Abstract
Dengue is a re-emerging disease, currently considered the most important mosquito-borne arbovirus infection affecting humankind, taking into account both its morbidity and mortality. Brazil is considered an endemic country for dengue, such that more than 1,544,987 confirmed cases were notified in 2019, which means an incidence rate of 735 for every 100 thousand inhabitants. Climate is an important factor in the temporal and spatial distribution of vector-borne diseases, such as dengue. Thus, rainfall and temperature are considered macro-factors determinants for dengue, since they directly influence the population density of Aedes aegypti, which is subject to seasonal fluctuations, mainly due to these variables. This study examined the incidence of dengue fever related to the climate influence by using temperature and rainfall variables data obtained from remote sensing via artificial satellites in the metropolitan region of Rio de Janeiro, Brazil. The mathematical model that best fits the data is based on an auto-regressive moving average with exogenous inputs (ARMAX). It reproduced the values of incidence rates in the study period and managed to predict with good precision in a one-year horizon. The approach described in present work may be replicated in cities around the world by the public health managers, to build auxiliary operational tools for control and prevention tasks of dengue, as well of other arbovirus diseases.
Introduction
Arthropod-borne diseases such as dengue, chikungunya, West Nile, Zika and yellow fever represent important emerging and re-emerging infectious diseases, which are considered serious global public health problems [1, 2]. Dengue is currently considered the most important mosquito-borne arbovirus infection to man, taking into account both its morbidity and mortality [3].
The World Health Organization (WHO) characterizes dengue as one of the world’s leading public health problems, with an estimated 50 to 100 million people infected annually, resulting in approximately 250,000 to 500,000 cases of haemorrhagic fever and 24,000 deaths / year in 100 countries (with the exception of Europe) [4–6]. In addition, total costs, totaling direct and indirect expenses resulting from this disease worldwide, reached 8.9 billion US dollars annually [7].
Dengue is an acute infectious disease caused by viruses (DENV) of the RNA genome, belonging to the Flaviviridae family and to the genus Flavivirus. To date, four antigenically distinct serotypes DENV-1, DENV-2, DENV-3, DENV-4 have been identified and a fifth serotype of dengue has been discovered in the State of Sarawak, located on the island of Borneo in Malaysia of wild transmission, DENV-5. This new serotype has been detected in humans and is associated with even severe cases [8, 9], but it has not yet been detected in Brazil.
The transmission of the dengue virus to man occurs through the bite of females of the genus Aedes, the main one being Aedes (Stegomyia) aegypti (Linnaeus, 1762), which presents urban and anthropophilic behavior, since it lives in cities and close to humans [10]. In addition to this species, in southeastern Asia, there is another primary vector of the dengue virus, Aedes (Stegomyia) albopictus (Skuse, 1894). In Brazil, Ae. albopictus is considered a potential vector of dengue viruses [11].
Aedes aegypti is associated to urban and suburban environments, where there is high human population concentration and high concentration of residences [12]. This species often shows an endophilic behavior, using the interior of the houses to be sheltered and, therefore, it is more often found in the intradomicile than in the peridomicile [12]. Their breeding grounds are usually artificial containers filled with rainwater or domestic water, including tires, cans, glasses, bottles, pots, water boxes, vats, brass, swimming pools, abandoned aquariums, among others [13–15].
The transmission of dengue is essentially urban. In this environment, all the key factors for its occurrence can coexist: man, the virus and the vector. In a situation where there is a simultaneous combination of socio-environmental and climatic conditions favorable to the mosquito’s reproduction, its population may explode [16].
Tropical countries are most affected because of their environmental, climatic and social conditions [17]. Climate is an important factor in the temporal and spatial distribution of vector-borne diseases such as dengue. Thus, rainfall and ambient temperature are called macro factors that are determinant for dengue, as they directly influence the population density of Ae. aegypti, which undergo seasonal fluctuations, due to these main variables. However, in areas of tropical or subtropical climate, its proliferation is continuous, although during the period of lower precipitation and lower average temperatures, the population density of this vector tends to reduce significantly [17, 18].
Studies of climatic variables can improve knowledge and prediction of epidemic seasonality because the vector-climate relationship is as important as vector-human interaction. Research in epidemiology has increasingly sought to find statistical and mathematical models based on climate factors that may explain the dynamics of dengue incidence. It is mainly tried to identify models with a strong future predictive power of dengue incidence to subsidize public health managers [3, 19]. In this sense, several authors study the relationship between climate variables and dengue, often using time-series analyzes to describe temporal trends, identify patterns and even make predictions [17, 20–24].
The present study has a nature similar to the works in the literature listed above, with the final objective of building a useful instrument for prediction of the disease and, in particular, of the occurrence of epidemics in the municipality of Duque de Caxias, in the Metropolitan Region of Rio de Janeiro, which borders the north of this city. The chosen climate data, namely temperature and rainfall, were obtained by remote sensing via artificial satellites. This type of data has important properties for epidemiological studies, especially in poor countries: they were previously available cost free for the whole world and they do not have deleterious missing values. To achieve this goal, we try to adjust an ARMAX model, the family of autoregressive models of moving averages (ARMA model) with exogenous variables, an approach for analysis and forecasting time series, which is an adequate way to treat the relationship between the incidence of dengue fever and climate variables, e.g., temperature and rainfall [25]. The ARMAX model is very flexible and can be extended to model non-stationary time series with seasonal components. In this case we have a SARIMAX model.
