Abstract
Purpose of review.
This review aims to discuss retinal diseases that may masquerade as neurological causes of vision loss and highlights modern ophthalmic ancillary testing that can help to establish these diagnoses.
Recent findings.
Retinal diseases with signs and symptoms overlapping with neurological causes of vision loss include central serous chorioretinopathy, retinal vascular insufficiency, acute macular neuroretinopathy, big blind spot syndrome, paraneoplastic retinopathy, retinal dystrophy, and toxic retinopathy. Diagnosis is facilitated by electrophysiologic studies and multimodal ophthalmic imaging including optical coherence tomography and fundus autofluorescence imaging. Looking into the future, translation of adaptive optics ophthalmoscopy into clinical practice may facilitate early detection of microscopic retinal abnormalities that characterize these conditions.
Summary.
With conventional methods of physical examination, diagnosis of retinal diseases that may masquerade as neurological causes of vision loss can challenging. Current advance in multimodal ophthalmic imaging along with electrophysiologic studies enhance the provider’s ability to make early diagnosis and monitor progression of these conditions.
Keywords: multimodal ophthalmic imaging, maculopathy, big blind spot syndrome, retinal dystrophy, retinal vascular insufficiency, toxic retinopathy
Introduction
Visual loss can result from any pathology along the visual pathway. A comprehensive ophthalmic examination usually identifies the cause, with common etiologies including refractive error, ocular surface dryness, cataract, macular degeneration and glaucoma. When the examination is unrevealing, patients are often referred to neurologists or neuro-ophthalmologists for evaluation of potential retrobulbar (optic nerve or cerebral) causes of visual loss. When doing these evaluations, it is important for neurologists to be aware of retinopathies which may be occult on physical examination so that they may be included in the differential diagnosis.(Table 1)
Table 1:
Differential diagnosis in patients presenting with vision loss
| Examination reveals apparent etiology of visual loss | |
|---|---|
| Category | Suggestive signs |
| Uncorrected refractive errors | ∘ Visual acuity improves with pinhole or glasses. |
| Ocular media opacity (e.g. corneal, lens or vitreous pathology | ∘ Abnormal findings on ophthalmic examination (e.g. slit-lamp examination), ∘ Diminished red-reflex. |
| Retinopathy or choroidopathy | ∘ Abnormal fundus findings |
| Optic neuropathy/chiasmopathy | ∘ Localizing visual field defects ∘ Dyschromatopsia ∘ Swelling or pallor of the optic nerve head ∘ rAPD present (asymmetric disease) |
| Retrochiasmal anterior optic pathway (optic tract)/ posterior visual pathway (retrogeniculate) lesion | ∘ Bilateral homonymous visual field defects ∘ Typically preserved visual acuity |
| Examination does not reveal apparent etiology of visual loss | |
| Category | Possible etiologies |
| Retinal disease with normal-appearing fundus or subtle abnormalities | ∘ Central serous chorioretinopathy ∘ Retinal vascular insufficiency, hyperacute/chronic (e.g. CRAO, BRAO) ∘ Acute macular neuroretinopathy ∘ AZOOR complex diseases (e.g. AIBES, MEWDS, AZOOR) ∘ Paraneoplastic retinopathy (e.g. CAR, MAR) ∘ Retinal dystrophy (e.g. Cone, cone-rod dystrophies, macular dystrophies) ∘ Toxic retinopathy (e.g. CQ or HCQ induced retinopathy, Vigabatrin induced retinopathy) |
| Neurological disease lacking ∘ Dyschromatopsia ∘ Swelling or pallor of the optic nerve head ∘ rAPD ∘ Localizing visual field defects |
∘ Optic neuropathy (early-stage or lacking axonal loss) ∘ Bilateral occipital lobe dysfunction ∘ Higher order visual processing disturbance ∘ Functional neurological symptom disorder |
Abbreviation: rAPD = relative afferent pupillary defect, CRAO = central retinal artery occlusion, BRAO = branch retinal artery occlusion, AIBES = acute idiopathic blind-spot enlargement syndrome, MEWDS = multiple evanescent white dot syndrome, AZOOR = acute zonal occult outer retinopathy, CQ = Chloroquine, HCQ = Hydroxychloroquine.
In the case of monocular vision loss, differentiating between optic neuropathy and retinopathy can be straightforward in patients with an obviously abnormal retina examination, an abnormal optic nerve head appearance, or a relative afferent pupillary defect, which is a difference in the direct and indirect pupillary response to light stimulation between both eyes, in the setting of mild to moderate vision loss. In the case of binocular vision loss, cerebral causes can often be identified by homonymous visual field defect patterns and bilateral optic neuropathies or retinopathies by the fundus appearance. However, several retinal diseases can present with symptoms overlapping with neurological causes of vision loss. These conditions may present with reduced visual acuity, visual field defects, and normal-appearing fundus examination because the fundus abnormality is below our visual detection threshold. A systematic approach with a broad differential diagnosis is crucial in order to localize and identify the etiology of visual loss.
Comprehensive review of all retinal diseases or the general approach to patient with visual loss are beyond the scope of this review.[1] This article will focus on retinal diseases that may commonly masquerade as optic neuropathies or cerebral causes of vision loss, and differentiate between maculopathies and optic neuropathies.
Differentiation between maculopathies and optic neuropathies
Signs and symptoms
Both maculopathies (diseases affecting the area of the retina responsible for central vision) and optic neuropathies can cause central scotomas presenting with altered visual acuity. Differentiation between maculopathies and optic neuropathies requires consideration of historical information, clinical examination, and ophthalmic investigations. Supplementary laboratory tests and neuroimaging may be warranted in selected cases.
Characteristics of visual loss and associated symptoms can aid in localization of pathology. Metamorphopsia (visual distortion) is commonly described in maculopathies but is rarely present in optic neuropathies. Loss of color perception is typically more severe than the degree of visual loss in optic neuropathies, whereas color perception in maculopathies usually correlates with the degree of visual loss. Cone photoreceptor diseases or preexisting congenital color blindness are exceptions to this. Pain during eye movements is associated with optic neuritis but is uncommon in other causes of optic neuropathy and maculopathies.
Monocular or asymmetric visual loss with a relative afferent pupillary defect (rAPD) is highly suggestive of an optic neuropathy. However, rAPD should be interpreted cautiously since rAPD only reflects the difference in optic nerve signal input between the eyes. Asymmetrical extensive retinal disease may produce an rAPD, whereas symmetrical bilateral optic neuropathies will not produce an rAPD.
The photostress test is useful for detecting maculopathies. This is performed by measuring best-corrected visual acuity(BCVA) with an undilated pupil, shining bright light in the eye being tested for 10 seconds (with the other eye covered), and then recording the time between removal of the light source, until the patient can read the letters one line above their BCVA on the eye chart. Normal recovery time, seen in patients with optic neuropathies is 27±11 seconds (mean±SD).[2] Maculopathies are associated with prolonged recovery times up to several minutes.[3] Clinically, comparison of recovery time between affected and unaffected eyes is most useful in distinguishing between asymmetric macular and optic nerve etiologies.[4]
Ophthalmoscopy provides the ability to visualize abnormalities of the macula or optic nerve head. Macular pathology, including subretinal fluid, exudates, hemorrhages, or pigmentary changes suggest maculopathy, while optic nerve head abnormalities ranging from subtle mild pallor to marked optic disc swelling suggest optic neuropathy. However, in both maculopathies and optic neuropathies, abnormalities might be below detection threshold or not present. Direct ophthalmoscopy through an undilated pupil as typically performed by neurologists further lowers detection threshold.[5, 6] These limitations can be overcome by ophthalmic ancillary testing.
Ophthalmic Ancillary Testing
Characteristic ophthalmic investigation findings in retinal diseases that may masquerade as neurological causes of vision loss are summarized in table 2.
