Abstract
The present investigation examined the ability of two threshold detection analyses (maximum distance, Dmax; modified maximum distance, mDmax) in identifying the near-infrared spectroscopy (NIRS) threshold, a lactate threshold (LT) estimate, from exercising tissue oxygen saturation (StO2) responses. Additionally, the test-retest reliability of exercising StO2 and total hemoglobin concentration (THC) responses were examined at moderate and peak cycling intensities. Fourteen healthy, recreationally active participants performed maximal incremental step cycling tests (+25 W / 3 minutes) to volitional fatigue on two separate occasions while StO2 and THC of the vastus lateralis were monitored. Exercising blood [lactate] was collected during Session One. LT and NIRS thresholds (NIRS1, NIRS2) were then determined using Dmax and mDmax threshold analyses. Significant (p < 0.05), moderate correlations were detected between LT and NIRS1 when using Dmax (LT = 130 ± 49 W, NIRS1 = 136 ± 34 W, r = 0.690), but not for mDmax (r = 0.487). No significant test-retest reliability for the NIRS thresholds were observed for Dmax (ICC = 0.351) or mDmax (ICC = 0.385). Exercising StO2 responses demonstrated good reliability (ICC = 0.841–0.873) while exercising THC responses demonstrated moderate-good reliability (ICC = 0.720–0.873) at moderate and peak exercise intensities. The results of this study suggest that neither the Dmax nor mDmax threshold analyses should be used to estimate the LT due to the unreliable detection of the NIRS threshold from session to session.
Keywords: Microvascular oxygenation, performance assessment, exercise intensity, cycling exercise, distance maximum, modified distance maximum
INTRODUCTION
The principle of specificity is frequently used in training programs, in which exercise is prescribed to produce desired training adaptations (15). In accordance with the principle of specificity, endurance exercise intensities should be prescribed via individualized physiological thresholds, so that specific training objectives can be emphasized and physiological responses are more consistent (18, 32). The lactate threshold (LT) is an example of a physiological threshold that can be monitored throughout training and used to prescribe individualized exercise intensities, such as in threshold and polarized training programs (28, 29). The American College of Sports Medicine recommends threshold methods as a preferred means of prescribing aerobic exercise intensities, as heart rate methods (percentage of heart rate maximum) may underestimate or overestimate exercise intensity (26). By identifying the LT, the point at which blood lactate levels rise rapidly as lactate production exceeds lactate clearance, practitioners could individualize aerobic exercise intensities for healthy adults in agreement with the American College of Sports Medicine’s guidelines (moderate intensity < LT < vigorous intensity) (21, 26). The LT has also been associated with peak oxygen uptake (VO2PEAK), endurance exercise performance, and training status, making it an important physiological metric for practitioners to monitor throughout a training program (1, 14, 19, 21, 33). Additionally, exercise below the LT is associated with more favorable affective responses than exercise above the LT, which may impact exercise adherence (8).
Traditionally, capillary blood samples have been used to assess the LT (4), however, other non-invasive surrogates have been validated in estimating the LT including a near-infrared spectroscopy (NIRS) threshold (2, 9, 13, 20, 25). NIRS is a non-invasive means to assess the local microvascular oxygenation responses reflecting the balance between oxygen delivery and oxygen utilization at the level of the microvasculature within skeletal muscle (10). The NIRS threshold can be identified when an abrupt deflection in the exercising tissue oxygen saturation (StO2) response occurs during a graded exercise test (GXT) (13). Some wireless NIRS devices have included proprietary algorithms to help detect the NIRS threshold in trained populations during running (2) and cycling (9) GXT. These findings along with the commercial availability and portability of wireless NIRS devices could provide practitioners with the opportunity for a cost-effective, versatile option for estimating the LT. However, it is important to investigate the validity and reliability of the NIRS threshold detection method as multiple analysis methods have been proposed (proprietary algorithms, visual inspection, segmented regression analysis) and to determine if the NIRS threshold assessments could be used in populations outside of athletics (2, 13, 20, 25). Lastly, StO2 has been shown to be more valid at monitoring exercise intensity during running compared to heart rate (3). Therefore, identifying the StO2 response at the NIRS threshold might provide a physiologically effective internal training load that can be prescribed during threshold training programs.
