Abstract
There has been an unprecedented increase in the number of research studies employing event-related potential (ERP) techniques to examine dynamic and rapidly-occurring neural processes with children during the preschool and early childhood years. Despite this, there has been little discussion of the methodological and procedural differences that exist for studies of young children versus older children and adults. That is, reviewers, editors, and consumers of this work often expect developmental studies to simply apply adult techniques and procedures to younger samples. Procedurally, this creates unrealistic expectations for research paradigms, data collection, and data reduction and analyses. Scientifically, this leads to inappropriate measures and methods that hinder drawing conclusions and advancing theory. Based on ERP work with preschoolers and young children from 10 laboratories across North America, we present a summary of the most common ERP components under study in the area of emotion and cognition in young children along with 13 realistic expectations for data collection and loss, laboratory procedures and paradigms, data processing, ERP averaging, and typical challenges for conducting this type of work. This work is intended to supplement previous guidelines for work with adults and offer insights to aid researchers, reviewers, and editors in the design and evaluation of developmental research using ERPs. Here we make recommendations for researchers who plan to conduct or who are conducting ERP studies in children between ages 2 and 12, focusing on studies of toddlers and preschoolers. Recommendations are based on both data and our cumulative experience and include guidelines for laboratory setup, equipment and recording settings, task design, and data processing.
1. Background and rationale
There has been a growing interest in using event-related potentials (ERPs) to examine socio-emotional and cognitive development in young children. ERPs are time-locked averages of neural activity recorded using electroencephalography (EEG). Because of their high temporal resolution, which is on the order of milliseconds, ERPs are an ideal approach for quantifying dynamic and rapidly-occurring neural activity, including cognitive processes involved in emotional reactivity and regulation (Derryberry & Rothbart, 1997; Kopp, 1982). ERP indices can be used to index both the amount of neural effort employed to complete a task, to the extent that such information is contained in observed increases/decreases in neural activity, the timing of cognitive processes, and/or to track the maturation of neural processes over time.
As ERP work extends to younger and younger populations, scientists have noted a need to identify developmentally-appropriate considerations for research using ERPs in samples of young children (Petersen, Hoyniak, McQuillan, Bates, & Staples, 2016; Schmidt & Segalowitz, 2008). Such items may include laboratory setup and décor, recommendations for hardware for use with developing populations, possible adjustments to protocols and procedures, standard procedures for data cleaning, and expectations for data loss. While some elements of ERP research with young children overlap with practices for adults, a developmentally-sensitive approach is critical for drawing valid conclusions about neural processes in early life. As one example, research conducted in 2009 revealed that the error negativity/error-related negativity (Ne/ERN), an ERP previously thought not to be reliably produced until age 12–13 (Davies, Segalowitz, & Gavin, 2004; Segalowitz & Davies, 2004), could be elicited in 5-year-old children when tasks were modified to be age appropriate (Torpey, Hajcak, & Klein, 2009). Specifically, relative to earlier studies, the paradigm used was simplified (go/no-go vs. flanker), stimuli were made more child-friendly (pictures rather than letters), participants were given more frequent breaks (fewer trials per block), extensive training was included, and stimulus presentation times were increased. Subsequent investigations have shown this component to be present even earlier in life (Canen & Brooker, 2017; Conejero, Guerra, Abundis-Gutiérrez, & Rueda, 2016; Grammer, Carrasco, Gehring, & Morrison, 2014), and a subset of work has linked the ERN to other early markers of risk for psychological disorders across childhood (Brooker & Buss, 2014; Jonkman, van Melis, Kemner, & Markus, 2007; Meyer, Weinberg, Klein, & Hajcak, 2012; Santesso, Segalowitz, & Schmidt, 2006). Critically, this series of discoveries regarding the presence and utility of the ERN in early life hinged on a developmentally-appropriate adaptation of a standard task used for ERN elicitation. In this case, the most common tasks used to elicit the ERN in adults were unsuccessful with younger populations but recording and interpreting the ERN could be done using a task that was age-appropriate.
ERP studies in very young children can be expected to continue, and grow, in the future given their potential implications for understanding the role of early neural processing for both child and adult mental health outcomes. At present, few guidelines exist specific to early childhood research using ERPs. Although recommendations for infant (DeBoer, Scott, & Nelson, 2007; Hoehl & Wahl, 2012) and adult (Keil et al., 2014; Picton et al., 2000) work exist, a simple extension of these guidelines to the toddler, preschool, and early childhood years is both impractical and improper; doing so can easily lead to compromised data quality, inappropriate methods and/or incorrect inferences based on results, and inconsistent findings across laboratories (Schmidt & Segalowitz, 2008). For example, tasks, hardware, and laboratory setups must be able to accommodate normative developmental changes in walking, talking, wake/feeding cycles, and social awareness that occur during early childhood. Valuable guidelines for other EEG work have been articulated but do not include ERP-specific considerations (Bell & Cuevas, 2012). In response to an absence of guidelines for research employing ERPs in the toddler, preschool, and early childhood years, we provide a summary of work from 10 laboratories across North America. For those who may be less familiar with the utility of ERPs in research with young children, we first provide a background summary of the most commonly used ERPs to date with young children. We then provide recommendations and offer insights into this domain of developmental neuroscience that can be of aid to researchers, reviewers, and editors in the design and evaluation of research. In general, our summary is intended to supplement previous descriptions, guidelines, and recommendations for ERP work with adults (Keil et al., 2014; Picton et al., 2000; Schmidt & Segalowitz, 2008). Although many of these recommendations for reporting remain unchanged, several caveats in approach and data analysis should be noted when ERP work is being done in an early childhood population.
2. A Review of ERP Components in Previous Work with Children
As the focus of this paper is on ERPs used in research on socio-emotional development during the toddler, preschool, and early-childhood years, a number of other fields that are also dependent on cognitive processes (e.g., language, memory, etc.) are not discussed. It is, therefore, prudent to mention the specific ERP components from which the following data and recommendations were drawn. This not only allows for the possibility that experiences and recommendations may be revised as new or other components come under study, but also acknowledges that necessary adjustments for developing populations may vary across fields of study. In what follows, we describe the five most commonly assessed ERPs to date in socioemotional research with young children. We focus primarily on research associated with ERP amplitude, which reflects postsynaptic potentials resulting from the synchronous firing of neurons in response to discrete external events. We focus on amplitudes given that associations between ERP latency, or the timing of neural processing, and socio-emotional development in early life has been relatively uninvestigated, or at least unreported.
Error-related Negativity.
The error-related negativity (ERN) is visible as a negative defection in the averaged EEG recording when is time-locked to a participant’s response. The ERN is typically peaks 50 to100 ms after an incorrect response and is maximal at frontocentral midline electrodes (FCz), though it is not uncommon to also see an ERN at posterior electrodes in children between 4 and 7 years of age (Brooker, Buss, & Dennis, 2011; Torpey et al., 2009). It is thought to reflect a general process of self-monitoring that signals the need for enhanced cognitive control (Gehring, Liu, Orr, & Carp, 2012). A large amount of work has been aimed at identifying the precise nature of the ERN, resulting in suggestions that it reflects error monitoring (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991), conflict detection (Botvinick, Braver, Barch, & Carter, 2001; Van Veen & Carter, 2006), reinforcement learning (Holroyd & Coles, 2002), motivation (Hajcak & Foti, 2008), neural “distress” (Bartholow et al., 2005), or compensatory processing (Moser, Moran, Schroder, Donnellan, & Yeung, 2013). Importantly, the premises of different theories are not necessarily at odds, but often reflect individual nuances regarding the elicitation of the ERN and its association with individual-difference variables. In nearly all cases, the ERN is visible as enhanced negative amplitudes following error commission relative to correct responses (see Vidal, Hasbroucq, Grapperon, & Bonnet, 2000, for an exception). To date, the ERN has been observed in both adults and young children (Brooker & Buss, 2014; Grammer et al., 2014; Torpey et al., 2009).
The ERN is modulated by individual difference characteristics that are of interest for emotion-cognition research. In adults, higher levels of worry are linked to enhanced ERN amplitudes (Hajcak, McDonald, & Simons, 2003; Moran, Taylor, & Moser, 2012; Moser, Moran, & Jendrusina, 2012), while greater impulsivity is associated with reductions in ERN amplitudes (de Bruijn et al., 2006; Potts, George, Martin, & Barratt, 2006). The ERN has been used, in adults, to proxy propensities for inhibition and withdrawal and is positively associated with sensitivity to punishment (Nash, Inzlicht, & McGregor, 2012; Potts et al., 2006).
From age three through adolescence, children at putative risk for anxiety problems (Brooker & Buss, 2014 [age 4.5]; McDermott et al., 2009 [age 15]), with heightened anxiety symptoms (Meyer et al., 2012 [ages 8–13]), or with a diagnosed anxiety disorder (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006 [ages 8–14]; Meyer et al., 2013 [age 6]) show augmented ERN amplitudes relative to typically-developing peers. Heightened ERN amplitudes, in turn, predict the subsequent onset of anxiety disorders (Meyer, Hajcak, Torpey-Newman, Kujawa, & Klein, 2015 [age 6–9]). Associations between impulsivity and the ERN are less consistent in children. To date, some studies with older children (i.e., ages 7+) report a reduced ERN during childhood in association with greater impulsivity and some studies report null (Jonkman et al., 2007; Wiersema, van der Meere, & Roeyers, 2007) or inverse (Burgio-Murphy et al., 2007) patterns of differences. One possible explanation for this is developmental in nature, suggesting that the direction of association between the ERN and risk for anxiety problems reverses between 6 and 9 years of age (Meyer et al., 2018).
Studies with older children have also implicated the ERN as a moderator, rather than direct correlate, of emotion-related outcomes. As one example, the ERN appears to modulate the association between early behavioral inhibition, an early marker of risk for anxiety problems (Biederman et al., 2001; Chronis-Tuscano et al., 2009), and the development of clinically-significant disorders. Specifically, by age 15, behaviorally at-risk children had greater odds of developing a clinically-significant disorder when ERN amplitudes were large (McDermott et al., 2009).
Anterior N2.
The anterior N2 (N2b) typically appears as a medial-frontal negativity (Fz, Cz) that is maximal 200 to 500 ms following the presentation of a cognitively-demanding visual stimulus (Falkenstein, Hoormann, & Hohnsbein, 1999; Lewis, Lamm, Segalowitz, Stieben, & Zelazo, 2006) (Falkenstein et al., 1999). The N2 has been proposed to index response inhibition (Falkenstein et al., 1999), attention shifting and focusing (Codispoti, Ferrari, Junghöfer, & Schupp, 2006), and conflict or mismatch detection for task-relevant stimuli (Van Veen & Carter, 2002, 2006), though important differences in interpretation arise depending on the task that is used (Hoyniak, 2017). In general, N2 amplitudes are enhanced when stimuli necessitate deliberate cognitive control, including response inhibition, conflict or action monitoring, selection among competing task-related schema, or cognitive adjustment to violations of expectations (Hoyniak, 2017; Lewis & Stieben, 2004; Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003; Van Veen & Carter, 2006). Important for emotion research, the N2 has been suggested as an index of neural effort during cognitive processes of emotion regulation (Lewis & Stieben, 2004).
In adults, greater negative affect is associated with reductions in N2 amplitude (Olofsson & Polich, 2007), although such effects may reflect the degree to which emotional arousal, more than negativity per se, interferes with cognitive processing. Greater differences in N2 amplitudes between emotion conditions are visible in the right (relative to left) hemisphere (Schupp, Junghöfer, Weike, & Hamm, 2003; Simon-Thomas, Role, & Knight, 2005), and in individuals who are low (versus high) in trait anxiety (Dennis & Chen, 2009). Findings are less consistent for depressed individuals, with some work suggesting enhanced and some work suggesting suppressed N2 amplitudes in depressed individuals. In a review of the literature including the N2 and depression, Bruder and colleagues (Bruder, Kayser, & Tenke, 2012) suggested that inconsistent findings resulted largely from methodological differences across studies. Upon closer inspection, they suggested that the N2 is increased in depressed, relative to nondepressed, individuals for simple discrimination tasks but that this effect was reversed (i.e., a greater N2 in depressed relative to nondepressed individuals) for complex decision-making or response inhibition tasks.