Table2:
Characteristic fundus findings and ophthalmic ancillary testing results in occult retinal diseases
| Disease | Characteristic fundus findings | Characteristic ophthalmic ancillary testing results |
|---|---|---|
| Central serous chorioretinopathy (CSCR) | •Round serous neurosensory retinal detachment in macula without hemorrhage |
Amsler grid: Metamorphopsia. OCT: Serous neurosensory retinal detachment or pigment epithelium detachment. FA: Leakage from level of RPE. |
| Retinal ischemia | ||
| Central retinal artery occlusion (CRAO) Branch retinal artery occlusion (BRAO) |
Hyper acute stage: •May appear normal. Embolus/plaque or attenuated arterioles may be observed. Acute stage: •CRAO – opacified and whitened retina in the posterior pole with characteristic cherry-red spot at fovea. •BRAO – whitened ischemic retina along distribution of occluded artery. •Retinal emboli may be observed. Chronic stage: •CRAO – attenuated retinal vessels, and optic disc pallor. •BRAO – affected arterial attenuation, and sectoral nerve fiber layer loss. |
OCT: Thickened hyperreflectivity of inner retina and loss of inner retina definition followed by thinning of ischemic inner retina in chronic stage. FA: Delay in retinal artery filling time and retinal arteriovenous transit time in acute stage. ffERG (CRAO): Diminished amplitude of b-wave with generally unaffected a-wave (“electronegative”). |
| Acute macular neuroretinopathy (AMN) | •Macular brown petaloid intraretinal lesions pointing to fovea |
HVF/Amsler grid: Small paracentral scotomata corresponds to retinal lesions. OCT: Initial hyperreflective band in outer nuclear and outer plexiform layers followed by focal disruption of ellipsoid zone. mfERG: Focal reduced responses corresponding to lesions. |
| AZOOR complex diseases | ||
| Acute idiopathic blind spot enlargement syndrome (AIBSE) | •Peripapillary pigmentary changes •Occasionally mild optic disc swelling. |
HVF: Enlarged physiologic blind spot. OCT: Disruption of peripapillary ellipsoid zone. FA: Optic disc staining may be present. mfERG: Abnormal responses from peripapillary area. |
| Multiple evanescent white dot syndrome (MEWDS) | •Initially, multiple small yellow-white lesions at the level of RPE or outer retina in perifoveal area •Later white dots fade and appear as granular macular pigmentary change. •Mild degree of optic disc swelling may be observed. |
HVF: Central, paracentral scotoma, or enlarged physiologic blind spot. OCT: Disruption of ellipsoid zone. FAF: Hyperautofluorescent dots correspond to lesions on fundus exam or even in the absence of fundus finding. FA: Punctate hyperfluorescent lesion in a wreath-like configuration on posterior pole. |
| Acute zonal occult outer retinopathy (AZOOR) | •Mostly normal fundus in early stage •Focal RPE pigmentary change and retinal atrophy (often peripapillary area) in later stage. |
HVF: Paracentral scotoma, or enlarged physiologic blind spot. OCT: Early disruption of ellipsoid zone, usually spare fovea. Chronically, thinning of affected retina and RPE. FAF: Hypoautofluorescent in atrophic area surrounding by area of speckled hyperautofluorescence reflects progressing area. ffERG: Delayed implicit time of photopic 30-Hz flicker response. mfERG: Attenuated responses corresponding to the affected area. |
| Paraneoplastic autoimmune retinopathy | ||
| Cancer-associated retinopathy (CAR) | •Mostly normal fundus in early stage •Optic nerve pallor, retinal vessels attenuation, and RPE abnormality in late stage. |
HVF: Ring scotoma, paracentral scotoma, or generalized depression. OCT: Thinning photoreceptor layer and loss of ellipsoid zone. FAF: Hyperautofluorescent ring around fovea. ffERG: Reduced a- and b-waves amplitude in both scotopic and photopic. |
| Melanoma-associated retinopathy (MAR) | •Mostly normal fundus, •Optic disc pallor, retinal vessels attenuation, and vitritis may be observed. |
HVF: Central, paracentral scotoma, generalized depression, or constricted visual field ffERG: Normal a-wave and reduced scotopic b-wave amplitude to bright flash (“electronegative”). |
| Retinal dystrophy | ||
| Cone, Cone-rod dystrophies | •Normal fundus in early stage •Macular pigmentary change developed as the disease progresses. |
ffERG: Cone dystrophies - reduced photopic a-wave and b-wave amplitude. Delayed 30-Hz flicker response. Cone-rod dystrophies - abnormal in both scotopic and photopic response. Photopic response being more affected than scotopic response. |
| Stargardt disease | •Normal fundus in early stage. •Macular atrophy with characteristic pisciform yellowish flecks lesion at RPE level in macula. |
OCT: Disruption of photoreceptor extends from central macular. FAF: Bull’s eye appearance, central hypoautofluorescence surrounded by area of hyperautofluorescence. FA: “Dark choroid” appearance PERG: Abnormal reduced amplitude. |
| Toxic retinopathy | ||
| CQ or HCQ retinopathy | •Bilateral bull’s eye maculopathy in late stage. |
HVF (10-2): Paracentral scotoma, ring scotoma. May consider 24-2 or 30-2 to detect toxicity in patents of Asian ancestry. OCT: Thinning of photoreceptor layers in parafoveal region. mfERG: Reduced responses in parafoveal region. |
| Vigabatrin retinopathy | •Peripheral retinal atrophy •Nasal optic disc pallor. |
HVF: Constricted visual field. ffERG: Reduced photopic 30-Hz flicker amplitude. |
(Abbreviation: OCT = optical coherence tomography, FA = fluorescein angiography, FAF = fundus autofluorescence, RPE = retinal pigment epithelium, HVF = Humphrey visual field, mfERG = multifocal electroretinogram, ffERG = full-field electroretinogram, PERG = pattern electroretinogram, CQ = Chloroquine, HCQ = Hydroxychloroquine)
Optical coherence tomography (OCT):
OCT is a non-invasive imaging method which provides in vivo high-resolution cross-sectional images of the retina and optic nerve head, with axial resolution ranging from 1-15μm.[7] Structural abnormalities such as cystoid macular edema, subretinal fluid, or photoreceptor abnormalities are readily visualized.(Figure1B, 1D, 1F, 1H, 1J, 1N) Cross sectional OCT may also be segmented to measure individual retinal layers of interest including the retinal nerve fiber layer(RNFL) and ganglion cell complex(GCC). Thinning of these layers can reflect optic neuropathy. Thinning of other layers or distortion of their anatomy suggests retinopathy.
Figure 1.
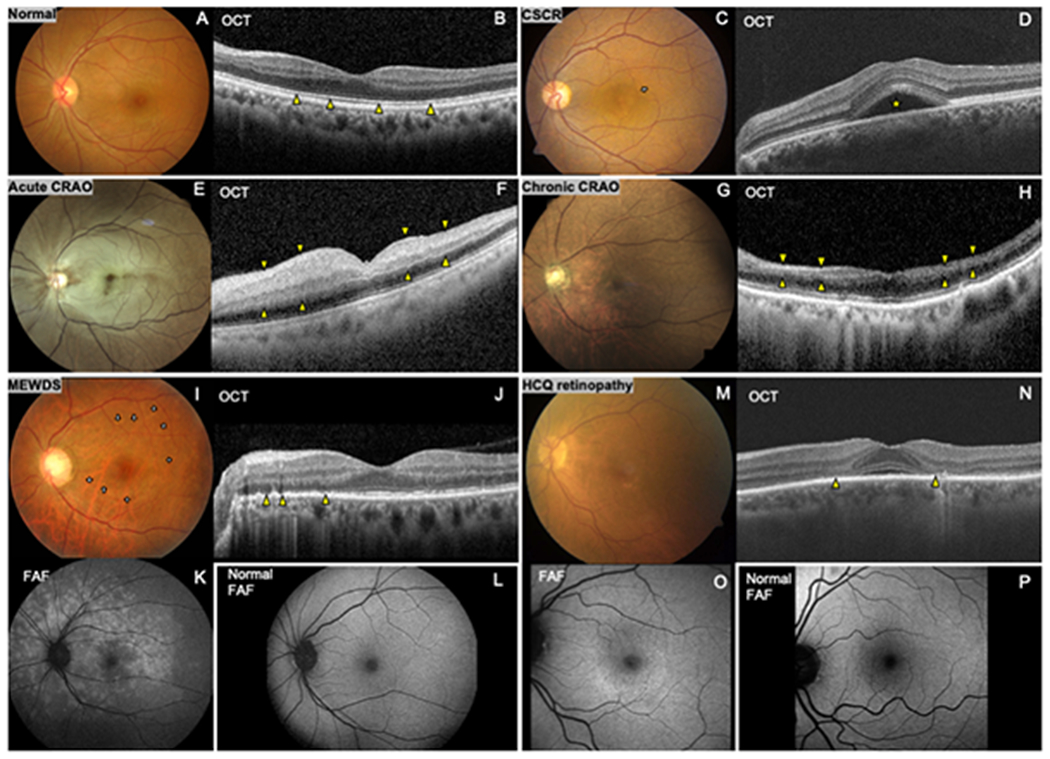
Characteristic ophthalmic imaging findings in retinal diseases that may masquerade as neurological cause of visual loss
(A) Normal fundus photo and (B) normal optical coherence tomography (OCT) demonstrating the cross-sectional structure of the retina with intact photoreceptor inner-outer segment junction (ellipsoid zone) (arrowheads). Central serous chorioretinopathy(CSCR): (C) Fundus photo reveals loss of foveal light reflex and subtle round serous macular detachment (arrow). (D) OCT shows corresponding subretinal fluid (star). Acute central retinal artery occlusion(CRAO): (E) Fundus photo shows diffuse opacified edematous retina with a characteristic ‘cherry-red spot’. (F) OCT shows thickened hyperreflective inner retina (area between arrowheads). Chronic CRAO: (G) Reperfused retina appears relatively normal except for optic disc pallor and attenuated retinal arterioles. (H) OCT shows thinning of ischemic inner retina (area between arrowheads). Multiple evanescent white dot syndrome (MEWDS): (I) Fundus photo shows subtle multiple small yellow-white perifoveal lesions (arrows). (K) Fundus autofluorescence (FAF) demonstrates numerous hyperautofluorescent lesions in the macula and peripapillary region (L) Normal FAF for comparison. (J) OCT shows disruption of the ellipsoid zone (arrowheads). Hydroxychloroquine (HCQ) retinopathy (late stage): (M) Fundus photo shows parafoveal retinal pigment epithelium depigmentation with (O) corresponding hyperautofluorescent parafoveal lesion on FAF. (P)Normal FAF for comparison. (N) OCT shows parafoveal photoreceptor loss (arrowheads).