However, previous investigations that have demonstrated valid NIRS threshold measurements, have done so in trained populations using proprietary algorithms that identify the NIRS threshold from the exercising StO2 data (2, 9). Previous research has demonstrated that trained individuals with greater aerobic capacity (higher VO2PEAK) have a larger deoxygenation ability (decrease in StO2) during maximal exercise than those with lower aerobic capacity (24, 27). These findings are consistent with the notion that the capacity for oxygen extraction between the capillaries and skeletal muscle relates to the aerobic capacity of active muscle, which may affect the validity of identifying the NIRS threshold when estimating the LT in less aerobically trained populations (24). Furthermore, the use of proprietary algorithms to identify the NIRS threshold may result in the data being analyzed differently between NIRS companies/devices and potentially limit the use of wireless NIRS technology among practitioners if a specific device does not include a LT estimation algorithm.
Therefore, the primary objective of this study was to assess the validity and test-retest reliability of two threshold detection analyses (distance maximum, Dmax; modified distance maximum, mDmax;) in estimating the LT by identifying the NIRS threshold in a healthy, recreationally active, adult population during cycling GXT (4). The Dmax and mDmax analysis techniques were investigated in the present study because if valid and reliable estimations are found, then these analysis techniques could be applied to the exercising StO2 data obtained from many wireless NIRS systems to estimate the LT, rather than only NIRS systems that include a proprietary algorithm. Additionally, the results of this investigation will aid practitioners in understanding the efficacy of two threshold detection analyses (Dmax, mDmax) that could help automate the identification of the NIRS threshold, potentially saving time in interpreting the assessment data. The secondary objective was to assess the day-to-day reliability of the exercising NIRS responses (StO2 and total hemoglobin concentration, THC) at moderate (50% of peak work rate, WRPEAK) and peak (100% of WRPEAK) cycling exercising intensities. This secondary objective will expand upon previous findings and help practitioners determine if NIRS techniques could be used as a reliable internal training load metric during exercise in less trained (recreationally active) populations (3, 6).
METHODS
Participants
Fourteen healthy, recreationally active adults volunteered for this investigation (Table 1). Recreationally active was defined as participating in exercise for at least two days per week for the last three months with no formal cycling experience (31). Any individual who self-reported a history of metabolic, pulmonary, or cardiovascular diseases, or an orthopedic related injury in the past 12 months was excluded. Based upon a previous NIRS threshold investigation, an a priori power analysis indicated that twelve participants were needed for the present investigation (power = 0.80, α = 0.05) (2). All participants provided written informed consent after having the purpose, experimental protocol, and possible risks associated with participation explained. The experimental protocol was approved by the university’s Institutional Review Board for Human Subject Research and was performed in accordance with the Declaration of Helsinki. Additionally, this research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (23).
Table 1.
Participant Demographics
| Demographic | Data |
|---|---|
| Sex | 9 Males, 5 Females |
| Age (years) | 27 ± 6 |
| Height (m) | 1.77 ± 0.07 |
| Weight (kg) | 77.5 ± 12.8 |
| Body Mass Index (kg/m2) | 24.5 ± 2.9 |
| Thigh Skinfold (mm) | 13 ± 4 |
| Maximal Heart Rate (bpm) | 180 ± 8 |
| Percentage of Age-Predicted Maximal Heart Rate | 94 ± 3% |
| Peak Work Rate (W) | 204 ± 29 |
| Estimated Peak Oxygen Uptake (VO2PEAK) (ml/kg/min) | 36.5 ± 4.9 |
| Peak Blood Lactate (mmol/L) | 12.5 ± 3.2 |
Protocol
Participants reported on two separate occasions, at approximately the same time of day (± 1 hour) and separated by 7–10 days, to perform a maximal cycling GXT. Participants were asked to refrain from exercise for 24 hours prior to each session. During the first session, the participants’ height, weight, and thigh skinfold were measured using a stadiometer, scale, and Lange skinfold calipers, respectively (Table 1). The thigh skinfold was measured twice over the muscle belly of the vastus lateralis at the midpoint of the thigh between the anterior superior iliac spine and the proximal patella. If the two skinfold measurements differed from each other by more than 2 mm then additional skinfold measurements were taken until two measurements were recorded that differed by no more than 2 mm from each other (26). The two skinfold measurements were then averaged for each participant. Participants were then outfitted with a heart rate monitor (Polar, Lake Success, New York, New York) around their chest and a wireless NIRS sensor on their dominant thigh during each session. The cycle ergometer (Monark 839E, Vansboro, Sweden) was adjusted to the participant according to the manufacturer’s recommendations and the settings were recorded for duplication during the subsequent session.