In children, N2 amplitudes also have been posited to index executive function (Lamm, Zelazo, & Lewis, 2006), including response inhibition (Hoyniak, 2017), and flexibility in regulatory behaviors (Lewis, Granic, & Lamm, 2006). Greater N2 amplitudes are associated with the elicitation of negative emotion (Lewis, Lamm, et al., 2006; Stieben et al., 2007), perhaps reflecting enhanced cognitive efforts to regulate affect. Consistent with this idea, an enhanced difference in N2 amplitudes for conflict relative to nonconflict trials by 4–8 years of age has been associated with diminished efficiency of executive attention (Buss, Dennis, Brooker, & Sippel, 2011). Associations between the N2 and externalizing problems in early life have been inconsistent, with some work suggesting that N2 amplitudes are substantially reduced in children who are high in impulsivity or aggression (Stieben et al., 2007 [age 10]), perhaps reflecting diminished cognitive control, and other work suggesting that the N2 is exacerbated in children who are high in impulsivity or externalizing behaviors (Gow et al., 2012 [ages 12–16]; Smith, Johnstone, & Barry, 2004 [age 7–12]; Woltering, Granic, Lamm, & Lewis, 2011 [ages 8–12]), perhaps reflecting inefficient neural processing. As with the ERN, studies in children have considered the N2 as a moderator of individual differences and emotion-related outcomes. For example, relatively recent work showed that behavioral inhibition in toddlers predicted social reticence at age 7, suggesting stability in anxiety risk, only when N2 amplitudes were more negative (Lamm et al., 2014).
P3/P300 and the P3b.
The P300 (Sutton, Braren, Zubin, & John, 1965) and its subcomponent the P3b are also targeted in studies of emotion-cognition interactions. Visible as a positive deflection in the ERP waveform between 250 to 500ms, the P300 is elicited during the processing of stimulus evaluation or categorization (McCarthy & Donchin, 1981) and has been posited to reflect neural activity associated with the revision of context information (Donchin, 1981), memory processing (Donchin, 1981), and the allocation of attentional resources (Polich & Kok, 1995). The P300 has two components, the P3a and the P3b (see also Polich, 2007, for descriptions of novelty P300 and no-go P300). The P3a is not task specific, is related to frontal focal attention, and typically has a central maximum (Cz) in adults, and is thought to be a signal for the generation of the P3b (Polich, 2003, 2007). The P3b, which is typically maximal at parietal (Pz) recording sites (Sutton et al., 1965) is thought to reflect neural effort related to attentional control (Polich, 2007) or context updating and memory storage (Donchin, 1981). Taken together, the P3a and the P3b may reflect a circuit pathway between frontal and temporal/parietal brain areas, resulting in the full-figure waveform visible as the P300.
The P3b is more commonly studied than the P3a in the domains of cognitive and emotional development. In both children and adults, P300 amplitudes are enhanced as target stimulus probability decreases (Ladish & Polich, 1989), although this effect is less robust in very young (age 4) compared to older children (age 7–12; Polich, Ladish, & Burns, 1990). In children, smaller P3b amplitudes are associated with externalizing problems (Gatzke-Kopp et al., 2015 [age 5]; Woltering et al., 2011 [age 8–12]), and symptoms of ADHD (Janssen, Geladé, van Mourik, Maras, & Oosterlaan, 2016 [age 7–14]; Overtoom et al., 1998 [age 6–14]).
Amplitude and latency change can be seen through maturation (Brown, Marsh, & LaRue, 1983). In a group of 5- to 19-year-olds, Ladish and Polich (1989) found amplitude tended to increase and peak latency tended to decrease as the children got older. It is typical to see a decrease in latency that starts by the age of 5 (Hill et al., 1999; Polich, Ladish, & Burns, 1990), and this decrease continues until the child begins puberty, around 15 years old (Polich, Ladish, & Burns, 1990), when the latency stabilizes (Ladish & Polich, 1989). After this, there is an increase in latency that continues through adulthood (Brown, Marsh, & LaRue, 1983; Polich, Ladish, & Burns, 1990). When comparing adults to children, the P300 latency is longer in adults than in children (Brown, March, & LaRue, 1983). Further, in adults, the P300 latency shows a significant positive correlation with age; this association is not seen in children. While there are differences in latency and amplitudes between children and adults are visible, the neural mechanisms that drive the function of the P300, such as stimulus probability, appear to be stable over time (Polich, Ladish, & Burns, 1990).
Late Positive Potential (LPP).
The late positive potential is a stimulus-locked, sustained midline positivity that is believed to reflect reactivity to salient or emotionally evocative stimuli at the neural level (Cacioppo, Crites, Berntson, & Coles, 1993; Cuthbert et al., 1995; Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000). The LPP becomes visible roughly 200 to 300 ms post-stimulus and is can be sustained for up to 6 seconds during picture viewing (Cuthbert et al., 2000) and 1000 ms after stimulus offset (Hajcak & Olvet, 2008). LPP amplitudes are typically maximal at posterior (Pz) electrode sites for adults and occipital sites (Oz) for children as young as age 6 (Kujawa, Klein, & Hajcak, 2012). Though the LPP is not associated with task difficulty (Hajcak, Dunning, & Foti, 2007), amplitudes appear to be sensitive to both “top-down” and “bottom-up” processing; for example, amplitudes are enhanced for motivationally or affectively salient relative to neutral stimuli (Cuthbert et al., 1995; Hajcak, Moser, & Simons, 2006; Moser, Hajcak, Bukay, & Simons, 2006) or for visual emotional stimuli that are inconsistent with emotional context (Cacioppo et al., 1993). Amplitudes are also enhanced for personally-relevant stimuli, such as a photo of one’s own (versus another) child (Grasso, Moser, Dozier, & Simons, 2009) or task demands (MacNamara, Foti, & Hajcak, 2009). Work with adults has suggested that the LPP is not one, but multiple dissociable components with separable spatial and temporal distributions (MacNamara et al., 2009) and similar spatiotemporal patterns appear to be present in young children as young as age 5 (Dennis & Hajcak, 2009; Hajcak & Dennis, 2009; Kujawa, Weinberg, Hajcak, & Klein, 2013).
Strategies of emotion regulation, and specifically the use of cognitive reappraisal during exposure to negative or unpleasant images, modulate LPP amplitudes in adults. Instructed or aided reappraisal appears to reduce LPP amplitudes to positive (Krompinger, Moser, & Simons, 2008) and negative images (Foti, Hajcak, & Dien, 2009; MacNamara et al., 2009; Moser et al., 2006; Moser, Krompinger, Dietz, & Simons, 2009). Although the LPP is visible in young children between ages 5 and 9 (DeCicco, O’Toole, & Dennis, 2014; DeCicco, Solomon, & Dennis, 2012; Dennis & Hajcak, 2009; Hajcak & Dennis, 2009), a similar pattern of modulation in association with reappraisal appears to depend on both age and sex. Specifically, a diminished LPP following neutral interpretations of negative images has been observed for boys (Dennis & Hajcak, 2009) and children older than age 7 (DeCicco et al., 2014; Dennis & Hajcak, 2009). Traditional LPP effects have not been visible in samples of girls younger than 7 years of age (Dennis & Hajcak, 2009). This work suggests that developmental differences in the maturation of the networks underlying cognitive reappraisal and/or observed LPP amplitudes will influence the degree to which the LPP is useful as an index of individual differences in regulation in young children.
Thus, the LPP may be a more closely tied to emotional reactivity, relative to regulation, in work that is intended to derive general conclusions across age and sex. The LPP has been linked to individual differences in fear and anxiety across development. Greater LPP amplitudes are visible during directed cognitive reappraisal (DeCicco et al., 2012 [age 5–7], 2014 [age 7–9] ) in children with greater levels of anxiety symptoms and who show greater temperamental fearfulness (DeCicco et al., 2012 [age 5–7]; Solomon, DeCicco, & Dennis, 2012 [age 5–7]). This is similar to work in adults that has shown greater LPP amplitudes to be associated with greater self-reported anxiety (MacNamara & Hajcak, 2010; Moser, Huppert, Duval, & Simons, 2008) and are significantly greater in participants who had been diagnosed with an anxiety disorder relative to healthy controls (MacNamara & Hajcak, 2010). In contrast, reduced LPP amplitudes are visible in children at risk for depression (Kujawa, Hajcak, Torpey, Kim, & Klein, 2012 [age 6]).
3. Recommendations for ERP Work with Toddlers, Preschoolers, and Young Children
The summary and recommendations below are based on 21 studies conducted with toddlers, preschoolers, and young children at 10 independent laboratories within the last 10 years across North America. To gather information, 29 investigators were identified, through a literature review, as having conducted ERP research with children between 2 and 12 years of age. We were interested in covering the range from toddlerhood to pre-adolescence. Each investigator was contacted via e-mail by the first and last authors and invited to contribute to the current project. Summaries of work were requested within approximately 2 months. Data included any project that collected EEG recordings in young children that were used to create ERPs reflecting emotional or cognitive processing. The majority of tasks presented visual stimuli during a cognitive task that targeted neural processes associated with reactivity to emotional stimuli, visual processing, cognitive control, inhibition, working memory, or self-monitoring. The total number of participants included in this summary was 3,251, although this number reflects unique ERPs, not necessarily unique individuals (i.e., the same person may have contributed data to multiple projects or contributed data to the same project at multiple time points). The final data set, summarized in Table 1, reflects 21 different projects that assessed ERPs in children. Participant ages ranged from 2 to 12 years of age (Mage = 6.55 years, MSD = 0.63; 46.6% female), and sample sizes ranged from 27 to 432 participants (M = 144.62).
Table 1.
Descriptive statistics for child samples from labs in the current report
| Lab | Original Sample size | Minimum Age (years) | Maximum age (years) | Mean Age | Age SD | % Female |
|---|---|---|---|---|---|---|
| 1 | 432 | 5 | 7 | 6.10 | 0.44 | 46.1 |
| 1 | 432 | 5 | 7 | 6.10 | 0.42 | 45.5 |
| 2 | 106 | 7 | 7 | 7.64 | 0.23 | 55.0 |
| 2 | 49 | 7 | 14 | 7.92 | * | 50.0 |
| 3 | 72 | 8 | 12 | 9.60 | 1.3 | 28.0 |
| 4 | 27 | 2 | 3 | 2.76 | 0.27 | 48.0 |
| 5 | 339 | 5 | 7 | 6.05 | 0.38 | 35.0 |
| 5 | 339 | 6 | 8 | 7.18 | 0.40 | 35.0 |
| 5 | 339 | 7 | 9 | 8.16 | 0.51 | 35.0 |
| 6 | 59 | 5 | 7 | 6.19 | 0.54 | 45.8 |
| 6 | 45 | 7 | 9 | 8.11 | 0.57 | 45.9 |
| 7 | 41 | 4 | 5 | 4.59 | 0.13 | 48.8 |
| 7 | 33 | 4 | 8 | 5.73 | 1.05 | 33.3 |
| 7 | 35 | 4 | 8 | 5.72 | 1.29 | 34.5 |
| 8 | 70 | 3 | 7 | 6.24 | 1.17 | 50.0 |
| 8 | 74 | 3 | 7 | 6.41 | 1.04 | 58.1 |
| 8 | 139 | 5 | 8 | 7.02 | 0.74 | 50.4 |
| 8 | 122 | 5 | 8 | 7.04 | 0.74 | 50.8 |
| 9 | 107 | 3 | 3 | 3.56 | 0.35 | 60.0 |
| 9 | 119 | 4 | 4 | 4.56 | 0.15 | 58.0 |
| 10 | 58 | 10 | 12 | 10.83 | 0.82 | 66.0 |
Note.
information was not provided by the PIs
Laboratory setup.