Fundus autofluorescence (FAF):
FAF is a non-invasive imaging method that provides an en face density map of lipofuscin in the retinal pigment epithelium(RPE) layer which plays an important role in the metabolism of the overlying neurosensory retina; FAF serves as a surrogate marker of the integrity and health status of RPE, and by extension, the retina. (Figure1L, 1L, 1O, 1P) It can demonstrate retinal abnormalities which may not visualized by fundus exam or OCT and it is useful in detection of variety of diseases such as early-stage macular dystrophies, inflammatory white dot syndromes, and toxic retinopathies.[8–10]
Retinal angiography:
Fluorescein angiography (FA), consists of time lapsed images of the retina following peripheral venous injection of fluorescein dye. It is useful for evaluation of ocular perfusion in both the retinal circulation which supplies the inner retina including retinal ganglion cells and bipolar cells, and the choroidal circulation which supplies the outer retina including the photoreceptors and RPE. For example, delayed arteriovenous transit time indicates abnormal retinal circulation, while delayed choroidal filling time is associated with choroidal ischemia in giant cell arteritis. Moreover, FA can identify retinal or choroidal vascular pathology causing central serous chorioretinopathy, diabetic macular edema, and retinal vasculitis.
Indocyanine green angiography (ICGA) is another modality of angiography with focus on choroidal circulation. Similar to FA, ICGA involves peripheral venous injection of dye while time lapsed fundus images are recorded. Indocyanine green (ICG) dye is almost completely protein-bound after peripheral venous injection. This enhances its retention in the choroidal circulation and facilitates visualization of the choroidal circulation imaging with less artifact from the retinal circulation which lies between the choroidal circulation and the camera recording the images.
Electroretinography (ERG):
ERG measures the retina electrical response to light stimuli using a superficial electrode and is particularly useful in detection of retinal diseases which have retinal dysfunction despite a normal-appearing fundus. The normal recording consists of a negative a-wave, which reflects photoreceptor (outer retina) function, followed by positive b-wave which reflects intermediate retina (bipolar cells and Mueller cells) integrity. Using different light stimuli, rod and cone photoreceptor-mediated systems can be investigated separately. Full-field ERG (ffERG), performed using full-field flash stimuli, is useful for detection of generalized retinal disease such as retinitis pigmentosa, cone-rod dystrophy, and paraneoplastic retinopathy.[11] Multifocal ERG (mfERG) is performed using patterned light stimuli to generate a map of macular function and is abnormal in maculopathies such as white dot syndromes and chloroquine-induced maculopathy.[12] Pattern ERG (PERG), performed using an alternating checkerboard light stimulus, also provides measurement of central retinal function and has components representing retinal ganglion cell (optic nerve) responses. mfERG and PERG are useful in differentiating optic neuropathy from macular disease in cases of occult central visual loss.
Adaptive optics (AO):
Looking into the future, advanced ophthalmic imaging techniques including adaptive optics (AO) ophthalmoscopy may improve not only the diagnostic capability but also the understanding of disease pathophysiology through high spatial resolution retinal imaging. AO, which are techniques for compensating for light scatter, combined with OCT or scanning laser ophthalmoscopy, provides in vivo imaging of retinal structures with cellular level resolution and has the potential to improve our ability to detect abnormalities beyond what is available with current clinical ophthalmic imaging.[13]
Additional testing
Localization of visual loss may be unrevealing despite comprehensive evaluations. Additional investigations, including brain/orbit neuroimaging, serology for autoantibodies, and nutritional screening, should be considered.
Retinal diseases masquerading as neurological causes of vision loss
In the remainder of this article we highlight the most common retinal disease that can masquerade as neurological causes of vision loss due to their subtle findings on retinal examination.
Central serous chorioretinopathy (CSCR)
CSCR occurs when leakage of fluid from choriocapillaris causes subfoveal neurosensory retinal detachment or pigment epithelium detachment. Despite an unclear mechanism, leakage is thought to be result of hyper-permeability of choriocapillaris and RPE dysfunction.[14] CSCR often affects young men and pregnant women.[15] Other described risk factors include “Type A” behavioral traits,[16] use of exogenous steroids,[17] and untreated hypertension.[18] CSCR may be confused with retrobulbar optic neuritis especially when fundus findings are subtle, since patients typically experience acute or subacute central visual loss with metamorphopsia. Reduced contrast sensitivity or color desaturation has also been described.[15]
Diagnosis of CSCR can be made by the fundus finding of round serous macular detachment with or without discrete detachment of the retinal pigment epithelium without hemorrhage.(Figure1C) However, this fundus finding can be subtle, which may also lead to consideration of retrobulbar optic neuropathy. OCT demonstrates serous detachment of neurosensory retina along with other subtle findings such as small pigmented epithelium detachments and choroidal thickening (by using enhanced depth imaging-OCT).(Figure1D)[19] FA generally reveals leakage from the level of RPE.
Risk factor modification, especially discontinuation of steroids, is strongly encouraged. CSCR is typically self-limited within 2-3 months. Early morphologic changes on OCT can predict visual outcome and persistence of fluid.[20] If fluid persists, laser photocoagulation or photodynamic therapy may be considered.[21, 22]
Retinal ischemia
The vascular supply of retina comes two circulations: the retinal circulation deriving from the central retinal artery for the inner retina and the choroidal circulation for the outer retina. The central retinal artery, derives from ophthalmic artery, and branches into 4 branch retinal arteries after exiting the optic nerve head to supply each quadrant of the retina. These branch retinal arteries are visible on fundoscopic examination. Occasionally, a cilioretinal artery, derived from choroidal circulation, may supply the inner portion of the fovea.
The most common clinical presentations of retinal ischemia are central retinal artery occlusion (CRAO) and branch retinal artery occlusion (BRAO). The most common cause of arterial occlusion is embolism from atherosclerotic carotid artery followed by aortic arch and cardiac emboli.[23] Other mechanisms of retinal ischemia other than emboli include hypercoagulable state,[24] vasculitis (especially giant cell arteritis(GCA)), and hypoperfusion. CRAO or BRAO is a stroke of the retina and must be considered as a cause for transient or sustained monocular visual loss due to the potential of devastating visual or neurological outcome if underlying causes are not promptly diagnosed and managed.
The clinical presentation depends on which territory is impacted. CRAO, in which occlusion occurs proximally at central retinal artery, disrupts wide-spread retinal circulation, typically causing sudden onset of painless complete severe visual loss in one eye. However, central visual acuity may be near normal in patients with a patent cilioretinal artery perfusing the fovea.[25] BRAO, in which occlusion occurs distally in a branch retinal artery, may be asymptomatic or only produce partial visual field loss and spare central vision if the central macula remains unaffected.
Inner retinal ischemia from CRAO or BRAO acutely results in whitened opacification of the retina in the distribution of the affected vessel. Classically, fundus examination of CRAO shows diffuse edematous and opacified retina with a characteristic cherry-red spot at the fovea.[26](Figure1E), while ophthalmoscopy in BRAO shows these inner retinal changes only in the distribution of the affected branch retinal arteriole. However, the degree of retinal opacification varies by the severity of the occlusion and the duration from onset. Retinal whitening may not become apparent for several hours after artery occlusion. Therefore, patients can present with less apparent fundus findings during the hyperacute stage of disease or with a partial occlusion. This situation can be confused with retrobulbar optic neuropathy, especially in CRAO when an rAPD is present from extensive inner retina infarction. Furthermore, retinal opacification typically resolves over a period of 4-6 weeks, usually leaving a pale optic disc, attenuated retinal vessels, and normal retina color.[26](Figure1G) This late stage of CRAO should be considered as a possible cause of unexplained inner retinal and optic atrophy. Though retinal emboli can be observed in 20-40% of CRAO and 60% of BRAO patients,[23, 27] absence of visible retinal emboli does not exclude an embolic cause of retinal artery occlusion.
