Abstract
Although rodents represent approximately 40% of all living mammalian species, our knowledge regarding their reproductive biology is still scarce. Due to their high vulnerability to environmental changes, wild rodents have become beneficial models for ecological studies. Thus, we aimed to comparatively investigate key functional testis parameters in four sexually mature wild rodent species (A. cursor, A. montensis, N. lasiurus, and O. nigripes). These species belong to the Cricetidae family, which is the most diverse family of rodents in South America, with a total of ~120 species in Brazil. The results found for the gonadosomatic index and the sickled sperm head shape observed strongly suggest that the species here evaluated are promiscuous, prolific, and short-lived. The duration of spermatogenesis was relatively short and varied from ~35–40 days. Both the percentage of seminiferous tubules (ST) in the testis parenchyma (~95–97%) and the number of Sertoli cells (SC) (~48–70 million) per testis gram were very high, whereas a fairly good SC efficiency (~8–13 round spermatids per SC) was observed. In comparison to other mammalian species studied, particularly the rodents of the suborder Myomorpha (i.e. hamsters, rats and mice), the rodents herein investigated exhibited very high (~62–80 million) daily sperm production per testis gram. This impressive spermatogenic efficiency resulted mainly from the short duration of spermatogenesis and quite high values found for the ST percentage in the testis and the SC number per testis gram. We expect that the knowledge here obtained will help conservation programs and the proper management of wildlife.
Introduction
Considering that wild rodents have restricted dispersion in forest regions and are highly sensitive to environmental changes, they have become valuable models for examining biodiversity alterations in the last decades [1, 2]. Besides that, this mammalian order is widely diverse and requires relatively small areas to maintain a viable population, which makes its sampling relatively easy [3]. The four species (Akodon cursor, Akodon montensis, Necromys lasiurus, and Oligoryzomys nigripes) investigated in the present study belong to the Cricetidae family, which is the most diverse family of rodents in South America and particularly in Brazil where they comprise a single neotropical Sigmodintinae subfamily with a total of 117 species and 36 genera [4].
The species A. cursor and A. montensis are insectivorous-omnivorous found in the Atlantic semi-deciduous forests [5–9], whereas N. lasiurus is a small rodent frequently found in savanna areas (also called Brazilian Cerrado) or ecotones with Atlantic Forest and feeds on insects, fruits and seeds [10]. This latter species has a smaller tail compared to its body and a distinctive periocular ring formed by light hair in each eye [4, 11]. The O. nigripes is a tiny rodent with a much larger tail compared to its body. It feeds on vegetables, commonly inhabiting open vegetation of the savanna or Atlantic Forest areas [12, 13]. As these species mentioned above inhabit a region with great mammalian diversity, they are important for the ecological interactions that occur among species [14–16].
Although often captured in areas of the Atlantic Forest and savanna, there are few studies related to the reproductive biology of these wild rodent species [17–20]. It is important to mention that these biomes have been severely fragmented and degraded by human activities for hundreds of years [21–24]. As a consequence, changes have occurred in the natural habitats and species diversity [25], which can favor generalist and opportunistic species to increase their densities and dispersion, as well as increasing the number of agricultural pests and changing the natural prevalence of zoonotic pathogens in wild reservoirs [26–28]. Moreover, the wild rodents herein investigated are classified as opportunistic species and reservoirs of zoonotic pathogens [2, 29–34]; understanding their reproductive behavior is very useful to develop conservation management strategies. For instance, the evaluation of reproductive organs in different species allows a better comprehension of distinct reproductive strategies and physiological specificities related to each species [35–42]. This evaluation is of particular interest when one considers that relatively few mammalian species have been investigated, and remarkable differences in their reproductive biology may exist [43–45]. Therefore, it cannot be assumed that reproductive mechanisms are uniform among species [46]. In this regard, the present study aimed to investigate several key morphofunctional testicular parameters and comparatively evaluate spermatogenesis in four wild Cricetidae rodent species already mentioned above.
Material and methods
Animals and tissue processing
The current study was performed in accordance with ethical and animal experiments regulations of the Brazilian Government (Law 11794/2008). All animal experiments were performed in strict accordance with the Guidelines for Animal Use and Experimentation. More specifically, this study was approved by the Animal Experimentation Ethics Committees of the Federal University of Minas Gerais (CEUA/UFMG, Belo Horizonte, Brazil; protocol # 94/2008). The corresponding author of this study, Dr. Luiz R. de França, who is a veterinarian (CRMV-MG #3980), gave the injections, did other necessary procedures and performed the euthanasia. During the time intervals between injection and euthanasia, the animals were maintained with ration, fruits, water ad libitum and housed in a photoperiod-controlled vivarium for wildlife species at the Department of Morphology of the Institute of Biological Sciences at the Federal University of Minas Gerais.
In the present investigation, testes from four sexually mature cricetid rodent species (A. cursor, n = 6; A. montensis, n = 9; N. Lasiurus, n = 13; and O. nigripes, n = 11) were evaluated. As these species are not seasonal [47–49], they were captured along the year in fragments of the Atlantic Forest and Brazilian savanna located in the state of Minas Gerais, Brazil (20°0′51″ S, 43°29′28″ W) during the rainy and dry seasons (S1 Table). The animals were captured using 100-wire mesh live traps placed on the ground at intervals of 15 meters along three lines spaced 20 meters apart. Traps were baited with a mixture of banana and peanut butter during four nights each month and checked in the morning.
All rodents were euthanized by anesthetic overdose [ketamine (300 mg/Kg BW) and xylazine (30 mg/Kg BW); Sigma-Aldrich, St. Louis, MO, USA]. Subsequently, testes were perfused-fixed by gravity-fed perfusion through the left ventricle with 0.9% saline and 4% buffered glutaraldehyde for 25-30min [50]. Following orchiectomy, testes were separated from the epididymis, weighed and cut longitudinally into small fragments (1-3mm thickness), which were routinely processed and embedded in glycol-methacrylate for histological and autoradiographic analysis as described below. The gonadosomatic index (GSI), which is the total testis weight divided by the BW, was also obtained for all animals.
Thymidine injections and autoradiographic analysis
In order to estimate the duration of spermatogenesis and before orchiectomy, intratesticular injections (50μCi per testis, n = 2 per each species) of tritiated thymidine [thymidine (methyl-3H), specific activity 82.0 Ci mmol-1; Amersham Life Science, UK] were given near the cauda of the epididymis. Two-time intervals (1 hour and approximately three weeks) were considered after thymidine injections for A. cursor, A. montensis, and N. Lasiurus, whereas for O. nigripes these intervals were 1 hour and approximately two weeks.
For the autoradiographic analysis, unstained testis sections (4μm) were dipped in autoradiographic emulsion (Kodak NTB-2, Eastman Kodak Company; Rochester, NY) at 43–45°C. After drying for approximately 1h at 25°C, testis sections were placed in sealed black boxes and stored in a refrigerator at 4°C for approximately four weeks. Subsequently, they were developed in Kodak D-19 (Eastman Kodak Company; Rochester, NY) solution at 15°C [51] and stained with toluidine blue. Aiming to detect the most advanced germ cell type labeled at the different time periods following thymidine injections, the analyses were performed using an Olympus microscope (BX60). Cells were considered labeled when four or more thymidine grains were present over the nucleus in a low to moderate background.
Testis morphometry
The volume densities of testicular tissue components were determined by light microscopy using a 441-intersection grid placed in the ocular. Fifteen randomly chosen fields (6,615 points) were scored for each animal at 400X magnification. The tubular diameter and seminiferous tubule epithelium height were measured at 200X magnification using an ocular micrometer. Thirty tubular profiles (round or nearly round) were chosen randomly and measured for each animal. The epithelium height was obtained in the same tubules used to determine tubular diameter. The total length of seminiferous tubules (meters) was obtained by dividing seminiferous tubule volume by the squared radius of the tubule multiplied by π [52].
Stages of the seminiferous epithelium cycle and duration of spermatogenesis
Seminiferous epithelium cycle (SEC) stages were characterized according to the development of the acrosome system and morphology of the developing spermatid nucleus [53]. The relative stage frequencies were determined by evaluating 250 seminiferous tubule cross-sections per animal at the magnification of 400x. The seminiferous tubules analyzed were randomly chosen, and both testes were examined for each animal.
The spermatogenic cycle length was estimated based on the stage frequencies and the most advanced germ cell type labeled at different periods following thymidine injections. The total duration of spermatogenesis took into account that approximately 4.5 cycles are necessary to complete the spermatogenic process [53, 54]. Since the nuclear volume of pachytene primary spermatocytes grows markedly during the meiotic prophase, their nuclei size was used as a reference in order to precisely determine the location of the most advanced labeled germ cell at a specific SEC stage.