The earliest-encountered, though least frequently discussed, element of ERP studies with children involves the setup of the laboratory environment. For most young children, all aspects of the EEG data collection procedures, including the equipment, the application procedures, and the look and feel of the process, will be a novel and foreign experience. For many young children, especially those who are highly fearful of novelty, this introduces the possibility that capping and/or task refusals will result from discomfort with the testing environment. As a result, laboratory environments are often modified to enhance the degree to which they appear friendly and inviting to young participants. In some cases, this may involve simply relaxing restrictions for individual testing or the use of sound-attenuating chambers while for some special populations, it may mean fully restructuring the laboratory setup. Changes may involve altering room décor to be more child friendly, either in general (Figure 1) or around a cohesive theme, such as a trip to the zoo or to outer space. Such efforts to introduce a theme are believed to organize the child’s experience in a sensible way. Child-friendly décor also minimizes visual emphasis on hardware (e.g., electrodes, wires, syringes for gel application, etc.) that are reminiscent of medical environments that tend not to be popular with young children.
Figure 1.

Example of child-friendly setup for EEG collection room
Anecdotal accounts suggest that it is advisable to allow a parent to remain present throughout the recording session for our youngest participants. Parents frequently help to reassure and encourage young children throughout the capping procedure(s), and most parents are able to restrict their involvement in testing procedures when instructed to do so. In addition, the mere presence of the parent protects against the task being confounded with the distress of a separation procedure at ages where separation distress is not uncommon (Francis, Last, & Strauss, 1987). In our work, parents are frequently present during testing with young children through age 4, having some level of interaction as needed during capping procedures and then sitting quietly behind children during laboratory tasks. Children older than age 4 have, in our experience, been able to complete tasks with only the experimenter in the room.
Recommendation #1: Create a child friendly atmosphere in the laboratory as part of the procedures. This may include structuring visits around child-friendly themes, adding child-friendly decorations, allowing a parent to remain present during testing, and/or making sure that experimenters are trained to interact with children.
Equipment and recording.
One of the first decisions to be made in study planning is how data will be acquired. As noted by Picton and colleagues (2000), a range of recording options are available, and it is important for the type of system to be reported as part of one’s methods section. However, working with young children leads to two special considerations: electrode application time must be minimized and scalp abrasion may be impractical. Toddlers and preschoolers, in particular, may not tolerate lengthy or aversive procedures. Similarly, although proper scalp abrasion procedures do not typically break the skin or lead to bleeding, a small risk for doing so is present when abrasion procedures are used. In these cases, the risk of the transmission of blood borne pathogens during abrasion increases when systems are not properly cleaned, augmenting the level of risk for the experiment as a whole. Increased risk is of particular concern in work with young children, who receive special protections in human subjects research (Department of Health and Human Services, 2009). One possible alternative to scalp abrasion procedures that are already becoming less common is to comb the scalp, although the generalizability of the effectiveness of this technique may be limited, as it was tested with older children who were primarily male (Mahajan & McArthur, 2010). As a result, most investigators who work with young children will want to employ EEG systems that can tolerate higher levels of input impedance. While high-impedance systems are commonplace in work with young children, a compromise is associated with their use. Specifically, higher impedances decrease common-mode rejection sensitivity, which minimizes artifacts that occur simultaneously across sites in the EEG recording. That is, higher impedance levels impair the ability to estimate noise that is common across all electrodes so that it can be (mathematically) removed from the data. This means that, in the final data, a greater proportion of the signal may be task-irrelevant noise rather than isolated task-related activity.
In our work with young children, we have employed primarily two systems for data acquisition: BioSemi’s Active Two system (n studies = 13) with an Active Two head cap, and the high-density recording system offered by Electrical Geodesics, Inc. (EGI; n studies = 8) with the Geodesic Sensor Net. Both systems boast a minimal need for scalp preparation and a short application time. In our work, 32 (n studies = 5), 64 (n studies = 9), and 128 channel systems (n studies = 7) have all been used. These have led to electrode application times ranging from roughly 10 to 30 minutes, with an average application time of roughly 15 minutes across setups. Unexpectedly, our laboratories report minimal differences in average application time for 32 (M = 12 min, SD = 2.74), 64 (M = 17.5 min, SD = 7.55), and 128-channel systems (M = 13.33 min, SD = 2.58), although times may be quite different for systems or caps that are not used by our group.
Recommendation #2: When possible, select a high impedance system.
Even with noninvasive, quickly-applied electrodes, it is not uncommon for children, particularly during the toddler and preschool years, to refuse application of the cap (Table 2). In these cases, a high rate of refusal of study procedures relative to work with adults should be considered a feature of the age group under study rather than a flaw in the research design or procedures. Across the 23 ERP studies included here, 0 (ages 4.5 and up, though variable by study) to 11 (age 5) percent of children who are consented for participation refuse to be capped for EEG sessions, a proportion in line with those noted in previous reports of other kinds of EEG work with young children (Bell & Cuevas, 2012). Refusal rates were slightly lower for the BioSemi than for the EGI system although average rates of refusal for both systems were relatively low (BioSemi = 2.12%; EGI = 7.69%). A correlation was also observed between child age and refusal of the cap application such that, not surprisingly, younger children may be slightly more likely to refuse capping (r = −0.53, p = 0.03). Note that this association controls for sample size, as the highest rates of refusal were observed in studies with the greatest numbers of participants. Refusal rates are likely higher, at all ages, in special populations (e.g., children who are highly fearful or hyperactive), though those data are not currently available for comparison.
Table 2.
Capping Statistics
| Child Refused Capping | EEG System | % refusing cap | Number of Channels Used | Approximate Mean Capping Time (min) |
|---|---|---|---|---|
| * | Biosemi | * | 32 | 15 |
| * | Biosemi | * | 32 | 15 |
| 0 | Biosemi | 0.00 | 64 | 10 |
| 0 | Biosemi | 0.00 | 64 | 20 |
| 0 | Biosemi | 0.00 | 64 | 20 |
| 0 | Biosemi | 0.00 | 64 | 30 |
| 1 | Biosemi | 0.72 | 64 | 25 |
| 1 | Biosemi | 0.82 | 64 | 25 |
| 2 | Biosemi | 3.39 | 64 | 30 |
| 3 | Biosemi | 2.80 | 64 | 10 |
| 8 | Biosemi | 2.36 | 32 | 15 |
| 11 | Biosemi | 2.55 | 32 | 10 |
| 11 | Biosemi | 2.55 | 32 | 10 |
| * | EGI | * | 64 | 15 |
| * | EGI | * | 128 | 15 |
| * | EGI | * | 128 | 10 |
| 0 | EGI | 0.00 | 128 | 15 |
| 0 | EGI | 0.00 | 128 | 15 |
| 2 | EGI | 6.06 | 128 | 15 |
| 2 | EGI | 7.41 | 128 | 10 |
| 4 | EGI | 11.43 | 128 | 15 |
Note.
information was not provided by the PIs
Recommendation #3: Expect that approximately 1% - 10% of children will refuse to be capped for data collection, with higher refusal rates at younger ages.
The association between the number of channels and refusals is more difficult to interpret from our data given that there was little variability among the number of channels used across studies. Systems containing 64 or more electrodes may be useful for at least partially offsetting a primary limitation of EEG recordings, spatial precision. However, techniques such as source localization, intended to resolve problems of spatial precision, have been criticized for the possibility that an infinite number of solutions may satisfy the inverse solution (Luck, 2005). That is, when the number of dipoles that contribute to an observed pattern of scalp-recorded activity is unknown, there is not a unique solution that accounts for the observed data. Though methods for source localization techniques are both improving and becoming more common in work with adults, knowledge about the applicability of such approaches to young children, who are undergoing rapid cortical development (Durston et al., 2002; Giedd et al., 1999), remains limited. For those laboratories who are not interested in source localization, a high volume of electrodes may be unnecessary. That is, the majority of ERP studies examine components along the midline, resulting in a maximum of 4 electrodes being the foci of most reports. More electrodes may be used when sites are pooled (e.g., Meyer et al., 2012). However, the majority of electrodes in a high-density system remain unexamined. This is true for all ERP studies, of course, but is particularly relevant in studies of children when the additional time spent applying electrodes and testing each location for clean contact may increase rates of data loss. Thus, the best practice for child studies is to use the minimum number of recording sites necessary for testing study hypotheses. At minimum, systems that include large numbers of electrodes (especially 128 channels or more), which may offer advantages for processes like channel interpolation or whole-head averaging, should be selected by researchers who have carefully considered the tradeoffs of increasing the number of recording sites. Ideally, ERP researchers who wish to also make conclusions regarding spatial aspects of processing can combine ERP with other techniques offering high levels of spatial resolution, such as Near Infrared Spectroscopy (NIRS) or Magnetic Resonance Imaging (MRI). Importantly, these techniques offer very different types of information; that is, EEG studies cannot simply be replaced by MRI studies. However, their combination can offer additional information for triangulating conclusions including temporal and spatial aspects of neural processing (e.g., Buzzell et al., 2017; Liu, Bai, & Pérez-Edgar, 2019).
Recommendation #4: Use the minimum number of recording sites necessary for testing study hypotheses. Our work suggests that 32 channels is optimal for the youngest participants (age 5 and younger) in studies that do not include source localization work.
In our work using BioSemi systems, we referenced to the Common Mode Sense and Driven Right Leg electrodes during recording; all EGI systems were referenced to the Cz electrode. Both of these settings are consistent with the recommendations of the manufacturers. Potential users and reviewers should note that impedance levels are calculated slightly differently across the two systems, although both systems offer benefits associated with their design to tolerate fairly high levels of impedance (Luck, 2014). The EGI system offers a traditional measure of impedance between each electrode and the recording reference and allows an opportunity to check impedance levels before each participant is tested. Most research with children follows the practice of reducing impedance values to less than 50–80 kΩ based either on previous work or recommendations from the manufacturer. The BioSemi system, in contrast, amplifies voltages between each electrode and a common mode sense electrode rather than a traditional recording reference. Prior to recording, electrode offsets, which provide an estimate of the deviation of individual channels from the common mode average of the recording system, are calculated in place of impedance values. Electrode offsets are typically reduced to less than 40mV, with an ideal value under 20mV. To our knowledge, there has not been a systematic investigation into possible differences in optimal impedance values for children versus adults. Additional recommendations for recording parameters in other child populations, including amplification factors, minimum sampling rates, headroom, and A/D units, have been previously specified (DeBoer et al., 2007) and do not, in our opinion, need revision for work with toddlers and preschoolers.
On a procedural note, we have also found it helpful to provide children with a moderately interesting activity in which to participate while the cap and electrodes are placed, provided the activity does not interfere with the capping itself. We and others have integrated activities such as allowing children to watch a video or film clip, free play with play dough, reading books, coloring, or using a LeapPad™ activity tablet (Bell & Cuevas, 2012) into the capping procedures. Although integrating these activities requires the presence of at least two experimenters during capping, their inclusion proves well worth the effort. These play activities provide an active distraction and keep from transforming capping procedures into a waiting or delay task for young participants, which may deplete patience and tolerance for subsequent procedures. Such considerations are important for studies of cognitive and emotional development, in particular, where regulatory resources may become depleted and result in an unintended induction of negative affect. Activities should be carefully selected such that they remain orthogonal to the questions to the study (i.e., ensuring that movie clips are neutral if they will precede a study of emotion) and reported in manuscripts in order to maintain transparency.
Recommendation #5: Add activities or games to the laboratory visit during capping. Report these procedures in manuscripts to maintain transparency about potential effects on experimental tasks.
Laboratory tasks.
