Abstract
The purpose of this study was to investigate the influence of acetic acid (apple cider vinegar; ACV) supplementation on resting and exercise energy expenditure and substrate utilization. Using a randomized, double blind, crossover design, 16 healthy subjects were supplemented for 4 d with either ACV (30-ml/d) mixed in 1 L of a non-nutritive lemon-flavored drink or a placebo (PLA). They were then assessed via indirect calorimetry for resting energy expenditure (REE) and substrate utilization. This was immediately followed by the assessment of steady state cycling exercise energy expenditure at 40 W (EEE-40) and 80 W (EEE-80) and substrate utilization. Results: Neither REE nor resting substrate utilization were significantly different between groups (p ≥ .05). During cycling exercise at both 40W and 80W, there were no significant differences observed between groups for energy expenditure (EEE-40: ACV 4.13 ± 0.79, PLA 4.37 ± 0.61 kcal/min; EEE-80: ACV 6.09 ± 0.87, PLA 6.26 ± 0.72 kcal/min) or substrate utilization (40W carbohydrate: ACV 0.72 ± 0.19, PLA 0.76 ± 0.16; fat: ACV 0.15 ± 0.07, PLA 0.16 ± 0.06 g/min), (80W carbohydrate: ACV 1.28 ± 0.32, PLA 1.34 ± 0.35; fat: ACV 0.14 ± 0.10, PLA 0.14 ± 0.10 g/min) (p ≥ .05). Conclusions: Recent findings suggest that chronic acetic acid supplementation is associated with significant reductions in body weight and body fat; however, the findings of the present study suggest that a semi-acute (4 d) acetic acid supplementation does not impact resting or exercise energy expenditure or substrate utilization.
INTRODUCTION
Vinegar’s origin is largely shrouded in mystery. Legend states that a courtier in Babylonia (5,000 BC) “discovered” wine, from forgotten grape juice, leading to the eventual discovery of both vinegar and its food preserving capabilities (7). Ancient civilizations used vinegar for a variety of purposes, with Hippocrates using it to treat wounds, fever, and sores (2). Early U.S. practitioners used it to treat poison ivy, croup, stomachache, high fever, and edema (2).
With the increasing popularity of at-home medicine, researchers have recently begun to investigate some of the purported health benefits of vinegar (4). Most notably, improved cardiovascular function (12, 18) and glycemic control (1, 8, 13). Additionally, vinegar has been shown to increase fatty acid oxidation, interrupt lipogenesis, increase energy expenditure, and reduce body mass and fat mass (10, 6, 21).
Investigation into vinegar’s role in weight loss has been primarily limited to animal models (6, 10, 21) and the mechanisms by which it stimulates weight loss are still unclear. Proposed mechanisms include an interruption of glucose stimulated lipogenesis (21), an increase in thermogenesis and fatty acid oxidation (10), and an increase in energy expenditure and alterations in substrate utilization (6). In the only two reported human trials, vinegar was shown to reduce both body mass and fat mass (9, 11). While the mechanisms by which vinegar may stimulate weight loss in humans are unclear, two notions have been posited. 1) it stimulates an increase in satiety (3, 15), and 2) it increases energy expenditure and substrate oxidation (6).
While there are several animal studies suggesting a positive effect of vinegar supplementation on energy balance and weight management (6, 10, 21), there is a paucity of investigations of its effects in humans (11). Therefore, the purpose of this study was to investigate the effect of semi-acute vinegar supplementation on resting and exercise energy expenditure, as well as substrate utilization at rest and during steady state exercise. It was hypothesized that a 3-day administration of vinegar would stimulate changes in resting and exercise metabolism and substrate utilization.
METHODS
This research was approved by the Institutional Review Board for the use of Human Subjects in Research (IRB-03379-2016) and was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (14). Inclusion criteria were non-smoking individuals over the age of 18, free of any metabolic altering medical conditions, and not consuming any nutritional/mineral supplements during the previous 30-d. Participants were required to maintain their normal physical activity levels through the duration of the study. For each laboratory testing session, subjects were requested to be at least 4-h post prandial, having refrained from exercise within the previous 12-h, and also having abstained from caffeine and alcohol for at least 24-h prior.