Cell numbers
Germ and Sertoli cells
The cells present in stage VIII of the cycle were counted in ten seminiferous tubule cross-sections per animal. These counts were corrected based on the method described by Abercrombie (1946) [55], as modified by Amann (1962) [56]. Cell ratios/proportions were achieved from these corrected counts. Assuming that no significant germ cell loss occurs during spermiogenesis, the number of round spermatids was considered as the total number of produced spermatozoa [53, 57].
The Sertoli cell numbers per testis and testis gram were estimated from the Sertoli cell nucleoli number per seminiferous tubule cross-section and the obtained seminiferous tubule total length [58, 59]. This methodology is based on the fact that Sertoli cell numbers are stable in adult animals and in seminiferous tubule cross-sections in the different spermatogenic cycle stages [43, 60–62]. Daily sperm production (DSP) per testis and gram of testis (spermatogenic efficiency) were obtained according to the formula described by França (1992) [63]: DSP = Sertoli cell number per testis x the ratio of round spermatids to Sertoli cells in stage VIII x stage VIII relative frequency (%)/stage VIII duration (days).
Leydig cells
Leydig cell volume was obtained using the nuclear volume as well as the proportion between the nucleus and cytoplasm. For this purpose, 30 nuclei were measured per animal. Leydig cell nuclear volume was obtained using the sphere formula (4/3πR3, in which R = nuclear diameter/2). The proportion between the nucleus and cytoplasm was estimated, scoring 1,000 points over Leydig cells per animal using a grid placed in the ocular at 400X magnification. The number of Leydig cells per testis was estimated from the individual Leydig cell volume and the total volume occupied by these cells in the testis parenchyma.
Results
Biometry and morphometry
The biometric data obtained from the rodent species evaluated in the present study are shown in Table 1. The highest testicular weight was observed in A. cursor, whereas O. nigripes exhibited the smallest value for this parameter and for the gonadosomatic index that was higher in both A. cursor and A. montensis.
Table 1. Biometric and testis morphometric data in A. cursor, A. montensis, N. Lasiurus and O. nigripes (mean ± SEM).
| Parameter | Species | |||
|---|---|---|---|---|
| A. cursor | A. montensis | N. lasiurus | O. nigripes | |
| Body weight (g) | 54 ± 3 | 37 ± 2 | 60 ± 3 | 23 ± 1 |
| Testis weight (mg) | 288 ± 27 | 217 ± 20 | 237 ± 15 | 63 ± 4 |
| Gonadosomatic índex (%) | 1.07 ± 0.1 | 1.1 ± 0.05 | 0.80 ± 0.04 | 0.5 ± 0.02 |
| Testis parenchyma volume density (%) | ||||
| Tubular compartment | 95.5 ± 0.7 | 96.6 ± 0.6 | 95.5 ± 0.4 | 96.3 ± 0.9 |
| Tunica própria | 3.1 ± 0.1 | 2.7 ± 0.1 | 3.5 ± 0.1 | 2.9 ± 0.1 |
| Seminiferous epithelium | 86.7 ± 1.1 | 86.1 ± 0.2 | 85.1 ± 0.6 | 87.1 ± 0.4 |
| Lumen | 5.7 ± 0.6 | 7.8 ± 0.2 | 6.9 ± 0.3 | 6.3 ± 0.3 |
| Intertubular compartment | 4.5 ± 0.3 | 3.4 ± 0.1 | 4.5 ± 0.3 | 3.7 ± 0.2 |
| Leydig cell | 2.0 ± 0.4 | 1.1 ± 0.1 | 1.6 ± 0.2 | 1.4 ± 0.1 |
| Blood vessels | 1.0 ± 0.3 | 1.0 ± 0.1 | 1.4 ± 0.3 | 1.0 ± 0.2 |
| Lymphatic space | 0.5 ± 0.2 | 0.3 ± 0.02 | 0.5 ± 0.1 | 0.4 ± 0.02 |
| Others | 1.0 ± 0.3 | 1.0 ± 0.04 | 1.0 ± 0.1 | 0.9 ± 0.08 |
| Tunica albugínea (%) | 4.1 ± 0.2 | 4.0 ± 0.6 | 3.8 ± 0.2 | 6.7 ± 0.2 |
| Tubular diameter (μm) | 245 ± 3 | 233 ± 3 | 246 ± 6 | 181 ± 5 |
| Seminiferous epithelium height (μm) | 92 ± 2 | 90 ± 2 | 86 ± 2 | 69 ± 1 |
| Tubular length per gram of testis (meters) | 20 ± 1 | 22 ± 1 | 21 ± 1 | 35 ± 3 |
| Total tubular length per testis (meters) | 5.7 ± 0.3 | 4.2 ± 0.6 | 4.7 ± 0.3 | 2.1 ± 0.1 |
Morphometric testicular parameters are also shown in Table 1. In all investigated species, the volume densities of seminiferous tubules and the seminiferous epithelium were very high and respectively in the range of ~95–97% and ~85–87%. In contrast, Leydig cells composed only 1–2% of the testicular parenchyma. In general, the values found for the tubular diameter (~235–245 um) and the seminiferous epithelium height (~85–90 um) were similar in A. cursor, A. montensis, and N. Lasiurus, whereas the values observed for these parameters were quite lower in O. nigripes, which presented almost 70% higher tubular length per gram of testis.
Stages of the seminiferous epithelium cycle
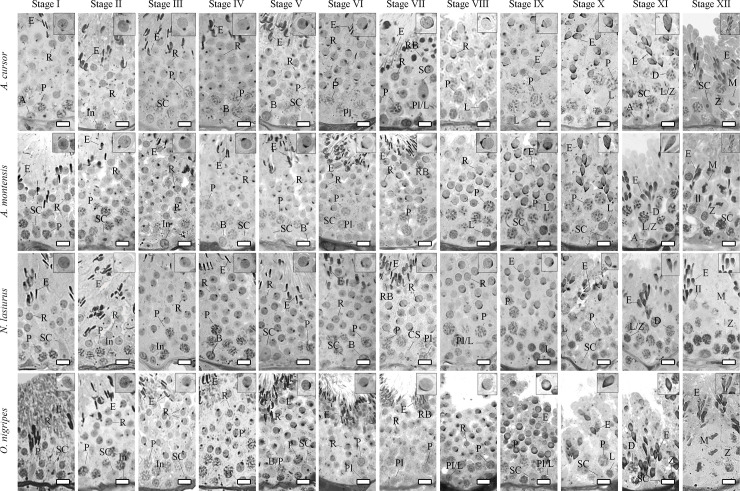
For all rodent species herein investigated, twelve stages were characterized according to the acrosomic system and morphology of the developing spermatid nucleus (Fig 1). Also, spermiation occurred in stage VII and the germ cell morphology was quite similar in all species. The characterized stages are briefly described below.
Fig 1. Different stages of the seminiferous epithelium cycle characterized according to the acrosome system in the four wild rodent species investigated.
Twelve stages were characterized. The inserts in the upper right corner show a representative spermatid step present in each stage. Abbreviations: type A spermatogonia (A); intermediate spermatogonia (In); type B spermatogonia (B); pre-leptotene spermatocytes (Pl); pre-leptotene in the transition to leptotene spermatocytes (Pl/L); leptotene spermatocytes (L); leptotene in the transition to zygotene spermatocytes (L/Z); zygotene spermatocytes (Z); pachytene spermatocytes (P); diplotene spermatocytes (D); secondary spermatocytes (II); meiotic divisions (M); round spermatids (R); elongating/elongated spermatids (E); Sertoli cells (SC); and residual bodies (RB). Bar = 10μm.
Stage I
Two generations of spermatids were present in this stage: early round spermatids and elongated spermatids. Since the proacrosomal granules cannot be observed by light microscopy in this stage, the newly generated round spermatids were characterized by the absence of a visible acrosome vesicle.
Stage II
Early round spermatids usually present a tiny acrosomal vesicle containing acrosomal granules in contact with the nucleus. Elongated spermatids were located closer to the tubular lumen.
Stage III
Acrosomal vesicles flatten over the nucleus of round spermatids. These acrosomal vesicles formed angles of approximately 28 ± 2°, 29 ± 2°, 41 ± 3°, and 44 ± 2° over the nuclear surface respectively in A. cursor, A. montensis, N. lasiurus and O. nigripes. Elongated spermatids were observed in bundles.
Stage IV
In this stage, the angles of the acrosomal vesicles over the round spermatids nuclear surface were 33 ± 1°, 34 ± 1°, 44 ± 2°, and 54 ± 2° respectively in A. cursor, A. montensis, N. lasiurus and O. nigripes. Elongated spermatid bundles slightly moved towards the seminiferous tubule lumen.
Stage V
Elongated spermatids were located very close to the tubular lumen. The angles of acrosomal vesicles over the round spermatids nuclear surface were 41 ± 3°, 44 ± 3°, 54 ± 2°, and 57 ± 2° in A. cursor, A. montensis, N. lasiurus and O. nigripes, respectively.