Ancillary studies may help diagnose CRAO or BRAO when the fundus examination is not diagnostic. During the acute stage, OCT demonstrates thickening, hyper-reflectivity, and loss of definition in the superficial and deep inner retinal layers.[28](Figure1F) With time, chronic ischemic inner retina results in thinning or disorganization within most or all of inner retinal layers on OCT.[29](Figure1H) This can be distinguished from OCT changes in chronic optic neuropathies cause isolated thinning in nerve fiber, ganglion cell and inner plexiform layers but spare deeper inner retinal layers. The layering architecture is also preserved. Acutely, FA typically shows delay in retinal artery filling time and retinal arteriovenous transit time.[30] ffERG in CRAO may reveal diminished amplitude of b-wave with generally unaffected a-wave, which reflects diffuse inner retina ischemia but spared photoreceptors function.[31] In contrast, optic neuropathies are unlikely to impact the ERG response.
Acute CRAO or BRAO is a medical emergency. In acute presentations of retinal infarction, patients should receive immediate emergency room or inpatient neurologic evaluation to diagnose concurrent ischemic stroke as well as diagnose and treat stroke risk factors due to the increased risk of ischemic stroke during first few days after the onset of visual loss.[32, 33] This includes actions to diagnose and treat GCA in patients greater than 50 years of age with compatible history.[34] In contrast, if the vision loss is long-standing and diagnostic testing supports chronic CRAO or BRAO, secondary stroke prevention work up can be done in the outpatient setting.
CRAO typically has a poor visual prognosis. Currently, there are insufficient data to decide if any beneficial treatments to restore perfusion exist for CRAO, including lowering intraocular pressure, ocular massage, surgical embolectomy,[35] or intraarterial fibrinolysis with recombinant tissue plasminogen activator.[36] On the other hand, BRAO may not warrant aggressive, invasive intervention if the vision is minimally affected, although systemic workup remains paramount.
Acute macular neuroretinopathy (AMN)
Although the exact pathophysiology of AMN is unknown, a microvascular abnormality in the deep capillary plexus of retina, which results in the pathology of perifoveal photoreceptors, is hypothesized. This rare condition is commonly described in young women in their 30s who take oral contraceptive pills. AMN may be misdiagnosed with retrobulbar optic neuritis.[37] Symptoms of AMN are the sudden onset of paracentral scotoma, often preceded by viral illness.[38] Visual field defects are typically small, with discrete, multifocal paracentral scotomas, which may be better demonstrated on Amsler grid or 10-2 Humphrey Visual Field. Fundus examination can reveal classic brown petaloid intraretinal lesions pointing to the fovea that correspond to scotomata, though these are often subtle. OCT through a lesion initially shows hyperreflective band in outer nuclear layer and outer plexiform layer followed by focal disruption of the inner segment-outer segment junction(ellipsoid zone) of the photoreceptors.[39] mfERG shows focal depressed signal in the area of the lesions. Although there is no proven treatment for AMN, spontaneous partial resolve of scotomas has been described.
Acute zonal occult outer retinopathy (AZOOR) complex disease
The term “AZOOR complex disease” refers to a group of disorders that share features of female predominance, unexplained visual field defects typically contiguous with the blind spot, photopsias, and reduced ERG amplitudes. [40] This grouping persists despite their distinct clinical presentations and proposed pathophysiologies. Current belief is that these disorders are a spectrum of outer retina inflammatory disorders with overlapping clinical presentations.[41–43] In this article we focus on disorders sometimes confused with neurological causes of vision loss. These disorders include acute idiopathic blind spot enlargement (AIBSE), multiple evanescent white dot syndrome (MEWDS), and acute zonal occult outer retinopathy (AZOOR). These disorders should be considered in patients with acute mild visual loss and a history of photopsia with minimal or no initial fundus abnormality. Additional multimodality investigations with OCT, FAF, FA, and ERG can improve diagnostic capability and help distinguish each disorder.
Symptoms of AZOOR complex diseases depend on the area of the retina and choroid affected. Of particular relevance to neurologist are those that involved the peripapillary retina presenting with enlargement of the physiologic blind spot . Such conditions causing dysfunction of the peripapillary retina or choroid should be considered in patients with blind spot enlargement who lack significant optic nerve swelling that displaces peripapillary photoreceptors or distorts the posterior globe to induce local refractive changes or peripapillary atrophy, two causes of blind spot enlargement that are apparent on fundus examination.[42]
Acute idiopathic blind spot enlargement (AIBSE) classically occurs in young adult patients with acute onset of photopsia and enlargement of blind spot without optic disc swelling or choroiditis.[44] Currently, the term AIBSE syndrome encompasses acute onset of positive visual phenomena (perception of flashing light, swirling, or colored lights) and enlargement of the blind spot. Most patients have good visual acuity.[42, 45] Visual field defects expanding from physiologic blind spot may vary in size but often have steep margins that can mimic temporal visual field defects from chiasmal lesions.[46] Fundus examination usually reveals peripapillary pigmentary changes with occasionally mild optic disc swelling, which may potentially confused with optic neuropathy with optic disc edema including papillitis. OCT is useful in detecting peripapillary photoreceptor inner segment-outer segment junction (ellipsoid zone) disruption. FAF shows peripapillary hyperautofluorescence. FA may demonstrate late staining of the optic disc, RPE and peripapillary region.[42] ffERG is typically normal, while mfERG can detect abnormal response from peripapillary retina.[47] Photopsias often spontaneously improve over a period of weeks, whereas visual field defects may persist.[42]
Multiple evanescent white dot syndrome (MEWDS) is distinguished from AIBSE by the fundus findings, which can be subtle.[48] Although pathogenesis remains unknown, about one-third of patients have a viral prodrome suggesting possible immune-mediated mechanism. MEWDS typically affects young, healthy, moderately myopic females in the second to fourth decades of life. Common presentation includes acute unilateral, blurred vision, photopsia, and scotoma corresponding to enlarged blind spot. Additionally, patient may present with RAPD and dyschromatopsia which indicate concurrent involvement of optic nerve by contiguous spread or vasculitis.[49] Visual field defects can be generalized depression, central, paracentral scotoma, or enlarged blind spot. Fundus exam during early course of disease may reveal multiple small yellow-white lesions at the level of RPE or outer retina, typically in perifoveal area.(Figure1I) As the disease progress, these white dots fade and appear as granular macular pigmentary change. A mild degree of optic disc swelling may be observed.[48, 50] FA shows early hyperfluorescent spots in wreath-like configuration on posterior pole. Optic disc and vascular leakage may also be seen.[51] FAF can help visualize white dot lesions as they appear hyperautofluorescent on FAF,(Figure1K) even in the absence of lesions on the fundus exam. Disruption of the ellipsoid zone on OCT localizes this condition to the outer retina and RPE.[52](Figure1J) Reduced a-wave amplitude on the ERG is present in the early stage of disease and resolve with time.[53]
MEWDS is a diagnosis of exclusion. Ocular syphilis, lymphoma, and tuberculosis should be considered even in patients with typical multimodal imaging of MEWDS.[54] The clinical course of MEWDS is usually self-limiting, with most patients having nearly complete recovery within weeks or months.[50]
Acute zonal occult outer retinopathy (AZOOR) is characterized by sudden onset of scotomas and photopsia (distinctively described as moving of colors or lights within area of scotomas) related to loss of outer retinal dysfunction. AZOOR typically affects young-adult, myopic females with unilateral disease being more common at onset but bilateral disease usually developing. [55] Visual loss is relatively mild with majority of the patients having VA of 20/40 or better.[56] RAPD may be found on the affected eye. Visual field defects include paracentral scotomas and enlargement of the blind spot. Despite often normal fundus exam in early stage, OCT can detect early disruption or loss of photoreceptor ellipsoid zone in areas corresponding to visual field defects, usually sparing the fovea. As the disease progresses, focal RPE pigmentary change and retinal atrophy, often in the peripapillary region, may be apparent on fundus exam with thinning of the affected retina and RPE on OCT.[57] ffERG is crucial and almost always reveals delayed implicit time of photopic 30-Hz flicker response reflecting diffuse photoreceptor dysfunction.[56] FA may be normal in the early stage of the disease. With time, abnormal findings corresponding to area of RPE disturbance become apparent. FAF is essential in monitoring progression of disease. In addition to demonstrating areas of hypoautofluorescence related to RPE atrophy, it also may demonstrate a border of speckled hyperautofluorescence reflecting expansion of the lesions and ongoing disease activity.[57, 58] Although there is no standard treatment of AZOOR, systemic corticosteroids are commonly used. During the course of disease, one-third of patients experience a recurrence. Approximately 75% of patients have stable visual field defects and 25% experience partial improvement.[56]
Paraneoplastic autoimmune retinopathy
Paraneoplastic autoimmune retinopathy (PAIR) is a rare entity which occurs remotely from primary malignancy or its metastases.[59] Various subtypes had been described including cancer-associated retinopathy (CAR), melanoma-associated retinopathy(MAR), cancer-associated cone dysfunction (CACD), and diffuse uveal melanocytic proliferation (DUMP). Although still poorly understood, this immune-mediated disease is often associated with circulating antibodies against retina. However, since the presence of antiretinal antibodies can be either pathogenic or an unrelated finding, it should only be used to support diagnosis and not as a sole diagnostic test.[46] Instead, the diagnosis of PAIR should be based on clinical findings and ophthalmologic investigations. Patients with PAIR should have carefully systemic evaluation for underlying malignancies.