The most innovative point in the study was the utilization of climate data coming from remote sensing via artificial satellites, which offers better accuracy and consistency properties with a low cost. Due to the good quality of the adjustment and good predictive capacity of the model, the forecasts provided by the developed model offer valuable information so that public managers can improve public health surveillance systems and act preventively in order to mitigate or avoid future dengue epidemics [19]. However, the most critical limitation of the study was to use data series with a monthly periodicity, because thirty days is a very long time interval, in which the vector population can explode. Thus, it is advisable to use a shorter time interval, as a weekly or daily one, to allow more efficient and fast prevention actions by the public health managers. Moreover, the inclusion of other explanatory variables, such as wind, population of mosquitoes or people flow, can improve the performance of the ARMAX model.
Materials and methods
Study area
The present study analyses the relationship between dengue incidence and climate variables, such as temperature and rainfall, in the municipality of Duque de Caxias, State of Rio de Janeiro. This is an statistical model based on historical series of cases of dengue infection confirmed and reported in this municipality from January 2007 to December 2016.
The choice of this municipality is due to its location in the Baixada Fluminense, a region of high population density and marked by recurrent dengue epidemics. Because it is a metropolitan municipality bordering the capital of Rio de Janeiro, there is a strong daily flow of people that circulate between the two cities, being able to be carriers of the dengue virus. Add to this the economic importance of Duque de Caxias, the third largest GDP of the state [26].
This municipality is located in coordinates 22°C 47’ 08” S and 43° 18’ 42” W and has an area of 467.6 km2, being at a distance of 12 km from the city of Rio de Janeiro.
The population of Duque de Caxias in 2010 was 855,048 inhabitants, with 99.7% living in the urban area, while only 2,910 (a 0.32% proportion) live in rural areas. This municipality is located in the metropolitan region of Rio de Janeiro, which has a total population of over 12.5 million inhabitants [27].
Data sources
Dengue is one of the notifiable diseases, according to the Brazilian Ministry of Health guidelines. Thus, any case of this disease in any public or private health unit must be notified [28].
The epidemiological data, the dengue cases reported in Duque de Caxias, comprising the period of 2007 to 2016, were obtained from the State Department of Health of the State of Rio de Janeiro (SES-RJ). Population data was obtained from IBGE (the Brazilian Institute of Geography and Statistics).
The climate data, namely temperature and rainfall, were obtained by the remotely sensed products here-in named as MODIS (Moderate Resolution Imaging Spectroradiometer), which is acquired from the satellites Aqua and Terra owned by NASA (North America Space Agency), and TRMM (Tropical Rainfall Measuring Mission), a collaboration of NASA with JAXA (Japan Aerospace Exploration Agency). Both products are available at NASA (https://mirador.gsfc.nasa.gov).
More specifically, the daily product MOD11A2 Version 6 at the 1-km spatial resolution has been used to address land surface temperature [29]. The spatio-temporal product is a result of 8-day per-pixel composition based on the product MOD11A1 (https://doi.org/10.5067/MODIS/MOD11A1.006). Images have been integrated and averaged at the monthly scale and subsequently interpolated at the level of the study area. The values are available Kelvin degree, which in turn are subsequently converted to Celsius degree.
On the other hand, the Tropical Rainfall Measuring Mission (TRMM) provides rainfall information by means of the so-called research product TRMM Multi-satellite Precipitation Analysis (TMPA) 3B42. The TMPA 3B42 was retrieved in this study at the daily time step with 0.25 degree spatial resolution. The 3B42 is based on TMPA 3B42RT (3-hourly near-real-time) with rain gauge data and is available since January 1st, 1998 [30]. More details concerning the TMPA algorithms can be found in Huffman et al. (2010) [31] and Huffman and Bolvin (2018) [32].
The works of consistency and formatting of the data captured by the artificial satellites were carried out by the Water Resources and Environmental Studies Laboratory (LABH2O) of the Civil Engineering Program at COPPE-UFRJ.
The ARMAX model
In order to model the relationship between the incidence rate of dengue cases (dependent variable y) and climate factors (explanatory variables x), we chose to specify the ARMAX model, the family of autoregressive models of moving averages with exogenous variables, see for example Hamilton (1994) [33] and that in its more general form assumes the expression:
| (1) |
In Eq (1) C is the intercept, ϵt is an unobservable random term, yt represents the value on month t of the monthly series of the logarithm of the incidence rate of dengue cases per 100,000 inhabitants and xt represents the climate variables precipitation and temperature in the month t. In addition, the three coefficients ϕ, θ, β are not known a priori, but can be estimated from the data by means of the conditional maximum likelihood method.
From the time series of monthly dengue occurrences in the municipality of Duque de Caxias, together with the respective population estimates for July 1 of each year and the monthly rainfall and temperature series (minimum, mean and maximum), ARMAX models were separately adjusted. The specification and estimation of the coefficients of the best ARMAX models in each municipality was performed with the help of the Gretl software (http://gretl.sourceforge.net/). In the selection of the best model we tried to minimize the information criterion of Akaike. The coefficients were estimated by means of the maximum likelihood method conditioned to the observations until December 2015. The data for the year 2016 were not used in the adjustment of the models, since they formed the out-of-pocket sets used to evaluate the predictive capacity of the adjusted models based on the following metrics:
Root of the Mean Square Error:
| (2) |
Theil’s U:
| (3) |
Results
Temporal series of dengue cases and circulating serotypes
The monthly time series of reported cases of dengue in Duque de Caxias during the period from 2007 to 2016 registers a total of 43,423 cases of dengue, including epidemic and non-epidemic periods (Fig (1)). Epidemic periods were observed from January to July 2008 (16,126 cases), January to June 2011 (9,554 cases), January to August 2012 (2,855 cases) and January to June 2013 (6,689 cases). The higher number of dengue cases took place in April in the epidemic years of 2008 and 2011 and May in 2012 and 2013.