Because ERPs must be time-locked to an event, their elicitation is often done through a synchronization of EEG recordings with computerized tasks. Tasks that have been or are currently being used with children in our target age range include passive viewing paradigms often involving emotional images (DeCicco et al., 2014, 2014; Kessel, Huselid, DeCicco, & Dennis, 2013; Kujawa, Hajcak, et al., 2012), go/no-go tasks (Canen & Brooker, 2017; DuPuis et al., 2015; Gatzke-Kopp et al., 2015; Lamm et al., 2014; Lamm & Lewis, 2010; Lo, Schroder, Moran, Durbin, & Moser, 2015; Torpey et al., 2013; Willner, Gatzke-Kopp, Bierman, Greenberg, & Segalowitz, 2015; Woltering et al., 2011), directed reappraisal of emotional stimuli (DeCicco et al., 2014, 2012), the auditory oddball (Hoyniak, Petersen, McQuillan, Staples, & Bates, 2015), emotional (Dennis, Amodio, & O’Toole, 2015; O’Toole, DeCicco, Berthod, & Dennis, 2013; Solomon, O’Toole, Hong, & Dennis, 2014) and non-emotional (Brooker & Buss, 2014; Brooker et al., 2011; Buss et al., 2011) versions of the Attention Network Test, cyberball (Tang, Lahat, Crowley, & Schmidt, 2017) modified flanker paradigms (Lo et al., 2015), and guessing games that produce win/loss outcomes (Belden et al., 2016). In nearly every case, the task being used has been modified from a procedure that was developed for use with adults in order to make it more appropriate for use with developing populations. Stimuli from two example tasks are shown in Figure 2.
Figure 2.

Example child friendly stimuli for go/no-go and flanker tasks
Practice trials.
To accommodate young children, modifications to laboratory tasks and procedures can and do take many forms. Arguably one of the easiest modifications is to include a set of practice trials for computerized response time tasks. Practice trials allow investigators to ensure that young children understand task instructions and are capable of completing the paradigm, a question that is often asked during the publication process. Practice trials also allow participants to familiarize themselves with the task, reducing the possibility of capturing the neural underpinnings of a learning process rather than the task of interest.
Only 5 studies included in the current summary did not report the inclusion of practice trials in computerized tasks that were used with children. Importantly, 4 of these non-practice protocols were passive-viewing tasks, for which practice may be less important. The number of practice trials ranged from 6 to 20, with more practice trials generally observed for younger children and/or putatively more difficult tasks. Practice trials always included an extra block in the computerized task and often also included preliminary exposure to and explanation of stimuli using cards or laminated photos. Certainly, it is important to remain cognizant of the overall time spent in the task when making decisions about the inclusion of practice trials. To date, there is no empirical work examining the effects of the inclusion of practice trials on cooperation, task completion, or data quality in young children; such work would be of great benefit to the field.
Practice trials might also include an opportunity to train participants on task-irrelevant features that will contribute to the collection of clean data. That is, participants can be trained to sit still, minimize blinking, or to relax their jaw during recording. Each of these can increase signal quality and would be particularly important for ERPs for which maximal amplitudes are relatively small. Such procedures are likely most appropriate for slightly older children; additional instructions may create secondary or tertiary tasks for very young participants, for whom task complexity will already be a primary issue.
Recommendation #6: Always include practice trials.
Trial number.
A second common modification is a decrease in the number of trials in each task. For the tasks listed above, the number of trials ranged from 20, for some of the youngest participants (ages 3 – 8), to 550, for some of the oldest participants (ages 7 – 14; Mtrials = 127.60, SD = 137.29). Moreover, nearly all of the researchers on this report divide trials into 1 to 8 blocks (M = 3.50, SD = 1.97) and allow breaks (92.31%) between blocks for as long as is needed by each participant. Minimizing trial numbers is thus the most optimal strategy, and this should be done with consideration of increasingly available information about the stability of ERP components in children at different numbers of trials (e.g., Meyer, Bress, & Proudfit, 2014; Pontifex et al., 2010). Decreasing the number of trials, dividing trials into greater numbers of blocks, and allowing for frequent breaks are essential aspects of data collection with young children, particularly when tasks require participants to restrict speech and movement, focus attention, and complete repetitive actions for long periods of time. Although adult researchers may perceive it as ideal to have all procedures, including rest periods, be identical across participants, the individual differences in tolerance of laboratory tasks vary much more in early childhood. That is, a scan of the literature suggests that a greater percentage of child participants – relative to adult participants – are likely to refuse participation and/or application of psychophysiological recording equipment. From our perspective, paradigms for participants younger than school age that require more than 10 to 15 minutes of uninterrupted focused attention pose the risk of creating secondary, largely regulatory, tasks for participants. Anecdotal evidence suggests that this can increase resistance to completing all trials, precipitate gross muscle movement artifacts in EEG recordings, introduce distress into the task itself, and clearly creates a potential confound for examinations of regulatory processes.
Recommendation #7: Minimize the number of trials in experimental paradigms while planning for a smaller proportion of usable data. Consider increasingly available reports on the stability of individual ERP components when deciding on trial numbers.
Task difficulty.
A third type of modification has been introduced that attempts to address the issue of equating task difficulty across participants. Research with children often relies on chronological age as a proxy for stage of cognitive or emotional development. Most frequently, children are recruited or divided into groups based on their age in years. Although this is a common and convenient measure, it is imperfect. There are vast individual differences in cognitive (Fischer & Silvern, 1985) and emotional (Goldsmith & Campos, 1982) stage, as well as neural development (Lenroot & Giedd, 2006), within any single grouping based on chronological age. There exists a concern that developmental neuroimaging work that collapses across ages may predominantly reflect neural activity in the oldest or youngest children (Crone & Ridderinkhof, 2011), who introduce statistical variance that results in findings of developmental differences relative to other age groups. Such concerns could logically be extended within age as well. For studies of neural development, in particular, might apparent differences result from tasks being easier for some participants in ways that are unrelated to the process under study? For example, performance differences, such as faster response times, on a go/no-go task may be attributable to individual differences in motor control and better hand-eye coordination rather than (or in addition to) improvements in cognitive control. This is particularly true for studies of children, who may develop these types of task-relevant skills at different rates. If such processes are of interest, then they may already be measured as part of data collection and can simply be controlled or included in statistical analyses. However, if performance differences reflect potential confounds likely to introduce variability that may obscure the process of interest, then additional adjustments may be needed.
One way researchers have attempted to address this issue is to make tasks iterative, such that performance and difficulty level are held somewhat constant across participants. One of the earliest examples of this in the context of a developmental neuroscience study came from the laboratory of Marc Lewis, where he, his students, and his colleagues created a go/no-go paradigm which adjusted stimulus presentation durations according to the performance of children between 8 and 12 years of age (Stieben et al., 2007). Specifically, error rates were maintained at roughly 50% by decreasing stimulus durations by 50 ms following correct responses and increasing stimulus durations by 50 ms following incorrect responses. Because researchers could specify error rates a priori, they were able to design the task such that it would elicit a desired number of correct versus incorrect responses and maintain a similar level of difficulty to participants at different developmental stages. This strategy also has been adopted in some of our laboratories, largely with success, though it should be noted that children may still time out of trials if the iterative procedure necessitates a response time that exceeds the length of the inter-trial interval (Canen & Brooker, 2017).
A common question about iterative procedures is whether they equate the task across participants or whether they introduce potentially problematic between-participant differences. On a technical level, the task certainly becomes unequal across participants. Most obviously, the duration of individual stimulus presentation times will fluctuate within whatever range is allowed by the program. However, an additional point for consideration is that forcing identical technical elements (e.g., task speed, trial duration, inter-trial interval lengths) on participants results in inter-individual variability with task difficulty. That is, older children are likely to find the task to be relatively easy, while younger children find the task to be difficult. This, of course, is problematic.
Recommendation #8: Use iterative procedures to equate task difficulty across participants. Avoid iterative procedures to equate task parameters across participants.
Even with appropriate adjustments, a subset of children will likely refuse to complete laboratory tasks. In general, children’s rates of refusal for a whole task are low (Figure 3; M = 3.59%, SD = 7.02, range: 0% - 29.63%), but the mean refusal rates are much higher and more variable when data include only children who are not yet school-aged (M = 7.24%, SD = 11.24, range: 0% - 29.63%) relative to samples for which the mean age is 5 years old or greater (M = 2.02%, SD = 3.83, range: 0% - 13.56%). Importantly, it is generally considered in line with “best practices” to explicitly note levels of data loss and/or refusal in published manuscripts rather than simply reporting the number of children with usable data.
Figure 3.
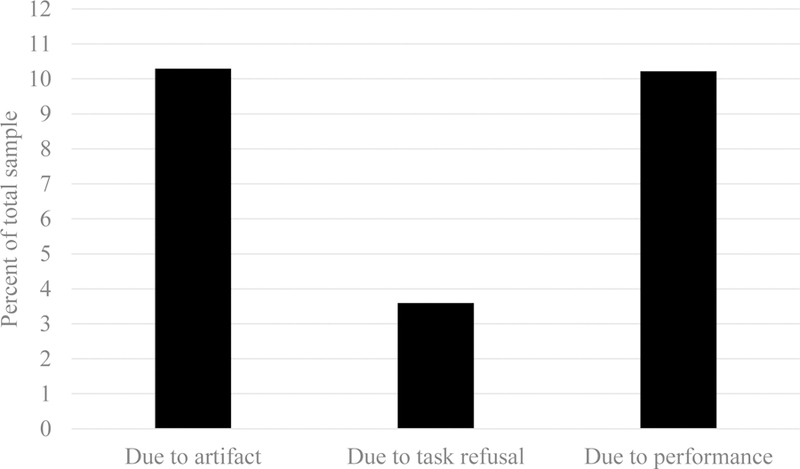
Most common reasons for data loss in child ERP studies
Feedback.
Anecdotal reports from our laboratories suggest that the use of trial-to-trial feedback is valuable for keeping young participants engaged. Specifically, children who are younger than school-aged appear to be able to persist longer in tasks when there is some element of feedback incorporated. However, empirical evidence suggests that feedback should be considered carefully, as it may influence the construct of interest under study. In adults, for example, trial-to-trial feedback appears not to influence the amplitude of the ERN, but does impact the association of the ERN with levels of anxiety such that an association between ERN and anxiety symptoms is visible only when feedback is absent (Olvet & Hajcak, 2009a). Clearly, empirical work is needed to assess the specific effects of feedback on ERPs in children; until these data are available, researchers should carefully consider both the possible benefits and drawbacks of incorporating feedback into work with early childhood samples. Thus our informal recommendation is to strongly consider including feedback in paradigms with very young children, though we are tentative to make this a general recommendation without additional empirical evidence of its effect on ERPs of interest.
Data processing.
Not only do those working with young children need to adjust tasks and make allowances for missing data, they also need to consider adjustments in data processing. In our work, we have used primarily two programs for cleaning ERP data, NetStation (Electrical Geodesics, Inc.; n = 6) and Brain Vision Analyzer (Brain Products GmbH; n = 13). For any system, a number of decisions need to be made that will impact the balance between data that are presumed to be primarily reflective of the neural process of interest (relative to artifact) and data that are believed to contain too many artifacts to remain in the dataset.
Referencing.
One of the first steps of data cleaning involves the selection of the offline reference. Other experts have written about the considerations that must be undertaken when selecting a reference site, which necessitates attention to the ERP of interest and the brain regions believed to be responsible (Hajcak, Weinberg, MacNamara, & Foti, 2012; Luck, 2014). At least one laboratory has compared reference sites for emotion-related ERPs in particular (Hajcak et al., 2012). Ultimately, one must keep in mind that because ERPs assess the potential for current to flow from one electrode site to another, the best reference sites will be neutral with regard to the anticipated scalp distribution of the ERP of interest. That is, re-referencing to an electrode that is proximal to the sites at which maximal amplitudes are expected can effectively subtract a large portion of the ERP voltage from the data and diminish the visibility of the effect. Therefore, although we describe the most typical procedures for re-referencing in our own laboratories, we also note that the most appropriate reference sites may differ based on the ERP(s) of interest. This is true for all ERP work, of course, but necessitates special consideration for developing populations in whom neural activity associated with emotional and cognitive regulation is often more posterior and more diffuse than is seen in adult samples (Durston et al., 2002; Lenroot & Giedd, 2006). Thus, the selection of reference site should be putatively neutral to the process under study at the developmental stage of interest. Though it is unlikely that any single site offers complete neutrality, investigators can compare potential offline reference sites and select the one that appears least influential on the component of interest. Ideally, the selection of the data cleaning reference would also allow for comparison with existing literature. Similar to adult work, we have most frequently used a whole-head average (n = 12) or average mastoid (n = 11) reference in our laboratories, although others (e.g., nose reference) have also been used.