Each participant arrived in the laboratory on three separate occasions, with a minimum of 2-d between visits. The first visit was a familiarization trial. The second and third visits were experimental trials, one being a treatment trial and the other being a placebo trial. These experimental and placebo trials were administered using a randomized, double blind, crossover design.
Protocol
Upon arrival in the laboratory, subjects completed an informed consent, Physical Activity Readiness Questionnaire (19) and a health history questionnaire, and were then assessed for measures of body mass, height, and body composition (Discovery DXA System, Marlborough, MA). They then underwent a familiarization trial during which resting energy expenditure (REE) was estimated via open-circuit indirect calorimetry (Vmax Encore Metabolic Cart; Yorba Linda, CA). Flow volume and gas calibrations were performed prior to each testing session according to the manufacturer’s instructions. Subjects were asked to assume a supine position and a Plexiglas ventilated hood was placed over their head. Expired gases were assessed for 30-min during which the flow rate was monitored to insure a FECO2 between 0.75–0.85%. The REE was determined from 5-min of steady state respiratory gas measurements assessed during minutes 20–30.
Upon completion of the REE assessment, the metabolic cart was immediately recalibrated to facilitate exercise testing and the subject was fitted with a facemask and mass flow sensor. The subject then performed 20-min of continuous exercise on a cycle ergometer (Monark 874 E, Vansbro, Sweden) with the first 10-min performed at 40-Watts and the last 10-min performed at 80-Watts. Heart rate was monitored via telemetry (Polar electro Inc., Lake success, NY) and recorded during the final 2-min of each workload. Expired gas volumes and fractional concentrations of O2 and CO2 were analyzed and used in the assessment of energy expenditure and substrate utilization. Energy expenditure was calculated as [3.94(VO2) + 1.1(VCO2)] (20) with resting data expressed as kcal/d and exercise data expressed as kcal/min. Substrate oxidation rates were calculated for fat as [1.695(VO2) − 1.701(VCO2)] and carbohydrate as [4.585(VCO2) − 3.226(VO2)] with resting data expressed as g/d and exercise data expressed as g/min (16).
Subsequent to completion of the familiarization trial, treatment and placebo trials were administered in a randomized, double blind fashion and the solutions were prepared by an individual not involved in data collection or having any participant interaction. For each of the trials, subjects were provided six drink bottles, each containing 500 ml of either the treatment or placebo solution. Subjects were instructed to drink the contents of one bottle in the morning and one bottle in the evening for three consecutive days. On the fourth day, they arrived in the laboratory and were instructed to consume an additional 500 ml of the respective solution. 1-h post consumption they were then assessed for REE and exercise energy expenditure (EEE) in the same fashion as described in the familiarization trial. Following a minimum of 2-d, subjects then completed the alternate trial condition.
The treatment and placebo solutions both consisted of a flavored, low-calorie drink mix (Crystal Light Lemonade, Kraft Foods, Chicago, Illinois). This was mixed at 150% of the manufacturer’s recommended concentration to provide a strong lemonade flavor and to increase tartness and sweetness in an attempt to disguise the vinegar taste. The treatment solution also contained an addition of 30-ml of apple cider vinegar (Double Shot - Apple Cider Vinegar with the Mother; Vermont Village, Village Cannery of Vermont, New Hampshire) in each liter of solution. While not directly assessed, informal debriefing of several subjects indicated that the drinks were indistinguishable from one another.
Statistical Analysis
A 3 x 9 Factorial Analysis of Variance was used to assess for differences between the three trials (familiarization, treatment, placebo) for REE (kcal/day), resting substrate utilization (g carbohydrates (CHO)/day and g fat/day), EEE (kcal/min) and substrate utilization (g CHO/min and g fat/min) at both 40-Watts and 80-Watts. All data were reported as mean ± SD and statistical significance was set at α ≤ .05.
RESULTS
Demographics (Table 1)
Table 1.
Subject demographics. Values are expressed as mean ± SD.
| Body weight (kg) | 72.2 ± 12.2 |
| Height (cm) | 168.7 ± 10.8 |
| Age (yr) | 22 ± 2 |
| Body fat (%) | 23.6 ± 5.5 |
A total of 21 participants volunteered to participate in the study and met the inclusion criteria; however, three were excluded due to noncompliance with supplementation or attendance, and there was corruption of the data for two additional subjects, leaving a final participant pool of n = 16 (5 males, 11 females). There were no adverse events reported during the course of the study.