Stage VI
Elongated spermatids were dissociated and located closer to the luminal border. The angles of acrosomal vesicles over the round spermatid nuclear surface were respectively 61 ± 4°, 61 ± 2°, 61 ± 3°, and 73 ± 2° in A. cursor, A. montensis, N. lasiurus and O. nigripes, respectively.
Stage VII
Elongated spermatids exhibiting sickle-shaped heads were located on the luminal border or undergoing spermiation towards the tubular lumen. Residual bodies were seen below these cells. Over the round spermatids nuclear surface, the angles formed by acrosomes were 97 ± 2°, 92 ± 4°, 78 ± 4°, and 83 ± 2° in A. cursor, A. montensis, N. lasiurus and O. nigripes, respectively.
Stage VIII
Since all elongated spermatids had spermiated, this stage had only one generation of spermatids. The angles of acrosomes over the round spermatids nuclear surface were 103 ± 3°, 102 ± 2°, 86 ± 4°, and 93 ± 4° in A. cursor, A. montensis, N. lasiurus and O. nigripes, respectively.
Stage IX
The nuclei of round spermatids started to elongate. The acrosomes also underwent an elongation process and formed angles over the nuclear surface of 105 ± 4°, 111 ± 2°, 95 ± 5°, and 106 ± 2° in A. cursor, A. montensis, N. lasiurus and O. nigripes, respectively.
Stage X
Nuclei of elongating spermatids started to form bundles. A ventral angle appeared in the elongating spermatid heads, and the ratio between the shortest and the longest longitudinal axis in A. cursor, A. montensis, N. lasiurus and O. nigripes was respectively 1.3 ± 0.1, 1.8 ± 0.4, 2.3 ± 0.1, and 1.5 ± 0.1. These nuclei also showed a polarization, and their heads were oriented toward the base of the tubule.
Stage XI
At this stage, spermatids completed their elongation process, and their nuclei were markedly grouped in bundles oriented toward Sertoli cell nuclei. The ratio between the shortest and the longest longitudinal axis in A. cursor, A. montensis, N. lasiurus and O. nigripes was 1.9 ± 0.2, 2.5 ± 0.1, 2.5 ± 0.1, and 1.8 ± 0.1, respectively.
Stage XII
The main feature of this stage was the presence of meiotic figures related to the first and the second meiotic divisions. Therefore, secondary spermatocytes were observed. Nuclei of elongated spermatids were more condensed. The ratio between the shortest and the longest longitudinal axis in A. cursor, A. montensis, N. lasiurus and O. nigripes was respectively 2.9 ± 0.2, 2.8 ± 0.2, 2.8 ± 0.1, and 2.5 ± 0.2.
Stage frequencies
The mean percentages of each of the twelve stages characterized, as well as the frequencies of pre-meiotic (stages VIII-XI), meiotic (stage XII) and post-meiotic (stages I-VII) phases, are displayed in Table 2. Stages III and VII were the most prevalent (~15 to ~20%) in A. cursor, A. montensis and N. lasiurus, whereas the frequencies of many stages (II, IV, V, VIII, X, and XI) were in the range of ~4–9% in all four species studied. The frequencies of pre-meiotic and post-meiotic phases were similar among A. cursor, A. montensis, N. lasiurus, and O. nigripes, and in the range of respectively 28–33% and 61–64%.
Table 2. Relative frequencies (%) of the stages and phases of the seminiferous epithelium cycle in A. cursor, A. montensis, N. Lasiurus and O. nigripes (mean ± SEM).
| Stages and phases of the seminiferous epithelium cycle | Species | |||
|---|---|---|---|---|
| A. cursor | A. montensis | N. lasiurus | O. nigripes | |
| Stage I | 4.2 ± 0.3 | 8.6 ± 1.4 | 4.6 ± 0.5 | 11.1 ± 1.2 |
| Stage II | 4.1 ± 0.4 | 6.4 ± 0.7 | 4.7 ± 0.3 | 6.1 ± 0.4 |
| Stage III | 15.7 ± 0.5 | 14.5 ± 1.1 | 16.3 ± 0.6 | 5.3 ± 0.7 |
| Stage IV | 6.3 ± 0.5 | 6.9 ± 1.5 | 6.1 ± 0.4 | 5.8 ± 0.1 |
| Stage V | 4.5 ± 0.5 | 4.0 ± 0.4 | 3.8 ± 0.4 | 7.5 ± 1.3 |
| Stage VI | 7.0 ± 1.0 | 6.5 ± 1.3 | 6.7 ± 0.6 | 12.9 ± 1.0 |
| Stage VII | 20.4 ± 0.9 | 17.5 ± 1.8 | 19.1 ± 0.8 | 10.9 ± 1.1 |
| Stage VIII | 7.1 ± 0.3 | 9.1 ± 1.0 | 6.5 ± 0.8 | 7.0 ± 1.1 |
| Stage IX | 12.6 ± 0.6 | 6.2 ± 0.9 | 12.7 ± 0.7 | 10.5 ± 0.8 |
| Stage X | 8.6 ± 0.9 | 5.9 ± 0.6 | 5.5 ± 0.6 | 6.3 ± 0.6 |
| Stage XI | 5.0 ± 0.6 | 6.2 ± 0.8 | 6.8 ± 0.7 | 6.5 ± 0.9 |
| Stage XII | 4.5 ± 0.4 | 8.2 ± 1.3 | 7.2 ± 0.7 | 10.1 ± 0.8 |
| Pre-meiotic phase1 | 33.3 ± 1.9 | 27.4 ± 3.4 | 31.5 ± 2.8 | 30.3 ± 3.4 |
| Meiotic phase2 | 4.5 ± 0.4 | 8.2 ± 1.3 | 7.2 ± 0.7 | 10.1 ± 0.8 |
| Post-meiotic phase3 | 62.2 ± 3.4 | 64.4 ± 3.1 | 61.3 ± 3.7 | 59.6 ± 7.1 |
1After spermiation and prior to metaphase.
2 Meiosis I through meiosis II.
3After completion of meiosis until spermiation.
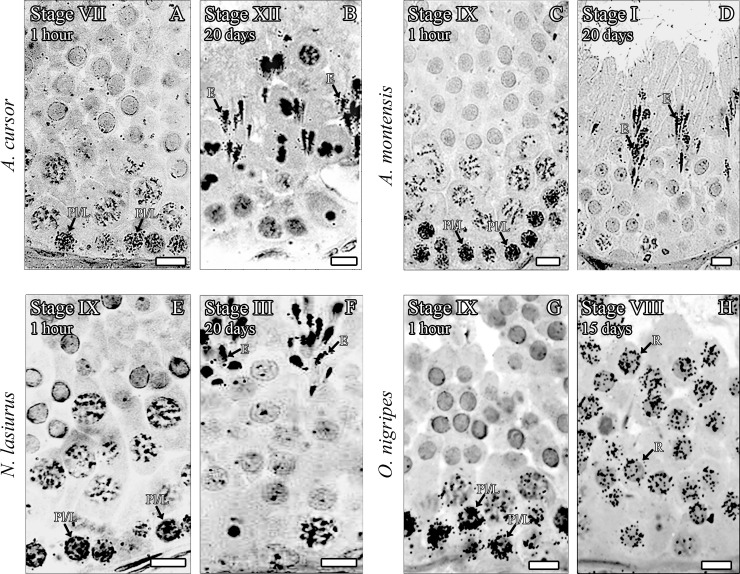
Germ cell labeling and seminiferous epithelium cycle length
As expected, approximately 1h after tritiated-thymidine injection, the most advanced labeled germ cells were identified as pre-leptotene in the transition to leptotene spermatocytes in all four species evaluated (Table 3 and Fig 2). These cells were found in the basal compartment of stage VII in A. cursor and stage IX in the other species (Table 3 and Fig 2).
