. 2019;14:1461–9. doi: 10.2147/CIA.S206522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gualano B, Neves M, Lima FR, Pinto ALDS, Laurentino G, Borges C, et al. Resistance training with vascular occlusion in inclusion body myositis: A case study. Med Sci Sports Exerc. 2010;42(2):250–4. doi: 10.1249/MSS.0b013e3181b18fb8. [DOI] [PubMed] [Google Scholar]
- 20.Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br J Sports Med. 2017;51(13):1003–11. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 21.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA - J Am Med Assoc. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. 2014. [DOI] [PubMed] [Google Scholar]
- 22.Kambic T, Novakovic M, Tomazin K, Strojnik V, Jug B. Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: A pilot randomized controlled trial. Front Physiol. 2019;10(JUN):1–11. doi: 10.3389/fphys.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SJ, Sherk VD, Bemben MG, Bemben DA. Effects of short-term, low-intensity resistance training with vascular restriction on arterial compliance in untrained young men. Int J KAATSU Train Res. 2009;5(1):1–8. [Google Scholar]
- 24.Liu S, Goodman J, Nolan R, Lacombe S, Thomas SG. Blood pressure responses to acute and chronic exercise are related in prehypertension. Med Sci Sports Exerc. 2012;44(9):1644–52. doi: 10.1249/MSS.0b013e31825408fb. [DOI] [PubMed] [Google Scholar]
- 25.Longo M, Bellastella G, Maiorino MI, Meier JJ, Esposito K, Giugliano D. Diabetes and aging: From treatment goals to pharmacologic therapy. Front Endocrinol (Lausanne) 2019;10(FEB) doi: 10.3389/fendo.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes KG, Bottino DA, Farinatti P, et al. Strength training with blood flow restriction – a novel therapeutic approach for older adults with sarcopenia? A case report. Clin Interv Aging. 2019;14:1461–1469. doi: 10.2147/CIA.S206522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald HV, Johnson BT, Huedo-Medina TB, Livingston J, Forsyth KC, Kraemer WJ, et al. Dynamic resistance training as stand-alone antihypertensive lifestyle therapy: A meta-analysis. J Am Heart Assoc. 2016;5(10) doi: 10.1161/JAHA.116.003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madarame H, Kurano M, Fukumura K, Fukuda T, Nakajima T. Haemostatic and inflammatory responses to blood flow-restricted exercise in patients with ischaemic heart disease: a pilot study. Clin Physiol Funct Imaging. 2013;33(1):11–7. doi: 10.1111/j.1475-097X.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 29.Navalta J, Stone W, Lyons S. Ethical Issues Relating to Scientific Discovery in Exercise Science. Int J Exerc Sci. 2019;12(1):1. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozaki H, Miyachi M, Nakajima T, Abe T. Effects of 10 Weeks Walk Training With Leg Blood Flow Reduction on Carotid Arterial Compliance and Muscle Size in the Elderly Adults. Angiology. 2011;62(1):81–6. doi: 10.1177/0003319710375942. [DOI] [PubMed] [Google Scholar]
- 31.Patterson SD, Hughes L, Warmington S, Burr J, Scott BR, Owens J, et al. Blood Flow Restriction Exercise Position Stand: Considerations of Methodology, Application, and Safety. Front Physiol. 2019;10(May):1–15. doi: 10.3389/fphys.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for Blood Pressure Measurement in Humans and Experimental Animals Part 1: Blood Pressure Measurement in Humans A Statement for Professionals From the Subcommittee of Professional and Public Education of the American Heart Association Counc. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 33.Pinto RR, Polito MD. Haemodynamic responses during resistance exercise with blood flow restriction in hypertensive subjects. Clin Physiol Funct Imaging. 2016;36(5):407–13. doi: 10.1111/cpf.12245. [DOI] [PubMed] [Google Scholar]
- 34.La Rovere MT, Pinna GD. Beneficial effects of physical activity on baroreflex control in the elderly. Ann Noninvasive Electrocardiol. 2014;19(4):303–10. doi: 10.1111/anec.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallinen J, Fogelholm M, Volek JS, Kraemer WJ, Alen M, Häkkinen K. Effects of strength training and reduced training on functional performance and metabolic health indicators in middle-aged men. Int J Sports Med. 2007;28(10):815–22. doi: 10.1055/s-2007-964901. [DOI] [PubMed] [Google Scholar]
- 36.Scott BR, Peiffer JJ, Thomas HJ, Marston KJ, Hill KD. Hemodynamic responses to low-load blood flow restriction and unrestricted high-load resistance exercise in older women. Front Physiol. 2018;9(OCT):1–9. doi: 10.3389/fphys.2018.01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu R, Hotta K, Yamamoto S, Matsumoto T, Kamiya K, Kato M, et al. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol. 2016;116(4):749–57. doi: 10.1007/s00421-016-3328-8. [DOI] [PubMed] [Google Scholar]
- 38.Spranger MD, Krishnan AC, Levy PD, O’Leary DS, Smith SA. Blood flow restriction training and the exercise pressor reflex: A call for concern. Am J Physiol - Hear Circ Physiol. 2015;309(9):H1440–52. doi: 10.1152/ajpheart.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai YL, Marshall EM, Glasgow A, Parks JC, Sensibello L, Kingsley JD. Autonomic modulation following an acute bout of bench press with and without blood flow restriction. Eur J Appl Physiol. 2019;119(10):2177–83. doi: 10.1007/s00421-019-04201-x. [DOI] [PubMed] [Google Scholar]
- 40.Tai YL, Marshall EM, Glasgow A, Parks JC, Sensibello L, Kingsley JD. Autonomic modulation following an acute bout of bench press with and without blood flow restriction. Eur J Appl Physiol. 2019;119(10):2177–83. doi: 10.1007/s00421-019-04201-x. [DOI] [PubMed] [Google Scholar]
- 41.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 42.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, et al. Immediate exercise hyperemia in humans is contraction intensity dependent: Evidence for rapid vasodilation. J Appl Physiol. 2004;96(2):639–44. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- 43.Vieira PJ, Chiappa GR, Umpierre D, Stein RRJ. Hemodynamic responses to resistance exercise with restricted blood flow in young and older men. J Strength Cond Res. 2013;27(8):2288–94. doi: 10.1519/JSC.0b013e318278f21f. [DOI] [PubMed] [Google Scholar]
- 44.Wood RH, Reyes R, Welsch MA, Favaloro-Sabatier J, Sabatier M, Lee CM, et al. Concurrent cardiovascular and resistance training in healthy older adults. Med Sci Sports Exerc. 2001;33(10):1751–8. doi: 10.1097/00005768-200110000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Zhou B, Bentham J, Di Cesare M, Bixby H, Danaei G, Cowan MJ, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. 2017;389(10064):37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]