Abstract
Background
This study provides detailed characteristics of vector populations in preparation for a three-arm cluster randomized controlled trial (RCT) aiming to compare the community impact of dual active-ingredient (AI) long-lasting insecticidal nets (LLINs) that combine two novel insecticide classes–chlorfenapyr or pyriproxifen–with alpha-cypermethrin to improve the prevention of malaria transmitted by insecticide-resistant vectors compared to standard pyrethroid LLINs.
Methods
The study was carried out in 60 villages across Cove, Zangnanando and Ouinhi districts, southern Benin. Mosquito collections were performed using human landing catches (HLCs). After morphological identification, a sub-sample of Anopheles gambiae s.l. were dissected for parity, analyzed by PCR for species and presence of L1014F kdr mutation and by ELISA-CSP to identify Plasmodium falciparum sporozoite infection. WHO susceptibility tube tests were performed by exposing adult An. gambiae s.l., collected as larvae from each district, to 0.05% alphacypermethrin, 0.75% permethrin, 0.1% bendiocarb and 0.25% pirimiphos-methyl. Synergist assays were also conducted with exposure first to 4% PBO followed by alpha-cypermethrin.
Results
An. gambiae s.l. (n = 10807) was the main malaria vector complex found followed by Anopheles funestus s.l. (n = 397) and Anopheles nili (n = 82). An. gambiae s.l. was comprised of An. coluzzii (53.9%) and An. gambiae s.s. (46.1%), both displaying a frequency of the L1014F kdr mutation >80%. Although more than 80% of people slept under standard LLIN, human biting rate (HBR) in An. gambiae s.l. was higher indoors [26.5 bite/person/night (95% CI: 25.2–27.9)] than outdoors [18.5 b/p/n (95% CI: 17.4–19.6)], as were the trends for sporozoite rate (SR) [2.9% (95% CI: 1.7–4.8) vs 1.8% (95% CI: 0.6–3.8)] and entomological inoculation rate (EIR) [21.6 infected bites/person/month (95% CI: 20.4–22.8) vs 5.4 (95% CI: 4.8–6.0)]. Parous rate was 81.6% (95%CI: 75.4–88.4). An. gambiae s.l. was resistant to alpha-cypermethrin and permethrin but, fully susceptible to bendiocarb and pirimiphos-methyl. PBO pre-exposure followed by alpha-cypermethrin treatment induced a higher 24 hours mortality compared to alphacypermethrin alone but not exceeding 40%.
Conclusions
Despite a high usage of standard pyrethroid LLINs, the study area is characterized by intense malaria transmission. The main vectors An. coluzzii and An. gambiae s.s. were both highly resistant to pyrethroids and displayed multiple resistance mechanisms, L1014F kdr mutation and mixed function oxidases. These conditions of the study area make it an appropriate site to conduct the trial that aims to assess the effect of novel dual-AI LLINs on malaria transmitted by insecticide-resistant vectors.
Background
Malaria remains a major public health issue in Benin, with prevalence of infection within the general population ranging from 11% to 51% depending on the region, with high burden in children under 5 years old [1]. Long-lasting insecticidal nets (LLINs) distributed at the national level every three years, and indoor residual spraying (IRS) in targeted districts, are the main pillars on which Benin’s National Malaria Control Programme (NMCP) relies for protection against malaria vectors. During the most recent mass distribution campaign in 2017, pyrethroid-treated LLINs (Yorkool, PermaNet 2.0 and Dawa Plus 2.0), were widely distributed across the country. In 2018, LLIN usage was relatively high, with 71% of the national population reporting sleeping under a net, and 92% of households owning at least one LLIN [1].
According to Bhatt et al., [2], malaria control interventions helped reduce malaria incidence by 40% in Africa between 2000 and 2015, with insecticide treated nets (ITNs) being the highest contributor (68% of cases averted). Similarly, reductions in malaria disease burden following the scale-up of LLINs have been routinely documented in trials conducted in several African countries including Kenya [3,4], and Benin [5,6]. Although LLINs are efficacious against susceptible vector populations, more recent studies have demonstrated that LLINs performed below expectations in areas where vectors are resistant to insecticides used for nets treatments, notably pyrethroids [7,8].
Insecticide resistance has emerged and spread across Africa, including Benin [9]. Between 2016 and 2018, progress in reducing malaria cases worldwide had stalled [9], with one of the likely reasons being pervasive insecticide resistance among malaria vector populations.
Researchers and decision-makers now have high expectations of next generation LLINs which the World Health Organization (WHO) has encouraged manufacturers to develop for the control of resistant mosquitoes. Currently, next generation LLINs under evaluation include nets which combine a pyrethroid insecticide with either piperonyl butoxide (PBO; a synergist that inhibits mono-oxygenases implicated in resistance) or pyriproxyfen (PPF; a growth regulator that inhibits fecundity and fertility in insects) or chlorfenapyr (a pyrrole insecticide that disrupts mitochondrial oxidative phosphorylation). Cluster randomized controlled trials (RCTs) conducted in Tanzania [8], Uganda [10], and Burkina Faso [11] demonstrated that pyrethroid-PBO LLINs and pyrethroid-PPF LLINs provide more protection against malaria than standard LLINs. Other insecticide mixture LLINs combining alpha-cypermethrin and chlorfenapyr [12] or pyriproxyfen [13], have showed superior efficacy compared to standard LLINs on entomological outcomes in experimental hut trials. However, there remains a dearth of evidence regarding the effectiveness of the latter two dual-AI LLINs on malaria infection and transmission at the community-level.
This study conducted in southern Benin presents baseline entomological data collected in preparation for an RCT assessing the effectiveness, of Interceptor G2 (a pyrethroid-chlorfenapyr LLIN) and Royal Guard (a pyrethroid-pyriproxifen LLIN) deployed in the community, on malaria incidence, prevalence and transmission.