Table 3. Length (days) of seminiferous epithelium cycle in A. cursor, A. montensis, N. Lasiurus and O. nigripes (mean ± SEM).
| Species | Animal | Interval after | Most advanced germ | Stage of the | Number of cycles | Cycle |
|---|---|---|---|---|---|---|
| Injection | cell type labeled | cycle | traversed | length | ||
| A. cursor | 1 | 1 hour | Pl/L | VII | - | - |
| 19.8 days* | E | XII | 2.33 | 8.49 | ||
| 2 | 1 hour | Pl/L | VII | - | - | |
| 19.8 days* | E | XII | 2.37 | 8.35 | ||
| Mean duration of the cycle based on Pl/L = 8.42 ± 0.1 days. | ||||||
| A. montensis | 1 | 1 hour | Pl/L | IX | - | - |
| 21.8 days* | E | I | 2.44 | 8.93 | ||
| 2 | 1 hour | Pl/L | IX | - | - | |
| 21.8 days* | E | I | 2.46 | 8.86 | ||
| Mean duration of the cycle based on Pl/L = 8.89 ± 0.04 days. | ||||||
| N. lasiurus | 1 | 1 hour | Pl/L | IX | - | - |
| 20.1 days* | E | III | 2.58 | 7.79 | ||
| 2 | 1 hour | Pl/L | IX | - | - | |
| 20.1 days* | E | III | 2.57 | 7.82 | ||
| Mean duration of the cycle based on Pl/L = 7.8 ± 0.02 days. | ||||||
| O. nigripes | 1 | 1 hour | Pl/L | IX | - | - |
| 14.7 days* | R | VIII | 1.83 | 8.00 | ||
| 2 | 1 hour | Pl/L | IX | - | - | |
| 14.6 days* | R | VIII | 1.86 | 7.87 | ||
| Mean duration of the cycle based on Pl/L = 7.94 ± 0.1 days. | ||||||
*Time after thymidine injection minus 1 hour. Pl/L = preleptotene/leptotene spermatocytes; R = round spermatids; E = elongated spermatids.
Fig 2. Evaluation of tritiated thymidine labeling.
The most advanced germ cell type labeled (arrows) at different time periods (1 hour, 15 and 20 days) in A. cursor (A and B), A. montensis (C and D), N. lasiurus (E and F) and O. nigripes (G and H), after intratesticular tritiated thymidine injections. Bar = 10μm. Abbreviations: pre-leptotene in the transition to leptotene spermatocytes (Pl/L); round spermatids (R); and elongating/elongated spermatids (E).
Fifteen days after injection, round spermatids were the most advanced germ cell type labeled at stage VIII in O. nigripes (Table 3 and Fig 2), whereas elongated spermatids were the most advanced germ cell labeled in stage XII, stage I and stage III in A. cursor, A. montensis and N. lasiurus, respectively, after 20 days (Table 3 and Fig 2).
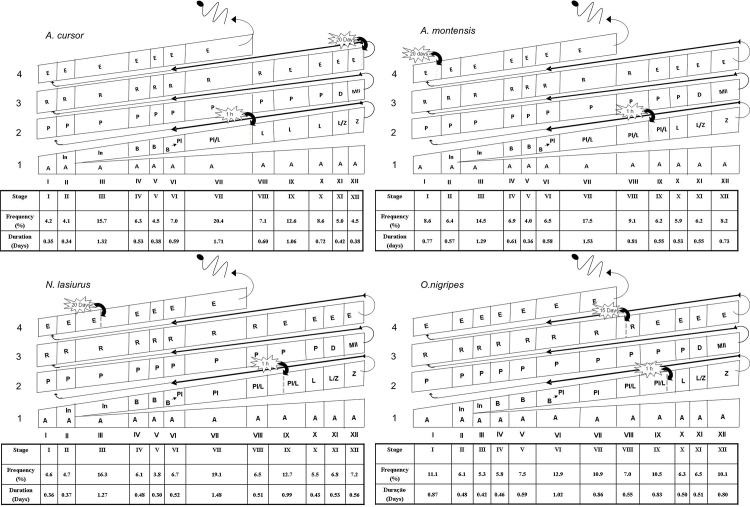
Based on the labeled germ cells observed in each period following thymidine injection and the stage frequencies (Tables 2 and 3 and Fig 2), the mean duration of the seminiferous epithelium cycle for A. cursor, A. montensis, N. lasiurus and O. nigripes was estimated, respectively as 8.4 ± 0.1, 8.9 ± 0.04, 7.8 ± 0.02 and 7.9 ± 0.1 days (Table 3). Since approximately 4.5 cycles are necessary for the entire spermatogenic process to be completed, the total length of spermatogenesis, also expressed in days, was estimated respectively as 37.9 ± 1.1 (A. cursor), 40 ± 0.9 (A. montensis), 35.1 ± 0.3 (N. lasiurus), and 35.6 ± 0.1 (O. nigripes). For each species, a schematic illustration of the stage frequencies and their duration is depicted in Fig 3.
Fig 3. Schematic illustration of the frequencies (%) and duration (days) of the twelve stages of the seminiferous epithelium cycle characterized according to the development of the acrosome in the spermatids in A. cursor, A. montensis, N. lasiurus and O. nigripes.
The most advanced germ cell labeled, one hour and 15 or 20 days after thymidine injection, is indicated by the thick arrow. The space corresponding to each stage of the cycle is proportional to its frequency and duration. Abbreviations: type A spermatogonia (A); intermediate spermatogonia (In); type B spermatogonia (B); pre-leptotene spermatocytes (Pl); pre-leptotene in the transition to leptotene spermatocytes (Pl/L); leptotene spermatocytes (L); leptotene in transition to zygotene spermatocytes (L/Z); zygotene spermatocytes (Z); pachytene spermatocytes (P); diplotene spermatocytes (D); secondary spermatocytes and meiotic divisions (MII); round spermatids (R); and elongating/elongated spermatids (E).
Cell counts and sperm production
The cell ratios and the Sertoli cell number and sperm production per gram of testis and testis are shown in Table 4. As observed in this table, except for O. nigripes, which presented a lower value, the meiotic index (number of round spermatids per primary spermatocyte) was approximately 3, meaning that the germ cell loss during meiosis was around 25%. Regarding the Sertoli cell efficiency (number of spermatids per Sertoli cell), N. lasiurus exhibited the highest value (~13:1), while O. nigripes showed the lowest efficiency (~8:1). The number of Sertoli cells per gram of testis in O. nigripes was almost 70 million, whereas in the other three rodent species the values found were in the range of ~48–55 million. Due to testis size, the total number of Sertoli cells per testis was ~4 million in O. nigripes and ~11–14 million in the other three species. In relation to the spermatogenic efficiency DSP/gram/testis, the values observed were ~62 million and ~66 million in A. montensis and A. cursor, respectively. This efficiency reached 74 million in O. nigripes and almost 80 million in N. lasiurus. Finally, the sperm production per testis was around 18 million in A. cursor and N. lasiurus, ~13 million in A. montensis, and less than 5 million in O. nigripes (Table 4).
Table 4. Cell ratios and sperm production in A. cursor, A. montensis, N. lasiurus and O. nigripes (mean ± SEM).
| Parameter | Species | |||
|---|---|---|---|---|
| A. cursor | A. montensis | N. lasiurus | O. nigripes | |
| Round spermatids: pachytene spermatocyte | 2.9 ± 0.2 | 3.1 ± 0.3 | 3.0 ± 0.1 | 2.6 ± 0.1 |
| Round spermatids: Sertoli cell nucleoli | 10.9 ± 0.8 | 10.3 ± 0.7 | 13.2 ± 0.5 | 8.4 ± 0.2 |
| Sertoli cell number per gram of testis (x 10 6) | 52.1 ± 4.3 | 54.7 ± 4.3 | 47.9 ± 3.7 | 69.2 ± 4.7 |
| Sertoli cell number per testis (x 10 6) | 14.4 ± 1.6 | 11.3 ± 0.9 | 10.9 ± 8.1 | 4.1 ± 0.3 |
| Daily sperm production per gram of testis (x 10 6) | 65.9 ± 3.8 | 62.0 ± 2.8 | 79.0 ± 5.1 | 74.0 ± 3 |
| Daily sperm production per testis (x 10 6) | 18.5 ± 2.2 | 13.2 ± 0.9 | 18.1 ± 1.3 | 4.8 ± 0.4 |
Leydig cell
All Leydig cell histomorphometric data are shown in Table 5. In comparison to the other three rodent species, O. nigripes showed the lowest individual Leydig cell volume (~400 um3 vs. ~800–1,100 um3). Because the Leydig cell individual volume is small in O. nigripes, overall, its number per testis gram (~33 million) was two-fold higher (~15–19 million) than in the three other species. However, as expected, its number per testis is much lower in O. nigripes (~2 vs. ~3–5 million; Table 5).