Fig 1. Dengue notification monthly series.
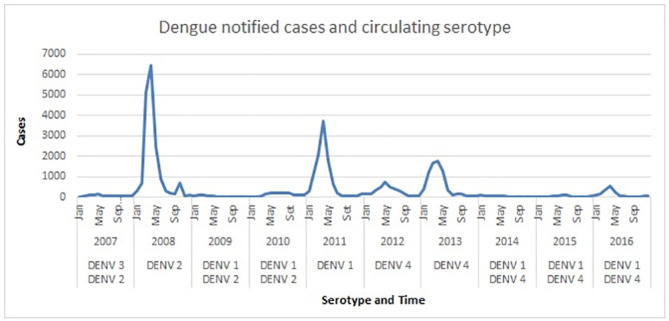
The analysis of the time series in the epidemic years shows two distinct periods. The first period is when the epidemic curve grows, coinciding with the warmest period in this region, until it reachs the apex, followed by a period of decrease of this curve. The growth rate is initially slow and, after four to six weeks, a strong increase occurs for ten to twelve weeks until it reaches the apex. Then there is a period of steep decline. Consequently, this period is always shorter, up to eight weeks. In summary, the analysis of the series of dengue cases in epidemic years shows a characteristic of seasonality, given its visible seasonal behavior, in a certain harmony with climate variables.
Analysing the information of circulating serotypes in the dengue time series, Fig 1, we observed that the DENV-3 serotype only circulated in 2007, a non-epidemic year. Thus, there is a generation of children born after that date, which is added to the adults susceptible to this serotype. Differently, the serotype DENV-1 circulated in the second major epidemic (2011) and in another five years of this time series. The DENV-2 serotype circulated in the largest epidemic (2008) and in the two following years of this time series. The DENV-4 serotype entered Duque de Caxias and caused a small epidemic in 2012 and a more intense one in 2013, and since then it still circulates. It should be noted that in the four epidemic years (namely: 2008, 2011, 2012, 2013), each year there was a dominance of only one of the four serotypes (namely: DENV-2, DENV-1, DENV-4, DENV-4).
Time series of incidence rate logarithms
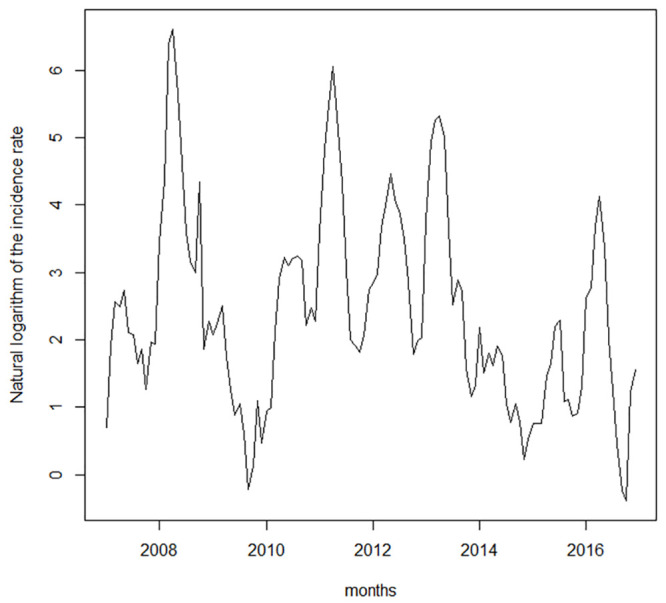
Fig 2 shows the time series of the incidence rate of dengue in Duque de Caxias per 100,000 inhabitants. The high peaks of occurrence suggest that the series exhibits heteroscedasticity, a characteristic that violates the premise of error homoscedasticity, which is a hypothesis assumed by the ARMAX model. For this reason, we took the natural logarithm of the incidence rate, the series of which is shown in Fig 3. The same transformation was applied by Gharbi et al. (2011) [21] and Guo et al. (2017) [22] in studies on dengue in Guadeloupe and Guangdong.
Fig 2. Incidence rate of dengue per 100000 inhabitants.
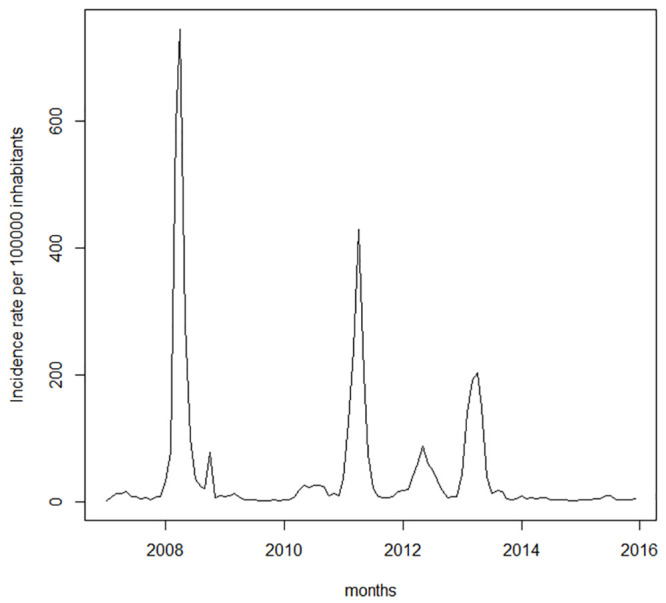
Fig 3. Logarithm of the incidence rate of dengue.