After five minutes of seated rest, heart rate and blood lactate ([La]) were measured. All [La] measurements were collected via a finger stick sample and analyzed immediately by a commercially available blood lactate analyzer (Nova Biomedical Lactate Plus Analyzer, Waltham, Massachusetts). Participants then completed a standardized 25-watt (W) warm-up for five minutes followed by the GXT (+25 W every 3 minutes) to volitional fatigue. Participants maintained the self-selected cycling cadence via visual feedback from a monitor. During the last 15 seconds of each stage, heart rate and [La] were measured. Microvascular oxygenation (StO2, THC) were measured continuously throughout the session.
During Session Two, participants completed the same 25 W warm-up and GXT that was completed during Session One. The GXT was terminated at the time to task failure recorded during Session One. During Session Two, exercising heart rate and microvascular oxygenation were recorded. A continuous-wave, wireless NIRS sensor (Moxy Muscle Oxygen Monitor, Fortiori Design, LLC, Hutchinson, Minnesota) was used to monitor StO2 and THC responses at wavelengths of 680, 720, 760, and 800 nanometers (11). The NIRS sensor was placed midway between the anterior superior iliac spine and the proximal patella over the dominant leg’s vastus lateralis and data was sampled at 2 Hz. Prior to sensor placement, the skin was shaved and cleansed with an alcohol preparation pad. To help improve data quality, the NIRS sensor was covered via a flexible plastic cover to prevent stray visible light from affecting the NIRS signal and secured with tape and elastic wraps to limit movement artifact.
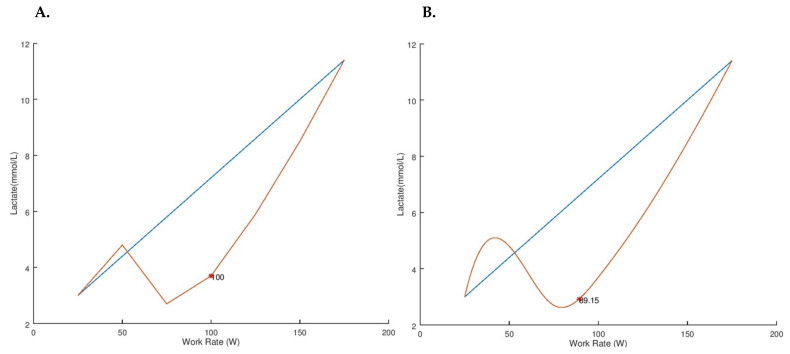
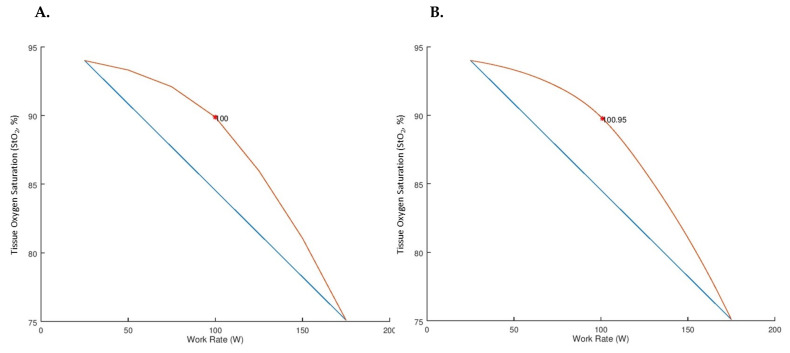
VO2PK was estimated from the time to task failure and peak work rate recorded at volitional fatigue (12). NIRS data were averaged over the last 15 seconds of each exercise stage and then normalized to the last 15 seconds of the warm-up, thus StO2 and THC data are presented as a change from baseline (0 ΔBSL = 25 W baseline) (13). Cycling work rates were identified for the LT (Session One) and NIRS thresholds (Session One, NIRS1; Session Two, NIRS2) using Dmax and mDmax threshold analyses (4). Dmax identifies the threshold at which a linear Lagrange interpolating polynomial has the maximal distance from the line formed by the endpoints of the curve (Figures 1 & 2). mDmax identifies the threshold at which a cubic spline has the maximal distance from the line formed by the endpoints of the curve (Figures 1 & 2).