Resting energy expenditure (REE) and substrate utilization (Table 2)
Table 2.
Resting energy expenditure and substrate utilization. Values are expressed as mean ± SD.
| Familiarization | Placebo | Vinegar | |
|---|---|---|---|
|
|
|||
| Resting energy expenditure (kcal/d) | 1629 ± 209 | 1654 ± 235 | 1669 ± 264 |
| Substrate utilization (g/d) | |||
| Fat | 114.8 ± 44.1 | 121.5 ± 36.8 | 118.4 ± 36.7 |
| Carbohydrate | 155.0 ± 147.7 | 135.2 ± 75.7 | 146.4 ± 65.8 |
REE was not significantly different between groups (p ≥ .05). Effect sizes calculated as Cohens d (placebo vs. treatment) REE: .06, Fat: .08, Carbohydrate: .15. There were also no significant differences in resting substrate utilization between treatments.
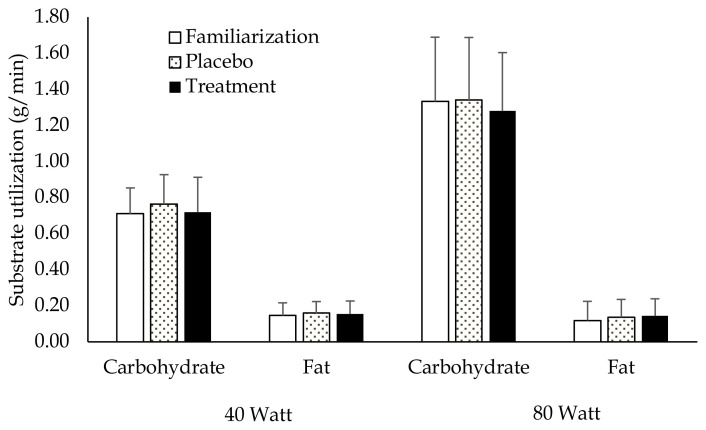
Exercise Energy Expenditure (EEE) and substrate utilization (Figure 1)
Figure 1.
Substrate utilization for Familiarization, Placebo and Treatments trials at cycling power outputs of 40-Watts and 80-Watts, respectively. There were no significant differences noted between trials for carbohydrate or fat utilization at either cycling intensity (p ≥ .05).
EEE was not significantly different between groups at cycling power outputs of either 40W or 80W (Table 3). Effect sizes calculated as Cohens d (placebo vs. treatment) 40W: .34, 80W: .21. Likewise, there were no significant between group differences in either fat or carbohydrate utilization at cycling power outputs of 40W or 80W.
The cycling power outputs employed in this study, 40-Watts and 80-Watts, were chosen to elicit light and moderate intensity efforts, respectively. The cycling power output of 40-Watts elicited a heart rate of 105 ± 15 b/min which equated to 53 ± 7% of age predicted maximal heart rate (APMHR) (220-age). The cycling power output of 80-Watts elicited a heart rate of 135 ± 17 b/min which equated to 67 ± 8% of APMHR.
DISCUSSION
The administration of acetic acid, the main component of vinegar, to mice was reported by Kondo et al. (10) to suppress fat deposition and weight gain, while at the same time maintaining skeletal muscle mass. Hattori et al. (6) supported these findings when they reported that acetic acid administration significantly increased energy expenditure and suppressed fat accumulation. While these reports were derived from animal work, it immediately drew interest in the potential use of vinegar in weight management in humans. Two human studies furthered this interest when it was reported that vinegar supplementation was associated with significant loss of both body mass and fat mass (9, 11). In light of these findings, the purpose of the present study was to investigate if semi-acute vinegar supplementation would have any impact on REE or steady state EEE, as well as substrate utilization both at rest and during exercise. In contrast with these previous studies, our findings indicate that vinegar supplementation does not increase energy expenditure or substrate utilization at rest or during light or moderate intensity exercise.