Material and methods
Study area
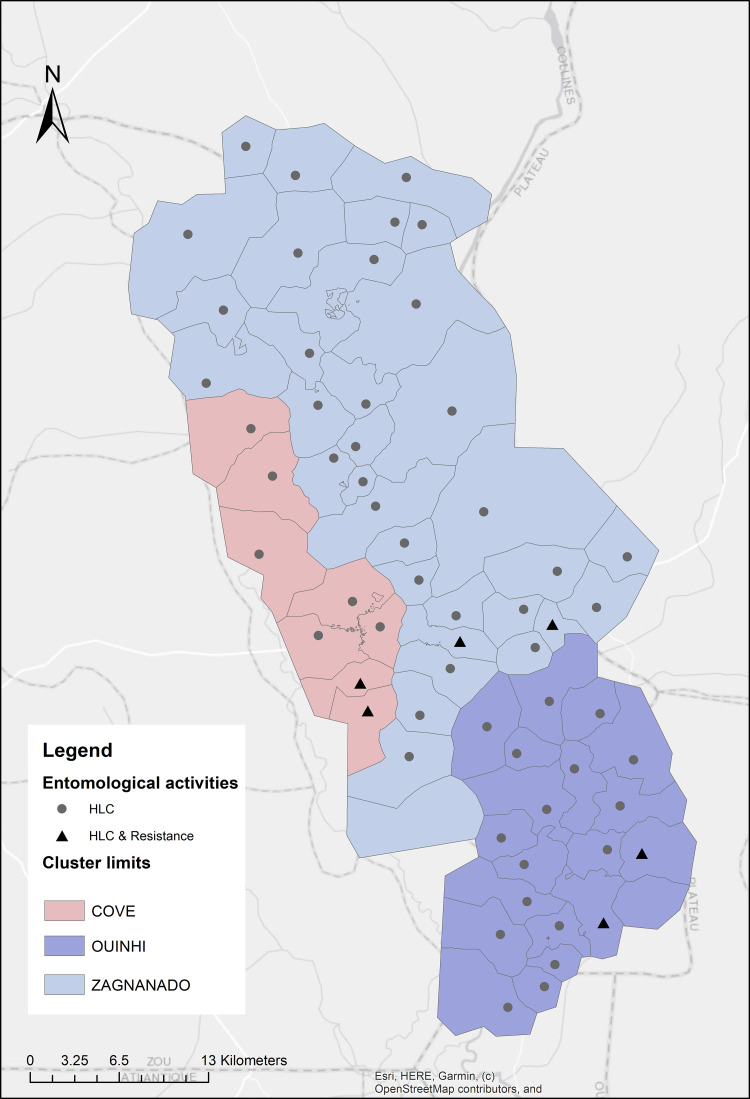
The study was carried out in three adjacent districts [Covè (07˚13’08.0400” N, 02˚20’21.8400” E), Ouinhi (07°05′00″ N, 02°29′00″ E) and, Zagnanando (07°16′00″ N, 02°21′00″ E)] located 150 kilometers away from Cotonou, the economic capital of Benin. The area has two rainy seasons (May-July and September-November) and was selected because of its high endemicity for malaria, with infection prevalence of 36.5% in children aged under 5 years old [1], intense pyrethroid resistance in the primary malaria vector species [14] and proximity to experimental huts sites. The main economic activities carried out by the population are agriculture, fishing, hunting, trade, and hospitality industry. The main crops produced are groundnuts, rice, maize, oranges, cassava, beans, oil palm, sorghum and millet [15]. According to the study census performed in 2019, the area population was approximately 220,000. A total of 60 clusters were formed from the 123 villages in the study area. Entomological monitoring was conducted in one village in each cluster, equating to a total of 60 villages with 8 in Cove district, 33 in Zagnanando and 19 in Ouinhi (Fig 1).
Fig 1. Map of the study area.
Human landing catches (HLC)
Mosquito sampling technique
One round of mosquito collections was undertaken across villages, between September and October 2019. In each village, four houses were selected for mosquito sampling using HLCs. To facilitate the supervision of mosquito collectors for HLCs, the first house was randomly selected from the study census list, while the other three were chosen by the field team, within a radius of 15–20 meters around the first one. Four collectors were required per house. Two collectors (one indoor and one outdoor) collected mosquitoes during 6 hours from 07:00 p.m. to 01:00 a.m. and the second group from 01:00 to 07:00 a.m. The collectors sat on a chair with their lower limbs exposed and collected all mosquitoes which landed on them using sucking tubes. To characterize Anopheles biting behaviour, collections were recorded per hour both indoors and outdoors.
Mosquito processing
Mosquito specimens collected in HLCs were morphologically identified to species-level using the Gillies and Meillon [16] taxonomic key. In each village, a sub-sample of An. gambiae s.l. from indoor and outdoor collection and across collection hours were randomly selected, dissected to determine parous status [17], and analyzed by ELISA-CSP to detect presence of Plasmodium falciparum, following the protocol of Wirtz et al. [18]. Abdomens, legs and wings of An. gambiae s.l., previously analysed by ELISA-CSP, were used for species identification and L1014F kdr mutation following the protocols of Santolamazza et al. [19] and Martinez-Torres et al. [20], respectively.
Household data collection
A short questionnaire about LLIN use was administered in houses where HLCs were conducted using Open Data Kit (ODK). The questionnaire recorded information about number of inhabitants in the surveyed households, number of people sleeping indoors and outdoors, number of people sleeping under nets, type of house (mud, cement, others), type of nets presents in the house, condition of nets, GPS coordinates of households and, other malaria prevention measures (IRS, coils, any others) used by household members.
WHO susceptibility tube tests
Mosquito larvae collections and rearing
Mosquito larvae and pupae were sampled from various breeding sites in 2 nearby villages in each district, using a larval dipper. They were transported to the field insectary for rearing until adulthood at 25°C ± 2°C and 80% ± 10% relative humidity. After emergence, morphological identification of adult mosquitoes was performed to species-level, and only An. gambiae s.l. individuals were tested.
Susceptibility testing
In each district, batches of 20–25 unfed females An. gambiae s.l. aged 3–5 days old were aspirated into four tubes containing WHO insecticide impregnated papers (0.75% permethrin, 0.05% alpha-cypermethrin, 0.1% bendiocarb or 0.25% pirimiphos-methyl) for one hour. Separate batches were exposed to a tube lined with a WHO control paper in parallel.
To evaluate the involvement of mixed function oxidases (MFOs) in pyrethroid resistance in populations of An. gambiae s.l., synergist assays with piperonyl butoxide (PBO; 4%) were performed. Mosquitoes were pre-exposed to PBO papers in WHO tubes for one hour, before transfer to different tubes with alpha-cypermethrin papers (0.05%) for a further hour.
The percentage mortality at 24, 48, and 72 hours post-exposure was recorded. All tests were performed following the WHO protocol [21].
