Abstract
Background
There is evidence that delays in treatment result in increased psychosocial morbidity for patients diagnosed with cancer. We evaluated waiting times for care among cancer patients treated by surgeons affiliated with regional cancer centres in Ontario.
Methods
Dates for 5 key events related to the surgical management of a patient with cancer were collected by a convenience sample of surgeons who treat breast, gynecologic, colorectal, head and neck, thoracic and urologic cancers. The key events were initial referral, first surgical visit, main treatment decision, major surgery and receipt of postoperative pathology report. The surgeons were also asked to judge the appropriateness of the waiting times for the intervals studied and to identify factors associated with inappropriate delays.
Results
A total of 62 surgeons affiliated with 8 regional cancer centres participated; data were collected for 1456 patients who underwent assessment and whose surgical visit occurred between Jan. 31 and May 31, 2000. The median waiting time from referral to first visit was 11.0 days, from first visit to treatment decision 0.0 days, from treatment decision to surgery 20.0 days and from surgery to receipt of the pathology report 8.0 days. The median waiting times for the 2 summary intervals (referral to surgery and referral to receipt of the pathology report) were 37.0 and 48.0 days respectively. The waiting times varied by cancer type; for example, the median time from referral to surgery varied from 29.0 days for colorectal cancers to 64.0 days for urologic cancers. The same interval varied from 19.0 to 43.0 days by treatment centre. The waiting times did not vary substantially by patient age. The surgeons judged that 344 (37.2%) of the 925 patients with dates for the referral-to-surgery interval had inappropriately long waiting times. They indicated that contributing factors to these inappropriate waits were shortage of operating room time (in 181 cases), lack of other resources such as diagnostic tests or allied health personnel (in 156) and patient preference or circumstance (in 28) (factors were not mutually exclusive).
Interpretation
Many of the patients with cancer seen by surgeons affiliated with regional cancer centres in Ontario may be experiencing significant delays in the assessment and treatment of their cancer.
Several days or weeks may elapse as a patient with possible cancer moves through the initial care path: from assessment to diagnosis to definitive treatment. Recently, for cancer surgery, expert groups have attempted to quantify the maximum length of time that should transpire between certain key events on this care path. For example, the Canadian Society of Surgical Oncology has stated that, for the average patient with cancer, the time from completion of diagnostic tests to definitive surgery should not exceed 2 weeks.1 The Canadian Strategy for Cancer Control has set 4 weeks as the maximum time needed to diagnose the most common cancers following patient presentation to a family physician.2 These recommendations are likely justified, since a number of papers have shown that diagnosis or treatment delays result in major psychosocial stresses for cancer patients,3,4,5,6,7,8,9 although there is a paucity of good-quality evidence that such delays result in worse clinical outcomes.10,11,12,13,14,15,16
We conducted a study to determine waiting times for care among patients treated by surgeons affiliated with regional cancer centres in Ontario. The project was designed to examine 4 questions: How long are cancer patients waiting for key events along their surgical care path? Do average waits vary by patient age? Do surgeons judge these waits to be appropriate or inappropriate? and What are the factors that contribute to inappropriate delays?
Methods
Our team developed a 1-page draft form for data collection and an accompanying instruction sheet in consultation with surgeons, administrators and researchers across Ontario. The form focused on dates for 5 key events that we felt summarized the initial care path followed by a patient when definitive treatment may involve major cancer surgery. These key events included initial referral to the treating surgeon, first surgical visit, main treatment decision, major surgery and receipt of the postoperative pathology report. Four key intervals resulted from a patient moving sequentially from event to event. We also considered the summary intervals of “referral to surgery” and “referral to receipt of the pathology report.” We assumed that the key interval “main treatment decision to surgery” was analogous to the interval “completion of diagnostic tests to definitive surgery” referred to by the Canadian Society of Surgical Oncology.1
Surgeons were instructed to decide in each case whether the length of time for each of the 4 key intervals was appropriate or inappropriate, and to mark the form accordingly. We did not define a priori appropriate versus inappropriate waits. If, for an individual case, one or more key intervals were marked as inappropriate, the summary intervals for that case were also classified as inappropriate. Surgeons were then asked to identify factors they felt contributed to an inappropriate wait. These factors were collapsed into 4 categories: patient preference, referring physician preference, lack of operative time and lack of other resources (e.g., preoperative diagnostic tests, allied health personnel and hospital beds). The form also requested the patient's date of birth, the final diagnosis and the type of major surgery. No data were collected that would allow the study team to identify individual patients.