There are several factors, at least in part, that may provide insight into the contrasting findings reported in the present study. The first factor to be considered is the choice of an acute or chronic vinegar administration period. The protocol of Hattori et al. (6) was an acute administration, via gastric tube, of a 1.5% acetic acid solution to laboratory mice. This protocol demonstrated an increase in energy expenditure and fat oxidation 1 h after administration. Kondo et al. (11) chose a chronic protocol consisting of 12 weeks of either a low dose (15 ml/d) or a high dose (30 ml/d) vinegar consumption delivered in a 0.5 L of solution. Their findings revealed a progressive and significant reduction in body mass, BMI, and body fat mass after 4-, 8-, and 12-weeks, respectively. Interestingly, four weeks after discontinuing the vinegar administration, all of these measures had returned to the pre-treatment values. Khezri et al. (9) also chose to investigate a chronic vinegar supplementation using the same quantity as Kondo et al. (11); however, in this instance, rather than subjects consuming the vinegar diluted in solution, subjects consumed 15-ml of vinegar with a salad at both lunch and dinner (30-ml daily). Similar to the findings of Kondo et al. (11), the vinegar treatment was associated with significant changes in body mass, BMI, and visceral adiposity.
The experimental design of the present study was a compromise of these previous studies in that a “semi-acute” administration was chosen. In this, each day the subjects consumed the same total volume of vinegar (30 ml/d) as Kherzi et al. (9) and Kondo et al. (11) with 15-ml of vinegar consumed in 0.5-L of solution two times per day. Subjects arrived in the laboratory and consumed a final 15-ml of vinegar in 0.5-L of solution. 1-h later they underwent assessment for indirect calorimetry which is similar in timing to the administration and assessment chosen by Hattori et al (6). Despite our experimental protocol attempting to maximize the variables previously associated with significant findings, our results did not support these earlier studies.
The mechanism by which acetic acid/vinegar impacts body mass and composition has yet to be fully elucidated; however, there are generally two schools of thought: 1) It enhances energy expenditure, suppresses carbohydrate utilization, and enhances fat oxidation (10, 21), or 2) It reduces hunger and hence, energy intake (2, 9, 15, 17). This study was designed to specifically investigate vinegar’s influence on energy expenditure and substrate utilization. While animal work has demonstrated vinegar to significantly impact these parameters (6, 10, 20), the fact that no statistically significant differences were revealed in our study suggests that, at least in humans, if vinegar is to have an impact on weight management and body composition, the research focus needs to be directed toward chronic supplementation and its effect on satiety and energy intake.
This study carried several limitations. First, there was no direct assessment of the effectiveness of the treatment masking protocol. However, during informal debriefing of subjects there were no reports of being able to distinguish between the treatment or placebo. Second, no direct assessments were taken of reported side effects during the treatment or placebo protocols. Past literature has reported the unpalatable nature of vinegar as a possible explanation for weight loss (3). However, considering some groups have controlled for energy intake, this notion is unlikely (11).
To the author’s knowledge this is the first study to investigate the effect of vinegar supplementation on resting and exercise energy expenditure and substrate utilization in humans. While previous vinegar supplementation studies have demonstrated body and fat mass loss, the present findings suggest this is not due to acute changes in metabolic rate or substrate utilization. Future vinegar supplementation research should be directed toward investigating the impact of chronic supplementation, specifically its effects on energy metabolism, and also its impact on satiety.