Figure 1.
Distance Maximum (Dmax) and Modified Distance Maximum (mDmax) of Blood Lactate ([La]) of a Representative Participant. The orange lines represent the blood lactate ([La]) response from a representative participant when analyzed via the distance maximum (Dmax, LT = 100 Watts, A.) and modified distance maximum (mDmax, LT = 89 Watts, B.). The blue lines represent the line formed by the endpoints of each curve.
Figure 2.
Distance Maximum (Dmax) and Modified Distance Maximum (mDmax) of Tissue Oxygen Saturation (StO2) of a Representative Participant. The orange lines represent the tissue oxygen saturation (StO2) response from a representative participant when analyzed via the distance maximum (Dmax, NIRS1 Threshold = 100 Watts, A.) and modified distance maximum (mDmax, NIRS1 Threshold = 101 Watts, B.). The blue lines represent the line formed by the endpoints of each curve.
Statistical Analysis
The validity of Dmax and mDmax threshold analyses were assessed using Pearson’s correlation coefficients and standard error of estimate (SEE) between the LT and NIRS1 threshold work rates. Mixed, absolute agreement intraclass correlation coefficients (ICC) were calculated between the NIRS1 and NIRS2 threshold work rates to assess test-retest reliability of Dmax and mDmax threshold analyses. The reliability of the exercising StO2 and THC responses were assessed by mixed, absolute agreement ICC at moderate (50% WRPEAK) and peak (100% WRPEAK) intensities between Sessions One and Two. Lastly, Pearson’s correlation coefficients (r) were used to examine the relationship between exercising heart rate and StO2 responses as well as exercising [La] and StO2 responses. NIRS, heart rate and [La] data points for the 250 W exercise stage (completed by two participants) were omitted from statistical analyses as they violated an assumption of the statistical reliability model. All ICC results were interpreted as: 0.90–1.00 = “excellent”, 0.75–0.90 = “good”, 0.50–0.75 = “moderate”, and < 0.50 = “poor” (17). All Pearson correlation coefficients were interpreted as: 1.00 = “perfect”, 0.75–0.99 = “strong”, 0.50–0.74 = “moderate”, and < 0.50 = “weak” correlations (6). All statistical analyses were performed using Statistical Package for Social Sciences, version 23 (IBM Corporation, Armonk, New York). Statistical significance was set a priori at p < 0.05.
RESULTS
Data were normally distributed according to the Shapiro-Wilk test. Moderate correlations were observed between LT and NIRS1 threshold (r = 0.690, p = 0.006) when analyzed using Dmax (Table 2). No significant correlations were observed between LT and NIRS1 threshold (r = 0.487, p = 0.078) when analyzed using mDmax (Table 2). No significant test-retest correlations were found between NIRS1 and NIRS2 thresholds when analyzed using Dmax (ICC = 0.351, p = 0.088) or mDmax (ICC = 0.385, p = 0.066) (Table 3). Exercising StO2 responses demonstrated good reliability at both moderate (ICC = 0.841, p < 0.001) and peak (ICC = 0.873, p < 0.001) exercise intensities (Table 4). Meanwhile, the exercising THC responses showed moderate reliability at moderate exercise intensities (ICC = 0.720, p < 0.001) and good reliability at peak intensities (ICC = 0.873, p < 0.001, Table 4). Lastly, moderate, negative relationships between exercising StO2 and exercising heart rate (r = −0.503, p < 0.001) as well as exercising StO2 and exercising [La] (r = −0.557, p < 0.001) were observed.