Recommendation #10: Select the offline reference that is most neutral to the process under study at the developmental stage of interest. This will likely necessitate the comparisons of different references and may differ from sites used in adult work.
Artifact detection.
Data from very young children will always contain more artifacts than data from adults; this difference is evident nearly from the moment that recordings begin. Thus, any researcher conducting ERP work in young, developing samples can expect high rates of artifact. We have used, a range of automatic, semiautomatic, and manual data cleaning techniques for identifying artifacts. Semi-automated procedures appear to be the most common, allowing for the employment of standard algorithms but also allowing researchers the freedom to examine data and make adjustments when algorithms are less successful at detecting data artifacts. To date, to our knowledge, there has not been an empirical comparison of the relative effects of these different approaches on the amplitudes and reliabilities of ERP components. Though this work is underway in at least two of our laboratories, a thorough comparison of data cleaning procedures for each ERP across ages would support the optimization of parameters for artifact detection and rejection. The absence of such data reflects a critical need in the field.
It is important to state explicitly that adult thresholds for artifact detection cannot simply be applied to data collected from young children. Infants and young children frequently show greater EEG power in lower frequency ranges relative to adults at baseline (Bell & Wolfe, 2008; Marshall, Bar-Haim, & Fox, 2002; Niedermeyer, 2005) and, as noted in the above review, frequently demonstrate higher amplitude ERPs. Thus, identifying artifact thresholds for children based on work with adults will result in the misidentification of artifacts, the rejection of usable data and increased data loss, as well as the potential mischaracterization of the ERP.
Scoring ERP amplitudes.
ERPs are, by definition, time-locked to a discrete event; for example, the N2, LPP, and P3 are time-locked to a stimulus presentation while the ERN is time-locked to a participant response. As part of the data reduction process, individual events are typically averaged together and the amplitude of the EEG within a specific time window is identified as the component of interest. Though these steps may seem straightforward, they necessitate several decisions from the investigator that may affect final scores.
Selection of time window.
In order to quantify the ERP of interest, a researcher must make a decision about the time window in which the component of interest is most likely to be found. For researchers using child samples, this can be a perplexing issue, because the established windows for any component are likely to be based on adult models. This is not ideal given that, as previously mentioned, latencies for ERP components often change as the brain matures. For example, as previously noted, both ERN and P3 latencies tend to decrease across development, putatively reflecting increasing neural efficiency as the brain matures. Thus, the younger the mean age of the sample, the more severe a deviation may occur in the timing of the component of interest relative to the windows established in adult samples. To date, researchers have largely used two methods to identify ERP time windows, neither of which completely resolves the issue related to developmental change.
One option for defining a time window for the component of interest is to visually examine the grand-averaged waveform, an average of all trials across all participants, to see where differences between conditions (or trial types) appear to be maximal. This region is then defined as the window of interest. Unfortunately, recent work has shown that this approach can lead to findings of significant condition (or trial type) differences under conditions when the null hypothesis is known to be true (Luck, 2014; Luck & Gaspelin, 2017).
A second option is to define windows of interest a priori based on previous work in the field. That is, one could presumably find enough published studies of the N2 using a visual go/no-go task in adolescents to identify the time window during which N2 amplitudes are expected to be maximal. An a priori definition helps to ensure that a researcher is not selecting a time window that simply suits his or her data. However, without empirical tests of the ways in which time windows may shift for across development, a priori definitions of time windows are difficult for components that have not yet been regularly examined in samples of young children. Similarly, even within samples of children who are all the same age, individual differences in the speed at which the brain matures may result in components for some individuals being clearly identifiable in the waveform, but outside of an a priori time window.
Additional approaches have been suggested by Steve Luck, a leading expert in the ERP technique, three of which are listed below. These approaches are defined in greater detail elsewhere (Luck, 2014). One possibility is the inclusion of a condition that is irrelevant to or nonoverlapping with the condition or comparison of interest for a given effect. For ERPs to be compared across emotion conditions, this may include a set of non-emotional trials in which a component may be defined (go/no-go N2 preceded by neutral faces) in order to be compared across emotion conditions (e.g., go/no-go N2 preceded by fear vs. happy faces). There is clear scientific benefit to this approach, but has a critical drawback for developmental researchers in that it likely necessitates the addition of trials. Thus, this is unlikely to be a feasible option for ERP studies with very young children.
Another more preferable option is to collapse across all conditions to select a time window of interest, such that the data used to identify the time window of interest does not reflect the comparison of interest. In the above example, this would mean collapsing N2 across fear and happy conditions to see, in general, where differences emerge between go and no-go trials. This would spare the researcher the task of adding neutral trials to the experiment, but also allow for a visualization of data that may help him or her select a developmentally-appropriate time window.
Finally, mathematical approaches such as temporal independent components analysis allow for a quantification of the magnitude of a component of interest across different trial types of conditions of interest. This type of approach may be best for eliminating the need to identify specific time windows of interest, although they work best with a large amount (i.e., many participants and many trials) of artifact-free data.
Ideally, researchers will find themselves identifying largely the same time windows for components no matter which approach is adopted. However, until these windows are well established in each developmental period, researchers should be diligent about reporting and justifying their approach to identifying time windows for ERP components. Thus, we recommend beginning with one of these two later approaches, but maintaining some flexibility and reporting in detail when and how adjustments are made and how adjustments are supported by developmental theory. This type of reporting will not only be critical for maintaining transparency in research, but also for ultimately building a literature containing appropriate time-windows for ERP studies of young children.
Selection of electrodes.
The selection of electrodes at which ERP amplitudes are scored gives rise to many of the same issues that are seen in the selection of a time window. That is, selecting for analysis only those electrodes where hypothesized differences appear to be present introduces the possibility that findings will be biased toward significant results.
However, developmental researchers, in particular, will find that scalp distributions for ERP components may change dramatically as children’s brains mature. As one example of this, children who are 10 years old or younger show greater neural reactivity to face stimuli and greater LPP to emotional pictures at occipital electrodes relative to older children (Kujawa, Klein, et al., 2012). Similarly, children older than age 10 show greater reactivity to faces at parietal sites and smaller LPP amplitudes to emotional pictures at occipital sites relative to younger children. Additional LPP work (Kujawa, Hajcak, et al., 2012) showed that at age 6, the LPP is maximal at occipital sites during an early time window (200 to 600 ms), but maximal at parietal electrodes in a later time window (600 to1000 ms). Thus, there appears to be a shift in the distribution of the LPP from occipital to frontoparietal regions during childhood. Moreover, this work suggests that the selection of electrodes may be interdependent with the selection of time window for scoring ERP components.
Possible resolutions to the issue of selecting electrode sites for analysis are similar to those for selecting a time window. We are hopeful that researchers will someday be able to base their choice(s) on a multitude of published empirical studies. Until that time, one will need to make selections carefully and scientifically while avoiding the problem of multiple implicit comparisons. One option is to average across electrode sites. This will not mean averaging across all electrodes used for data collection (especially for high-density systems), but rather averaging across electrodes in the scalp region where ERP effects are anticipated based on previous work and good developmental theory.
Spatial principal components analyses aim to identify the spatial distribution of latent ERP components from the EEG data. The recommendation for researchers wishing to use the principal components analysis approach to identify both the time window and electrode sites of interest for an ERP component is a temporal-spatial principal components analysis, in which the results from an initial temporal PCA, which identifies the time course of latent components, are rearranged and submitted to a spatial PCA for the identification of the most relevant electrode sites (Dien, Frishkoff, & Handy, 2005).
Clearly, more empirical work is needed to compare the above approaches and understand how and under what conditions differences may arise. Again, however, until the corpus of published ERP studies is established across developmental periods, researchers will need to report and justify their approach to identifying the electrodes at which ERP components were scored.
Recommendation #11: Initiate the selection of electrodes of interest and time windows using established methods that do not introduce statistical bias. Report any adjustments that are made and their justification per developmental theory.
ERP averaging.
How many trials are necessary to generate a stable ERP? The answer to this question undoubtedly differs based on the ERP of interest and the age group under study. Larger-amplitude ERPs can be more easily distinguished from artifact in the recordings with a smaller number of trials. In many cases, mean amplitudes of ERPs differ in children relative to adults, with some ERPs increasing in amplitude and some ERPs decreasing in amplitude as the brain’s neural networks mature. Thus, researchers must consider not only the number of trials that children can reasonably be expected to complete, but also rates of data loss and expected amplitudes of ERPs. Relatively few ERP components have been systematically tested to find the point at which averages generate stable estimates of amplitude. Pontifex and colleagues (Pontifex et al., 2010) suggested that a minimum of 6 trials were necessary to generate stable estimates of the error-related negativity (ERN) in children and adults ranging in age from 8 to 73 years. More recent work suggests that the number of trials necessary to achieve sufficient internal reliability with children may differ across tasks, with a minimum number of trials of 8 and 20 for flanker and go/no-go tasks, respectively (Meyer et al., 2014). Additional analyses in this work suggested that further nuances may exist based on child age (i.e., younger vs. older children) given that amplitudes tend to decline over time.
In either case, estimates are similar to the conclusion reached when the same analyses were performed in adult-only samples (Olvet & Hajcak, 2009b; Rietdijk, Franken, & Thurik, 2014). The number of necessary trials for the N2, P3, error positivity (Pe), and LPP in adult samples has been reported as 20–30, 14–20, 8, and 8–12, respectively (Clayson & Larson, 2013; Cohen & Polich, 1997; Moran, Jendrusina, & Moser, 2013; Rietdijk et al., 2014). To our knowledge, the internal consistency of ERPs during toddlerhood and preschool, including differences of these from adult estimates of internal consistency, has gone largely unexamined (see Tomarken, 1995), though work done in samples of older children suggests similar stability relative to adults (Hämmerer, Li, Völkle, Müller, & Lindenberger, 2013; Taylor, Gavin, & Davies, 2016).
The issue of the number of trials needed to generate a stable ERP introduces a caveat to our recommendation to keep the number of total trials in any task to a minimum. Specifically, the minimum number of trials needed for a task must be sensitive to the number of trials needed for a stable ERP. Once again, then, developmental researchers must aim to strike a balance between creating a child-friendly paradigm that is low in number of trials with a task that will produce a sufficient number of trials for a reliable ERP. As the number of ERP studies in children continues to increase, a critical avenue for future work will be to identify the minimum number of trials needed for stability in each ERP component, acknowledging that this number is likely to change across developmental periods.
Mean vs. peak amplitudes.
In both child and adult studies, ERP amplitudes may be scored as either mean or maximum amplitudes. For mean amplitude scoring, each sample in the EEG recording during the time window of interest is used to create a mean average, resulting in a composite level of activity. Experts suggest prioritizing mean amplitudes, when possible, in order to prevent possible biasing of ERP scores from a variety of sources (Luck, 2014). Mean amplitude measures certainly make intuitive sense for components that have a protracted time course, such as the LPP. However, an important drawback of mean amplitude is that it cannot completely accommodate the broad individual differences in the timing and shape of components across participants that are observed in the toddler, preschool, and early childhood years. Individual components appear to be more variable in young children relative to adults (Brooker, 2018; Hoyniak, 2017), likely reflecting a relative inadequacy of age as a proxy for stage of neural development. In practice, this means that those of us working with the youngest participants have found it is somewhat more feasible to select a time window that includes the peak amplitude of components for all participants than to select a window that is wide enough to include the full component (capturing both the increase in amplitude and return to baseline) but so narrow that it does not include activity that is clearly not component-related.
Ideally, the selection of mean or peak amplitudes will include a series of checks to make sure that the selection of measure does not bias the conclusions drawn from the findings. First, we suggest that researchers score both peak and area measures in order to ascertain whether substituting one measure for another changes the pattern of results. If similar results are not obtained through both methods, then it is the duty of the researcher to try to understand whether the reason is that one measure biases findings. When space allows, both peak and area measures can be presented to show that results replicate through both methods.