REFERENCES
- 1.Brighenti F, Castellani G, Benini L, Casiraghi MC, Leopardi E, Crovetti R, Testolin G. Effect of neutralized and native vinegar on blood glucose and acetate responses to a mixed meal in healthy subjects. European Journal of Clinical Nutrition. 1995;49(4):242–247. [PubMed] [Google Scholar]
- 2.Budak NH, Aykin E, Seydim AC, Greene AK, Guzel-Seydim ZB. Functional properties of vinegar. Journal of Food Science. 2014;79(5):R757–764. doi: 10.1111/1750-3841.12434. [DOI] [PubMed] [Google Scholar]
- 3.Darzi J, Frost GS, Montaser R, Yap J, Robertson MD. Influence of the tolerability of vinegar as an oral source of short-chain fatty acids on appetite control and food intake. International Journal of Obesity. 2014;38(5):675–681. doi: 10.1038/ijo.2013.157. [DOI] [PubMed] [Google Scholar]
- 4.Ernst E. Rise in popularity of complementary and alternative medicine: Reasons and consequences for vaccination. Vaccine. 2001;20:S90–S93. doi: 10.1016/S0264-410X(01)00290-0. [DOI] [PubMed] [Google Scholar]
- 5.Fushimi T, Sato Y. Effect of acetic acid feeding on the circadian changes in glycogen and metabolites of glucose and lipid in liver and skeletal muscle of rats. Br J Nutr. 2005;94:714–719. doi: 10.1079/bjn20051545. [DOI] [PubMed] [Google Scholar]
- 6.Hattori M, Kondo T, Kishi M, Yamagami K. A single oral administration of acetic acid increased energy expenditure in C57BL/6J mice. Bioscience, Biotechnology, and Biochemistry. 2010;74(10):2158–2159. doi: 10.1271/bbb.100486. [DOI] [PubMed] [Google Scholar]
- 7.Johnston C. Medicinal Uses of Vinegar. Complementary and Alternative Therapies and the Aging Population. 2009:433–443. doi: 10.1016/B978-0-12-374228-5.00022-6. [DOI] [Google Scholar]
- 8.Johnston CS, Steplewska I, Long CA, Harris LN, Ryals RH. Examination of the antiglycemic properties of vinegar in healthy adults. Annals of Nutrition & Metabolism. 2010;56(1):74–79. doi: 10.1159/000272133. [DOI] [PubMed] [Google Scholar]
- 9.Khezri SS, Saidpour A, Hosseinzadeh N, Amiri Z. Beneficial effects of Apple Cider Vinegar on weight management, Visceral Adiposity Index and lipid profile in overweight or obese subjects receiving restricted calorie diet: A randomized clinical trial. Journal of Functional Foods. 2018;43:95–102. doi: 10.1016/j.jff.2018.02.003. [DOI] [Google Scholar]
- 10.Kondo T, Kishi M, Fushimi T, Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. Journal of Agricultural and Food Chemistry. 2009;57(13):5982–5986. doi: 10.1021/jf900470c. [DOI] [PubMed] [Google Scholar]
- 11.Kondo T, Kishi M, Fushimi T, Ugajin S, Kaga T. Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Bioscience, Biotechnology, and Biochemistry. 2009;73(8):1837–1843. doi: 10.1271/bbb.90231. [DOI] [PubMed] [Google Scholar]
- 12.Lerman A, Zeiher AM. Endothelial Function: Cardiac Events. Circulation. 2005;111(3):363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 13.Liatis S, Grammatikou S, Poulia K-A, Perrea D, Makrilakis K, Diakoumopoulou E, Katsilambros N. Vinegar reduces postprandial hyperglycaemia in patients with type II diabetes when added to a high, but not to a low, glycaemic index meal. European Journal of Clinical Nutrition. 2010;64(7):727–732. doi: 10.1038/ejcn.2010.89. [DOI] [PubMed] [Google Scholar]
- 14.Navalta JW, Stone WJ, Lyons TS. Ethical Issues Relating to Scientific Discovery in Exercise Science. Int J Exerc Sci. 2019;12(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Ostman E, Granfeldt Y, Persson L, Björck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. European Journal of Clinical Nutrition. 2005;59(9):983–988. doi: 10.1038/sj.ejcn.1602197. [DOI] [PubMed] [Google Scholar]
- 16.Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16(1):23–9. [PubMed] [Google Scholar]
- 17.Petsiou EI, Mitrou PI, Raptis SA, Dimitriadis GD. Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight. Nutrition Reviews. 2014;72(10):651–661. doi: 10.1111/nure.12125. [DOI] [PubMed] [Google Scholar]
- 18.Sakakibara S, Murakami R, Takahashi M, Fushimi T, Murohara T, Kishi M, Kaga T. Vinegar intake enhances flow-mediated vasodilatation via upregulation of endothelial nitric oxide synthase activity. Bioscience, Biotechnology, and Biochemistry. 2010;74(5):1055–1061. doi: 10.1271/bbb.90953. [DOI] [PubMed] [Google Scholar]
- 19.Shephard RJ. PAR-Q, Canadian Home Fitness Test and Exercise Screening Alternatives. Sports Medicine. 1988;5(3):185–195. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 20.Weir JB, de V. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of Physiology. 1949;109(1–2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita H, Fujisawa K, Ito E, Idei S, Kawaguchi N, Kimoto M, Tsuji H. Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Bioscience, Biotechnology, and Biochemistry. 2007;71(5):1236–1243. doi: 10.1271/bbb.60668. [DOI] [PubMed] [Google Scholar]