Ethical considerations
The protocol of the present study has been reviewed and approved by the Benin national ethics committee for health research (N°30/MS/DC/SGM/DRFMT/CNERS/SA, Approval n°6 of 04/03/2019) and the ethics committee at the London School of Hygiene and Tropical Medicine (16237–1). Written consent to participate in the study was taken from head of households and adult volunteers who performed HLCs after they have been fully informed of the risks of the study. Collectors were trained to collect any mosquito that landed on them before being bitten. All fieldworkers have been vaccinated against yellow fever. When they experienced malaria symptoms, they were immediately provided with anti-malarial medication such as Artemisinin-based Combination Therapy in the nearest health facility.
Data management and analysis
Entomological monitoring data were double entered into databases designed in CS Pro 7.2 software and, cleaned with Stata 15.0 (Stata Corp., College Station, TX).
Entomological indicators of malaria transmission measured both indoors and outdoors, were determined as mentioned in the Table 1 below.
Table 1. Formulas of entomological indicators of malaria transmission.
| Indicators | Formulas |
|---|---|
| Human biting rate (HBR) | Total An. gambiae s.l./number of collector night |
| Sporozoite rate (SR) | Number of positive mosquitoes/Total number tested |
| Parous rate | Number of parous mosquitoes/Total number dissected |
| Monthly EIR | HBR x SR x 30 |
As all An. gambiae s.l. positive for CSP ELISA and approximately 50% of the negative ones were tested for molecular species identification, the SR per molecular species (An. coluzzii and An. gambiae s.s.) was weighted to account for proportion of collected Anopheles processed for CSP. This allowed taking into account the unequal sampling.
The mean of the household results for HBR, SR and EIR were used to generate a village level result. The mean of the village results is presented by district and their confidence intervals were calculated using the Poisson distribution.
According to the WHO guidelines [21], resistance status of populations of An. gambiae s.l. was determined after exposure to the discriminating insecticide dose, as follows:
Susceptible (mortality rate ≥ 98%)
Possible resistance (mortality rate between 90–97%)
Resistance (mortality <90%)
According to the WHO guidelines [21], involvement of metabolic mechanisms in insecticide resistance in populations of An. gambiae s.l. was determined as follows:
Metabolic mechanism not involved (insecticide-synergist mortality not higher than for insecticide-only)
Metabolic mechanism partially involved (insecticide-synergist <98% mortality but higher than for insecticide-only)
Metabolic mechanism fully involved (insecticide-synergist ≥98% and higher than for insecticide-only)
Confidence intervals of mortality rates were determined using the exact binomial test. All statistical analyses were performed using Stata version 15.0 (Stata Corp., College Station, TX).
Results
Household and individual characteristics of study population
A total of 240 households were visited for HLCs, the average number of habitants per household was 4.5 (95% confidence interval (CI): 4.2–4.8) (Table 2). The majority (92.8%, 95% CI: 87.2–98.8) of the population slept indoors with no difference between districts. All visited households owned at least one LLIN. The proportion of people that report to sleep under nets the previous night was similar across districts with a mean of 82.7% (95% CI: 77.3–88.3) for the study area.
Table 2. Household and individual characteristics of study population.
| Indicators | Cove | Zangnanado | Ouinhi | Study area |
|---|---|---|---|---|
| (95% CI), N | (95% CI), N | (95% CI), N | (95% CI), N | |
| Households (HH) | ||||
| Total N of people | 69960 | 73733 | 72596 | 216289 |
| Total N of HH | 16941 | 18470 | 18732 | 54143 |
| N of visited HH | 32 | 132 | 76 | 240 |
| Mean N of people per visited HH | 4.3 (3.5–5.1), 137 | 4.7 (4.3–5.1), 624 | 4.4 (3.8–4.9), 331 | 4.5 (4.2–4.8), 1092 |
| Proportion of visited HH with at least one LLIN | 100% | 100% | 100% | 100% |
| People sleeping indoors in the visited HH | 97.1% (81.2–100), 133 | 92.9% (85.5–100), 580 | 90.9% (80.9–100), 301 | 92.8% (87.2–98.8), 1014 |
| People sleeping under nets the previous night in the visited HH | 89.1% (73.9–100), 122 | 81.9% (74.9–89.3), 511 | 81.6% (72.1–91.9), 270 | 82.7% (77.3–88.3), 903 |
| Type of housing | ||||
| Cement wall | 40.6% (21.6–69.5), 13 | 23.5% (15.9–33.3), 31 | 26.3% (16.0–40.6), 20 | 26.7% (20.5–34.1), 64 |
| Mud wall | 43.8% (23.9–73.4), 14 | 72% (58.2–87.9), 95 | 64.5% (47.6–85.2), 49 | 65.8% (55.9–76.9), 158 |
| Other type of wall | 15.6% (5.0–36.5), 5 | 4.5% (1.6–9.9), 6 | 9.2% (3.7–18.9), 7 | 7.5% (4.4–11.9), 18 |
| LLINs | ||||
| Total number of LLINs | 62 | 237 | 148 | 447 |
| Mean N of LLINs per HH | 1.9 (1.4–2.5) | 1.8 (1.5–2.0) | 1.9 (1.6–2.3) | 1.9 (1.6–2.0) |
| Permanet 2.0 | 79% (58.4–100), 49 | 88.6% (77.0–100), 210 | 90.5 (75.8–100), 134 | 87.9% (79.4–97.1), 393 |
| Other LLINs | 21% (11.1–35.9), 13 | 11.4% (7.5–16.6), 27 | 9.5 (5.1–15.9), 14 | 12.1(9.8–15.8), 54 |
| Holed nets | 45.2% (30–65.3), 28 | 43.9% (35.8–53.2), 104 | 51.4 (40–64.3), 76 | 46.5% (40.4–53.3), 208 |
N: Number of, HH: Household.
Overall, the majority of houses were made of mud (65.8%, 95% CI: 55.9–76.9) and cement (26.7%, 95% CI: 20.5–34.1). At the district level, while most houses were made of mud in Zangnanando and Ouinhi, mud and cement made houses were found in similar proportions in the more urban district of Cove.The mean number of LLINs per household was 1.9 (95% CI: 1.6–2.0) in the study area and, similar across districts. The majority (87.9%, 95% CI: 79.4–97.1) of nets used were PermaNet 2.0. The other types of LLINs used were: OlysetNet, DawaPlus, DuraNet and Yorkool. On average, 46.5% (95% CI: 40.4–53.3) of LLINs had holes with no difference between districts (Table 2).