Analysis of the influence of climate variables
In Eq (1) the time series y and X must be stationary. Therefore, based on the Augmented Dickey-Fuller Test (ADF) and KPSS test [34], a diagnosis was made to assess the stationarity of the available monthly time series, i.e., the time series of dengue incidence, temperature and rainfall. The ADF and KPSS tests indicated that the time series available are stationary. Therefore, in this case, there is no need to take time series differences. Then, after rejecting the hypothesis of non-stationarity (unit root), we started the search for the best specification of the ARMAX model capable of relating the incidence of dengue with the available climate variables. In this stage we select the explanatory variables and their respective temporal lag (lags). The identification of the best specification passes through the examination of some alternative specifications that differ in relation to the set of explanatory variables (between available variables) and their temporal lags. The best specification should meet some criteria, for example, in this work it was considered that the best specification is the one with the lowest Akaike index.
Thus, from the data from January 2007 to December 2015 (in-sample period) several specifications for the ARMAX model were evaluated. The best result specification for the ARMAX model is summarized in Table 1, from which it can be concluded that the incidence rate in one month t is explained by the rainfall (PLUV1 and PLUV2) and the logarithm of incidence rates (y1 and y2) in the immediately preceding two months (t − 1) and (t − 2).
Table 1. Estimated coefficients for the best ARMAX mode.
| Coeficient | Standart error | Z | p-value | |
|---|---|---|---|---|
| Const | 0.0433966 | 0.147424 | 0.2944 | 0.7685 |
| Phi1 | 1.01665 | 0.0954977 | 10.65 | 1.82E-26 |
| Phi2 | -0.189711 | 0.0924959 | -2.051 | 0.0403 |
| PLUV1 | 0.00126183 | 0.000478276 | 2.638 | 0.0083 |
| PLUV2 | 0.000917676 | 0.000498571 | 1.841 | 0.0657 |
From the coefficients estimated in Table 1, we obtain the following prediction equation:
| (4) |
which can be interpreted as follows: the incidence rate yt in month t is explained by the rates occurring in the previous two months, yt−1 and yt−2, with great predominance of the first one, as well as by the rainfall occurred during the same two months, Pluvt−1 and Pluvt−2.
As illustrated by the residual ACF (Residual Autocorrelation Function) and residual PACF (Residual Partial Autocorrelation Function) in Fig 4, the residuals autocorrelations are not significant, so the model was considered satisfactory.
Fig 4. Residual ACF and residual PACF.
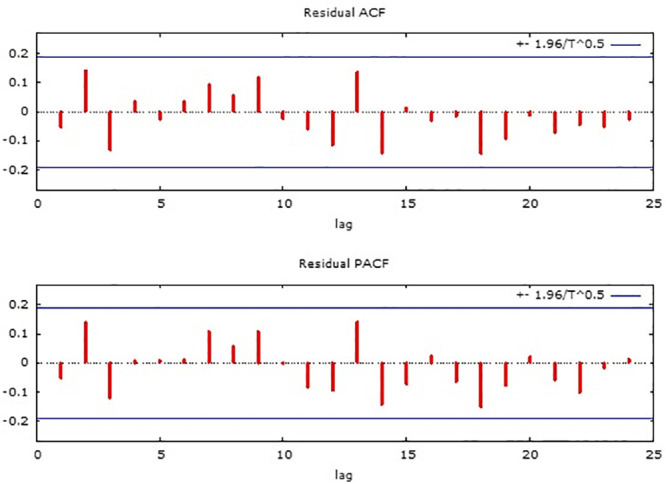
Table 2 shows some performance statistics of the adjusted model in the forecasted one month ahead in the out-sample period (from January to December 2016). The results for the RMSE indicators are of the same order of magnitude as other studies on the theme, such as Gharbi et al. (2011) [21] and Guo et al. (2017) [22] and the value for Theil’s U, below one, indicates that the adjusted model provides better predictions than those obtained by the naive predictor, i.e., a prediction in which the incidence rate is assumed equal to the last observed value.
Table 2. Performance in the out-sample period for the incidence rate.
| Metric | Value |
|---|---|
| Root Mean Square Error (RMSE) | 0.6744 |
| Theil’s U | 0.8597 |
In Fig 5, the estimated cases comprise those from the beginning of the series through December 2015, where all data from that period are used in the adjustment of the equation of the ARMAX model, with which the estimated values are calculated.
Fig 5. Number of cases observed versus fitted (2008-2015).