Table 5. Leydig cell morphometric parameters in A. cursor, A. montensis, N. lasiurus and O. nigripes (mean ± SEM).
| Parameter | Species | |||
|---|---|---|---|---|
| A. cursor | A. montensis | N. lasiurus | O. nigripes | |
| Nuclear diameter (μm) | 8.0 ± 0.2 | 7.3 ± 0.1 | 7.6 ± 0.1 | 6.5 ± 0.1 |
| Leydig cell volume (μm3) | 1092 ± 131 | 807 ± 49 | 967 ± 21 | 393 ± 18 |
| Nucleus volume (μm3) | 275 ± 17 | 204 ± 11 | 235 ± 7 | 146 ± 4 |
| Cytoplasm volume (μm3) | 817 ± 115 | 603 ± 40 | 732 ± 52 | 247 ± 15 |
| Leydig cell number per gram of testis (x106) | 19.4 ± 4.2 | 14.6 ± 1.8 | 17.1 ± 2.3 | 33.2 ± 2.9 |
| Leydig cell number per testis (x106) | 5.2 ± 1.0 | 3.1 ± 0.5 | 4.0 ± 0.7 | 1.9 ± 0.1 |
Discussion
Although rodents represent approximately 40% of all mammalian species alive, our knowledge regarding their reproductive biology is still incipient [19, 64–66]. In the present study, key testis morphofunctional parameters of four small rodent species from the Cricetidae family, inhabiting an ecotone of high biodiversity [14, 67], were evaluated. All four species herein studied exhibited high spermatogenic efficiency that resulted from a combination of several key testis functional parameters, such as the short duration of spermatogenesis and the very high values found for the percentage of seminiferous tubules in the testis parenchyma. The very high reproductive efficiency herein demonstrated can be a problem in an ecologically disturbed environment, leading to overpopulation of these four rodent species and potentially disseminating diseases among humans [2].
In general, several testis parameters were very similar among A. cursor, A. montensis and N. lasiurus. Interestingly, these species belong to the same clade, known as Akodontini [68]. However, O. nigripes, from the Oryzomyini clade [68], presented distinct features, such as reduced gonadossomatic index, lower Leydig cell size, higher incidence of apoptosis in the seminiferous epithelium and an increased number of Sertoli cell per testis gram. Nevertheless, to maintain high daily sperm production, the high Sertoli cell number observed for this particular species somehow compensated the germ cell loss.
In mammals, although under the control of the germ cell genotype [69], the length of the seminiferous epithelium cycle is not phylogenetically determined [53, 69–71] Nevertheless, similar to what has already been already described in fish [72], recent studies from our laboratory have shown that the duration of spermatogenesis in mice is accelerated by higher environmental temperatures [59]. For most mammalian species already investigated, the duration of one spermatogenic cycle is in the range of 9 to 12 days, and, overall, wild rodents have a faster spermatogenic cycle length [53, 60, 73, 74]. In fact, in the species herein evaluated, one spermatogenic cycle lasted only ~8–9 days and, taking into account that spermatogenesis in mammals requires approximately 4.5 cycles to be completed, N. lasiurus and O. nigripes exhibited one of the shortest durations of spermatogenesis (~35–36 days). An interesting aspect suggested in the literature, particularly in studies conducted in our laboratory investigating about twenty different species [38, 44, 75, 76], is the observation that the frequencies of pre-meiotic and post-meiotic phases of spermatogenesis are similar among phylogenetically closely related species, and in particular for those belonging to the same family. Therefore, confirming our hypothesis, in the present study, the four rodent species that belong to the Cricetidae family presented values following the pattern in which the post-meiotic frequency is markedly higher (see comparative Table 6).
Table 6. Comparative parameters related to biometry, testis stereology and spermatogenic events in well-investigated sexually mature rodent species from the Myomorpha and Hystricomorpha suborders.
| Suborder Myomorpha | Suborder Hystricomorpha | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. cursor | A. montensis | N. lasiurus | O. nigripes | Gerbila | Hamsterb | Ratc | Moused | P. guyannensise | T. moojenif | |
| Body weight (g) | 54 | 37 | 60 | 23 | 80 | 160 | 414 | 26–39 | 288 | 207 |
| Testis weight (g) | 0.29 | 0.22 | 0.24 | 0.06 | 0.54 | 1.7 | 1.57 | 0.095–0.113 | 1.63 | 0.97 |
| Gonadosomatic Index (%) | 1.07 | 1.1 | 0.8 | 0.5 | 0.72 | 2.13 | 0.76 | 0.76–0.55 | 1.15 | 0.93 |
| Seminiferous tubules (%) | 96 | 97 | 96 | 96 | 92 | 93 | 89 | 91–93 | 96 | 98 |
| LC (%) | 2 | 1.1 | 1.6 | 1.4 | 3 | 2.7 | 1.4 | 3.7–5.3 | 1.5 | 0,3 |
| LC size (μm3) | 1092 | 807 | 967 | 393 | 1148 | 1092 | 1207 | 1021–1450 | 746 | 799 |
| LC number per gram of testis (106) | 19 | 15 | 17 | 33 | 28 | 55 | 13 | 29–49 | 19 | 4 |
| Pre-meiotic phasec (%) | 33 | 28 | 32 | 30 | 36 | 26 | 24 | 22 | 49 | 40 |
| Meiotic phased (%) | 5 | 8 | 7 | 10 | 9 | 8 | 6 | 9 | 10 | 7 |
| Post-meiotic phasee (%) | 62 | 64 | 61 | 61 | 55 | 67 | 71 | 69 | 41 | 53 |
| Meiotic indexf | 2.9 | 3.1 | 3 | 2.6 | 2.8 | 3.3 | 3.4 | 2.3–2.8 | 2.7 | 3 |
| Rate of apoptosis during meiosis | 25% | 22% | 25% | 35% | 30% | 17% | 15% | 43–30% | 33% | 26% |
| Spermatogenic cycle length (days) | 8.42 | 8.89 | 7.8 | 7.94 | 10.6 | 8.7 | 12.9 | 8.6–8.9 | 7.5 | 8.6 |
| Duration of spermatogenesis (days) | 37.9 | 40 | 35.1 | 35.7 | 47.7 | 39.2 | 58 | 38.7–40 | 33.6 | 38.7 |
| Sertoli cells per gram of testis (x106) | 52 | 55 | 48 | 69 | 28 | 45 | 27 | 39–41 | 78 | 53 |
| Round spermatids per Sertoli cell | 10.9 | 10.3 | 13.2 | 8.4 | 12.6 | 8.2 | 8.0–10.3 | 10.5–11.5 | 7.9 | 14.7 |
| DSP per gram of testis (x106) | 66 | 62 | 79 | 74 | 33 | 24 | 17–24 | 45–48 | 78 | 82 |
Similar to the mice strains usually utilized in laboratory studies (Table 6), the species from the Cricetidae family herein investigated presented low body weight and a gonadosomatic index considered relatively high for mammals [86], particularly in A. cursor and A. montensis. According to the literature [87], relative testis size and sperm morphology are indicators of the mating system, sexual behavior, and reproductive strategies related to sperm competition. Moreover, in order to survive, species with lower body weight are energetically more committed to reproduction [86]. In general, and in contrast to the Hystrichomorpha suborder, rodent species belonging to the Myomorpha suborder (including rats, hamsters and mice) are characterized by lower life expectancy, promiscuous mating system, multiple offspring per litter and per year and sperm with falciform heads instead of spatula shape [88, 89]. In fact, although there is still very little data available in the literature on the species herein investigated, the latter are known to be prolific and present more than one annual reproductive cycle [4]. Moreover, similar to the findings observed for other species from the Cricetidae family [90, 91], the qualitative and quantitative data herein obtained for all four species, such as spermatozoa with sickle-shaped/falciform heads and high DSP per testis gram, suggest that they might reproduce faster and efficiently in order to ensure their survival as a species.
In the present study, the percentage of the seminiferous tubules in the testis parenchyma was very high and similar to most rodents from the Myomorpha and Hystrichomorpha suborders investigated (see Table 6). It means that both Leydig cells’ volume density (%) and the number of these steroidogenic cells per testis gram are expected to be low, particularly compared to most mammalian species already investigated [44, 53, 60, 92].
We did not evaluate the individual Sertoli cell size and its occupancy (%) in the seminiferous epithelium in the present investigation. However, based on the Sertoli cell results herein obtained, such as their comparatively high number per testis gram and their good support capacity for germ cells (Table 6), according to the literature [73], we can assume that their individual size and consequently their occupancy in the seminiferous epithelium is not big. For instance, in mice, which in comparison to the four rodents herein investigated have close values for the seminiferous volume density, tubular diameter and Sertoli cell support capacity, the Sertoli cell percentage in the seminiferous tubule was the smallest (~14%) among the twelve mammalian species investigated [73]. Most importantly, in association with the short duration of spermatogenesis, the Sertoli cells findings herein obtained greatly accounted for the very high sperm production per testis gram (~60–80 million). To our knowledge, up-to-date this range of spermatogenic efficiency is only smaller than or similar to that observed in P. guyannensis and T. moojeni (~78–82 million) that belong to the Hystricomorpha suborder (Table 6). Also contributing to the very high spermatogenic efficiency, except for O. nigripes, the germ cell loss observed in our study was not expressive [53].
Conclusion
In summary, in comparison to the other mammalian species already investigated and, in particular, with other rodents from the suborder Myomorpha, the four cricetid rodents herein evaluated exhibited very high spermatogenic efficiency. This efficiency is mainly related to the short duration of spermatogenesis, the high values found for the percentage of seminiferous tubules in the testis parenchyma and the number of Sertoli cells per testis gram. We expect that, besides contributing to comparative and evolutionary reproductive biology studies, the knowledge herein generated will help to advance the conservation programs and the proper management of wildlife.