Second, it will be necessary for future work to consider possible differences in internal consistency for peak and area measures. Differences in reliability, particularly if one method appears not to be reliable, can lead to sharp differences in one’s ability to draw conclusions from their data. Measures of internal consistency have been examined in slightly older samples (Olvet & Hajcak, 2009b) and have begun to be reported in work with preschoolers (Brooker, 2018). However, reliabilities are rarely reported for psychophysiological measures despite their importance for developmental research. As an example of this, multiple studies have shown an instability in ERN amplitudes during the preschool years (Brooker, 2018; Grammer et al., 2014). Reliability estimates are critical for this type of work to show that instability is not simply a function of poor measurement at one age. Thus, we suggest that authors use reliability estimates to inform choices regarding mean vs. amplitude measures and regularly report reliability measures to inform future work.
Recommendation #12: Include both peak and area measures when possible and report internal consistency for ERPs under study.
Data loss.
As previously suggested, researchers can anticipate relatively high rates of data loss in samples of children relative to what is typically found in adult samples. When coupled with an overall fewer number of trials for averaging, this can result in ERPs that look quite different for young children than for adults. Does this mean that ERPs with children are inherently hopeless avenues for reaching empirically-based conclusions? Certainly not. It does, however, require researchers, reviewers, and editors to adjust their expectations for the number of artifacts and the amount of lost data that will be reported in developing populations, and for atypical populations in particular. This is not a reflection on the researcher or quality of the research study but is an anticipated result of working with child populations of interest. It is for the very reason that young children are challenging to study that makes studying them important and interesting.
Artifact.
In our work, we have observed numbers of participants excluded due to excessive artifact1 ranging from less than 1% to more than 36% (M = 10.29, SD = 10.37), with the range of differences likely reflecting the range of tasks, participant ages, and laboratory procedures being utilized. Even with relatively large samples, one can still end up with ERP averages created from relatively small numbers of trials if data contain more artifact overall, resulting in a larger number of individual segments that contain artifacts. In the 23 studies reviewed for this report, an average of 35.18 trials were used for ERP averaging in preschoolers. In the complete dataset, the range of usable trials, after artifact rejection, ranged from 0 to 199. Thus, a substantial amount of data loss can be expected during the artifact rejection process in work with young children.
Insufficient performance.
A second common reason for data loss is due to insufficient performance. For example, even iterative tasks may fail if an individual’s performance is poor enough that stimulus durations begin to approach (or need to exceed) the full length of the inter-trial interval. Another portion of data are lost because of inadequate performance, with some children performing below a preset threshold (M = 5.93%, SD = 13.46, range: 0% - 50.46%). Again, the broad range observed in our work likely reflects laboratory differences in tasks, sample ages, protocols, and components of interest. For us, this is often tolerable, but nonetheless, reviewers often note that this is more than would typically be anticipated based on research with adults.
Recommendation #13: For each study, report the amount of data lost, reasons for data loss, and any correlations between missing data and other variables of interest.
Other developmental considerations.
Although the ERP technique is unique for its utility in isolating rapidly-unfolding neural processes, one must remain cognizant that neural activity recorded using EEG does not occur in isolation. Paradigm design, recording, and data processing procedures should always include a careful consider other factors that may influence the final waveforms. For example, because the ERN component is time-locked to the participant’s response, which occurs near the end of a trial, it may be impacted by the underlying stimulus-locked waveform. That is, faster responses to task stimuli may occur before neural processes of attention (involved in stimulus processing) or cognitive control (involved in response selection) are fully complete. Thus, response-locked components, such as the ERN, may overlap with other ERP components, introducing non-random artifact into the data. This is also important to consider in longitudinal designs that are hallmarks of developmental science, given that response times for numerous components decrease across development. Decreased response times coincide with shorter latencies for response-locked ERPs, ultimately “shifting” the entire waveform toward earlier components. Waveforms should be carefully inspected at each stage and decisions about experimental design, data cleaning procedures, and interpretations of final data should be made after considering this possibility with care and caution.
An additional consideration that is relevant for multiple aspects of the ERP technique with children is the potential confound of developmental differences with methodological differences. As noted above, mean amplitudes for several ERP components appear to change across development. Increases in amplitude over time are generally thought to reflect physical maturational changes (e.g., synaptic pruning, etc.) and increases in neural processing efficiency. While there is evidence that both of these types of developmental change occur, age-related changes also include an increased tolerance for experimental procedures. Increased cooperation ultimately leads to greater numbers of usable trials and less “noisy” waveforms for each participant, which may present in the data as smaller amplitudes. Researchers should take care to understand whether variability in the number of usable trials is correlated with variability in the ERP amplitudes and be prepared to make the appropriate design or statistical adjustments.
4. Conclusion
The last decade has witnessed unprecedented growth in the use of brain imaging and electrocortical techniques world-wide. A recent report by Sapien Labs noted that, in the year 2015 alone, there were a total of 5,979 EEG papers published. Although the vast majority of these studies were with adults, it illustrates the vitality of the field that is beginning to take hold in laboratories who study brain development in children. Children are not “little adults” and they present with their own unique set of circumstances when collecting electrocortical measures. However, there is no go-to guide in the field for ERP work with young children (e.g., Schmidt & Segalowitz, 2008) similar to guideline papers written by the Society of Psychophysiological Research for the use of psychophysiological methods with adults. This results in developmental researchers frequently being met with the challenge of educating readers and reviewers on the unique issues associated with applying ERP methods to each empirical study conducted in a sample of young children.
In this paper, we have listed some of the unique circumstances and challenges of measuring electrocortical event-related potentials in young children. We surveyed 10 leading EEG research laboratories in North America who are doing ERP work with young children and who have also published empirical data on findings from these studies. We examined commonalities among the laboratories in terms of EEG acquisition systems, laboratory tasks used, data reduction and analytic techniques, and data interpretation in ERP work with very young children.
Although the present report may be limited by possible selection biases of laboratories solicited for contributing data, it does represent a first step in attempting to provide the field with useful guidelines for ERP research with child samples. Based on these comparisons, we provided recommendations (summarized in Figure 4) to the research community based on this information that we believe might be of use to investigators and reviewers alike as we move to developing standard guidelines for the nascent field of developmental psychophysiology in general and ERP work with very young children in particular.
Figure 4.
Summary of recommendations for conducting ERP work with young children
Footnotes
Data loss percentages at the trial level could not be calculated from the data set created for this manuscript.
Contributor Information
Rebecca J. Brooker, Texas A&M University
John E. Bates, Indiana University – Bloomington
Kristin A. Buss, The Pennsylvania State University
Mara J. Canen, Montana State University
Tracy A. Dennis-Tiwary, Hunter College of the City University of New York
Lisa M. Gatzke-Kopp, The Pennsylvania State University
Caroline Hoyniak, Indiana University – Bloomington.
Daniel N. Klein, Stony Brook University
Autumn Kujawa, Vanderbilt University.
Ayelet Lahat, McMaster University.
Connie Lamm, University of Arkansas.
Jason S. Moser, Michigan State University
Isaac T. Petersen, University of Iowa
Alva Tang, McMaster University.
Steven Woltering, Texas A&M University.
Louis A. Schmidt, McMaster University
References
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, & Gratton G (2005). Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology, 42(1), 33–42. doi: 10.1111/j.1469-8986.2005.00258.x [DOI] [PubMed] [Google Scholar]
- Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, … Barch DM (2016). Neural correlates of reward processing in depressed and healthy preschool-age children. Journal of the American Academy of Child & Adolescent Psychiatry, 55(12), 1081–1089. doi: 10.1016/j.jaac.2016.09.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, & Cuevas K (2012). Using EEG to study cognitive development: Issues and practices. Journal of Cognition and Development, 13(3), 281–294. doi: 10.1080/15248372.2012.691143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, & Wolfe CD (2008). The use of the electroencephalogram in research on cognitive development. In Schmidt LA & Segalowitz SJ (Eds.), Developmental Psychophysiology (pp. 150–170). New York, New York: Cambridge University Press. [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, … Faraone SV (2001). Further evidence of association between behavioral inhibition and social anxiety in children. American Journal of Psychiatry, 158(10), 1673–1679. doi: 10.1176/appi.ajp.158.10.1673 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, & Carter CS (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. doi: 10.1037/0033-295X.108.3.624 [DOI] [PubMed] [Google Scholar]
- Brooker RJ (2018). Maternal behavior and socioeconomic status predict longitudinal changes in error-related negativity in preschoolers. Child Development, 89(3), 725–733. doi: 10.1111/cdev.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, & Buss KA (2014). Toddler fearfulness is linked to individual differences in error-related negativity during preschool. Developmental Neuropsychology, 39(1), 1–8. doi: 10.1080/87565641.2013.826661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, & Dennis TA (2011). Error-monitoring brain activity is associated with affective behaviors in young children. Developmental Cognitive Neuroscience, 1(2), 141–152. doi: 10.1016/j.dcn.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WS, Marsh JT, & LaRue A (1983). Exponential electrophysiological aging: P3 latency. Electroencephalography and Clinical Neurophysiology, 55(3), 277–285. doi: 10.1016/0013-4694(83)90205-5 [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, & Tenke CE (2012). Event-related brain potentials in depression: Clinical, cognitive, and neurophysiological implications. In Luck SJ & Kappenman ES (Eds.), The Oxford handbook of event-related potential componenets (pp. 563–592). New York, New York: Oxford University Press. [Google Scholar]
- Burgio-Murphy A, Klorman R, Shaywitz SE, Fletcher JM, Marchione KE, Holahan J, … Shaywitz BA (2007). Error-related event-related potentials in children with attention-deficit hyperactivity disorder, oppositional defiant disorder, reading disorder, and math disorder. Biological Psychology, 75(1), 75–86. doi: 10.1016/j.biopsycho.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Dennis TA, Brooker RJ, & Sippel LM (2011). An ERP study of conflict monitoring in 4–8-year old children: Associations with temperament. Developmental Cognitive Neuroscience, 1(2), 131–140. doi: 10.1016/j.dcn.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Richards JE, White LK, Barker TV, Pine DS, & Fox NA (2017). Development of the error-monitoring system from ages 9–35: Unique insight provided by MRI-constrained source localization of EEG. NeuroImage, 157, 13–26. h doi: 10.1016/j.neuroimage.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Crites SL, Berntson GG, & Coles MGH (1993). If attitudes affect how stimuli are processed, should they not affect the event-related brain potential? Psychological Science, 4(2), 108–112. doi: 10.1111/j.1467-9280.1993.tb00470.x [DOI] [Google Scholar]