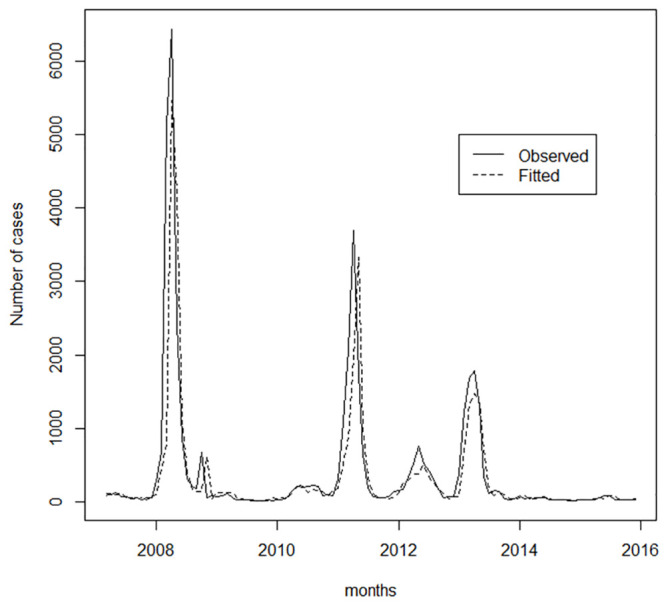
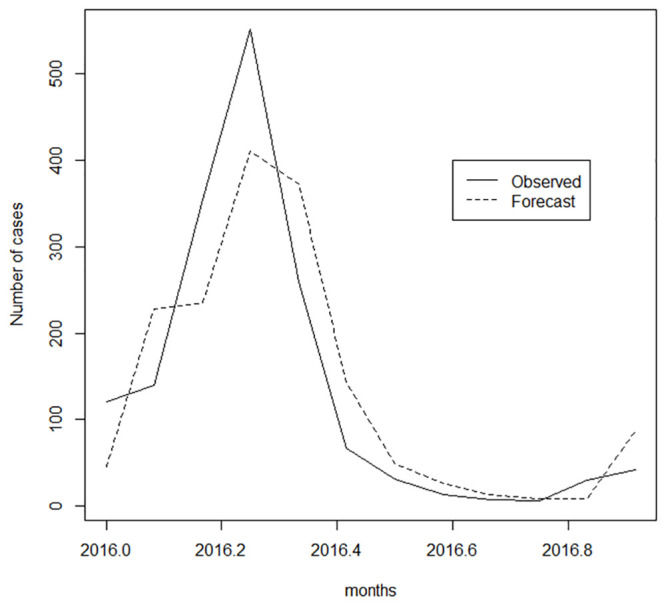
In Fig 6, the expected cases include the out-sample period, for the months of January to December 2016, in which the ARMAX model equation is used in the calculation of forecasts one month ahead from January 2016 to December 2016. The good adherence of the model to the observed data can be visualized in Figs 5 and 6, which show the monthly observed totals of dengue occurrence and the respective fitted/forecasted values obtained from the results provided by the ARMAX model using Eq 4.
Fig 6. Number of cases observed versus forecast (2016).
Discussion
First, the time series of incidence rates of dengue and of rainfall are discussed separately, then a multivariate relationship between the climate variables and the incidence rates of dengue is studied. A few similar works were selected from the broad literature for a short and simplified comparison with the results shown here. All of these works show in common the importance of climate data.
Analyzing time series of the present study, it can be observed that the highest incidence rates of dengue happen in the first semester of each year. The months with the highest numbers of cases are April and May, in the Autumn season. Analyzing the rainfall chart, it can be seen that the first semester of each year is the period of greatest rainfall, the rainy season, in which the deposits of water necessary for Aedes to reproduce are created.
Gomes et al. (2012) studied the correlation between climate variables and the risk for dengue, analysing the period from 2001 to 2009 in the city of Rio de Janeiro. They also identified that most cases occur in the first semester, particularly between the months of March and May [17]. Their records are similar to those found in the analysis of time series of incidence rates of dengue above.
Analysing the information about circulating serotypes in the dengue time series, we observed that the DENV-3 serotype only circulated in 2007, a non-epidemic year. Thus, there is a generation of children born after that date who make up the largest contingent of people susceptible to contagion, together with the adults susceptible to this serotype. Therefore, among the four different serotypes, DENV-3 has the greater potential, in thesis, for propagation in the population and may even produce an epidemic. The diversity of serotypes of the dengue virus, the efficiency of the Aedes aegypti vector and their anthropophilic characteristics, coupled with the disorderly and precarious urbanization pattern of large cities in developing countries, mostly located in tropical and subtropical areas of the planet, make the control of this arbovirus infection extremely difficult and complex [13, 35].
Several studies show that breeding occurs mainly during the months of higher rainfall. Studies carried out in the Paraíba Valley (São Paulo), in São José do Rio Preto (São Paulo), in the State of Maranhão and of Paraíba, in a protected area in the metropolitan region of São Paulo, in Uberlândia (Minas Gerais) and Boa Vista (Roraima) showed that, even though there is a difference in rainfall dynamics in the various regions of Brazil, the highest incidence rates of dengue coincide with the rainy months, the hottest months of the year in the country [36–44]. The density and availability of breeding sites for Ae. Aegypti, determined by the regime of rainy and dry periods, exert great influence in the dispersion of this species [3].
Honorio et al. (2009) offer explanations for the conclusions of previous studies. They report that the occurrence of weekly average temperatures above 22°–24°C is strongly associated with high Aedes aegypti population and consequently with an increased risk of dengue transmission [45]. The rainfall factor is important for the production of larvae, pupae and mosquitoes, influencing the occurrence of dengue. The variability of precipitation interferes in the breeding of larvae and pupae of Aedes aegypti. Rainfall interacting with temperature affects evaporation, thus affecting the breeding water viability, see also Morin et al. (2013) [46].
Viana and Ignotti (2013) systematically review 31 articles published in databases (Scielo, PubMed and Lilacs) concerning dengue and meteorological variations in Brazil between 1991 and 2010. In a summary of the 31 papers, the authors record that in ten of these articles there is a correlation between the occurrence of dengue only with the variable rainfall, whereas in only four articles, there is correlation only with the temperature variable [13].
Struchiner et al. (2015) specified a multivariate Poisson regression model to explain the incidence of dengue fever in Singapore. Population growth, Ae aegypti population, climate parameters such as temperature and rainfall, and population arrivals of endemic dengue regions are defined as variables for the model. This model estimates the values of the contribution of these individual factors in increasing the incidence of dengue. The model concludes that the predominant individual factor for the occurrence of dengue was population growth, followed by climate factors [47].
Sharmin et al. (2015) in modelling the dengue occurrence series, in the Dhaka metropolitan region, Bangladesh, apply a generalized (binomial negative) linear regression model. The authors analyse the relationship between dengue and climate. To do so, they include temperature and rainfall as explanatory variables in the prediction equation of dengue incidence [48].