Cancer-associated retinopathy (CAR) is the most common type of PAIR. Due to shared epitopes between tumor antigens and with retinal antigens there is resulting in cross-reaction between autoantibodies produced as part of an immune response to tumor antigens and retinal antigens.[60] Recoverin, an antibody that reacts with 23-kDa CAR antigen in the retina and results in loss of photoreceptors, was the first anti-retinal antibody identified. Other antibodies, including anti-enolase and anti-TRMP1, have been reported association with CAR and may affect the clinical course.[61, 62] This condition is twice as common in females as in males. Average age at diagnosis is 65 years.[63] The most common cancers associated with CAR are breast cancer and small cell lung cancer, followed by gynecological, hematologic, prostate, and colon cancer. Onset of CAR can precede the diagnosis of cancer in some cases, originally reported as high as half of the patients.[64]
Patients typically present with progressive painless vision loss along with visual phenomena associated with dysfunction of both rod and cone systems including night blindness, light sensitivity, and dyschromatopsia. Visual field defects are progressive and patterns may vary from generalized depression, paracentral scotoma, to ring scotoma.[65] Fundus examination in early stage is often normal. While optic nerve pallor, arteriolar attenuation, and RPE changes are present in later stages of CAR.
Ophthalmic investigations reflect retinal degenerative changes. OCT shows thinning of the photoreceptor layer and loss of ellipsoid zone. FAF reveals an abnormal hyperautofluorescent ring around the fovea which reflects loss of RPE function.[66] ERG demonstrates severely reduced scotopic and photopic a- and b-waves reflecting diffuse retinal dysfunction in both rod and cone system. ERG is a crucial diagnostic tool in early stages when fundus exam is unrevealing.
Currently, there is no effective treatment for CAR. Retinal degeneration tends to be progressive despite treatment of the underlying cancer. However, various immunosuppressive regimens, including systemic corticosteroids, plasmapheresis, and monoclonal antibodies, have been reported improvement in visual function.[67, 68] Testing for serum retinal autoantibodies may be beneficial not only for supporting the diagnosis of the condition but also to detect autoantibodies that are strongly associated with a particular cancer which could aid in identification of a previously undiagnosed malignancy. However, it is noteworthy that the presence of antiretinal antibodies alone does not confirm the diagnosis of CAR, since antiretinal antibodies can also be found in general population and other retinal diseases. [69]
Melanoma-associated retinopathy (MAR) impacts the bipolar cells in the intermediate retina in patients with melanoma.[70] Various circulating antibodies, including antibodies against S-arrestin, aldolase A, α-enolase and recoverin, have been associated with MAR.[71] Average age of onset is approximately 60 years-old and males are more common affected.[72] MAR is typically observed in patients with an established diagnosis of melanoma that is often metastatic.[73]
Patients typically experience night blindness with positive visual phenomena described as shimmering or flickering light. Visual acuity and color perception are relatively preserved compared to other autoimmune retinopathies, with 80% of patients having visual acuity of 20/60 or better, which rarely progresses to blindness. Different patterns of visual field defects are noted including central, paracentral scotoma, generalized depression, or peripheral constriction. The fundus examination is usually normal at presentation, though optic disc pallor, retinal vessel attenuation, and vitritis may be observed.[73]
Ophthalmic imaging including OCT and FAF are often normal in early MAR, but ERG is a very sensitive modality in detection of compromised bipolar cell function. Dark-adapted (scotopic) ERG is characterized by an electronegative pattern similar to congenital stationary night blindness or CRAO. Under scotopic conditions a bright flash stimulus produces a normal a-wave with a markedly attenuated b-wave, indicating dysfunction of the bipolar cells, and the rod-mediated system in particular.[73] Dim-flash responses are typically absent.
Treatment for MAR is mostly ineffective. Current strategies consist of two main measures; cytoreduction to reduce tumor burden and antigen production, and immunotherapy to reduce circulating antibodies.
Retinal dystrophy
Though these are inherited diseases, symptoms can develop later in life. The early stage of certain diseases such as cone, cone-rod dystrophies, and Stargardt disease may have central visual loss and dyschromatopsia with a nearly normal-appearing fundus, making them difficult to distinguish from optic neuropathy.
Cone or cone-rod dystrophies.
Patients with cone dystrophies typically exhibit symptoms related to cone dysfunction include gradual onset of central vision loss, dyschromatopsia, visual field defects, and light-induced blindness (hemeralopia). Onset of disease is between first and third decade of life with unexplained subnormal vision. Symptoms usually progress over years. Fundus exam can be normal in early stage with bilateral symmetric macular pigmentary change or bull’s eye maculopathy developing as the disease progress. Mild to severe temporal optic disc pallor may also be present. Visual field defects usually affect central vision while peripheral vision remains normal. Patients with cone-rod dystrophies may also report progressive night blindness (nyctalopia), which is a symptom of rod dysfunction. Fundus exam in later stages may reveal peripheral retinal atrophy.[74]
With regards to ancillary tests, OCT can demonstrate thinning of the outer retina in the macula [75] with corresponding abnormal hypoautofluorescence on FAF images. ffERG is considered a crucial study for cone or cone-rod dystrophies. Cone dystrophies produce abnormal or undetectable light-adapted (photopic) ERG response. 30-Hz flicker, which is sensitive to generalized cone dysfunction, shows abnormal delay in response time, whereas the dark-adapted(scotopic) rod-mediated ERG response, remains relatively normal.[76] Cone-rod dystrophies will cause abnormalities in both rod- and cone-derived responses with cone-derived ERG being more affected than rod responses.
Stargardt disease is the most common inherited macular dystrophy, often caused by mutations in the ABCA4 gene. The spectrum of presentations is broad ranging from mild visual loss with less apparent fundus findings to more severe cone or cone-rod dystrophies depending on the genotype. Onset of symptoms most commonly occurs in childhood and early adulthood. Patients typically present with bilateral central scotomas, dyschromatopsia and characteristic macular changes with yellowish flecked lesion at the RPE level in the macula.[77] However, the fundus exam can be normal appearing in early stages. OCT will reveal photoreceptor disruption extending from the central macula and (often) relatively spared peripheral macula and retina. FAF can demonstrate fundus changes before they are clinically evident on ophthalmoscopy. Abnormal findings from FAF includes bull’s eye appearance, central hypoautofluorescence surrounded by area of hyperautofluorescence, and expanding of hyperautofluorescence flecks.[78] Retina adjacent to the optic nerve is characteristically spared throughout all disease stages which can be demonstrated on FAF and OCT. FA reveals characteristic ‘dark choroid’ due to blockage of signal from lipofuscin deposition in RPE.[79] ffERG can be normal in certain phenotypic subtype of Stargardt disease with macular dysfunction but preserve generalized cone and rod function. While PERG, which provides measurement of central retinal function, is invariably abnormal.[80]
Toxic retinopathy
Various medications used in treatment of systemic diseases can have adverse effects on vision, and on the retina in particular. Although most toxic retinopathies can be detected by fundus examination, some may have normal-appearing fundus in the early stage. This section covers toxic retinopathy commonly encounter in neuro-ophthalmology practice in which fundus finding may be subtle or absent.