Table 2.
Near-Infrared Spectroscopy (NIRS) Threshold and Lactate Threshold (LT) Validity During Session One
| Variable | Distance Maximum (Dmax) | Modified Distance Maximum (mDmax) |
|---|---|---|
| Lactate Threshold (LT) Work Rate | 130 ± 49 W | 135 ± 41 W |
| Near-Infrared Spectroscopy (NIRS) Threshold Work Rate | 136 ± 34 W | 136 ± 37 W |
| Correlation Coefficient | r = 0.690* | r = 0.487 |
| Standard Error of Estimate | 25 W | 34 W |
Significant (p < 0.05) Pearson’s correlation coefficient (r).
Table 3.
Near-Infrared Spectroscopy (NIRS) Threshold Test-Retest Reliability
| Variable | Distance Maximum (Dmax) | Modified Distance Maximum (mDmax) |
|---|---|---|
| Session One | 136 ± 34 W | 136 ± 37 W |
| Session Two | 120 ± 39 W | 120 ± 35 W |
| Intraclass Correlation Coefficient (ICC) | ICC = 0.351 95% CI = −0.149–0.724 |
ICC = 0.385 95% CI = −0.109–0.742 |
Table 4.
Reliability of Exercising Microvascular Oxygenation Responses (StO2 & THC) at Moderate (50% WRPEAK) and Peak (100% WRPEAK) Work Rates
| Variable | Session One | Session Two | Intraclass Correlation Coefficient (ICC) |
|---|---|---|---|
| StO2 (50% WRPEAK) | −11.1 ± 10.9 ΔBSL | −12.3 ± 12.4 ΔBSL | ICC = 0.841+ 95% CI = 0.582–0.946 |
| THC (50% WRPEAK) | −0.03 ± 0.13 ΔBSL | −0.09 ± 0.15 ΔBSL | ICC = 0.720+ 95% CI = 0.316–0.902 |
| StO2 (100% WRPEAK) | −30.0 ± 18.5 ΔBSL | −31.5 ± 19.3 ΔBSL | ICC = 0.873+ 95% CI = 0.653–0.957 |
| THC (100% WRPEAK) | −0.08 ± 0.24 ΔBSL | −0.09 ± 0.22 ΔBSL | ICC = 0.873+ 95% CI = 0.655–0.957 |
Significant (p < 0.05) intraclass correlation coefficient (ICC).
DISCUSSION
The primary purpose of the present investigation was to assess the validity and test-retest reliability of two threshold detection analyses in identifying the NIRS threshold in recreationally active participants. The Dmax and mDmax analysis techniques were investigated to determine if either analysis technique could provide a valid, reliable, and objective estimation of the LT by identifying the StO2 threshold. The present results suggest that when the Dmax analysis is applied to exercising StO2 data it provides a moderately valid estimation of the LT, while the mDmax analysis demonstrated no significant relationship with the LT (Table 2). Additionally, the standard error of estimate for the Dmax analysis during the present investigation was 25 W. According to this investigation’s methods, this error accounts for approximately one exercise stage (+25 W / Stage) during the GXT. Therefore, it might be of interest to further investigate if the use of a slope function GXT or smaller step-wise (<25 W transitions per stage) GXT would yield smaller measurement errors compared to the present findings. Higher correlations (r2 = 0.95) have been reported between the LT and NIRS threshold during cycling GXT (13). The discrepancies between the previous and present findings could be due to the data analysis method (visual inspection, Dmax, mDmax) and/or participant training status, as the present investigation recruited recreationally active participants whereas the previous investigation recruited highly trained mountain climbers (13). Trained individuals have a greater ability to deoxygenate the exercising muscle at maximal intensities compared to less trained individuals (24). This deoxygenation difference in relation to training status could potentially affect the endpoints of the exercising NIRS curve used in the Dmax and mDmax analyses (blue lines in Figure 2) and lead to the contended findings.