Mosquito species composition
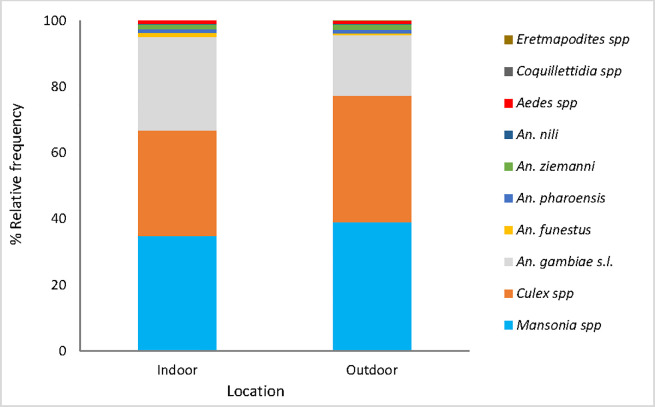
A total of 46,613 mosquitoes were collected with HLCs. Culex and Mansonia accounted for 35.3% and 36.9% of all the collection respectively, and their proportions were slightly higher outdoors than indoors. An. gambiae s.l. was the most abundant of the Anopheles species and represented 28.2% and 18.4% of the collection indoors and outdoors respectively. Other species found in lower density were An. funestus, An. nili, An. ziemanni, and Aedes spp. collected both indoors and outdoors. Coquilletidia spp. and Eretmapodites spp. were captured only indoors but at very low frequencies (<1%) (Fig 2).
Fig 2. Mosquito species composition in the study area.
Molecular species identification performed on 1797 specimens showed that 53.9% (N = 968) were An. coluzzii and the remaining were An. gambiae s.s. Indoors, both species were in similar proportions [50.9%, 95% CI: 48.0–53.9 for An. coluzzii vs 49.1%, 95% CI: 46.1–52.0 for An. gambiae s.s.]. Outdoors, An. coluzzii predominated (59.3%, 95% CI: 55.3–63.1) over An. gambiae s.s. (40.7%, 95% CI: 36.8–44.7).
HBR, SR and monthly EIR in An. gambiae s.l.
Overall, the mean HBR was higher indoors with 26.5 bites per person per night (b/p/n) (95% CI: 25.2–27.9), n = 6373] compared to outdoors [18.5 b/p/n (95% CI: 17.4–19.6), n = 4434] in the study area. The same trend was observed in all three study districts (Table 3).
Table 3. HBR, SR and EIR in An. gambiae s.l. in Cove, Ouinhi and Zangnanado.
| Districts | Biting location | N of An collected | Person night | HBR (95%CI) | N of An tested | SR (95%CI) | EIR (95%CI) |
|---|---|---|---|---|---|---|---|
| Cove | Indoor | 975 | 32 | 30.5a (26.7–34.6) | 366 | 3.3a (0.6–9.5) | 21.4a (18.2–24.8) |
| Outdoor | 556 | 32 | 17.4b (14.6–20.5) | 163 | 2.4a (0.06–13.4) | 6.4b (4.7–8.4) | |
| Zangnanado | Indoor | 2792 | 132 | 21.2a (19.6–22.8) | 1187 | 3.7a (1.8–6.5) | 24.1a (22.4–25.9) |
| Outdoor | 2134 | 132 | 16.2b (14.8–17.6) | 719 | 2.1a (0.5–5.4) | 5.9b (4.9–6.7) | |
| Ouinhi | Indoor | 2606 | 76 | 34.3a (31.7–37.1) | 711 | 1.7a (0.3–4.9) | 17.3a (15.4–19.3) |
| Outdoor | 1744 | 76 | 22.9b (20.8–25.2) | 459 | 1.7a (0.2–6.3) | 4.3b (3.4–5.4) | |
| Study area | Indoor | 6373 | 240 | 26.5a (25.2–27.9) | 2264 | 2.9a (1.7–4.8) | 21.6a (20.4–22.8) |
| Outdoor | 4434 | 240 | 18.5b (17.4–19.6) | 1341 | 1.8a (0.6–3.8) | 5.4b (4.8–6.0) |
An: An. gambiae s.l., The SR is expressed in percentage (%), The HBR was expressed in the number of bites/person/night (b/p/n), The EIR is expressed in number of infected bites/person/month (ib/p/m)
a,b: Indicator values with different superscripts within a same district are significantly different (p<0.05).
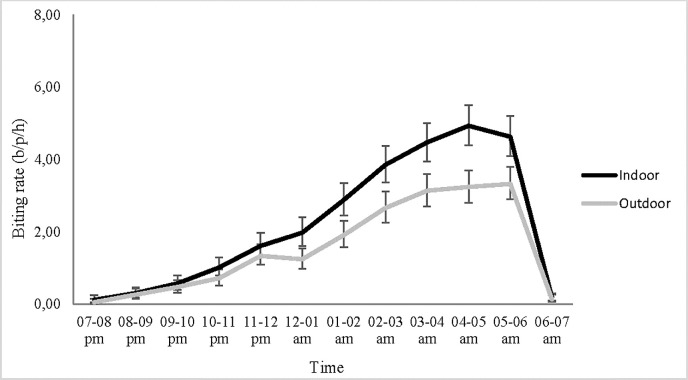
Biting of An. gambiae s.l. was more intense late at night both indoors and outdoors, with the lowest density before 10 pm. The peak in biting was 4.9 b/p/h (95% CI: 4.4–5.5) indoor and 3.3 b/p/h (95% CI: 2.9–3.8) outdoor and occurred between 4 and 6 a.m (Fig 3). The trend was the same at the study district level (Supporting information files, S1 Fig).
Fig 3. An. gambiae s.l. hourly biting rates in the study area (N = 6373 indoors, N = 4434 outdoors), b/p/h: Bite/person/hour, the error bars indicate the confidence intervals.
In An. gambiae s.l., the SR was higher indoors [2.9% (95% CI: 1.7–4.8), n = 2264] than outdoors [1.8% (95% CI: 0.6–3.8), n = 1341] in the study area with no significant difference, so was the trend in each district (Table 3).
At the molecular species level, the SR was 3.1% (95% CI: 2.3–4.0) in An. gambiae s.s. compared to 2.1% (95% CI: 1.5–2.8) in An. coluzzii.
Combined data revealed a higher monthly EIR indoors [21.6 b/p/m (95% CI: 20.4–22.8)] compared to outdoors [5.4 b/p/m (95% CI: 4.8–6.0)] in the study area, with the same trend in the three study districts (Table 3).