Chuang et al. (2017) model the dengue time series in Taiwan using distributed lag non-linear regression models. The authors evaluated several variable specifications for the prediction equation [25]. The best model found has as explanatory variables: minimum temperature and precipitation.
Koh et al. (2018) adjusted a neural network and a Poisson regression model to weekly data on dengue incidence and precipitation in Singapore. In the models presented by the authors, the incidence of dengue is explained according to the past values of the incidence rate and precipitation [49].
The present study a priori is also based on the temperature and precipitation climate variables, ubiquitous in the set of works listed above. However, the importance of the remaining variables cannot be ruled out, such as, for example, the already commented expressive flow of people in Duque de Caxias, which was not verified due to the absolute absence of data.
The statistical model used in this work for joint analysis of the incidence series and climate variables was the ARMAX (Auto-regression Integrated Moving Average Cause Effect), which is a hybrid model, since it has characteristics of strictly temporal model conjugated with multiple regression, to contemplate other explanatory variables.
In an initial exploratory phase, the low explanatory power of the temperature variable was identified. After a few attempts of using only the effect of precipitation, the final formulation definition was obtained, having as explanatory variables the rainfall and the incidence rates of dengue in the two previous months.
As shown in Fig 1, the highest incidences of dengue occurs up to 2 months after the end of summer, a result consistent with the lags (t − 1) and (t − 2) of rainfall, identified in the final formulation of the ARMAX model.
The final formulation reproduced the incidence series of dengue with very good accuracy. As a more remarkable result, this same formulation was also able to produce forecasts a month ahead in the out-sample period (from January to December 2016), which were very close to the values actually observed in the step ahead.
In order to show the strength of the approach developed in the present study, some of its properties and results should be highlighted.
The first property is simplicity, since the model is simple, natural, orthodox, because it is based on climate variables, and very accessible to a good range of health managers, even in small municipalities. Likewise, the mathematical formulation employs one of the most widespread techniques in statistics and multivariate analysis, available through several providers of free software through the internet, which facilitates its use.
Since this work was based on climate data coming from remote sensing via artificial satellites, previously available cost-free for the whole world, without the deleterious feature of having missing values, this approach offers a great range of applicability, in particular for poor countries located in tropical regions where dengue is widespread.
Regarding adhesion to field data, the developed model was able to overcome difficulties common to other alternatives, producing predictions with an adequate precision. As to its predictive capacity, it should be highlighted that the developed model was able to accurately predict the incidence rate with a horizon of one month ahead during a period of twelve months.
Regarding the possibility of generalization, the developed model can also be applied to other arbovirus diseases, in particular chikungunya and zika, which have Aedes as a vector.
Taking into account that there are historical deficiencies in the mechanisms of arbovirus surveillance, this work could be replicated in several cities around the world by public health managers, to build auxiliary operational tools for the tasks of control and prevention.
The ARMAX model was adjusted from the dengue incidence rates, rainfall and temperature. The most critical limitation of the study is the monthly periodicity of series. In epidemic periods, the variation of incidence rates can be large, which can produce extremely high incidence rates values, as shown by Fig 1 in the year of 2008. Therefore, thirty days is a very long time interval, one in which the vector population can explode. Thus, it is advisable to adopt a shorter time interval, as a weekly or daily one, to allow more efficient and fast prevention actions by the public health managers. Moreover, the inclusion of other explanatory variables can improve the performance the ARMAX model.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors would like to thank the Water Resources and Environmental Studies Laboratory (LABH2O), Civil Engineering Program, Alberto Luiz Coimbra Institute for Graduate Studies and Research in Engineering (COPPE), Federal University of Rio de Janeiro for the helpful assistance in the tasks associated to consistency and formatting of the temperature and rainfall climate data captured by artificial satellites.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1. Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Reseach. 2010. February; 85(2):328–345 10.1016/j.antiviral.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donalisio MR, Freitas ARR, Zuben APBV. Arboviruses emerging in Brazil: challenges for clinic and implications for public health. Revista de Saúde Pública. 2017. 51(30):1–6 10.1590/S1518-8787.2017051006889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Viana DV, Ignotti E. The ocorrence of dengue and weather changes in Brazil; A systematic review. Rev.Bras Epidemiol. 2013. June;16(2):240–256. 10.1590/S1415-790X2013000200002 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. World Health Organization. 1997. 2ed. https://apps.who.int/iris/handle/10665/41988 [Google Scholar]