Ontario's 8 regional cancer centres and the Princess Margaret Hospital are an important component of the province's health care system providing treatment and support for cancer patients.17 These centres deliver all radiotherapy and a major portion of chemotherapy in the province. Eight surgeons (from 6 of the regional cancer centres, the Princess Margaret Hospital and a community hospital) agreed to coordinate our study at their respective sites. The community hospital, which added 2 surgeons to the study, will soon be designated as a regional cancer centre; therefore, we refer to all of the participating sites as regional cancer centres. Site leaders were meant to approach and obtain the participation of other surgeons at their site, answer questions locally about the study, and distribute and collect data forms. Site leaders and other surgeons were asked to participate if they treated any of the following types of cancer: breast, gynecologic, colorectal, head and neck, thoracic and urologic. Ultimately, 62 surgeons contributed data to the study.
Data were collected for patients whose surgical visit — the second key event — occurred between Jan. 31 and May 31, 2000. Participating surgeons were instructed to include patients if their signs or symptoms were consistent with a diagnosis of cancer and, assuming this diagnosis, that a curative resection was possible. Thus, many patients contributed data for our first 3 key events but did not undergo major surgery owing to findings on further investigation (e.g., widely metastatic disease, no evidence of cancer or a prohibitive operative risk). Before calculating the time intervals, we divided the study cohort into 3 groups of similar size on the basis of age at first visit. We used these groups to measure differences in time intervals by age group.
We used descriptive statistics and box plots to show the data. The Mann–Whitney test for 2 independent groups was used to compare surgeon-designated appropriateness or inappropriateness of waiting times.
The study did not receive ethical approval because the chair of the Hamilton Health Sciences Corporation Research Ethics Board considered it to be a quality-assurance study, with no risks to subjects and no concerns about confidentiality.
Results
Of the 1620 data forms submitted, 164 were rejected because the referral date was outside the designated 4-month study period (141 forms); the time intervals between key events yielded negative values, which indicated incorrect dates (12 forms); or the forms were duplicates (11 forms). This left 1456 cases that were eligible for data analysis. Of these cases breast cancer (440 cases) was the most frequent and colorectal cancer (100 cases) the least frequent. The volume of cases managed at the cancer centres ranged from 14 to 809.
Table 1 provides descriptive data for the 4 key and 2 summary intervals. To be included in an interval, a case needed dates marked for both key events within that interval. Thus, the number of valid cases dropped across the 4 key intervals, from 1429 to 744. The median waiting time from referral to first visit was 11.0 days, from first visit to treatment decision 0.0 days, from treatment decision to surgery 20.0 days and from surgery to receipt of the pathology report 8.0 days. The median waiting times from referral to surgery and from referral to receipt of the pathology report were 37.0 and 48.0 days respectively. The proportion of patients undergoing surgery within 14 days of the main treatment decision, as recommended by the Canadian Society of Surgical Oncology,1 was 32.5%. The longest median waiting time from referral to surgery was 64.0 days, for patients undergoing surgery for urologic cancers; the range for the remaining cancer types was 29.0 to 40.0 days (Fig. 1A). The range for this interval by cancer centre was 19.0 to 43.0 days (Fig. 1B). For patients aged 50 years or less, 51 to 65, and 66 years or more, the median waiting times from referral to surgery were 36.0 (mean 41.9, 95% confidence interval [CI] 38.7–45.1), 39.0 (mean 43.6, 95% CI 40.7–46.5) and 36.5 (mean 42.7, 95% CI 40.0–45.3) days respectively (p = 0.49) (data not shown).
