Abstract
Alterations in cardiac energy metabolism contribute to the severity of heart failure. However, the energy metabolic changes that occur in heart failure are complex, and are dependent not only on the severity and type of heart failure present, but also on the co-existence of common co-morbidities such as obesity and type 2 diabetes. The failing heart faces an energy deficit, primarily due to a decrease in mitochondrial oxidative capacity. This is partly compensated for by an increase in ATP production from glycolysis. The relative contribution of the different fuels for mitochondrial ATP production also changes, including a decrease in glucose and amino acid oxidation, and an increase in ketone oxidation. The oxidation of fatty acids by the heart increases or decreases, depending on the type of heart failure. For instance, in heart failure associated with diabetes and obesity, myocardial fatty acid oxidation increases, while in heart failure associated with hypertension or ischemia, myocardial fatty acid oxidation decreases. Combined, these energy metabolic changes result in the failing heart becoming less efficient (i.e., a decrease in cardiac work/O2 consumed). The alterations in both glycolysis and mitochondrial oxidative metabolism in the failing heart are due to both transcriptional changes in key enzymes involved in these metabolic pathways, as well as alterations in redox state (NAD+ and NADH levels) and metabolite signaling that contribute to post-translational epigenetic changes in the control of expression of genes encoding energy metabolic enzymes. Alterations in the fate of glucose, beyond flux through glycolysis or glucose oxidation, also contribute to the pathology of heart failure. Of importance, pharmacological targeting of the energy metabolic pathways has emerged as a novel therapeutic approach to improving cardiac efficiency, decreasing the energy deficit and improving cardiac function in the failing heart.
Keywords: mitochondria, glucose oxidation, fatty acid oxidation, ketones, insulin resistance, diabetic cardiomyopathy, lipotoxicity, branched chain amino acids, acetylation, NAD+, NADH
Introduction
The heart has a very high energy demand, and must continuously produce large amounts of ATP to sustain contractile function 1, 2. For instance, if not replaced, the heart would run out of ATP in 2–10 seconds, resulting in contractile failure. As a result, the continuous production of ATP must occur in order to maintain cardiac function. The heart achieves this by metabolising a variety of fuels (including fatty acids, glucose, lactate, ketones, pyruvate, and amino acids), primarily by mitochondrial oxidative phosphorylation. This process requires large amounts of oxygen, resulting in the heart consuming more oxygen/unit weight than any other organ in the body. Any disruptions in the energy metabolic pathways that produce ATP, or in oxygen supply to the heart, can have catastrophic consequences on cardiac function. As a result, compromised energy production by the heart is an important contributor to most forms of heart disease 1, 3.
Heart failure is a debilitating disease that has a major clinical and economic impact on the world’s population 4. The inability of the heart to adequately pump enough blood to meet the body’s needs for nutrients and oxygen results in heart failure patients having significant disabilities and a high mortality rate 5. Heart failure presents primarily as two major types, heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). Both types of heart failure, and their associated co-morbidities and mortality, have a comparable prevalence in our society 6–8. Heart failure is a heterogenous clinical syndrome, that is caused by multiple different co-morbidities, with ischemic heart disease and hypertension being prominent contributors to heart failure development. It is generally accepted that altered energy metabolism characterizes the failing heart, resulting in an “energy deficit” which contributes to the severity of heart failure (see 3 for review). This compromised energy production results from a number of factors, which includes impaired mitochondrial oxidative metabolism, alterations in energy substrate preference by the heart, and a decrease in cardiac efficiency 9, 10. More recently, altered flux via diverse metabolic pathways have been shown to generate metabolites or redox alterations that activate pathways that promote myocardial injury and may contribute to ventricular dysfunction 11. Here we review the current knowledge of the energy metabolic changes that occur in heart failure. This includes distinguishing the cardiac energetic changes that occur in HFrEF, as well as heart failure associated with diabetes (i.e., diabetic cardiomyopathy). Although we briefly address the cardiac metabolic changes that occur in HFpEF the majority of the review will focus on cardiac energy metabolic changes seen in HFrEF, given that to date the majority of studies have examined HFrEF and a consensus on metabolic changes associated with HFpEF has not yet emerged. We also address the underlying mechanisms responsible for these changes in cardiac energy metabolism in heart failure, and how targeting cardiac energy metabolism may be a novel approach to treating heart failure.