Supporting information
(DOCX)
Acknowledgments
We are very grateful to the Pontifical Catholic University of Minas Gerais (PUC-Minas), the Reserva Particular do Patrimônio Natural Santuário do Caraça and to the Image Acquisition and Processing Center (CAPI- ICB/UFMG) for the valuable support. The scientific and technical assistance from Dr. Sônia Aparecida Talamoni, Dr. André Felipe de Figueiredo Almeida and Mara Lívia dos Santos are also highly appreciated.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
We are very thankful to the National Council for Scientific and Technological Development (CNPq) [LRF], the National Council for the Improvement of Higher Education Personnel (CAPES) [DAC], the Foundation for Research Support of Minas Gerais (FAPEMIG) [LRF], the Pro-Rectory of Research of the Federal University of Minas Gerais (PRPq-UFMG) [GMJC] for their financial support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lizzarralde M, Bianchi NO, Goldemberg JB. Ecologia de dos poblaciones de roedores de Tierra Del Fuego. II. Area habitacional, movilidad y su heredabilidade. Physis (Bueno Aires). 1986; 44: 73–81. [Google Scholar]
- 2.Prist PR, Prado A, Tambosi LR, Umetsu F, de Arruda Bueno A, Pardini R, et al. Moving to healthier landscapes: Forest restoration decreases the abundance of Hantavirus reservoir rodents in tropical forests. Sci Total Environ. 2021; 752:141967. 10.1016/j.scitotenv.2020.141967 [DOI] [PubMed] [Google Scholar]
- 3.Herrmann G. Estrutura de comunidades de pequenos mamíferos em áreas secundárias de mata atlântica. Belo Horizonte: UFMG; 1991. [Google Scholar]
- 4.Oliveira J, Bonvicino C, 2006. Ordem Rodentia. In: Reis N, Peracchi A, Pedro W, Lima I, editors, Mamíferos do Brasil. Londrina: Imprensa da UEL, p.347–406. [Google Scholar]
- 5.Redford K, Eisenberg J. Mammals of the Neotropics. The Southern Cone. Chile, Argentina, Uruguay, Paraguay. Illinois: University of Chicago Press; 1992. [Google Scholar]
- 6.Gentile R. Population Dynamics and Reproduction of Marsupials and Rodents in a Brazilian Rural Study: A Five Year Study. Stud. Neotrop. Fauna. E. 2000; 35: 1–9. 10.1076/0165-0521(200004)35:1;1-M;FT001 [DOI] [Google Scholar]
- 7.Patton J, Smith F. Diversification in the Genus Akodon (Rodentia: Sigmodontiane) in Southeastern South America: Mitochondrial DNA Sequence Analysis. J. Mammal. 2001; 82: 92–101. [DOI] [Google Scholar]
- 8.Graipel ME, Miller PRM, Glock L. Padrão de atividade de A. montensis e Oryzomys russatus na reserva de Volta Velha, Santa Catarina, sul do Brasil. Mastozool. Neotrop. 2003; 10: 255–260. [Google Scholar]
- 9.Scheibler DR, Christoff AU. Habitat associations of small mammals in southern Brazil and use of regurgitated pellets of birds of prey for inventorying a local fauna. Braz. J. Biol. 2007; 67: 619–625. 10.1590/s1519-69842007000400005 [DOI] [PubMed] [Google Scholar]
- 10.Vieira EM, Baumgarten LC. Daily activity patterns of small mammal in a cerrado area from cetral Brazil. J. Trop. Ecol. 1995; 11: 255–262. 10.1017/S0266467400008725 [DOI] [Google Scholar]
- 11.Bonvicino CR, Lemos B, Weksler M. Small mammals of Chapada dos Vedeiros national park (Cerrado of central Brazil). Ecologic, Karyologic and taxonomic considerations. Braz. J. Biol. 2005; 65: 395–406. 10.1590/s1519-69842005000300004 [DOI] [PubMed] [Google Scholar]
- 12.Andrades-Miranda J, Nunes AP, Zanchin NIT, Oliveira LFB, Langguth AR, Mattevi MS. Cytogenetic studies in nine taxa of the genus Oryzomys (Rodentia, Sigmodontinae) from Brazil. Mammalia. 2001; 65: 461–472. 10.1515/mamm.2001.65.4.461 [DOI] [Google Scholar]
- 13.Weksler M, Bonvicino CR. Taxonomy of pigmy rice rats genus Oligoryzomys Bangs, 1900 (Rodentia, Sigmodontinae) of the Brazilian Cerrado, with the description of two new species. Arq. Mus. Nac. 2005; 63: 113–130. [Google Scholar]
- 14.Silva JA, Talamoni SA. Core area and centre of activity of maned wolves, Chrysocyon brachyurus (Illiger, 1815) (Mammalia, Canidae), submitted to supplemental feeding. Rev. Bras. Zool. 2004; 21: 391–395. 10.1590/S0101-81752004000200038 [DOI] [Google Scholar]
- 15.Cordeiro DA Jr, Talamoni SA. New data on the life history and occurrence of spiny rats T. moojeni (Rodentia: Echimyidae), in southeastern Brazil. Acta Theriol. 2006; 51: 163–168. [Google Scholar]
- 16.Talamoni SA, Amaro BD, Cordeiro DA Jr, Maciel CEMA. Mammals of Reserva Particular do Patrimônio Natural Santuário do Caraça, state of Minas Gerais, Brazil. Check List (São Paulo. Online). 2014; 10: 1005–1013. 10.15560/10.5.1005 [DOI] [Google Scholar]
- 17.Parreira GG, Cardoso FM. Biologia reprodutiva de machos Bolomys lasiurus Lund, 1841 (Rodentia, Cricetidae). I. Morfologia da espermatogênese e ciclo do epitélio seminífero. Rev. Bras. Biol. 1991; 5: 639–646. [Google Scholar]
- 18.Couto D, Talamoni AS. Reproductive condition of Akodon montensis Thomas and Bolomys lasiurus (Lund) (Rodentia, Muridae) based on histological and histometric analyses of testes and external characteristics of gonads. Acta Zool. 2005; 86: 111–118. 10.1111/j.1463-6395.2005.00191 [DOI] [Google Scholar]
- 19.Duarte APG. Aspectos reprodutvos de Akodon montensis (Rodentia, Muridae) e Artibeus lituratus (Chiroptera, Phyllostomidae) em áreas de mata no sudeste do Brasil. Belo Horizonte: PUC-Minas; 2003. [Google Scholar]
- 20.D’elía G, Mora I, Myers P, Owen RD. New and noteworthy records of Rodentia (Erethizontidae, Sciuridae, and Cricetidae) from Paraguay. Zootaxa. 2008; 1784: 39–57. 10.5281/zenodo.182407 [DOI] [Google Scholar]
- 21.INSTITUTO NACIONAL DE PESQUISAS ESPACIAIS (INPE). PRODES–Amazônia [internet]. São José dos Campos: Instituto Nacional de Pesquisas Espaciais; 2019. Available from: http://www.inpe.br/noticias/noticia.php?Cod_Noticia=5320 [Google Scholar]
- 22.Castellar A, Bertoli PC, Galdino LH, Domeniconi RF, Cruz-Neto AP. Differences in physiological traits associated with water balance among rodents, and their relationship to tolerance of habitat fragmentation. J Exp Zool A Ecol Genet Physiol. 2015; 323: 731–744. 10.1002/jez.1966 [DOI] [PubMed] [Google Scholar]
- 23.Bovendorp RS, Villar N, de Abreu-Junior EF, Bello C, Regolin AL, Percequillo AR, et al. Atlantic small-mammal: a dataset of communities of rodents and marsupials of the Atlantic forests of South America. Ecology. 2017; 98: 2226. 10.1002/ecy.1893 [DOI] [PubMed] [Google Scholar]
- 24.Owen RD, Camp JV, Jonsson CB. Sigmodontine community and species responses to El Niño and precipitation in different levels of forest degradation. Therya. 2019; 10: 255–265. 10.12933/therya-19-899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devictor V, Julliard R, Couvet D, Jiguet F. Birds are tracking climate warming, but not fast enough. Proc. R. Soc. B. 2008; 275: 2743–2748. 10.1098/rspb.2008.0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenseth NC, Ottersen G, James W. Hurrell JW, Mysterud A, Lima M, et al. Studying climate effects on ecology through the use of climate indices: The North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc Biol Sci. 2003; 270: 2087–2096. 10.1098/rspb.2003.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umetsu F, Pardini R. Small mammals in a mosaic of forest remnants and anthropogenic habitats—evaluating matrix quality in an Atlantic forest landscape. Lands Ecol. 2007; 22: 517–530. 10.1007/s10980-006-9041-y [DOI] [Google Scholar]