Table 1
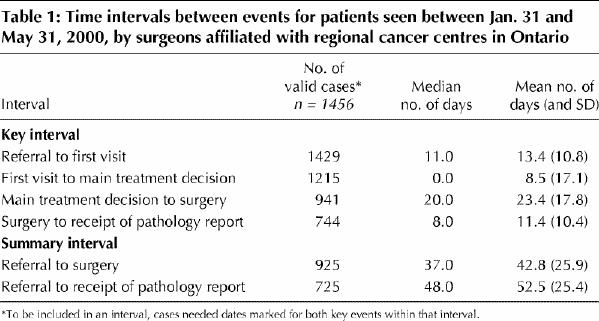
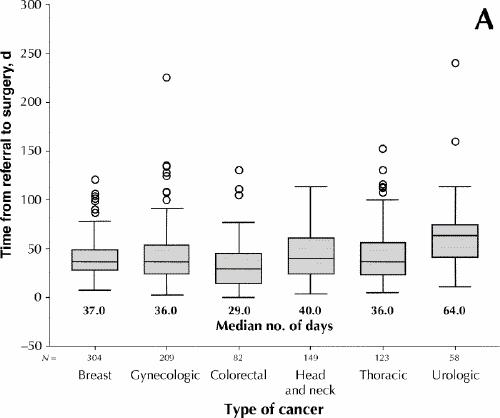
Fig. 1: Waiting times from referral to surgery, by type of cancer (A) and by cancer centre (B), for 925 cases treated by surgeons affiliated with regional cancer centres in Ontario. Lower and upper portions of the boxes represent the 25th and 75th percentiles, respectively; the midline marks the median; the projecting lines represent the most extreme values in the data set that were not more than 1.5 times the height of the box beyond either quartile; the circles represent outliers.
Fig. 1: Waiting times from referral to surgery, by type of cancer (A) and by cancer centre (B), for 925 cases treated by surgeons affiliated with regional cancer centres in Ontario. Lower and upper portions of the boxes represent the 25th and 75th percentiles, respectively; the midline marks the median; the projecting lines represent the most extreme values in the data set that were not more than 1.5 times the height of the box beyond either quartile; the circles represent outliers.
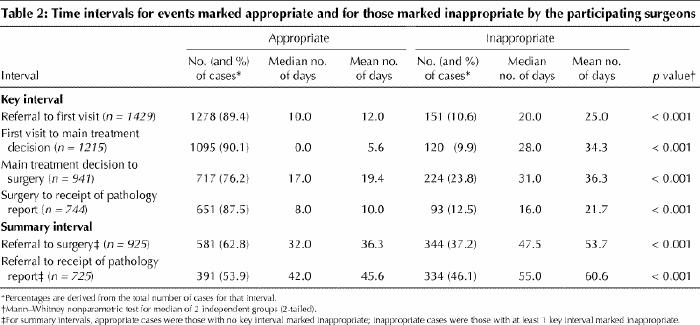
The median values for all of the time intervals marked inappropriate were significantly greater than those marked appropriate (Table 2). Of the 925 patients with dates for the referral-to-surgery interval, 344 (37.2%) were judged by their surgeons to have had an inappropriate wait. The surgeons indicated that the factors associated with these inappropriate delays were shortage of operating room time (in 181 cases), lack of other resources such as diagnostic tests or allied health personnel (in 156) and patient preference or circumstance (in 28) (factors were not mutually exclusive).
Table 2
Interpretation
Our study provides a snapshot of waiting times along the initial care path followed by cancer patients referred to surgeons affiliated with regional cancer centres in Ontario. The median wait from main treatment decision to surgery was 20.0 days, well above the 14-day wait recommended by the Canadian Society of Surgical Oncology.1 The median wait from first visit to main treatment decision was 0.0 days, which suggests that most patients were informed of their treatment options at the time of the initial surgical assessment. In our patient cohort, there were no important differences in waiting times when the data were analyzed by age group.
The median waiting times from referral to surgery were similar for all cancer types except urologic cancers (64 days). This longer median wait may reflect the difficult treatment choices patients with prostate cancer must make: choices that include watchful waiting, hormone therapy, radiation therapy or surgery.18 A crude comparison with a study of waiting times for cancer patients in England indicates that waits in our study were similar to those in the other study for major urologic procedures, slightly longer for breast cancer surgery and shorter for gynecologic, colorectal and thoracic procedures.19 In a recent study of waiting times to breast cancer surgery in Quebec, the median wait was 42 days in 1998.20 Unfortunately, it is difficult to compare this finding with the 37-day wait for breast cancer surgery observed in our study. The starting point in the Quebec study was the first related diagnostic procedure rather than referral to a surgeon; the first diagnostic procedures for breast cancer (e.g., mammography) typically precede the date of referral to a surgeon.