- 5. Halstead SB. Dengue virus mosquito interactions. Annual Review of Entomology. 2008. January 53:273–291. 10.1146/annurev.ento.53.103106.093326 [DOI] [PubMed] [Google Scholar]
- 6. Gurugama P, Gard P, Perera J, Wijewickrama A, Seneviratne SL. Dengue viral infections. Indian Journal of Dermatology. 2010. March;55(1):68–78. 10.4103/0019-5154.60357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. The Lancet Infectious Diseases. 2016. April;16(8):935–941 10.1016/S1473-3099(16)00146-8 [DOI] [PubMed] [Google Scholar]
- 8. Normile D. Surprising New Dengue Virus Throws a Spanner in Disease Control Efforts. Tropical Medicine. 2013. OTC;342(6157):45 [DOI] [PubMed] [Google Scholar]
- 9. Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Medical Journal Armed Forces India. 2015. January;71(1):67–70. 10.1016/j.mjafi.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mc Bride CS, Baier FB, Omondi AB, Spitzer SA, Lutomiah J, Sang R, et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature Internation Journal of Science. 2014. 10.1038/nature13964;515:222-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carvalho RG, Lourenço-de-Oliveira R and Braga IA. Updating the geographical distribution and frequency of Aedes albopictus in Brazil with remarks regarding its range in the Americas. Mem Inst Oswaldo Cruz. 2014. September;109(6):787–796. 10.1590/0074-0276140304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lima-Camara TN, Urbinatti PR, Chiaravalloti-Neto F. Finding Aedes aegypti in a natural breeding site in an urban zone, Sao Paulo, Southeastern Brazil. Rev Saúde Pública. 2014. March;50(3):1–4. 10.1590/S1518-8787.2016050006245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tauil PL. Urbanização e ecologia do dengue. Rev Saúde Pública. 2001;17(suppl):99–102. 10.1590/S0102-311X2001000700018 [DOI] [PubMed] [Google Scholar]
- 14. Braks MAH, Honório NA, Lourenço-De-Oliveira R, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. Journal of Medical Entomogy. 2003. November;40(6):785–794. 10.1603/0022-2585-40.6.785 [DOI] [PubMed] [Google Scholar]
- 15. Abreu FVS, Morais MM, Ribeiro SP, Eiras AE. Influence of breeding site availability on the oviposition behavior of Aedes aegypti. Mem Inst Oswaldo Cruz. 2015; 110: 669–676. 10.1590/0074-02760140490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santos SL, Augusto LGS. Modelo multidimensional para o controle da dengue: uma proposta com base na reprodução social e situações de risco. Physis: Revista de Saúde Coletiva. 2011; 21(1):177–196. 10.1590/S0103-73312011000100011 [DOI] [Google Scholar]
- 17. Gomes AF, Nobre AA, Cruz OG. Temporal analysis of the relationship between dengue and meteorological variables in the city of Rio de Janeiro, 2001-2009. Cadernos Saúde Pública. 2012. November; 28(1):177–196. 10.1590/S0103-73312011000100011 [DOI] [PubMed] [Google Scholar]
- 18. Lourenço-de-Oliveira R. Biologia e Comportamento do Vetor. In: Valle D, Pimenta D, Cunha R. Dengue: Teorias e Práticas. Editora FIOCRUZ. 2015;1:75–92 [Google Scholar]
- 19. Racloz V, Ramsey R, Tong S, Hu W. Surveillance of dengue fever virus: a review of epidemiological models and early warning systems. PLoS Neglected Tropical Diseases. 2012. May; 6(5):e1648. 10.1371/journal.pntd.0001648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phung D, Huang C, Rutherford S, Chu C, Wang X, Nguyen M, et al. Identification of the prediction model for dengue incidence in Can Tho city, a Mekong Delta area in Vietnam. Acta Tropica. 2015. January; 141, Part A:88–96 10.1016/j.actatropica.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 21. Gharbi M, Quenel P, Gustave J, Cassadou S, La Ruche G, Girdary L, et al. Time series analysis of dengue incidence in Guadeloupe, French West Indies: Forecasting models using climate variables as predictor. BMC Infectious Diseases 2011, 11:166 10.1186/1471-2334-11-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo P, Liu T, Zhang Q, Wang L, Xiao J, Zhang Q, et al. Developing a dengue forecast model using machine learning: A case study in China. PLOS Neglected Tropical Diseases, October 16, 2017. 10.1371/journal.pntd.0005973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng Q, Jing Q, Spear RC, Marshall JM, Yang Z, Gong P. Climate and the Timing of Imported Cases as Determinants of the Dengue Outbreak in Guangzhou, 2014: Evidence from a Mathematical Model. Plos Neglected Tropical Diseases. 2016. February; 10:1–22. 10.1371/journal.pntd.0004417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chuang TW, Chaves LF, Chen PJ. Effects of local and regional climatic fluctuations on dengue outbreaks in Southern Taiwan. Plos One. 2017. July; 12(7):e0181638. 10.1371/journal.pone.0178698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silva JLR Júnior, Padilha TF, Rezende JE, Rabelo ECA, Ferreira ACG e Rabahi MF Efeito da sazonalidade climática na ocorrência de sintomas respiratórios em uma cidade de clima tropical. Jornal Brasileiro de Pneumologia, 2011, vol.37 no.6 São Paulo 10.1590/S1806-37132011000600009 [DOI] [Google Scholar]
- 26.CEPERJ- Fundação Centro Estadual de Estatísticas Pesquisas e Formação de Servidores Públicos do Rio de Janeiro. Produto interno bruto do Estado do Rio de Janeiro [Internet]. Governo do Estado do Rio de Janeiro. 2015 Available at: http://www.ceperj.rj.gov.br/ceep/pib/pib.html [accessed on Octuber 18, 2019].