- Canen MJ, & Brooker RJ (2017). ERN, theta power, and risk for anxiety problems in preschoolers. Biological Psychiatry, 123, 103–110. 10.1016/j.biopsycho.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, … Fox NA (2009). Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 48(9), 928–935. 10.1097/CHI.0b013e3181ae09df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson PE, & Larson MJ (2013). Psychometric properties of conflict monitoring and conflict adaptation indices: Response time and conflict N2 event-related potentials. Psychophysiology, 50(12), 1209–1219. h doi: 10.1111/psyp.12138 [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Junghöfer M, & Schupp HT (2006). The categorization of natural scenes: Brain attention networks revealed by dense sensor ERPs. NeuroImage, 32(2), 583–591. doi: 10.1016/j.neuroimage.2006.04.180 [DOI] [PubMed] [Google Scholar]
- Cohen J, & Polich J (1997). On the number of trials needed for P300. International Journal of Psychophysiology, 25(3), 249–255. doi: 10.1016/S0167-8760(96)00743-X [DOI] [PubMed] [Google Scholar]
- Conejero Á, Guerra S, Abundis-Gutiérrez A, & Rueda MR (2018). Frontal theta activation associated with error detection in toddlers: influence of familial socioeconomic status. Developmental Science, 21, 1–10. doi: 10.1111/desc.12494 [DOI] [PubMed] [Google Scholar]
- Crone EA, & Ridderinkhof KR (2011). The developing brain: From theory to neuroimaging and back. Developmental Cognitive Neuroscience, 1(2), 101–109. doi: 10.1016/j.dcn.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. doi: 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- Cuthbert B, Schupp H, McManis M, Hillman C, Bradley MM, & Lang PJ (1995). Abstracts of Papers to be Presented at the Thirty-Fifth Annual Meeting of the Society for Psychophysiological Research. Psychophysiology, 32, S26. doi: 10.1111/j.1469-8986.1995.tb02379.x [DOI] [Google Scholar]
- Davies PL, Segalowitz SJ, & Gavin WJ (2004). Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology, 25(3), 355–376. doi: 10.1207/s15326942dn2503_6 [DOI] [PubMed] [Google Scholar]
- de Bruijn ERA, Grootens KP, Verkes RJ, Buchholz V, Hummelen JW, & Hulstijn W (2006). Neural correlates of impulsive responding in borderline personality disorder: ERP evidence for reduced action monitoring. Journal of Psychiatric Research, 40(5), 428–437. doi: 10.1016/j.jpsychires.2005.09.004 [DOI] [PubMed] [Google Scholar]
- DeBoer T, Scott LA, & Nelson CA (2007). Methods for acquiring and analyzing infant event-related potentials. In DeHaan M (Ed.), Infant EEG and Event-Related Potentials (pp. 5–38). New York: Psychology Press. [Google Scholar]
- DeCicco JM, O’Toole LJ, & Dennis TA (2014). The late positive potential as a neural signature for cognitive reappraisal in children. Developmental Neuropsychology, 39(7), 497–515. doi: 10.1080/87565641.2014.959171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCicco JM, Solomon B, & Dennis TA (2012). Neural correlates of cognitive reappraisal in children: An ERP study. Developmental Cognitive Neuroscience, 2(1), 70–80. doi: 10.1016/j.dcn.2011.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Amodio DM, & O’Toole LJ (2015). Associations between parental ideology and neural sensitivity to cognitive conflict in children. Social Neuroscience, 10(2), 206–217. doi: 10.1080/17470919.2014.968290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, & Chen C-C (2009). Trait anxiety and conflict monitoring following threat: an ERP study. Psychophysiology, 46(1), 122–131. doi: 10.1111/j.1469-8986.2008.00758.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, & Hajcak G (2009). The late positive potential: a neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry, 50(11), 1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, & Rothbart MK (1997). Reactive and effortful processes in the organization of temperament. Development and Psychopathology, 9(04), 633–652. [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff GA, & Handy TC (2005). Principal components analysis of ERP data. In Event-related potentials: A methods handbook Cambridge, MA: MIT Press. [Google Scholar]
- Donchin E (1981). Surprise!… Surprise? Psychophysiology, 18(5), 493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x [DOI] [PubMed] [Google Scholar]
- DuPuis D, Ram N, Willner CJ, Karalunas S, Segalowitz SJ, & Gatzke-Kopp LM (2015). Implications of ongoing neural development for the measurement of the error-related negativity in childhood. Developmental Science, 18(3), 452–468. doi: 10.1111/desc.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Uluğ AM, Zimmerman RD, & Casey BJ (2002). A neural basis for the development of inhibitory control. Developmental Science, 5(4), F9–F16. doi: 10.1111/1467-7687.00235 [DOI] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, & Blanke L (1991). Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology, 78(6), 447–455. doi: 10.1016/0013-4694(91)90062-9 [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, & Hohnsbein J (1999). ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica, 101(2–3), 267–291. doi: 10.1016/S0001-6918(99)00008-6 [DOI] [PubMed] [Google Scholar]
- Fischer KW, & Silvern L (1985). Stages and Individual Differences in Cognitive Development. Annual Review of Psychology, 36(1), 613–648. doi: 10.1146/annurev.ps.36.020185.003145 [DOI] [Google Scholar]
- Foti D, Hajcak G, & Dien J (2009). Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology, 46(3), 521–530. [DOI] [PubMed] [Google Scholar]
- Francis G, Last CG, & Strauss CC (1987). Expression of separation anxiety disorder: The roles of age and gender. Child Psychiatry and Human Development, 18(2), 82–89. doi: 10.1007/BF00709952 [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Willner CJ, Jetha MK, Abenavoli RM, DuPuis D, & Segalowitz SJ (2015). How does reactivity to frustrative non-reward increase risk for externalizing symptoms? Psychophysiological Science and the Research Domain Criteria, 98(2, Part 2), 300–309. doi: 10.1016/j.ijpsycho.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, & Carp J (2012). The error-related negativity (ERN/Ne). In Luck SJ & Kappenman ES (Eds.), The oxford handbook of event-related potential components (pp. 231–291). New York, New York: Oxford University Press. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2(10), 861. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, & Campos JJ (1982). Toward a theory of infant temperament. In Emde RN & Harmon RJ (Eds.), The development of attachment and affiliative systems (pp. 161–193). New York, New York: Plenum. [Google Scholar]
- Gow RV, Rubia K, Taylor E, Vallée-Tourangeau F, Matsudaira T, Ibrahimovic A, & Sumich A (2012). Abnormal centroparietal ERP response in predominantly medication-naive adolescent boys with ADHD during both response inhibition and execution. Journal of Clinical Neurophysiology, 29, 181–189. doi: 10.1097/WNP.0b013e31824e1025 [DOI] [PubMed] [Google Scholar]
- Grammer JK, Carrasco M, Gehring WJ, & Morrison FJ (2014). Age-related changes in error processing in young children: A school-based investigation. Developmental Cognitive Neuroscience, 9, 93–105. doi: 10.1016/j.dcn.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso DJ, Moser JS, Dozier M, & Simons R (2009). ERP correlates of attention allocation in mothers processing faces of their children. Biological Psychology, 81(2), 95–102. doi: 10.1016/j.biopsycho.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, & Dennis TA (2009). Brain potentials during affective picture processing in children. Biological Psychology, 80(3), 333–338. doi: 10.1016/j.biopsycho.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, & Foti D (2007). Neural response to emotional pictures is unaffaected by concurrent task difficutly: An event-related potential study. Behavioral Neuroscience, 121(6), 1156–1162. doi: 10.1037/0735-7044.121.6.1156 [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Foti D (2008). Errors are aversive: Defensive motivation and the error-related negativity. Psychological Science, 19(2), 103–108. doi: 10.1111/j.1467-9280.2008.02053.x [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, & Simons RF (2003). Anxiety and error-related brain activity. Biological Psychology, 64(1–2), 77–90. doi: 10.1016/S0301-0511(03)00103-0 [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, & Simons RF (2006). Attending to affect: Appraisal strategies modulate the electrocortical response to arousing pictures. Emotion, 6(3), 517–522. doi: 10.1037/1528-3542.6.3.517 [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Olvet DM (2008). The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion, 82(2), 250–255. doi: 10.1037/1528-3542.8.2.250 [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, & Foti D (2012). ERPs and the study of emotion. In The OxfordHhandbook of Event-Related Potential Components (pp. 441–472). New York, New York: Oxford University Press. [Google Scholar]
- Hämmerer D, Li S-C, Völkle M, Müller V, & Lindenberger U (2013). A lifespan comparison of the reliability, test-retest stability, and signal-to-noise ratio of event-related potentials assessed during performance monitoring. Psychophysiology, 50(1), 111–123. doi: 10.1111/j.1469-8986.2012.01476.x [DOI] [PubMed] [Google Scholar]
- Hoehl S, & Wahl S (2012). Recording Infant ERP Data for Cognitive Research. Developmental Neuropsychology, 37(3), 187–209. doi: 10.1080/87565641.2011.627958 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, & Coles MGH (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. doi: 10.1037/0033-295X.109.4.679 [DOI] [PubMed] [Google Scholar]
- Hoyniak C (2017). Changes in the nogo N2 event-related potential component across childhood: A systematic review and meta-analysis. Developmental Neuropsychology, 42(1), 1–24. doi: 10.1080/87565641.2016.1247162 [DOI] [PubMed] [Google Scholar]
- Hoyniak CP, Petersen IT, McQuillan ME, Staples AD, & Bates JE (2015). Less Efficient Neural Processing Related to Irregular Sleep and Less Sustained Attention in Toddlers. Developmental Neuropsychology, 40(3), 155–166. doi: 10.1080/87565641.2015.1016162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen TWP, Geladé K, van Mourik R, Maras A, & Oosterlaan J (2016). An ERP source imaging study of the oddball task in children with Attention Deficit/Hyperactivity Disorder. Clinical Neurophysiology, 127(2), 1351–1357. doi: 10.1016/j.clinph.2015.10.051 [DOI] [PubMed] [Google Scholar]
- Jonkman LM, van Melis JJM, Kemner C, & Markus CR (2007). Methylphenidate improves deficient error evaluation in children with ADHD: An event-related brain potential study. Biological Psychology, 76(3), 217–229. doi: 10.1016/j.biopsycho.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Keil A, Debener S, Gratton G, Junghöfer M, Kappenman ES, Luck SJ, … Yee CM (2014). Committee report: Publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology, 51(1), 1–21. doi: 10.1111/psyp.12147 [DOI] [PubMed] [Google Scholar]
- Kessel EM, Huselid RF, DeCicco JM, & Dennis TA (2013). Neurophysiological processing of emotion and parenting interact to predict inhibited behavior: An affective-motivational framework. Frontiers in Human Neuroscience, 7, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp CB (1982). Antecedents of self-regulation: A developmental perspective. Developmental Psychology, 18(2), 199–214. [Google Scholar]
- Krompinger JW, Moser JS, & Simons RF (2008). Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion, 8(1), 132–137. doi: 10.1037/1528-3542.8.1.132 [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, & Klein DN (2012). Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry, 53(2), 207–215. doi: 10.1111/j.1469-7610.2011.02461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, & Hajcak G (2012). Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience, 2, 458–467. doi: 10.1016/j.dcn.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Weinberg A, Hajcak G, & Klein DN (2013). Differentiating event-related potential components sensitive to emotion in middle childhood: Evidence from temporal-spatial PCA. Developmental Psychobiology, 55(5), 539–550. doi: 10.002/dev.21058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, & Ryan ND (2006). Increased error‐related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry, 47(10), 1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x [DOI] [PubMed] [Google Scholar]
- Lamm C, & Lewis MD (2010). Developmental Change in the Neurophysiological Correlates of Self-Regulation in High- and Low-Emotion Conditions. Developmental Neuropsychology, 35(2), 156–176. doi: 10.1080/87565640903526512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, McDermott JM, & Fox NA (2014). Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Developmental Science, 17(5), 667–681. doi: 10.1111/desc.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Zelazo PD, & Lewis MD (2006). Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Advances in Developmental Cognitive Neuroscience, 44(11), 2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013 [DOI] [PubMed] [Google Scholar]
- Lenroot RK, & Giedd JN (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Methodological and Conceptual Advances in the Study of Brain-Behavior Dynamics: A Multivariate Lifespan Perspective, 30(6), 718–729. doi: 10.1016/j.neubiorev.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Lewis MD, Granic I, & Lamm C (2006). Behavioral Differences in Aggressive Children Linked with Neural Mechanisms of Emotion Regulation. Annals of the New York Academy of Sciences, 1094(1), 164–177. doi: 10.1196/annals.1376.017 [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, & Zelazo PD (2006). Neurophysiological Correlates of Emotion Regulation in Children and Adolescents. Journal of Cognitive Neuroscience, 18(3), 430–443. [DOI] [PubMed] [Google Scholar]