The surgeons in our study marked as inappropriate a high proportion of waits from referral to surgery (344 cases [37.2%]) and from referral to receipt of the pathology report (334 cases [46.1%]). They indicated that shortage of operating room time and lack of other resources were the most common factors leading to inappropriate waiting times. For the waits from main treatment decision to surgery that were marked appropriate by the surgeons, the median wait was 17 days, still longer than the 14-day maximum recommended by the Canadian Society of Surgical Oncology.
There are limitations to our study. First, the participating surgeons were affiliated with regional cancer centres, and most were with teaching hospitals; thus, our results may not be generalizable to all surgeons and hospitals in Ontario. This is especially important for common cancers such as breast and colorectal cancers, because nearly 70% of operations for these disease sites are done in nonteaching hospitals.21,22 Second, the quality of the data was dependent on our participating surgeons. For example, despite instructing the surgeons to enroll consecutive patients who may undergo major curative cancer surgery, we know this did not always occur, which may have resulted in selection bias. A review of operative data at 4 of the 8 participating centres indicated that 72% of the cancer-directed surgeries were included in our study. Response bias may have affected the appropriateness ratings if a surgeon completing a form contributed to a patient delay. We have already noted that surgeons did not always complete dates for the 5 key events, even for patients who underwent major surgery. Third, we were unable to comment on the variation in intervals among the cancer centres because we did not control for the cancer type or number of cases contributed from each centre. Finally, because of resource constraints, we could not check the accuracy of data entered by the surgeons against actual chart-based information. Our definition of “inappropriate” waits can also be criticized, since it relied on the subjective perceptions of the participating surgeons. Unfortunately, objective benchmarks derived from evidence for this portion of the study do not exist. As well, our results suggest that the surgeons were fair arbiters in deciding which intervals were inappropriate, because for each of the 4 key intervals few of the waits were marked inappropriate and because the inappropriate waits were significantly longer than the appropriate waits.
In conclusion, when we compare the waiting times from main treatment decision to surgery with the recommendations of an expert group and we consider the large number of patients with waits for the summary intervals deemed to be inappropriate by the surgeons, we suggest that many cancer patients treated by surgeons affiliated with Ontario regional cancer centres are experiencing significant delays in the assessment and surgical treatment of their cancer.
Acknowledgments
We thank the members of the Provincial Advisory Committee for Surgical Oncology who provided helpful suggestions during the planning, execution, analysis and write-up of this study. The assistance of Amanda Heydon, Eddy Rempel and Stefan Powell in entering, summarizing and verifying the data was greatly valued. The efforts of the participating surgeons and the following site coordinators were especially appreciated: Ms. Anita Jean (Northwestern Ontario Regional Cancer Centre), Mr. Allan Katz (Northeastern Ontario Regional Cancer Centre), Dr. Michael Fung Kee Fung (Ottawa Regional Cancer Centre), Ms. Lisa Johnston (Breast Health Centre, Ottawa Hospital), Dr. Jay Engel (London Regional Cancer Centre), Dr. Ray Osborne (Toronto–Sunnybrook Regional Cancer Centre), Ms. Jane Hanes (Princess Margaret Hospital) and Dr. Ken Woolfson (Lakeridge Health, Oshawa).
Footnotes
This article has been peer reviewed.
This research was supported by the Ontario Ministry of Health and Long Term Care through Cancer Care Ontario.
Competing interests: None declared.
Correspondence to: Dr. Marko Simunovic, Hamilton Regional Cancer Centre, 699 Concession St., Hamilton ON L8V 5C2
References
- 1.Canadian Society for Surgical Oncology position statement. Available: www.cos.ca/csso/policy.htm (accessed 2001 July 10).
- 2.Draft synthesis report. Canadian Strategy for Cancer Control; 2001 Jan 18. Available: www.hc-sc.gc.ca/hppb/cscc/work_reports.html (accessed 2001 July 10).