Despite the valid results of the Dmax, both Dmax and mDmax analyses yielded poor, non-significant test-retest reliability (Table 3). The lack of test-retest reliability in quantifying the NIRS threshold using Dmax or mDmax decreases the practicality of a practitioner using these data analysis methods to monitor potential training adaptations of a client. However, the exercising StO2 responses demonstrated good reliability at both moderate and peak exercise intensities between session one and session two (Table 4). The exercising THC responses showed moderate reliability at moderate exercise intensities and good reliability at peak intensities between exercise sessions (Table 4). These findings extend the existing knowledge, that wireless NIRS can provide reliable monitoring of exercising microvascular oxygenation (StO2, THC) responses in trained and now recreationally active populations (6). However, the StO2 reliability has been shown to decrease when absolute work rates increase above 250 W in well-trained cyclists, potentially due to more intense muscular contractions impairing muscle perfusion (6, 30). Therefore, the previous and present findings together, would suggest that wireless NIRS can provide reliable StO2 and THC measurements at absolute exercise intensities ( ≤ 250 W) that would most commonly be used for recreationally active participants (6).
The reliability of the microvascular oxygenation measurements (StO2, [THC]) observed in the present and previous (6) investigations, along with the portability of the wireless NIRS device suggest that it may be useful for measuring changes in local microvascular oxygenation in diverse settings. Previous findings in elite cyclists have demonstrated significant negative correlations between exercising StO2 and heart rate (r = −0.71), and exercising StO2 and oxygen uptake (VO2, r = −0.73) (6). When investigating healthy adults, the present investigation observed moderate, negative relationships between exercising StO2 and exercising heart rate (r = −0.503, p < 0.001) as well as exercising StO2 and exercising [La] (r = −0.557, p < 0.001). It is important to note that the participants of the previous and present investigations performed cycling exercise, and therefore it is currently unknown if similar relationships would be observed during different exercise modes (6).
Despite the interesting findings, the participants in the current investigation had higher adipose tissue thicknesses (thigh skinfold = 13 ± 4 mm) at the NIRS site compared to a previous NIRS threshold investigation using trained individuals (thigh skinfold = 5 ± 1 mm) (13). This should be considered when using NIRS in diverse populations as higher adipose tissue thicknesses could attenuate the scattering of the NIRS signal, thus potentially affecting the StO2 measurements (5). Additionally, it is important to consider the potential change in skin blood flow associated with prolonged exercise, as increased skin blood flow has been associated with increased StO2 values (6, 7). During the present investigation, all testing was performed in a climate-controlled laboratory to minimize potential differences in skin blood flow. However, it is currently unknown how uncontrolled climates (outdoor setting) may affect the validity and reliability of the microvascular oxygenation data. Furthermore, the microvascular oxygenation data was only recorded at the midpoint of the dominant thigh during the present investigation. This is also important to consider as the exercising microvascular oxygenation response may vary at different locations within the same muscle and/or within the contralateral muscle (16, 22). Lastly, the participants’ VO2PEAK was estimated in the present investigation using the time to task failure and peak work rates recorded at volitional fatigue during the GXT (12). These estimated VO2PEAK values could vary from a directly measured VO2PEAK. However, based on participants’ heart rate responses at volitional fatigue, (180 ± 8 bpm, 94 ± 3 % age-predicted maximal heart rate) (Table 1), participants were exercising at near-maximal/maximal intensity.
In conclusion, the results of the present investigation do not support the use of the two threshold detection analyses to non-invasively estimate the LT from the exercising StO2 response due to the unreliable detection of the NIRS threshold from session to session. However, similar to trained populations, the present results demonstrate moderate to good reliability of the exercising microvascular oxygenation responses (StO2, THC) at moderate (50% WRpeak) and peak (100% WRpeak) cycling intensities in healthy adults when recorded using wireless NIRS (6).
ACKNOWLEDGEMENTS
The authors would like to sincerely thank the participants for volunteering their time and efforts during the research investigation.