Chloroquine (CQ) or Hydroxychloroquine (HCQ), an anti-malarial drug that is also used in collagen vascular diseases, cause dose- and duration-related retinopathy. The mechanism is not well understood. Daily dose is the most critical determinant of risk in developing HCQ retinopathy. Patients treated with HCQ daily dose≤5mg/kg of real body weight have less than 1% risk in first 5 years. The risk of HCQ retinopathy is significantly higher with a higher daily dose. In addition, the risk also increases with duration of treatment, concomitant Tamoxifen use, or pre-existing renal disease or macular disease. Patients typically have no visual symptoms. Visual acuity is preserved until late stage. The classic fundus appearance is bilateral bull’s eye maculopathy, parafoveal ring of RPE depigmentation with spared central fovea.(Figure1M) However, this finding reflects late stage of HCQ induced maculopathy and should rarely be found in clinical practice since the current recommended screening protocol facilitates early detection of maculopathy. Screening protocol should include automated visual fields test and macular OCT.[81] Early signs of CQ or HCQ induced maculopathy include parafoveal thinning of photoreceptor layers on OCT imaging.(Figure1N) This localized loss of photoreceptors is strong indicator of toxicity. Automated threshold visual field testing of the central 10 degrees of vision is very sensitive in the detection of paracentral scotoma or ring scotoma. However, patients of East Asian ethnicity often manifest toxicity beyond the macula. Therefore, wider test area of OCT and automated visual fields test (24-2 or 30-2 study) should be considered in these patients. Other useful tests include mfERG and FAF.[81](Figure1O) Treatment is cessation of drug. HCQ toxic effects may continue after drug cessation for a certain period of time depending on the severity of toxic retinopathy at the time of cessation.[82] In general, HCQ maculopathy are irreversible. However, if the retinopathy is detected in early stage, there is a better chance to preserve the vision.
Vigabatrin, an antiepileptic drug, is used in treatment of infantile spasm. Vigabatrin induced retinopathy is manifested by visual field constriction, which is found in up to 52% of adults and 34% of children who received the medication.[83] Depending on the age of patient, Screening protocol typically includes complete ophthalmic examination, visual field testing, and ffERG or OCT. Screening visual field testing may be challenging in young children. Therefore, ffERG is recommended for screening in patients age≤2year-old at baseline and at 3-month intervals.[84] Abnormal peripheral retinal atrophy and nasal optic disc pallor can be observed from fundus examination. OCT can demonstrate thinning of nasal retinal nerve fiber layer (RNFL). Reduction in amplitude of light-adapted (photopic) 30-Hz flicker ERG is a suggested predictor of vigabatrin induced visual field defect.[85] Visual field losses and abnormal ERG tend to persist and also show progression with exposure continue.
Pentosan polysulfate sodium (PPS) is an only oral therapy approved by US Food and Drug Administration for interstitial cystitis. Recently, it has been associated with pigmentary maculopathy resembling pattern macular dystrophy and age-related macular degeneration. Patients typically have long-term exposure to PPS with median exposure duration of 14.5 years but other risk factors including dosage have not yet identified. Currently, there is no formal screening guideline. Although the most common visual symptoms are blurred vision (49%) and prolonged dark adaptation (49%), visual acuity is relatively preserved with 86% have best-corrected visual acuity 20/40 or better. [86] Visual field is generally normal unless RPE atrophy is presented. Fundus examination may reveal paracentral hyperpigmented spot on a yellow-orange subretinal deposits background and, later, progress to RPE atrophy. FAF can demonstrate a densely packed pattern of hyper- and hypoautofluorescent spots centered on fovea and improve visualization of disease extension. OCT reveals focal elevation or thickening of the RPE and loss of definition of interdigitation zone or ellipsoid zone. ffERG demonstrates variable attenuation of amplitude consistent with macular disease. While mfERG demonstrates attenuation of response amplitudes from affected area. Treatment is cessation of drug. [86] Currently, there is lack of information on visual prognosis after drug cessation.
Conclusion
Retinal disease should be included in the differential diagnosis alongside optic neuropathy and cerebral vision loss in patients with “unexplained visual loss”. In addition to clinical characteristics of visual loss pattern and fundus findings, electrophysiologic studies and multimodal ophthalmic imaging including OCT, FAF, FA play a crucial role in diagnosis of retinal diseases with subtle or normal-appearing fundus exam. Timely diagnosis with proper intervention may prevent permanent visual loss or even lead to a diagnosis of an undiagnosed malignancy or intervenable stroke risk factor.
Acknowledgement
We thank Tharikarn Sujirakul, MD., Wimwipa Dieosuthichat,MD., Atit Koovisitsopit, MD. for contributing clinical images.
Unrestricted grant from Research to Prevent Blindness, NIH P30 026877
Footnotes
Conflicts of interest: All authors have no conflicts of interest.
Human and Animal Rights: This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
- 1.Newman N, Biousse V. Diagnostic approach to vision loss. Continuum (Minneap Minn). 2014;20(4 Neuro-ophthalmology):785–815. doi: 10.1212/01.Con.0000453317.67637.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glaser JS, Savino PJ, Sumers KD, McDonald SA, Knighton RW. The photostress recovery test in the clinical assessment of visual function. Am J Ophthalmol. 1977;83(2):255–60. doi: 10.1016/0002-9394(77)90624-9. [DOI] [PubMed] [Google Scholar]
- 3.Wu G, Weiter JJ, Santos S, Ginsburg L, Villalobos R. The macular photostress test in diabetic retinopathy and age-related macular degeneration. Arch Ophthalmol. 1990;108(11):1556–8. doi: 10.1001/archopht.1990.01070130058030. [DOI] [PubMed] [Google Scholar]
- 4.Liu G, Volpe NJ, Galetta SL. The neuro-ophthalmic examination. In: Liu G, Volpe NJ, Galetta SL, editors. Liu, Volpe, and Galetta’s Neuro-Ophthalmology Diagnosis and Management. Edinburgh; New York: Elsevier; 2018. p. 7–36. [Google Scholar]
- 5.Kelly LP, Garza PS, Bruce BB, Graubart EB, Newman NJ, Biousse V. Teaching ophthalmoscopy to medical students (the TOTeMS study). Am J Ophthalmol. 2013;156(5):1056–61.e10. doi: 10.1016/j.ajo.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay DD, Garza PS, Bruce BB, Newman NJ, Biousse V. The demise of direct ophthalmoscopy: A modern clinical challenge. Neurol Clin Pract. 2015;5(2):150–7. doi: 10.1212/cpj.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000;2(1-2):9–25. doi: 10.1038/sj.neo.7900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cukras C, Huynh N, Vitale S, Wong WT, Ferris FL 3rd, Sieving PA. Subjective and objective screening tests for hydroxychloroquine toxicity. Ophthalmology. 2015;122(2):356–66. doi: 10.1016/j.ophtha.2014.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph A, Rahimy E, Freund KB, Sorenson JA, Sarraf D. Fundus autofluorescence and photoreceptor bleaching in multiple evanescent white dot syndrome. Ophthalmic Surg Lasers Imaging Retina. 2013;44(6):588–92. doi: 10.3928/23258160-20131105-08. [DOI] [PubMed] [Google Scholar]
- 10.Lois N, Halfyard AS, Bird AC, Holder GE, Fitzke FW. Fundus autofluorescence in Stargardt macular dystrophy-fundus flavimaculatus. Am J Ophthalmol. 2004;138(1):55–63. doi: 10.1016/j.ajo.2004.02.056. [DOI] [PubMed] [Google Scholar]
- 11.Miyake Y, Shinoda K. Clinical Electrophysiology. In: Schachat AP, Sadda SR, Hinton DR, Wilkinson CP, Wiedemann P, editors. Ryan’s Retina. 6th ed. Amsterdam, Netherland: Elsevier; 2018. p. 249–72. [Google Scholar]
- 12.Young B, Eggenberger E, Kaufman D. Current electrophysiology in ophthalmology: a review. Curr Opin Ophthalmol. 2012;23(6):497–505. doi: 10.1097/ICU.0b013e328359045e. [DOI] [PubMed] [Google Scholar]
- 13.•.Georgiou M, Kalitzeos A, Patterson EJ, Dubra A, Carroll J, Michaelides M. Adaptive optics imaging of inherited retinal diseases. British Journal of Ophthalmology. 2018;102(8):1028–35. doi: 10.1136/bjophthalmol-2017-311328. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review article briefly describes adaptive optics retinal imaging. Article describes adaptive optics retinal imaging findings along with multimodal ophthalmic imaging in inherited retinal diseases with correlation to the pathophysiology.