Parous rate in An. gambiae s.l.
Of the 2,843 specimens of An. gambiae s.l. dissected in the study area, 2,327 were parous equating to a parous rate of 81.6% (95% CI: 75.4–88.4). Similar parous rates were observed indoors (82.2%, 95% CI: 74.0–91.0) and outdoors (80.7%, 95% CI: 70.7–91.8) in the study area. The same trend was observed in all three study districts (Supporting information files, S1 Table).
WHO susceptibility tube tests and L1014F kdr mutation frequency
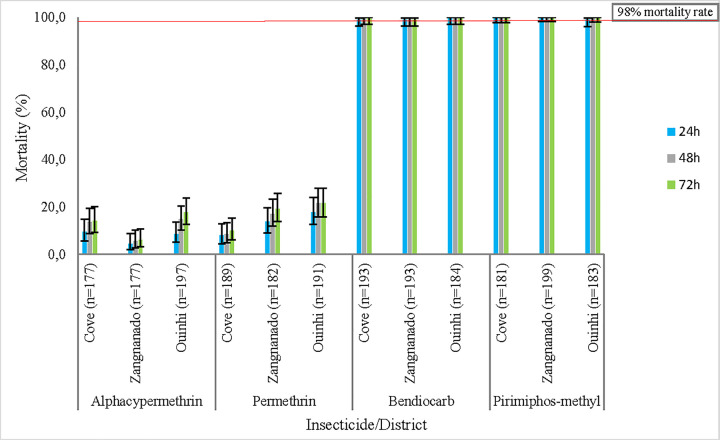
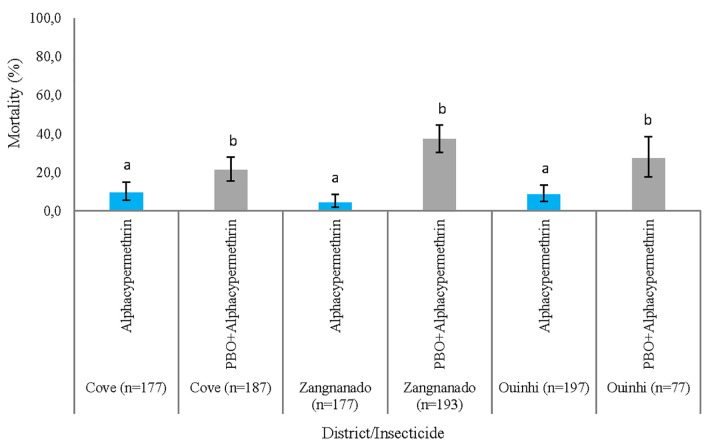
Susceptibility tube testing showed that An. gambiae s.l. populations were resistant to pyrethroid insecticides (alpha-cypermethrin and permethrin). By comparison, full susceptibility was observed to bendiocarb and pirimiphos-methyl. No significant difference was observed between the mortality rates at 24h, 48h and 72h, post-exposure (Fig 4) per district or per insecticide. While full susceptibility was not reached, pre-exposure to PBO increased mortality to alpha-cypermethrin from 9.6% (95% CI: 5.7–14.9), 4.5% (95% CI: 2–8.7), 8.6 (95% CI: 5.1–13.5) to 21.4% (95% CI: 15.7–28), 37.3% (95% CI: 30.5–44.5), 27.3% (95% CI: 17.7–38.6), respectively in Cove, Zangnanando and Ouinhi (Fig 5). This indicates partial involvement of mixed function oxidases (MFOs) in pyrethroid resistance in these An. gambiae s.l. populations.
Fig 4. Mortality rates of An. gambiae s.l. to 0.05% alpha-cypermethrin, 0.75% permethrin, 0.1% Bendiocarb and, 0.25% Pirimiphos-methyl, the error bars indicate the confidence intervals.
Fig 5. 24 hours Mortality of An. gambiae s.l. to alpha-cypermethrin and PBO+alpha-cypermethrin in Cove, Ouinhi and Zangnanado, the error bars indicate the confidence intervals.
Frequency of the L1014F kdr mutation in An. gambiae s.s. was 89.8% (95% CI: 88.2–91.2, n = 829) compared to 84.3% (95% CI:82.5–85.9, n = 968) in An. coluzzii collected during HLC. At the district level, the same trend was observed in Zangnanando, while the frequency of the mutation was similar in both species in Cove and Ouinhi (Supporting information files, S2 Table).
Discussion
The current study provides detailed characteristics of vector populations in the study area in preparation for a cluster RCT which will assess the impact of community use of two dual-AI LLINs in the Zou region, Benin.
Overall, Anopheles gambiae s.l. was the major vector complex, followed by Anopheles funestus and Anopheles nili, as previously reported in other regions of Benin [22–24]. Mosquito species from the Mansonia and Culex genera were also collected at moderate frequency (<40%), unlike Aedes, Coquilletidia and Eretmapodites which were found in low proportions (<2%). All collected mosquito species were found both indoors and outdoors except for Coquilletidia spp. and Eretmapodites spp. which were only collected indoors. The strong presence of mosquito species from the Culex and Aedes genera suggests a high potential for transmission of lymphatic filariasis and arbovirus such as dengue and yellow fever as observed in the Ouinhi [25] and Abomey-Calavi [26] districts. For that, apart from the LLINs whose distribution occurred as part of the RCT, a complementary strategy such as a larval source management program that will help discarding the majority of productive mosquito breeding sites would be needed.
Molecular species identification performed within the An. gambiae s.l. complex revealed the presence of a mixture of An. coluzzii (53.9%) and An. gambiae s.s. (46.1%). This is consistent with results from previous studies carried out in the same area by Ngufor et al. [14] and, in the neighbouring Plateau region by Sovi et al. [27]. According to Diabate et al. [28], permanent and semi-permanent breeding sites are conducive to the development of An. coluzzii while, temporary breeding site are favorable to the emergence of An. gambiae s.s. Indeed, several semi-permanent breeding sites have been created by the numerous tributaries of the Oueme and Zou rivers that water the study area as well as the presence of many rice growing areas. By comparison, temporary breeding sites were usually created by rain. The variation observed in the composition of the two molecular species between indoor and outdoor suggests that a scrutiny survey investigating the host-seeking behaviour of each species would be of interest. Despite the large number of specimens of An. gambiae s.l. analysed by PCR, An. arabiensis was not detected, although its presence in the neighbouring districts of Allada has been documented [29]. This could be due to the short mosquito sampling period and, the zoophilic behaviour of this species.