- 28.Thompson RCA. Parasite zoonoses and wildlife: one health, spillover and human activity. Int. J. Parasitol. 2013; 43: 1079–1088. 10.1016/j.ijpara.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaz VC, D’Andrea PS, Jansen AM. Effects of habitat fragmentation on wild mammal infection by Trypanosoma cruzi. Parasitology. 2007; 134: 1785–1793. 10.1017/S003118200700323X [DOI] [PubMed] [Google Scholar]
- 30.Oliveira RC, Teixeira BR, Mello FCA, Pereira AP, Duarte AS, Bonaldo M C, et al. Genetic characterization of a Juquitiba-like viral lineage in Oligoryzomys nigripes in Rio de Janeiro, RJ, Brazil. Acta Trop. 2009; 112: 212–218. 10.1016/j.actatropica.2009.07.029 [DOI] [PubMed] [Google Scholar]
- 31.Cardoso TS, Simões RO, Luque JLF, Maldonado A Jr, Gentile R. The influence of habitat fragmentation on helminth communities in rodent populations from Brazilian Mountain Atlantic Forest. J. Helminthol. 2016; 90: 460–468. 10.1017/S0022149X15000589 [DOI] [PubMed] [Google Scholar]
- 32.Gonçalves LR, Favacho ARM, Roque ALR, Mendes NS, Junior OLF, Benevenute JL, et al. Association of Bartonella species with wild and synanthropic rodents in different brazilian biomes. Appl. Environ. Microbiol. 2016; 82: 7154–7164. 10.1128/AEM.02447-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira CNS, Carvalho RF, Pacheco MACM., Lessa LG. A new record of Calassomys apicalis (Rodentia, Cricetidae) in the Espinhaço Mountain Range, Brazil. R. bras. Zoo. 2017; 18: 45–50. 10.34019/2596-3325.2017.v18.24672 [DOI] [Google Scholar]
- 34.Gentile R, Cardoso TS, Costa-Neto SF, Teixeira BR, D’Andrea PS, Community structure and population dynamics of small mammals in an urban-sylvatic interface area in Rio de Janeiro, Brazil. Zoologia. 2018; 35: e13465. 10.3897/zoologia.35.e13465 [DOI] [Google Scholar]
- 35.França LR. Análise morfofuncional da espermatogênese de suínos adultos da raça Piau. Belo Horizonte: UFMG; 1991. [Google Scholar]
- 36.França LR, Godinho CL. Testis morphometry, seminiferous spithelium cycle length, and daily sperm production in domestic cats (Felis catus). Biol. Reprod. 2003; 68: 1554–1561. 10.1095/biolreprod.102.010652 [DOI] [PubMed] [Google Scholar]
- 37.Leal MC, França LR. The Seminiferous Epithelium Cycle Length in the Black Tufted-Ear Marmoset (Callithrix penicillata) is Similar to Humans. Biol. Reprod. 2006; 74: 616–624. 10.1095/biolreprod.105.048074 [DOI] [PubMed] [Google Scholar]
- 38.Costa GMJ, Garcia HC, Morato RG, Alvarenga RLLS, França LR. Duration of spermatogenesis and daily sperm production in the jaguar (Panthera onca). Theriogenology. 2008; 70: 1136–1146. 10.1016/j.theriogenology.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 39.Leal MC, França LR. Slow increase of Sertoli cell efficiency and daily sperm production causes delayed establishment of full sexual maturity in the rodent Chinchilla lanigera. Theriogenology. 2009; 71: 509–518. 10.1016/j.theriogenology.2008.08.017 [DOI] [PubMed] [Google Scholar]
- 40.Menezes TP, Hill E, de Alencar Moura A, Lobo MDP, Monteiro-Moreira ACO, Breton S, et al. Pattern of protein expression in the epididymis of Oligoryzomys nigripes (Cricetidae, Sigmodontinae). Cell Tissue Res. 2018; 372: 135–147. 10.1007/s00441-017-2714-9 [DOI] [PubMed] [Google Scholar]
- 41.Menezes TP, Castro MM, Vale JA, Moura AA, Lessa G, Machado-Neves M, Proteomes and morphological features of Calomys tener and Necromys lasiurus (Cricetidae, Sigmodontinae) epididymides. J. Mammal. 2017; 98: 579–590 10.1093/jmammal/gyw201 [DOI] [Google Scholar]
- 42.Morais AC, Balarini MK, Menezes TP, Ferraz FS, Gomes ML, Morais DB, et al. Germ cells and the seminiferous epithelium cycle in the wild rodent Oxymycterus rufus (Rodentia: Cricetidae). IOSR J Pharm Biol Sci. 2016;11: 61–71. [Google Scholar]
- 43.Johnson L, Varner DD, Roberts ME, Smith TL, Keillor GE, Scrutchfield WL. Efficiency of spermatogenesis: a comparative approach. Anim. Reprod. Sci. 2000; 60–61: 471–480. 10.1016/s0378-4320(00)00108-1 [DOI] [PubMed] [Google Scholar]
- 44.França LR, Avelar GF, Almeida FF. Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology. 2005; 63: 300–318. 10.1016/j.theriogenology.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 45.Setchell BP, Breed WG. Anatomy, vasculature and innervation of the male reproductive tract. In: Neil JD, editor. Physiology of Reproduction. Pittsburgh: Elsevier; 2006. p. 771–825. [Google Scholar]
- 46.Wildt DE. Lions, Tigers, and Pandas, Oh My. J. Androl. 2005; 26: 452–454. 10.2164/jandrol.05046 [DOI] [PubMed] [Google Scholar]
- 47.Iaeger CT, Toni JV, Marinho JR. Sexual dimorphism and seasonal variation in a rodent community in southern Brazil. Perspectiva. 2015; 39: 53–60. [Google Scholar]
- 48.Moojen J. Os Roedores do Brasil. Rio de Janeiro: Instituto Nacional do Livro; 1952. [Google Scholar]
- 49.Fonseca GAB, Kierulff MCM. Biology and natural history of Brazilian Atlantic Forest small mammals. Bull Fla Mus Nat Hist Biol Sci; 1988. 34: 99–152 [Google Scholar]
- 50.Sprando RL. Perfusion of the rat testis through the heart using heparin. In: Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED, editors. Histological and Histopathological Evaluation of the Testis. Vienna: Cache River Press; 1990. p. 277–280. [Google Scholar]
- 51.Bundy DC. Photografic emulsions and processing. In: Stumpf WE, Solomon HF, editors. Autoradiography and correlative imaging. San Diego: Academic Press; 1995. p. 49–57. [Google Scholar]
- 52.Johnson L, Neaves WB. Age-related changes in the Leydig cell population, seminiferous tubules, and sperm production in stallions. Biol. Reprod. 1981; 24: 703–712. 10.1095/biolreprod24.3.703 [DOI] [PubMed] [Google Scholar]
- 53.Hess RA, França LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008; 636:1–15. 10.1007/978-0-387-09597-4_1 [DOI] [PubMed] [Google Scholar]
- 54.Amann RP, Schanbacher BD. Physiology of male reproduction. J. Anim. Sci. Suppl. 1983; 57: 380–403. 10.2527/animalsci1983.57Supplement_2380x [DOI] [PubMed] [Google Scholar]
- 55.Abercrombie M, Johnson ML. Quantitative histology of Wallerian degeneration I. Nuclear population in rabbit sciatic nerve. J. Anat. Lond. 1946; 80: 37–50. 10.1136/jnnp.9.4.113 [DOI] [PubMed] [Google Scholar]
- 56.Amann RP, Almquist JO. Reproductive capacity of dairy bulls. VIII. Direct and indirect measurement of testicular sperm production. J. Dairy Sci. 1962; 45: 774–781. 10.3168/jds.S0022-0302(62)89487-9 [DOI] [Google Scholar]
- 57.Russell LD, Clermont Y. Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat. Rec. 1977; 187: 347–366. 10.1002/ar.1091870307 [DOI] [PubMed] [Google Scholar]
- 58.Hochereau-De-Reviers MT, Lincoln GA. Seasonal-variation in histology of testis of red deer, Cervus-elaphus. J. Reprod. Fertil. 1978; 54: 209–213. 10.1530/jrf.0.0540209 [DOI] [PubMed] [Google Scholar]
- 59.Costa GMJ, Lacerda SMSN, Figueiredo AFA, Leal MC, Rezende-Neto JV, França LR. Higher environmental temperatures promote acceleration of spermatogenesis in vivo in mice (Mus musculus). J. Therm. Biol. 2018; 77: 14–23. 10.1016/j.jtherbio.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 60.França LR, Russel LD. The testis of domestic animals. In: Regadera J, Martinez-Garcia F, editors. Male reproduction: a multidisciplinary overview, Madrid: Churchill Livingstone; 1998. p.197–219. [Google Scholar]