- 14.Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121(1):26–34. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 15.Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41(2):201–14. doi: 10.1111/j.1442-9071.2012.02848.x. [DOI] [PubMed] [Google Scholar]
- 16.Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc. 1986;84:799–845. [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho-Recchia CA, Yannuzzi LA, Negrao S, Spaide RF, Freund KB, Rodriguez-Coleman H, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834–7. doi: 10.1016/s0161-6420(02)01117-x. [DOI] [PubMed] [Google Scholar]
- 18.Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244–9. doi: 10.1016/j.ophtha.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–73. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 20.Suwal B, Khadka D, Shrestha A, Shrestha S, Shrestha N, Khatri B. Baseline Predictive Factors of Visual Outcome and Persistence of Subretinal Fluid Based on Morphologic Changes in Spectral Domain Optical Coherence Tomography in Patients with Idiopathic Central Serous Chorioretinopathy. Clin Ophthalmol. 2019;13:2439–44. doi: 10.2147/opth.S233273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park YJ, Kim YK, Park KH, Woo SJ. Long-Term Efficacy and Safety of Photodynamic Therapy in Patients With Chronic Central Serous Chorioretinopathy. Ophthalmic Surg Lasers Imaging Retina. 2019;50(12):760–70. doi: 10.3928/23258160-20191119-03. [DOI] [PubMed] [Google Scholar]
- 22.Scholz P, Altay L, Fauser S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye (Lond). 2016;30(10):1371–7. doi: 10.1038/eye.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown GC, Magargal LE. Central retinal artery obstruction and visual acuity. Ophthalmology. 1982;89(1):14–9. doi: 10.1016/s0161-6420(82)34853-8. [DOI] [PubMed] [Google Scholar]
- 24.Bertram B, Remky A, Arend O, Wolf S, Reim M. Protein C, protein S, and antithrombin III in acute ocular occlusive diseases. Ger J Ophthalmol. 1995;4(6):332–5. [PubMed] [Google Scholar]
- 25.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140(3):376–91. doi: 10.1016/j.ajo.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 26.Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina. 2007;27(3):276–89. doi: 10.1097/01.iae.0000238095.97104.9b. [DOI] [PubMed] [Google Scholar]
- 27.Ros MA, Magargal LE, Uram M. Branch retinal-artery obstruction: a review of 201 eyes. Ann Ophthalmol. 1989;21(3):103–7. [PubMed] [Google Scholar]
- 28.•.Furashova O, Matthé E. Retinal Changes in Different Grades of Retinal Artery Occlusion: An Optical Coherence Tomography Study. Investigative Ophthalmology & Visual Science. 2017;58(12):5209–16. doi: 10.1167/iovs.17-22411. [DOI] [PubMed] [Google Scholar]; Article objectively compares layer by layer OCT thickness and reflectivity changes between severity grades of acute retinal artery occlusion.
- 29.Chen SN, Hwang JF, Chen YT. Macular thickness measurements in central retinal artery occlusion by optical coherence tomography. Retina. 2011;31(4):730–7. doi: 10.1097/IAE.0b013e3181f2a15c. [DOI] [PubMed] [Google Scholar]
- 30.David NJ, Norton EW, Gass JD, Beauchamp J. Fluorescein angiography in central retinal artery occlusion. Arch Ophthalmol. 1967;77(5):619–29. doi: 10.1001/archopht.1967.00980020621010. [DOI] [PubMed] [Google Scholar]
- 31.Henkes HE. Electroretinography in circulatory disturbances of the retina. II. The electroretinogram in cases of occlusion of the central retinal artery or of its branches. AMA Arch Ophthalmol. 1954;51(1):42–53. doi: 10.1001/archopht.1954.00920040044006. [DOI] [PubMed] [Google Scholar]
- 32.••.Biousse V, Nahab F, Newman NJ. Management of Acute Retinal Ischemia: Follow the Guidelines! Ophthalmology. 2018;125(10):1597–607. doi: 10.1016/j.ophtha.2018.03.054. [DOI] [PubMed] [Google Scholar]; Article reviews risk of stroke and acute coronary syndrome in patients with acute retinal ischemia and provides recommended urgent managements.
- 33.Park SJ, Choi NK, Yang BR, Park KH, Lee J, Jung SY, et al. Risk and Risk Periods for Stroke and Acute Myocardial Infarction in Patients with Central Retinal Artery Occlusion. Ophthalmology. 2015;122(11):2336–43.e2. doi: 10.1016/j.ophtha.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Biousse V, Newman NJ. Ischemic Optic Neuropathies. N Engl J Med. 2015;372(25):2428–36. doi: 10.1056/NEJMra1413352. [DOI] [PubMed] [Google Scholar]
- 35.Almeida DR, Mammo Z, Chin EK, Mahajan VB. SURGICAL EMBOLECTOMY FOR FOVEA-THREATENING ACUTE RETINAL ARTERY OCCLUSION. Retin Cases Brief Rep. 2016;10(4):331–3. doi: 10.1097/icb.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schumacher M, Schmidt D, Jurklies B, Gall C, Wanke I, Schmoor C, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010;117(7):1367–75.e1. doi: 10.1016/j.ophtha.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Cebeci Z, Bayraktar erife, Oray M, K1r N. Acute macular neuroretinopathy misdiagnosed as optic neuritis. International Ophthalmology. 2014;35:125–9. [DOI] [PubMed] [Google Scholar]
- 38.Turbeville SD, Cowan LD, Gass JD. Acute macular neuroretinopathy: a review of the literature. Surv Ophthalmol. 2003;48(1):1–11. doi: 10.1016/s0039-6257(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 39.Maschi C, Schneider-Lise B, Paoli V, Gastaud P. Acute macular neuroretinopathy: contribution of spectral-domain optical coherence tomography and multifocal ERG. Graefes Arch Clin Exp Ophthalmol. 2011;249(6):827–31. doi: 10.1007/s00417-010-1560-1. [DOI] [PubMed] [Google Scholar]
- 40.Gass JDM. Acute zonal occult outer retinopathy. Donders lecture: The Netherlands ophthalmological society, Maastricht, Holland, June 19, 1992. Journal of Neuro-Ophthalmology. 1993;13(2):79–97. [PubMed] [Google Scholar]
- 41.Gass JD. Overlap among acute idiopathic blind spot enlargement syndrome and other conditions. Arch Ophthalmol. 2001;119(11):1729–31. doi: 10.1001/archopht.119.11.1729. [DOI] [PubMed] [Google Scholar]
- 42.Volpe NJ, Rizzo JF 3rd, Lessell S. Acute idiopathic blind spot enlargement syndrome: a review of 27 new cases. Arch Ophthalmol. 2001;119(1):59–63. [PubMed] [Google Scholar]
- 43.Watzke RC, Shults WT. Clinical features and natural history of the acute idiopathic enlarged blind spot syndrome. Ophthalmology. 2002;109(7):1326–35. doi: 10.1016/s0161-6420(02)01066-7. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher WA, Imes RK, Goodman D, Hoyt WF. Acute idiopathic blind spot enlargement. A big blind spot syndrome without optic disc edema. Arch Ophthalmol. 1988;106(1):44–9. doi: 10.1001/archopht.1988.01060130050026. [DOI] [PubMed] [Google Scholar]
- 45.Callanan D, Gass JD. Multifocal choroiditis and choroidal neovascularization associated with the multiple evanescent white dot and acute idiopathic blind spot enlargement syndrome. Ophthalmology. 1992;99(11):1678–85. doi: 10.1016/s0161-6420(92)31755-5. [DOI] [PubMed] [Google Scholar]
- 46.Tamhankar MA. Visual loss: retinal disorders of neuro-ophthalmic interest. In: Liu G, Volpe NJ, Galetta SL, editors. Liu, Volpe, and Galetta’s Neuro-Ophthalmology Diagnosis and Management. 3rd ed. Edinburgh; New York: Elsevier; 2018. p. 53–99. [Google Scholar]
- 47.Kondo N, Kondo M, Miyake Y. Acute idiopathic blind spot enlargement syndrome: prolonged retinal dysfunction revealed by multifocal electroretinogram technique. Am J Ophthalmol. 2001;132(1):126–8. doi: 10.1016/s0002-9394(00)00932-6. [DOI] [PubMed] [Google Scholar]
- 48.Jampol LM, Sieving PA, Pugh D, Fishman GA, Gilbert H. Multiple evanescent white dot syndrome. I. Clinical findings. Arch Ophthalmol. 1984;102(5):671–4. doi: 10.1001/archopht.1984.01040030527008. [DOI] [PubMed] [Google Scholar]
- 49.Dodwell DG, Jampol LM, Rosenberg M, Berman A, Zaret CR. Optic nerve involvement associated with the multiple evanescent white-dot syndrome. Ophthalmology. 1990;97(7):862–8. doi: 10.1016/s0161-6420(90)32489-2. [DOI] [PubMed] [Google Scholar]
- 50.Quillen DA, Davis JB, Gottlieb JL, Blodi BA, Callanan DG, Chang TS, et al. The white dot syndromes. Am J Ophthalmol. 2004;137(3):538–50. doi: 10.1016/j.ajo.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 51.Gross NE, Yannuzzi LA, Freund KB, Spaide RF, Amato GP, Sigal R. Multiple evanescent white dot syndrome. Arch Ophthalmol. 2006;124(4):493–500. doi: 10.1001/archopht.124.4.493. [DOI] [PubMed] [Google Scholar]
- 52.Marsiglia M, Gallego-Pinazo R, Cunha de Souza E, Munk MR, Yu S, Mrejen S, et al. EXPANDED CLINICAL SPECTRUM OF MULTIPLE EVANESCENT WHITE DOT SYNDROME WITH MULTIMODAL IMAGING. Retina. 2016;36(1):64–74. doi: 10.1097/iae.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 53.Sieving PA, Fishman GA, Jampol LM, Pugh D. Multiple Evanescent White Dot Syndrome: II. Electrophysiology of the Photoreceptors During Retinal Pigment Epithelial Disease. Archives of Ophthalmology. 1984;102(5):675–9. doi: 10.1001/archopht.1984.01040030531009. [DOI] [PubMed] [Google Scholar]
- 54.•.Russell JF, Pichi F, Scott NL, Hartley MJ, Bell D, Agarwal A, et al. Masqueraders of multiple evanescent white dot syndrome (MEWDS). Int Ophthalmol. 2020;40(3):627–38. doi: 10.1007/s10792-019-01223-4. [DOI] [PubMed] [Google Scholar]; Article reports cases with typical presentation of MEWDS. However, suspicious clinical findings lead to investigation which revealed alternative diagnosis masquerading as MEWDS.