A large variability in the density of An. gambiae s.l. was observed among villages both indoors and outdoors in each district. This could be attributed to the disparity in the distribution of breeding sites from village to village. Despite the presence of LLINs in the majority of houses, and that more than 80% of household members slept under nets, the human biting rate in An. gambiae s.l was higher indoors than outdoors. This confirms the classical endophagic and anthropophagic behaviour which is observed in this mosquito species [30,31]. Indeed, this behaviour could have been facilitated by vector resistance to pyrethroids incorporated on the LLINs in use in the study area [14]. In addition, most biting was recorded late at night (from 11 p.m.), with peaks early in the early morning (around 4–5 a.m. or 5–6 a.m.). This suggests that the use of non-holed mosquito nets overnight can provide substantial protection to sleepers by significantly reducing the man-vector contact. According to a socio-anthropological study by N’tcha et al. [32], Benin’s rural populations usually perform various nightly activities (children’s play-activities, night talks, cooking, washing the dishes, eating, resting) outdoors and sleep under their nets indoors from 10 p.m. Thus, the little biting activity observed in An. gambiae s.l. early in the night (between 7-10pm) is not sufficient to put people at substantial elevated risk of receiving infected bites.
Populations of An. gambiae s.l. from all three study districts were resistant to pyrethroids but fully susceptible to bendiocarb and pirimiphos-methyl. A similar trend was observed by Gnanguenon et al. [33] and Sovi et al. [34] in the neighbouring districts of Allada and Pobe/Ketou, respectively. High use of LLINs over years, as well as the uncontrolled spray of insecticides for agricultural purposes throughout the region might have contributed to vector resistance to pyrethroids. While emergence of bendiocarb resistance occurred in some northern regions of the country where a carbamate-based IRS was implemented [35,36], continued susceptibility to the same product and to pirimiphos-methyl was observed in the Cove, Ouinhi and, Zangnanando districts located in the south. This stresses the need for a judicious application of non-pyrethroid insecticides to delay the onset of resistance and preserve their efficacy.
The resistance genotyping revealed that the high frequency of L1014 kdr mutation could be partly responsible for pyrethroid resistance in An. gambiae s.l. Indeed, the frequency was high at 89.8% in An. gambiae s.s. and 84.3% in An. coluzzii. In addition, the synergistic assay data suggests the partial involvement of MFOs that confer pyrethroid resistance, which confirms previous work performed in the region by Ngufor et al. [14].
Aggregated data in the study area shows a higher SR indoors than outdoors in An. gambiae s.l., although no significant difference was observed. This might be due to the difference in the molecular species composition between indoor and outdoor. Indeed, the relative high ability of An. gambiae s.s. to get infected could have increased the SR indoors where it was in similar proportion with An. coluzzii, contrary to outdoors where its proportion was significantly lower. Similarly, indoor biting vectors were slightly older as shown by the parity rates observed. This is reminiscent of works by Machani et al. [37] who observed similar trend for SR in Bungoma and Kisian, Western Kenya. Although not significant, the SR was higher in An. coluzzii than in An. gambiae s.s. in the Alibori and Donga regions, Northern Benin [38]. The opposite trend observed in the present study might indicate the relative time in the age structure of the mosquito population, since sampling was performed only once.
The large variability in the EIR between villages confirms the heterogeneity in malaria transmission. Moreover, malaria transmission occurred mostly indoors than outdoors despite the use of conventional LLINs. This is in agreement with previous reports by Degefa et al. [39] in western Kenya, and might be due to the fact that most people slept indoors overnight and also the spread of resistance to pyrethroids incorporated in the conventional nets in use, as observed by Trape et al. [40] and, Ndiath et al. [41]. This emphasizes the need for assessing at the community level, the efficacy of dual-AI LLINs currently developed by the manufacturers, to control resistant mosquitoes.
Outdoor malaria transmission is a well-documented phenomenon in several other countries, including Cambodia [42], Peru [43], and Kenya [36]. According to Sherrard-Smith et al. [44], with a scenario of universal LLIN and IRS coverage (indoor control tools) across Africa, outdoor transmission could result in an estimated 10.6 million additional malaria cases over a year. In future, a major challenge to malaria control and elimination will likely be residual transmission as it remains a serious threat to the effectiveness of vector control tools that mostly target indoor malaria transmission.
Compared to An. gambiae s.l, An. funestus and An. nili likely play a minor role (lower EIR) in local malaria transmission due to their very low frequency (<2%), as observed in some Benin northern districts (Kerou and Pehunco) by Osse et al. [24]. However, the contribution of An. funestus and An. nili to malaria transmission was not evaluated in the present study. The seasonality and the single session of night sampling per village were also study limitations.
High resistance of malaria vectors to pyrethroids, as well as persistence of the disease transmission despite the strong culture of conventional LLINs use make the study area suitable for the implementation of the RCT that aims at assessing the efficacy of two dual-AI LLINs on malaria incidence, prevalence and transmission. For this assessment, baseline data gathered over the present study will serve for comparisons with the post-intervention ones.
Conclusion
The present cross-sectional study provides information on key entomological indicators of malaria transmission prior to the implementation of the RCT. The mosquito relative abundance shows that An. gambiae s.l. was the primary malaria vector followed by An. funestus and An. nili. An. gambiae s.l. was both highly resistant to pyrethroids and displayed multiple resistance mechanisms, L1014F kdr mutation and mixed function oxidases. HBR and EIR were higher indoors than outdoors in An. gambiae s.l. despite the high usage of conventional LLINs. This stresses the need for evaluating novel types of dual-AI LLINs that could help to better tackle malaria transmitted by pyrethroid resistant vectors.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We acknowledge the populations of the Cove, Ouinhi and Zangnanando districts as well as the local authorities who facilitated the implementation of the present study, through their close collaboration. The technicians who conducted mosquito processing and bioassays are thanked for their dedicated work, so is the LSHTM ODK support team that provided Electronic data solutions through LSHTM Open Research Kits (http://odk.lshtm.ac.uk/).