- 27.IBGE-Instituto de Geografia e Estatística. Censo Demográfico 2010. https://censo2010.ibge.gov.br/resultados.html
- 28.Ministry of Health Brazil-Ministerial Ordinance N 1271, 6 July de 2014. Defines National List of Compulsory Notification of Diseases, Health Conditions and Health Events public and private health services throughout the national territory, in accordance with annex, and other arrangements. http://www.pncq.org.br/uploads/2014/qualinews/portaria_1271_6jun2014.pdf
- 29. Wan Z., 2008. New refinements and validation of the MODIS Land-Surface Temperature/Emissivity products. Remote Sens. Environ. 112, 59–74. 10.1016/j.rse.2006.06.026 [DOI] [Google Scholar]
- 30. Liu Z. 2015. Comparison of precipitation estimates between Version 7 3-hourly TRMM Multi-Satellite Precipitation Analysis (TMPA) near-real-time and research products. Atmospheric Research 153: 119–133. 10.1016/j.atmosres.2014.07.032 [DOI] [Google Scholar]
- 31. Huffman GJ, Adler RF, Bolvin DT, Nelkin EJ. 2010. The TRMM Multi-Satellite Precipitation Analysis (TMPA). In: Gebremichael M and Hossain F (eds) Satellite Rainfall Applications for Surface Hydrology. Springer Netherlands: Dordrecht, 3–22. [Google Scholar]
- 32. Huffman GJ, Bolvin DT. 2018. Real-Time TRMM Multi-Satellite Precipitation Analysis Data Set Documentation. NASA Goddard Space Flight Center. Greenbelt. [Google Scholar]
- 33. Hamilton J. D. Time Series Analysis. Princeton University Press; 1994 [Google Scholar]
- 34. Verbeek M. A guide to modern econometrics 4th ed, Willey, New York, 2012 [Google Scholar]
- 35. Medronho RA. Dengue fever and the urban environment. Revista Brasileira de Epidemiologia 2006. June; 9(2):159–161. 10.1590/S1415-790X2006000200002 [DOI] [Google Scholar]
- 36. Gomes AC, Forattini OP, Kakitami I, Marques GRAM, Marques CCA, Marucci D et al. Microhabitats de Aedes albopictus (Skuse) na região do Vale do Paraíba, Estado de São Paulo, Brasil. Revista de Saúde Pública 1992. April; 26(2): 108–118. 10.1590/S0034-89101992000200007 [DOI] [PubMed] [Google Scholar]
- 37. Chiaravalloti Neto F. Descrição da colonização de Aedes aegypti na região de São José do Rio Preto, São Paulo. Revista da Sociedade Brasileira de Medicina Tropical 1997. Jul-Aug; 30(4):279–285. 10.1590/S0034-89101992000200007 [DOI] [PubMed] [Google Scholar]
- 38. Rebêlo JMM, Costa JML, Silva FS, Pereira YNO, Silva JM. Distribution of Aedes aegypti and dengue in the State of Maranhão, Brazil. Cadernos de Saúde Pública 1999; 15(3): 447–486. 10.1590/S0102-311X1999000300004 [DOI] [PubMed] [Google Scholar]
- 39. Forattini OP, Kakitania I, Santos RLC, Kobayashib KM, Uenob HM e Fernandez Z. Distribution of Aedes aegypti and dengue in the State of Maranhão, Brazil. Revista de Saúde Pública 2000. October; 34(5):461–467. 10.1590/S0034-89102000000500005 [DOI] [PubMed] [Google Scholar]
- 40. Pinheiro VCS, Tadei WP. Frequency, diversity, and productivity study on the Aedes aegypti most preferred containers in the city of Manaus, Amazonas, Brazil. Revista do Instituto de Medicina Tropical de São Paulo 2002. Sep-Oct; 44(5):245–250. 10.1590/S0036-46652002000500002 [DOI] [PubMed] [Google Scholar]
- 41. Souza ICA, Vianna RPT, Moraes RM. MModelagem da incidência do dengue na Paraíba, Brasil, por modelos de defasagem distribuída. Cadernos de Saúde Pública 2007; 23(11): 2623–2630. 10.1590/S0102-311X2007001100010 [DOI] [PubMed] [Google Scholar]
- 42. Urbinatti PR, Menezes RMT, Nata D. Sazonalidade de Aedes albopictus em área protegida na cidade de São Paulo, Brasil. Revista de Saúde Pública 2007. May; 41(3):478–481 [PubMed] [Google Scholar]
- 43. Costa FS, Silva JJ, Souza CM, Mendes J. Population dynamics of Aedes aegypti (L) in an urban area with high incidence of dengue. Revista da Sociedade Brasileira de Medicina Tropical 2008. May-Jun; 41(3):309–312. 10.1590/S0037-86822008000300018 [DOI] [PubMed] [Google Scholar]
- 44. Zeidler JD, Acosta POA, Barrêto PP, Cordeiro JS. Dengue virus in Aedes aegypti larvae and infestation dynamics in Roraima, Brazil. Revista de Saúde Pública 2008. December; 42(6):1–6. 10.1590/S0034-89102008005000055 [DOI] [PubMed] [Google Scholar]
- 45. Honório NA, Codeço CT, Alves FC, Magalhães MAFM, Lourenço-de-Oliveira R. Temporal distribution of Aedes aegypti in different districts of Rio de Janeiro, Brazil, measured by two types of traps. Journal of Medical Entomology 2009. September; 46(5):1001–1014. 10.1603/033.046.0505 [DOI] [PubMed] [Google Scholar]
- 46. Morim CW, Comrie AC, Ernst K. Climate and dengue transmission: evidence and implications. Environmental Heath Perspectives 2013. January; 121(11-12): 1264–1272. 10.1289/ehp.1306556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Struchiner CJ, Rocklöv J, Smith AW, Massad E. Increasing dengue incidence in Singapore over the past 40 years: population growth, climate and mobility. PLoS ONE 2015. August; 10(8): e0136286. 10.1371/journal.pone.0136286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sharmin S, Glass K, Viennet E, Harley D. Interaction of mean temperature and daily fluctuation influences dengue incidence in Dhaka, Bangladesh. Plos Neglected Tropical Disease 2015. July; 9(7): e0003901. 10.1371/journal.pntd.0003901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koh YM, Spindler R, Sandgren M, Jiang J. A model comparison algorithm for increased forecast accuracy of dengue fever incidence in Singapore and the auxiliary role of total precipitation information. International Journal of Environmental Health Research 2018. June 28:5, 535–552, 10.1080/09603123.2018.1496234 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.