REFERENCES
- 1.Amann M, Subudhi A, Foster C. Predictive validity of ventilatory and lactate thresholds for cycling time trial performance. Scand J Med Sci Sports. 2006;16:27–34. doi: 10.1111/j.1600-0838.2004.00424.x. [DOI] [PubMed] [Google Scholar]
- 2.Borges N, Driller M. Wearable lactate threshold predicting device is valid and reliable in runners. J Strength Cond Res. 2016;30:2212–2218. doi: 10.1519/JSC.0000000000001307. [DOI] [PubMed] [Google Scholar]
- 3.Born D, Stoggl T, Swaren M, Bjorklund G. Near-infrared spectroscopy: More accurate than heart rate for monitoring intensity in running in hilly terrain. Int J Sports Physiol Perform. 2017;12:440–447. doi: 10.1123/ijspp.2016-0101. [DOI] [PubMed] [Google Scholar]
- 4.Cerda-Kohler H, Burgos-Jara C, Ramirez-Campillo R, Valdes-Cerda M, Baez E, Zapata-Gomez D, Andrade D, Izquierdo M. Analysis of agreement between four lactate threshold measurements methods in professional soccer players. J Strength Cond Res. 2016;30:2864–2870. doi: 10.1519/JSC.0000000000001368. [DOI] [PubMed] [Google Scholar]
- 5.Craig J, Broxterman R, Wilcox S, Chen C, Barstow T. Effect of adipose tissue thickness, muscle site, and sex on near-infrared spectroscopy derived total-[hemoglobin + myoglobin] J Appl Physiol. 2017;123:1571–1578. doi: 10.1152/japplphysiol.00207.2017. [DOI] [PubMed] [Google Scholar]
- 6.Crum E, O'Connor W, Van Loo L, Valckx M, Stannard S. Validity and reliability of the moxy oxygen monitor during incremental cycling exercise. Euro J Sport Sci. 2017;17:1037–1043. doi: 10.1080/17461391.2017.1330899. [DOI] [PubMed] [Google Scholar]
- 7.Davis SL, Fadel PJ, Cui J, Thomas GD, Crandall CG. Skin blood flow influences near-infrared spectroscopy-derived measurements of tissue oxygenation during heat stress. J Appl Physiol. 1985;100(1):221–224. doi: 10.1152/japplphysiol.00867.2005. 2006. [DOI] [PubMed] [Google Scholar]
- 8.Ekkekakis P, Parfitt G, Petruzzello S. The pleasure and displeasure people feel when they exercise at different intensities: Decennial update and progress towards a tripartite rationale for exercise intensity prescription. Sports Med. 2011;41:641–671. doi: 10.2165/11590680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Farzam P, Starkweather Z, Franceschini M. Validation of a novel wearable, wireless technology to estimate oxygen levels and lactate threshold power in the exercising muscle. Physiol Rep. 2018;6:e13664. doi: 10.14814/phy2.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle phyiology: Recent developments. Philos Trans A Math Phys Eng Sci. 2011;369:4577–4590. doi: 10.1098/rsta.2011.0230. [DOI] [PubMed] [Google Scholar]
- 11.Fortiori Design L. Moxy muscle oxygen monitor owner's manual. 2015. Available at: https://www.moxymonitor.com/wp-content/uploads/Moxy-Owners-Manual-Online-Version-Rev-8.pdf.