Abbreviations
- LLINs
Long lasting insecticidal nets
- RCT
Randomized controlled trial
- AI
Active ingredient
- HLC
Human landing catches
- PCR
Polymerase chain reaction
- CSP
Circumsporozoite protein
- HBR
Human biting rate
- b/p/n
bite/person/night
- MFOs
mixed function oxidases
- ib/p/m
infected bite/person/night
- PBO
Piperonyl butoxide
- kdr
Knock down resistance
- ITN
Insecticide treated nets
- WHO
World Health Organization
- PPF
Piriproxyfen
- HLC
Human landing catch
- ODK
Open Data Kit
- GPS
Global Positioning System
- IRS
Indoor residual spraying
- HBR
Human biting rate
- SR
Sporozoite rate
- EIR
Entomological inoculation rate
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research is supported by a grant to the London School of Hygiene and Tropical Medicine from UNITAID and Global Fund via the Innovative Vector Control Consortium (IVCC). This cluster-randomized clinical trial is part of a larger project, “The New Nets Project”.
References
- 1.Institut National de la Statistique et de l’Analyse Économique (INSAE) et ICF. Enquête Démographique et de Santé au Bénin, 2017–2018: Indicateurs Clés. Cotonou, Bénin et Rockville, Maryland, USA: INSAE et ICF; ; 2018. https://insae.bj/images/docs/insae-statistiques/sociales/Sante/Enqu%C3%AAte%20D%C3%A9mographique%20et%20de%20Sant%C3%A9%20au%20B%C3%A9nin%20(EDSB)%20de%202017-2018.pdf. [Google Scholar]
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015; 526:207–211. Epub 2015 Sep 16. 10.1038/nature15535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindblade KA, Eisele TP, Gimnig JE, Alaii JA, Odhiambo F, et al. Sustainability of reductions in malaria transmission and infant mortality in western Kenya with use of insecticide-treated bednets: 4 to 6 years of follow-up. J Am Med Assoc. 2004; 291: 2571–2580. 10.1001/jama.291.21.2571 . [DOI] [PubMed] [Google Scholar]
- 4.Ochomo E, Chahilu M, Cook J, Kinyari T, Bayoh NM, et al. Insecticide-Treated Nets and Protection against Insecticide-Resistant Malaria Vectors in Western Kenya. Emerg Infect Dis. 2017; 23: 758–764. 10.3201/eid2305.161315 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damien GB, Djènontin A, Chaffa E, Yamadjako S, Drame PM, et al. Effectiveness of insecticidal nets on uncomplicated clinical malaria: a case-control study for operational evaluation. Malar J. 2016; 15: 102. 10.1186/s12936-016-1156-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley J, Ogouyèmi-Hounto A, Cornélie S, Fassinou J, de Tove YSS, et al. Insecticide-treated nets provide protection against malaria to children in an area of insecticide resistance in Southern Benin. Malar J. 2017;16: 225. 10.1186/s12936-017-1873-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asidi A, N’Guessan R, Akogbeto M, Curtis C, Rowland M. Loss of household protection from use of insecticide-treated nets against pyrethroid-resistant mosquitoes, Benin. Emerg Infect Dis. 2012; 18: 1101–1106. 10.3201/eid1807.120218 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Protopopoff N, Mosha JF, Lukole E, Charlwood JD, Wright A, et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet. 2018; 391:1577–1588. 10.1016/S0140-6736(18)30427-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. World Malaria Report, 2019. Geneva: World Health Organization; 2019. https://www.who.int/malaria/publications/world-malaria-report-2019/World-Malaria-Report-2019-briefing-kit-eng.pdf. [Google Scholar]
- 10.Staedke SG, Gonahasa S, Dorsey G, Kamya MR, Maiteki-Sebuguzi C, et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): a pragmatic, cluster-randomised trial embedded in a national LLIN distribution campaign. Lancet. 2020; 395:1292–1303. 10.1016/S0140-6736(20)30214-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiono AB, Ouédraogo A, Ouattara D, Bougouma EC, Coulibaly S, et al. Efficacy of Olyset Duo, a bednet containing pyriproxyfen and permethrin, versus a permethrin-only net against clinical malaria in an area with highly pyrethroid-resistant vectors in rural Burkina Faso: a cluster-randomised controlled trial. Lancet. 2018; 392:569–580. 10.1016/S0140-6736(18)31711-2 . [DOI] [PubMed] [Google Scholar]
- 12.N’Guessan R, Odjo A, Ngufor C, Malone D, Rowland M. A Chlorfenapyr Mixture Net Interceptor® G2 Shows High Efficacy and Wash Durability against Resistant Mosquitoes in West Africa. PLoS One. 2016;11(11): e0165925. 10.1371/journal.pone.0165925 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngufor C, Agbevo A, Fagbohoun J, Fongnikin A, Rowland M. Efficacy of Royal Guard, a new alpha-cypermethrin and pyriproxyfen treated mosquito net, against pyrethroid-resistant malaria vectors. Sci Rep. 2020; 10:12227. 10.1038/s41598-020-69109-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngufor C, N’Guessan R, Fagbohoun J, Subramaniam K, Odjo A, et al. Insecticide resistance profile of Anopheles gambiae from a phase II field station in Cové, southern Benin: implications for the evaluation of novel vector control products. Malar J. 2015; 14:464. 10.1186/s12936-015-0981-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institut National de la Statistique et de l’Analyse Economique (INSAE): Cahiers des villages et quartiers de ville du département du Zou, Recensement général de la population 2013–4, Août 2016. https://www.insae-bj.org/images/docs/insae-statistiques/enquetes-recensements/RGPH/1.RGPH_4/resultats%20finaux/Cahiers%20villages/Cahier%20des%20villages%20et%20quartiers%20de%20ville%20du%20zou.pdf.