- Lewis MD, & Stieben J (2004). Emotion regulation in the brain: Conceptual issues and directions for developmental research. Child Development, 75(2), 371–376. doi: 10.1111/j.1467-8624.2004.00680.x [DOI] [PubMed] [Google Scholar]
- Liu P, Bai X, & Pérez-Edgar KE (2019). Integrating high-density ERP and fMRI measures of face-elicited brain activity in 9–12-year-old children: An ERP source localization study. NeuroImage, 184, 599–608. doi: 10.1016/j.neuroimage.2018.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SL, Schroder HS, Moran TP, Durbin CE, & Moser JS (2015). Neurophysiological evidence of an association between cognitive control and defensive reactivity processes in young children. Developmental Cognitive Neuroscience, 15, 35–47. doi: 10.1016/j.dcn.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2014). An introduction to the event-related potential technique (Second). Cambridge, MA: MIT Press. [Google Scholar]
- Luck SJ, & Gaspelin N (2017). How to get statistically significant effects in any ERP experiment (and why you shouldn’t). Psychophysiology, 54(1), 146–157. doi: 10.1111/psyp.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Foti D, & Hajcak G (2009). Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion, 9(4), 531–543. doi: 10.1037/a0016251 [DOI] [PubMed] [Google Scholar]
- MacNamara A, & Hajcak G (2010). Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depression and Anxiety, 27(3), 234–243. doi: 10.1002/da.20679 [DOI] [PubMed] [Google Scholar]
- Mahajan Y, & McArthur G (2010). Does combing the scalp reduce scalp electrode impedances? Journal of Neuroscience Methods, 188(2), 287–289. doi: 10.1016/j.jneumeth.2010.02.024 [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113(8), 1199–1208. doi: 10.1016/S1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- McCarthy G, & Donchin E (1981). A metric for thought: a comparison of P300 latency and reaction time. Science, 211(4477), 77. doi: 10.1126/science.7444452 [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, & Fox NA (2009). A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry, 65(5), 445–448. doi: 10.1016/j.biopsych.2008.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress JN, & Proudfit GH (2014). Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology, 51(7), 602–610. doi: 10.1111/psyp.12208 [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, … Klein DN (2013). Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of Abnormal Child Psychology, 41(8), 1257–1266. doi: 10.1007/s10802-013-9762-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, & Klein DN (2015). Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. Journal of Abnormal Psychology, 124(2), 266–274. doi: 10.1037/abn0000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak, Greg H, Torpey-Newman D, Kujawa A, Olino TM, Dyson M, & Klein DN (2018). Early temperamental fearfulness and the developmental trajectory of error‐related brain activity. Developmental Psychobiology, 60(2), 224–231. doi: 10.1002/dev.21605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, & Hajcak G (2012). The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience, 2(1), 152–161. doi: 10.1016/j.dcn.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TP, Jendrusina AA, & Moser JS (2013). The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research, 1516, 66–75. doi: 10.1016/j.brainres.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Moran TP, Taylor D, & Moser JS (2012). Sex moderates the relationship between worry and performance monitoring brain activity in undergraduates. International Journal of Psychophysiology, 85(2), 188–194. doi: 10.1016/j.ijpsycho.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Moser J, Moran T, Schroder H, Donnellan B, & Yeung N (2013). On the relationship between anxiety and error monitoring: A meta-analysis and conceptual framework. Frontiers in Human Neuroscience, 4, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, & Simons RF (2006). Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology, 43(3), 292–296. [DOI] [PubMed] [Google Scholar]
- Moser JS, Huppert JD, Duval E, & Simons RF (2008). Face processing biases in social anxiety: An electrophysiological study. Biological Psychology, 78(1), 93–103. doi: 10.1016/j.biopsycho.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Moser JS, Krompinger JW, Dietz J, & Simons RF (2009). Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology, 46(1), 17–27. doi: 10.1111/j.1469-8986.2008.00721.x [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, & Jendrusina AA (2012). Parsing relationships between dimensions of anxiety and action monitoring brain potentials in female undergraduates. Psychophysiology, 49(1), 3–10. doi: 10.1111/j.1469-8986.2011.01279.x [DOI] [PubMed] [Google Scholar]
- Nash K, Inzlicht M, & McGregor I (2012). Approach-related left prefrontal EEG asymmetry predicts muted error-related negativity. Biological Psychology, 91(1), 96–102. doi: 10.1016/j.biopsycho.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Niedermeyer E (2005). The normal EEG of the waking adult. In Niedermeyer E & Lopes da Silva FH (Eds.), Electroencephalography: Basic principles, Clinical Applications, and Related Fields (Fifth Edition, pp. 167–192). Philadelphia, PA: Lippincott, Williams, & Wilkins. [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, & Ridderinkhof K (2003). Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience, 3(1), 17–26. doi: 10.3758/CABN.3.1.17 [DOI] [PubMed] [Google Scholar]
- Olofsson JK, & Polich J (2007). Affective visual event-related potentials: Arousal, repetition, and time-on-task. Biological Psychology, 75(1), 101–108. doi: 10.1016/j.biopsycho.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, & Hajcak G (2009a). The effect of trial-to-trial feedback on the error-related negativity and its relationship with anxiety. Cognitive, Affective, & Behavioral Neuroscience, 9(4), 427–433. doi: 10.3758/CABN.9.4.427 [DOI] [PubMed] [Google Scholar]
- Olvet DM, & Hajcak G (2009b). The stability of error‐related brain activity with increasing trials. Psychophysiology, 46(5), 957–961. doi: 10.1111/j.1469-8986.2009.00848.x [DOI] [PubMed] [Google Scholar]
- O’Toole LJ, DeCicco JM, Berthod S, & Dennis TA (2013). The N170 to Angry Faces Predicts Anxiety in Typically Developing Children Over a Two-Year Period. Developmental Neuropsychology, 38(5), 352–363. doi: 10.1080/87565641.2013.802321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overtoom CCE, Verbaten MN, Kemner C, Kenemans JL, Van Engeland H, Buitelaar JK, … Koelega HS (1998). Associations between event-related potentials and measures of attention and inhibition in the continuous performance task in children with ADHD and normal controls. Journal of the American Academy of Child & Adolescent Psychiatry, 37(9), 977–985. h doi: 10.1097/00004583-199809000-00018 [DOI] [PubMed] [Google Scholar]
- Petersen IT, Hoyniak CP, McQuillan ME, Bates JE, & Staples AD (2016). Measuring the development of inhibitory control: The challenge of heterotypic continuity. Developmental Review, 40, 25–71. doi: 10.1016/j.dr.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, … Taylor MJ (2000). Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology, 37(02), 127–152. [PubMed] [Google Scholar]
- Pliszka SR (2000). Patterns of psychiatric comorbidity with attention-deficit/hyperactivity disorder. Child and Adolescent Psychiatric Clinics of North America, 9(3), 525–540. [PubMed] [Google Scholar]
- Polich J (2003). Theoretical Overview of P3a and P3b. In Polich J (Ed.), Detection of Change: Event-Related Potential and fMRI Findings (pp. 83–98). Boston, MA: Springer US. R; doi: 10.1007/978-1-4615-0294-4_5 [DOI] [Google Scholar]
- Polich J (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–2148. doi: 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, & Kok A (1995). Cognitive and biological determinants of P300: an integrative review. Biological Psychology, 41(2), 103–146. doi: 10.1016/0301-0511(95)05130-9 [DOI] [PubMed] [Google Scholar]
- Polich J, Ladish C, & Burns T (1990). Normal variation of P300 in children: Age, memory span, and head size. International Journal of Psychophysiology, 9(3), 237–248. doi: 10.1016/0167-8760(90)90056-J [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu C, Themanson JR, & Hillman CH (2010). On the number of trials necessary for stabilization of error‐related brain activity across the life span. Psychophysiology, 47(4), 767–773. doi: 10.1111/j.1469-8986.2010.00974.x, [DOI] [PubMed] [Google Scholar]
- Potts GF, George MRM, Martin LE, & Barratt ES (2006). Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neuroscience Letters, 397(1–2), 130–134. doi: 10.1016/j.neulet.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Rietdijk WJR, Franken IHA, & Thurik AR (2014). Internal Consistency of Event-Related Potentials Associated with Cognitive Control: N2/P3 and ERN/Pe. PLoS ONE, 9(7), e102672. doi: 10.1371/journal.pone.0102672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, & Schmidt LA (2006). Error-Related Electrocortical Responses Are Enhanced in Children With Obsessive–Compulsive Behaviors. Developmental Neuropsychology, 29(3), 431–445. doi: 10.1207/s15326942dn2903_3 [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, & Hamm AO (2003). Attention and emotion: an ERP analysis of facilitated emotional stimulus processing. NeuroReport, 14(8), 1107–1110. [DOI] [PubMed] [Google Scholar]
- Segalowitz S ., & Davies PL (2004). Charting the maturation of the frontal lobe: An electrophysiological strategy. Development of Orbitofrontal Function, 55(1), 116–133. doi: 10.1016/S0278-2626(03)00283-5 [DOI] [PubMed] [Google Scholar]
- Simon-Thomas ER, Role KO, & Knight RT (2005). Behavioral and Electrophysiological Evidence of a Right Hemisphere Bias for the Influence of Negative Emotion on Higher Cognition. Journal of Cognitive Neuroscience, 17(3), 518–529. doi: 10.1162/0898929053279504 [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, & Barry RJ (2004). Inhibitory processing during the go/no-go task: An ERP analysis of children with attention-deficit/hyperactivity disorder. Clinical Neurophysiology, 115, 1320–1331. doi: 10.1016/j.clinph.2003.12.027 [DOI] [PubMed] [Google Scholar]
- Solomon B, DeCicco JM, & Dennis TA (2012). Emotional picture processing in children: An ERP study. Developmental Cognitive Neuroscience, 2(1), 110–119. doi: 10.1016/j.dcn.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, O’Toole L, Hong M, & Dennis TA (2014). Negative affectivity and EEG asymmetry interact to predict emotional interference on attention in early school-aged children. Brain and Cognition, 87(0), 173–180. doi: 10.1016/j.bandc.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, & Pepler D (2007). Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology, 19(02), 455–480. doi: 10.1017/S0954579407070228 [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, & John ER (1965). Evoked-Potential Correlates of Stimulus Uncertainty. Science, 150(3700), 1187. doi: 10.1126/science.150.3700.1187 [DOI] [PubMed] [Google Scholar]
- Tang A, Lahat A, Crowley MJ, & Schmidt LA (2017). Developmental differences in event-related potentials to social exclusion in children, adolescents, and adults. Presented at the Annual meeting of the International Society for Developmental Psychobiology, Washington DC. [Google Scholar]
- Taylor BK, Gavin WJ, & Davies PL (2016). The test–retest reliability of the visually evoked contingent negative variation (CNV) in children and adults. Developmental Neuropsychology, 41(3), 162–175. doi: 10.1080/87565641.2016.1170835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa AJ, Dyson MW, Olino TM, & Klein DN (2013). Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. Journal of Child Psychology and Psychiatry, 54(8), 854–862. doi: 10.1111/jcpp.12041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, & Klein DN (2009). An examination of error-related brain activity and its modulation by error value in young children. Developmental Neuropsychology, 34(6), 749–761. doi: 10.1080/87565640903265103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen V, & Carter CS (2002). The Timing of Action-Monitoring Processes in the Anterior Cingulate Cortex. Journal of Cognitive Neuroscience, 14(4), 593–602. doi: 10.1162/08989290260045837 [DOI] [PubMed] [Google Scholar]
- Van Veen V, & Carter CS (2006). Conflict and Cognitive Control in the Brain. Current Directions in Psychological Science, 15(5), 237–240. doi: 10.1111/j.1467-8721.2006.00443.x [DOI] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, & Bonnet M (2000). Is the ‘error negativity’ specific to errors? Biological Psychology, 51(2–3), 109–128. doi: 10.1016/S0301-0511(99)00032-0 [DOI] [PubMed] [Google Scholar]
- Wiersema JR, van der Meere JJ, & Roeyers H (2007). Developmental changes in error monitoring: An event-related potential study. Neuropsychologia, 45(8), 1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Willner CJ, Gatzke-Kopp LM, Bierman KL, Greenberg MT, & Segalowitz SJ (2015). Relevance of a neurophysiological marker of attention allocation for children’s learning-related behaviors and academic performance. Developmental Psychology, 51(8), 1148–1162. doi: 10.1037/a0039311 [DOI] [PubMed] [Google Scholar]
- Woltering S, Granic I, Lamm C, & Lewis MD (2011). Neural Changes Associated with Treatment Outcome in Children with Externalizing Problems. Biological Psychiatry, 70(9), 873–879. doi: 10.1016/j.biopsych.2011.05.029 [DOI] [PubMed] [Google Scholar]