- 3.Benedict S, Williams RD, Baron PL. Recalled anxiety: from discovery to diagnosis of a benign breast mass. Oncol Nurs Forum 1994;21(10):1723-7. [PubMed]
- 4.Risberg T, Sorbye SW, Norum J, Wist EA. Diagnostic delay causes more psychological distress in female than in male breast cancer patients. Anticancer Res 1996;16(2):995-9. [PubMed]
- 5.Jones RV, Greenwood B. Breast cancer: causes of patients' distress identified by qualitative analysis. Br J Gen Pract 1994;44(385):370-1. [PMC free article] [PubMed]
- 6.Gray RE, Fitch MI, Phillips C, Labrecquie M, Klotz L. Presurgery experiences of prostate cancer patients and their spouses. Cancer Pract 1999;7(3):130-5. [DOI] [PubMed]
- 7.Julian-Reynier C, Eisinger F, Chabal F, Aurran Y, Bignon YJ, Nogues C, et al. Time elapsing from cancer diagnosis and anxiety in women attending cancer genetic clinics. Oncol Rep 1998;5(4):885-8. [DOI] [PubMed]
- 8.Rapoport Y, Kreitler S, Chaitchik S, Algor R, Wiessler K. Psychosocial problems in head-and-neck cancer patients and their change with time since diagnosis. Ann Oncol 1993;4(1):69-73. [DOI] [PubMed]
- 9.Weisman AD, Worden JW. The existential plight in cancer: significance of the first 100 days. Int J Psychiatry Med 1976-77;7(1):1-15. [DOI] [PubMed]
- 10.Caplan LS, Heszlsouer KJ. Delay in breast cancer: a review of the literature. Public Heath Rev 1992-93;20(3-4):187-214. [PubMed]
- 11.Sainsbury R, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast cancer symptoms: a retrospective analysis. Lancet 1999; 353(9159):1132-5. [DOI] [PubMed]
- 12.Coates AS. Breast cancer: delays, dilemmas, and delusions. Lancet 1999;353 (9159):1112-3. [DOI] [PubMed]
- 13.Richard MA, Grob JJ, Avril MF, Delaunay M, Gouvernet J, Wolkenstein P, et al. Delay in diagnosis and melanoma prognosis: I. The role of patients. Int J Cancer 2000;89(3):271-9. [PubMed]
- 14.Roncoroni L, Pietra N, Violi V, Sarli L, Choua O, Peracchia A. Delay in the diagnosis and outcome of colorectal cancer: a prospective study. Eur J Surg Oncol 1999;25(2):173-8. [DOI] [PubMed]
- 15.Wurtz LD, Peabody TD, Simon MA. Delay in the diagnosis and treatment of primary bone sarcoma of the pelvis. J Bone Joint Surg Am 1999;81(3):317-25. [DOI] [PubMed]
- 16.Christensen ED, Harvald T, Jendresen M, Aggestrup S, Petterson G. The impact of delayed diagnosis of lung cancer on the stage at the time of operation. Eur J Cardiothorac Surg 1997;12(6):880-4. [DOI] [PubMed]
- 17.About Cancer Care Ontario: an overview. Toronto: Cancer Care Ontario. Available: www.cancercare.on.ca/about/home.html#overview (accessed 2001 July 10).
- 18.Pentyala SN, Lee J, Hsieh K, Waltzer WC, Trocchia A, Musacchia L, et al. Prostate cancer: a comprehensive review. Med Oncol 2000;17(2):85-105. [DOI] [PubMed]
- 19.Spurgeon P, Barwell F, Kerr D. Waiting times for cancer patients in England after general practitioners' referrals: retrospective national survey. BMJ 2000;320(7238):838-9. [PMC free article] [PubMed]
- 20.Mayo NE, Scott SC, Shen N, Hanley J, Goldberg MS, MacDonald N. Waiting time for breast cancer surgery in Quebec. CMAJ 2001;164(8):1133-8. Available: www.cma.ca/cmaj/vol-164/issue-8/1133.asp [PMC free article] [PubMed]
- 21.Chaudhry R, Goel V, Sawka C. Breast cancer survival by teaching status of the initial treating hospital. CMAJ 2001;164(2):183-8. Available: www.cma.ca/cmaj/vol-164/issue-2/0183.htm [PMC free article] [PubMed]
- 22.Simunovic M, To T, Baxter N, Balshem A, Ross E, Cohen Z, et al. Hospital procedure volume and teaching status do not influence treatment and outcome measures of rectal cancer surgery in a large general population. J Gastrointest Surg 2000;4(3):324-30. [DOI] [PubMed]