- 55.Gass JD, Agarwal A, Scott IU. Acute zonal occult outer retinopathy: a long-term follow-up study. Am J Ophthalmol. 2002;134(3):329–39. doi: 10.1016/s0002-9394(02)01640-9. [DOI] [PubMed] [Google Scholar]
- 56.Monson DM, Smith JR. Acute zonal occult outer retinopathy. Surv Ophthalmol. 2011;56(1):23–35. doi: 10.1016/j.survophthal.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Mrejen S, Khan S, Gallego-Pinazo R, Jampol LM, Yannuzzi LA. Acute zonal occult outer retinopathy: a classification based on multimodal imaging. JAMA Ophthalmol. 2014;132(9):1089–98. doi: 10.1001/jamaophthalmol.2014.1683. [DOI] [PubMed] [Google Scholar]
- 58.Fujiwara T, Imamura Y, Giovinazzo VJ, Spaide RF. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina. 2010;30(8):1206–16. doi: 10.1097/IAE.0b013e3181e097f0. [DOI] [PubMed] [Google Scholar]
- 59.Sawyer RA, Selhorst JB, Zimmerman LE, Hoyt WF. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;81(5):606–13. doi: 10.1016/0002-9394(76)90125-2. [DOI] [PubMed] [Google Scholar]
- 60.Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. 1987;105:372–5. [DOI] [PubMed] [Google Scholar]
- 61.Weleber RG, Watzke RC, Shults WT, Trzupek KM, Heckenlively JR, Egan RA, et al. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol. 2005;139(5):780–94. doi: 10.1016/j.ajo.2004.12.104. [DOI] [PubMed] [Google Scholar]
- 62.Ueno S, Ito Y, Maruko R, Kondo M, Terasaki H. Choroidal atrophy in a patient with paraneoplastic retinopathy and anti-TRPM1 antibody. Clin Ophthalmol. 2014;8:369–73. doi: 10.2147/opth.S55124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adamus G Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev. 2009;8(5):410–4. doi: 10.1016/j.autrev.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol. 2003;48(1):12–38. doi: 10.1016/s0039-6257(02)00416-2. [DOI] [PubMed] [Google Scholar]
- 65.Ohguro H, Yokoi Y, Ohguro I, Mamiya K, Ishikawa F, Yamazaki H, et al. Clinical and immunologic aspects of cancer-associated retinopathy. Am J Ophthalmol. 2004;137(6):1117–9. doi: 10.1016/j.ajo.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 66.Lima LH, Greenberg JP, Greenstein VC, Smith RT, Sallum JM, Thirkill C, et al. Hyperautofluorescent ring in autoimmune retinopathy. Retina. 2012;32(7):1385–94. doi: 10.1097/IAE.0b013e3182398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dy I, Chintapatla R, Preeshagul I, Becker D. Treatment of cancer-associated retinopathy with rituximab. J Natl Compr Canc Netw. 2013;11(11):1320–4. doi: 10.6004/jnccn.2013.0156. [DOI] [PubMed] [Google Scholar]
- 68.Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127(4):390–7. doi: 10.1001/archophthalmol.2009.24. [DOI] [PubMed] [Google Scholar]
- 69.•.Adamus G, Champaigne R, Yang S. Occurrence of major anti-retinal autoantibodies associated with paraneoplastic autoimmune retinopathy. Clin Immunol. 2020;210:108317. doi: 10.1016/j.clim.2019.108317. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large study describes association of major anti-retinal autoantibodies with ocular symptoms and correlation of autoantibodies with type of tumors in CAR.
- 70.Potter MJ, Thirkill CE, Dam OM, Lee AS, Milam AH. Clinical and immunocytochemical findings in a case of melanoma-associated retinopathy. Ophthalmology. 1999;106(11):2121–5. doi: 10.1016/s0161-6420(99)90493-1. [DOI] [PubMed] [Google Scholar]
- 71.Lu Y, Jia L, He S, Hurley MC, Leys MJ, Jayasundera T, et al. Melanoma-associated retinopathy: a paraneoplastic autoimmune complication. Arch Ophthalmol. 2009;127(12):1572–80. doi: 10.1001/archophthalmol.2009.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–78. doi: . [DOI] [PubMed] [Google Scholar]
- 73.Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol. 2001;21(3):173–87. doi: 10.1097/00041327-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis. Surv Ophthalmol. 2006;51(3):232–58. doi: 10.1016/j.survophthal.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 75.Lima LH, Sallum JM, Spaide RF. Outer retina analysis by optical coherence tomography in cone-rod dystrophy patients. Retina. 2013;33(9):1877–80. doi: 10.1097/IAE.0b013e31829234e6. [DOI] [PubMed] [Google Scholar]
- 76.Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michaelides M, Hunt DM, Moore AT. The genetics of inherited macular dystrophies. J Med Genet. 2003;40(9):641–50. doi: 10.1136/jmg.40.9.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2017;101(1):25–30. doi: 10.1136/bjophthalmol-2016-308823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lambertus S, van Huet RA, Bax NM, Hoefsloot LH, Cremers FP, Boon CJ, et al. Early-onset stargardt disease: phenotypic and genotypic characteristics. Ophthalmology. 2015;122(2):335–44. doi: 10.1016/j.ophtha.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 80.Lois N, Holder GE, Bunce C, Fitzke FW, Bird AC. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch Ophthalmol. 2001;119(3):359–69. doi: 10.1001/archopht.119.3.359. [DOI] [PubMed] [Google Scholar]
- 81.••.Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology. 2016;123(6):1386–94. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]; Article update on important risk factors and practical recommendations on screening for Chloroquine and Hydroxychloroquine Retinopathy.
- 82.Marmor MF, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014;132(9):1105–12. doi: 10.1001/jamaophthalmol.2014.1099. [DOI] [PubMed] [Google Scholar]
- 83.Maguire MJ, Hemming K, Wild JM, Hutton JL, Marson AG. Prevalence of visual field loss following exposure to vigabatrin therapy: a systematic review. Epilepsia. 2010;51(12):2423–31. doi: 10.1111/j.1528-1167.2010.02772.x. [DOI] [PubMed] [Google Scholar]
- 84.Sergott RC, Wheless JW, Smith MC, Westall CA, Kardon RH, Arnold A, et al. Evidence-based Review of Recommendations for Visual Function Testing in Patients Treated with Vigabatrin. Neuro-Ophthalmology. 2010;34(1):20–35. doi: 10.3109/01658100903582498. [DOI] [Google Scholar]
- 85.Harding GFA, Robertson K, Spencer EL, Holliday I. Vigabatrin; its effect on the electrophysiology of vision. Doc Ophthalmol. 2002;104(2):213–29. doi: 10.1023/a:1014643528474. [DOI] [PubMed] [Google Scholar]
- 86.••.Hanif AM, Armenti ST, Taylor SC, Shah RA, Igelman AD, Jayasundera KT, et al. Phenotypic Spectrum of Pentosan Polysulfate Sodium-Associated Maculopathy: A Multicenter Study. JAMA Ophthalmol. 2019;137(11):1275–82. doi: 10.1001/jamaophthalmol.2019.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large multicenter study described clinical spectrum and multimodal imaging findings of pentosan polysulfate sodium-associated maculopathy.