- 61.França LR, Hess RA. Structure of the Sertoli cell. In: Skinner M, Griswold M, editors. Sertoli cell Biology. San Diego: Elsevier Academic Press; 2005. p.19–40. [Google Scholar]
- 62.Neves EM, Costa GMJ, França LR. Sertoli cell and spermatogenic efficiencies in Pêga Donkey (Equus asinus). Anim. Reprod. 2014; 11: 517–525. [Google Scholar]
- 63.França LR. Daily sperm production in Piau boars estimated from Sertoli cell population and Sertoli cell index. In: Dieleman SJ, editor. Proceedings of the 12th International Congress on Animal Reproduction and Artificial Insemination. The Hague: ICAR; 1992. p.1716–1718. [Google Scholar]
- 64.Nowak RM. Walker’s Mammals of the World. 6th ed. London: Johns Hopkins University Press; 1999. [Google Scholar]
- 65.Boiani L, Casanova G, Oliveira A, Berois N. Seminiferous epithelium cycle of a hantavirus reservoir, the long-tailed mouse Oligoryzomys flavescens (Rodentia—Cricetidae). Tissue Cell. 2007; 39: 267–275. 10.1016/j.tice.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 66.Lage RR, Schimidt SEM. Rodentia–Roedores Silvestres (Capivara, Cutia, Paca, Ouriço). In: Cubas ZS, Silva JCR, Catão-Dias JL, editors. Tratado de Animais Selvagens Medicina Veterinária. São Paulo: Roca; 2007. p.475–491. [Google Scholar]
- 67.Cordeiro DA Jr, Costa GMJ, Talamoni AS, França LR. Spermatogenic efficiency in the spiny rat, Trinomys moojeni (Rodentia: Echimyidae). Anim. Reprod. Sci. 2010; 119: 97–105. 10.1016/j.anireprosci.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 68.Salazar‐Bravo J, Pardiñas UF, D’Elía G. A phylogenetic appraisal of Sigmodontinae (Rodentia, Cricetidae) with emphasis on phyllotine genera: systematics and biogeography. The Norwegian Academy of Science and Letter 2013;42: 250–261. [Google Scholar]
- 69.França LR, Ogawa T, Avarbock MR, Brinter RL, Russell LD. Germ cell genotype control cells cycle during spermatogenesis in the rat. Biol. Reprod. 1998; 59: 1371–1377. 10.1095/biolreprod59.6.1371 [DOI] [PubMed] [Google Scholar]
- 70.Clermont Y. Kinetics of spermatogenesis in mammals, seminiferous epithelium cycle and spermatogonial review. Physiol. Rev. 1972; 52: 198–236. 10.1152/physrev.1972.52.1.198 [DOI] [PubMed] [Google Scholar]
- 71.Ortavant R. Autoradiographie des cellules germinales du testicule de bélier durée des phénomènes spermatogénétiques. Arch. Anat. Microsc. Morphol. Exp. 1956; 45: 1–10. [PubMed] [Google Scholar]
- 72.Lacerda SMSN, Batlouni SR, Silva SBG,Homem CSP, França LR. Germ cells transplantation in fish: the Nile-tilapia model. Anim. Reprod. 2006; 3: 146–159. [Google Scholar]
- 73.Russell LD, Ettlin RA, Sinha-Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River Press; 1990. p. 1–286. [Google Scholar]
- 74.Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956; 99: 507–516. 10.1002/aja.1000990307 [DOI] [PubMed] [Google Scholar]
- 75.Costa GM, Leal MC, Ferreira CS, Guimarães DA, França LR. Duration of spermatogenesis and spermatogenic efficiency in 2 large neotropical rodent species: the agouti (Dasyprocta leporina) and paca (Agouti paca). J Androl. 2009; 31: 489–499. 10.2164/jandrol.109.009787 [DOI] [PubMed] [Google Scholar]
- 76.Silva RC, Costa GMJ, Andrade LM, França LR. Testis stereology, seminiferous epithelium cycle length, and daily sperm production in the ocelot (Leopardus pardalis). Theriogenology. 2010; 73: 157–167. 10.1016/j.theriogenology.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 77.Segatelli TM, Franca LR, Pinheiro PF, Alemida CC, Martinez M, Martinez FE. Spermatogenic cycle length and spermatogenic efficiency in the gerbil (Meriones unguiculatus). J Androl. 2004; 25: 872–880. 10.1002/j.1939-4640.2004.tb03156.x [DOI] [PubMed] [Google Scholar]
- 78.Sinha-Hikim AP, Bartke A, Russell, LD. Morphometric studies on hamster testes in gonadally active and inactive states: light microscope findings. Biol. Reprod. 1988; 39: 1225–1237. 10.1095/biolreprod39.5.1225 [DOI] [PubMed] [Google Scholar]
- 79.Van Haaster LH, De Rooij DG. Cycle of the seminiferous epithelium in the Djungarian hamster (Phodopus sungorus sungorus). Biol. Reprod. 1993; 48: 515–521. 10.1095/biolreprod48.3.515 [DOI] [PubMed] [Google Scholar]
- 80.Clermont Y, Harvey SC. Duration of the cycle of the seminiferous epithelium of normal, hypophysectomized and hypophysectomized-hormone treated albino rat. Endocrinology. 1965; 76: 80–89. 10.1210/endo-76-1-80 [DOI] [PubMed] [Google Scholar]
- 81.Rocha DCM, Debeljuk L, França LR. Exposure to constant light during testis development increase daily sperm production in adult Wistar rats. Tissue Cell. 1999; 31: 372–379. 10.1054/tice.1999.0043 [DOI] [PubMed] [Google Scholar]
- 82.França LR. Espermatogénesis (Espermatogénesis, producción y tránsito del esperma a través del epidídimo). [Spermatogenesis (Spermatogenesis, sperm production and transit through the epididymis).] Separata. 2007; 15: 16–27. [Google Scholar]
- 83.Clermont Y, Trott M. Duration of the cycle of the seminiferous epithelium in the mouse and hamster determined by means of 3H-thymidine and radioautography. Fert. Steril. 1969; 20: 805–817. 10.1016/S0015-0282(16)37153-9. [DOI] [PubMed] [Google Scholar]
- 84.Oliveira CFA, Lara NLM, Cardoso BRL, França LR, Avelar GF. Comparative testis structure and function in three representative mice strains. Cell Tissue Res. 2020; 382: 391–404. 10.1007/s00441-020-03239-0 [DOI] [PubMed] [Google Scholar]
- 85.Lara NLM, Santos IC, Costa GMJ, Cordeiro-Junior DA, Almeida ACG, Madureira AP, et al. Duration of spermatogenesis and daily sperm production in the rodent Proechimys guyannensis. Zygote. 2016; 16: 1–11. 10.1017/S0967199416000137 [DOI] [PubMed] [Google Scholar]
- 86.Kenagy GJ, Trombulak SC. Size of mammalian testes in relation to body size. J. Mammal. 1986; 67: 1–22. [Google Scholar]
- 87.Short RV. The testis: the witness of the mating system, the site of mutation and the engine of desire. Acta Paediatr Suppl. 1997; 422: 3–7. 10.1111/j.1651-2227.1997.tb18336.x [DOI] [PubMed] [Google Scholar]
- 88.Eisenberg JF. The mammalian radiations: An analysis of trends in evolution, adaptation and behavior. Chicago: University of Chicago Press; 1981. p. 1–610. [Google Scholar]
- 89.Eddy EM, O’Brien DA, The spermatozoon. In: Knobil E, Neil JD, editors. The physiology of reproduction. New York: Raven; 1994. p. 29–77. [Google Scholar]
- 90.Melo FCSA, Sousa TP, Costa KLC, Matta SLP, Melo FR, Santa-Rita RM. Descriptive morphometry and stereology of the tubular compartment in the wild rodent Hylaeamys megacephalus (Rodentia: Cricetidae) from Central Brazil. Anim. Reprod. Sci. 2013; 138: 110–117. 10.1016/j.anireprosci.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 91.Morais ACT, Balarini MK, Lopes EO. Menezes TP, Quintela FM, Morais DB, et al. “The tubular compartment and the spermatogenic dynamics of the wild rodent Oxymycterus nasutus (Rodentia: Cricetidae),” Anim. Reprod. Sci. 2014; 149: 249–258. 10.1016/j.anireprosci.2014.06.027 [DOI] [PubMed] [Google Scholar]
- 92.Russell L.D. Mammalian Leydig cell structure. In: Payne AH, Hardy MP, Russell LD, editors. The Leydig cell. Vienna: Cache River; 1996. p. 218–222. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.