- 16.Gillies M, De Meillon B. The Anophelinae of Africa south of the Sahara. Publ South Afri Inst Med Res. 1968; 54:343. http://mosquito-taxonomic-inventory.info/anophelinae-africa-south-sahara-ethiopian-zoogeographical-region. [Google Scholar]
- 17.Detinova TS. The determination of the physiological age of the females of Anopheles gambiae by changes in the tracheal system of the ovaries. Med Parasitol. 1945; 45–49. https://pubmed.ncbi.nlm.nih.gov/20280604/ . [PubMed] [Google Scholar]
- 18.Wirtz R, Zavala F, Charoenvit Y, Campbell G, Burkot T, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987; 65:39. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2490858/ PMCID: PMC2490858. [PMC free article] [PubMed] [Google Scholar]
- 19.Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008; 7:163. 10.1186/1475-2875-7-163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998; 7:179–184. 10.1046/j.1365-2583.1998.72062.x . [DOI] [PubMed] [Google Scholar]
- 21.WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes, second edition. Geneva: World Health Organization; 2016. https://www.who.int/malaria/publications/atoz/WHO-insecticide-resistance-test-procedures-2016-presentation-en.pdf?ua=1. [Google Scholar]
- 22.Djènontin A, Bio-Bangana S, Moiroux N, Henry MC, Bousari O, et al. Culicidae diversity, malaria transmission and insecticide resistance alleles in malaria vectors in Ouidah-Kpomasse-Tori district from Benin (West Africa): A pre-intervention study. Parasit Vectors. 2010; 3:83. 10.1186/1756-3305-3-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salako AS, Dagnon F, Sovi A, Padonou GG, Aïkpon R, et al. Efficacy of Actellic 300 CS-based indoor residual spraying on key entomological indicators of malaria transmission in Alibori and Donga, two regions of northern Benin. Parasit Vectors. 2019; 12:612. 10.1186/s13071-019-3865-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ossè RA, Tokponnon F, Padonou GG, Sidick A, Aïkpon R, et al. Involvement of Anopheles nili in Plasmodium falciparum transmission in North Benin. Malar J. 2019; 18:152. 10.1186/s12936-019-2792-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boko-Collins PM, Ogouyemi-Hounto A, Adjinacou-Badou EG, Gbaguidi-Saizonou L, Dossa NI, et al. Assessment of treatment impact on lymphatic filariasis in 13 districts of Benin: progress toward elimination in nine districts despite persistence of transmission in some areas. Parasit Vectors. 2019; 12:276. 10.1186/s13071-019-3525-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padonou GG, Ossè R, Salako AS, Aikpon R, Sovi A, et al. Entomological assessment of the risk of dengue outbreak in Abomey-Calavi Commune, Benin. Trop Med Health. 2020; 48:20. 10.1186/s41182-020-00207-w . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sovi A, Djègbè I, Soumanou L, Tokponnon F, Gnanguenon V, et al. Microdistribution of the resistance of malaria vectors to deltamethrin in the region of Plateau (southeastern Benin) in preparation for an assessment of the impact of resistance on the effectiveness of Long Lasting Insecticidal Nets (LLINs). BMC Infect Dis. 2014; 14:103. 10.1186/1471-2334-14-103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diabate A, Dabire RK, Heidenberger K, Crawford J, Lamp WO, et al. Evidence for divergent selection between the molecular forms of Anopheles gambiae: Role of predation. BMC Evol Biol. 2008; 8:5. 10.1186/1471-2148-8-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gnanguenon V, Govoetchan R, Agossa FR, Ossè R, Oke-Agbo F, et al. Transmission patterns of Plasmodium falciparum by Anopheles gambiae in Benin. Malar J. 2014; 13:444. 10.1186/1475-2875-13-444 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayoh MN, Walker ED, Kosgei J, Ombok M, Olang GB, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit Vectors. 2014; 7:380. 10.1186/1756-3305-7-380 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, et al. ‘A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015; 14:259. 10.1186/s12936-015-0766-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.N’tcha L, Akogbeto M. Socio-behavioral study on population sleeping patterns in northern Benin for Indoor Residual Spraying decision. Unpublished USAID Annual Report, IL#31; 2019.
- 33.Gnanguenon V, Agossa FR, Badirou K, Govoetchan R, Anagonou R, et al. Malaria vectors resistance to insecticides in Benin: current trends and mechanisms involved. Parasit Vectors. 2015; 8:223. 10.1186/s13071-015-0833-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sovi A, Govoétchan R, Ossé R, Koukpo CZ, Salako AS, et al. Resistance status of Anopheles gambiae s.l. to insecticides following the 2011 mass distribution campaign of long-lasting insecticidal nets (LLINs) in the Plateau Department, south-eastern Benin. Malar J. 2020; 19:26. 10.1186/s12936-020-3116-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aïkpon R, Sèzonlin M, Ossè R, Akogbéto M. Evidence of multiple mechanisms providing carbamate and organophosphate resistance in field An. gambiae population from Atacora in Benin. Parasit Vectors. 2014; 7:568. 10.1186/s13071-014-0568-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salako AS, Ahogni I, Aïkpon R, Sidick A, Dagnon F, et al. Insecticide resistance status, frequency of L1014F Kdr and G119S Ace-1 mutations, and expression of detoxification enzymes in Anopheles gambiae (s.l.) in two regions of northern Benin in preparation for indoor residual spraying. Parasit Vectors. 2018; 11:618. 10.1186/s13071-018-3180-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machani MG, Ochomo E, Amimo F, Kosgei J, Munga S, et al. Resting behaviour of malaria vectors in highland and lowland sites of western Kenya: Implication on malaria vector control measures. PLoS One. 2020;15(2): e0224718. 10.1371/journal.pone.0224718 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akogbéto MC, Salako AS, Dagnon F, Aïkpon R, Kouletio M, et al. Blood feeding behaviour comparison and contribution of Anopheles coluzzii and Anopheles gambiae, two sibling species living in sympatry, to malaria transmission in Alibori and Donga region, northern Benin, West Africa. Malar J. 2018; 17:307. 10.1186/s12936-018-2452-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degefa T, Yewhalaw D, Zhou G, Lee M-c, Atieli H, et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malaria J. 2017;16: 443. 10.1186/s12936-017-2098-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trape JF, Tall A, Diagne N, Ndiath O, Ly AB, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet. 2011; 11: 925–932. 10.1016/S1473-3099(11)70194-3 . [DOI] [PubMed] [Google Scholar]
- 41.Ndiath MO, Mazenot C, Sokhna C, Trape JF. How the malaria vector Anopheles gambiae adapts to the use of insecticide-treated nets by African populations. PLoS One. 2014; 9(6): e97700. 10.1371/journal.pone.0097700 . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Durnez L, Mao S, Denis L, Roelants P, Sochantha T, et al. Outdoor malaria transmission in forested villages of Cambodia. Malar J. 2013; 12:329. 10.1186/1475-2875-12-329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saavedra MP, Conn JE, Alava F, Carrasco-Escobar G, Prussing C, et al. Higher Risk of Malaria Transmission Outdoors Than Indoors by Nyssorhynchus Darlingi in Riverine Communities in the Peruvian Amazon. Parasit Vectors. 2019; 12:374. 10.1186/s13071-019-3619-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci U S A. 2019; 116:15086–15095. 10.1073/pnas.1820646116 . [DOI] [PMC free article] [PubMed] [Google Scholar]