- 12.Glass S, Dwyer G. ACSM’s metabolic calculations handbook. Baltimore, MD: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 13.Grassi B, Quaresima V, Marconi C, Ferrari M, Cerretelli P. Blood lactate accumulation and muscle dexoygenation during incremental exercise. J Appl Physiol. 1999;87:348–355. doi: 10.1152/jappl.1999.87.1.348. [DOI] [PubMed] [Google Scholar]
- 14.Green J, Hornsby J, Pritchett R, Pritchett K. Lactate threshold comparison in anaerobic vs. aerobic athletes and untrained participants. Int J Exerc Sci. 2014;7:329–338. [Google Scholar]
- 15.Haff G, Triplett N. Essentials of strength training and conditioning. Champaign, IL: Human Kinetics; 2016. [Google Scholar]
- 16.Hesford C, Laing S, Cardinale M, Cooper C. Asymmetry of quadriceps muscle oxygenation during elite short-track speed skating. Med Sci Sports Exerc. 2012;44:501–508. doi: 10.1249/MSS.0b013e31822f8942. [DOI] [PubMed] [Google Scholar]
- 17.Koo T, Li M. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lansley K, DiMenna F, Bailey S, Jones A. A "new" method to normalise exercise intensity. Int J Sports Med. 2011;32:535–541. doi: 10.1055/s-0031-1273754. [DOI] [PubMed] [Google Scholar]
- 19.Larsson P, Olofsson P, Jakobsson E, Burlin L, Henriksson-Larsen K. Physiological predictors of performance in cross-country skiing from treadmill tests in male and females subjects. Scand J Med Sci Sports. 2002;12:347–353. doi: 10.1034/j.1600-0838.2002.01161.x. [DOI] [PubMed] [Google Scholar]
- 20.McMorries R, Joubert D, Jones E, Faries M. A validation study of a non-invasive lactate threshold device. Int J Exerc Sci. 2019;12:221–232. doi: 10.70252/LOED6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messonnier L, Emhoff C, Fattor J, Horning M, Carlson T, Brooks G. Lactate kinetics at the lactate threshold in trained and untrained men. J Appl Physiol. 2013;114:1593–1602. doi: 10.1152/japplphysiol.00043.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto N, Wakahara T, Ema R, Kawakami Y. Non-uniform muscle oxygenation despite uniform neuromuscular activity within the vastus lateralis during fatiguing heavy resistance exercise. Clin Physiol Funct Imaging. 2013;33:463–469. doi: 10.1111/cpf.12054. [DOI] [PubMed] [Google Scholar]
- 23.Navalta J, Stone W, Lyons T. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okushima D, Poole D, Barstow T, Rossiter H, Kondo N, Bowen T, Amano T, Koga S. Greater VO2peak is correlated with greater skeletal muscle deoxygenation amplitude and hemoglobin concentration within individual muscles during ramp-incremental cycle exercise. Physiol Rep. 2016;4:e13065. doi: 10.14814/phy2.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raleigh C, Donne B, Fleming N. Association between different non-invasively derived thresholds with lactate threshold during graded incremental exercise. Int J Exerc Sci. 2018;11:391–403. doi: 10.70252/BUCT5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riebe D, Ehrman J, Liguori G, Magal M. ACSM's guidelines for exercise testing and prescription. Philadelphia, PA: Wolters Kluwer; 2018. [Google Scholar]
- 27.Salvadego D, Keramidas M, Brocca L, Domenis R, Mavelli I, Rittweger J, Eiken O, Mekjavic I, Grassi B. Separate and combined effects of a 10-d exposure to hypoxia and inactivity on oxidative function in vivo and mitochondrial respiration ex vivo in humans. J Appl Physiol. 2016;121:154–163. doi: 10.1152/japplphysiol.00832.2015. [DOI] [PubMed] [Google Scholar]
- 28.Seiler K, Kjerland G. Quantifying training intensity distribution in elite endurance athletes: Is there evidence for an "optimal" distribution? Scand J Med Sci Sports. 2006;16:49–56. doi: 10.1111/j.1600-0838.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 29.Stoggl T, Sperlich B. Polarized training has greater impact on key endurance variables than threshold, high intensity, or high volume training. Front Physiol. 2014;5(33):1–9. doi: 10.3389/fphys.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiel C, Vogt L, Himmelreich H, Hubscher M, Banzer W. Reproducibility of muscle oxygen saturation. Int J Sports Med. 2011;32:277–280. doi: 10.1055/s-0030-1269922. [DOI] [PubMed] [Google Scholar]
- 31.Thompson W, Gordon N, Pescatello L. ACSM's guidelines for exercise testing and prescription. Baltimore, MD: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 32.Tonnessen E, Sylta O, Haugen T, Hem E, Svendsen I, Seiler S. The road to gold: Training and peaking characteristics in the year prior to a gold medal endurance performance. PloS one. 2014;9(7):e101796. doi: 10.1371/journal.pone.0101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida T, Chida M, Ichioka M, Suda Y. Blood lactate parameters related to aerobic capacity and endurance performance. Eur J Appl Physiol. 1987;56:7–11. doi: 10.1007/BF00696368. [DOI] [PubMed] [Google Scholar]