Energy metabolism in the normal heart
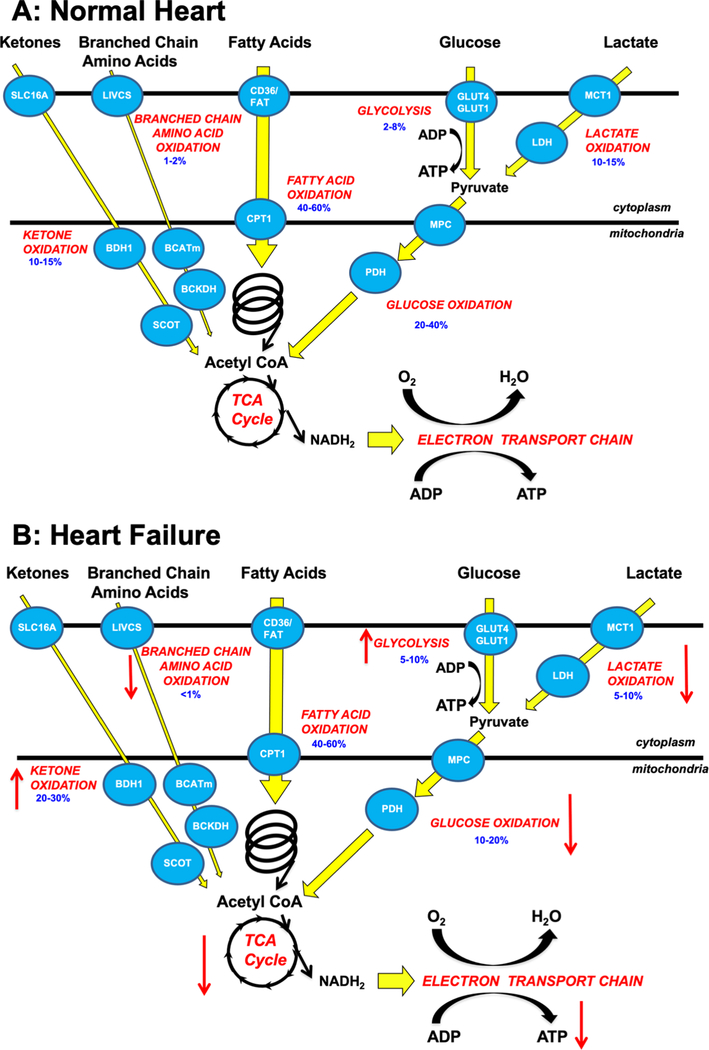
The adult heart generates enormous quantities of ATP necessary to sustain contractile function from two primary sources: mitochondrial oxidative phosphorylation and glycolysis. Mitochondrial oxidative phosphorylation normally contributes ~95% of myocardial ATP requirements, with glycolysis providing the remaining 5% 12, 13. The healthy heart is also “metabolically flexible”, and can readily shift between different energy substrates to maintain ATP production 2. The main fuels of the heart are fatty acids, lactate, glucose, ketones, and amino acids, which must be acquired continuously from the blood due to a low ability of the heart to store these energy substrates intracellularly. The majority of mitochondrial ATP production, ~40–60%, is derived from the oxidation of fatty acids and the remainder originating from the oxidation of pyruvate (originating from glucose and lactate), ketone bodies and amino acids (Figure 1). The majority of the oxygen consumed by the heart is used for mitochondrial oxidative phosphorylation by the electron transport chain (ETC), while the synthesis of ATP derived from glycolysis does not require oxygen (Figure 1A).
Figure 1: Overview of energy metabolism in the normal heart and failing heart.
A: Glucose is transported into the cell via glucose transporter 1 or 4 (GLUT1, GLUT4), it then undergoes glycolysis to produce pyruvate. Lactate is taken up by the cardiomyocytes via monocarboxylic acid transporters (MCT) and converted to pyruvate by lactate dehydrogenase (LDH). The pyruvate from glucose and lactate is transported into the mitochondria via the mitochondrial pyruvate carrier (MPC) and is converted to acetyl CoA by pyruvate dehydrogenase (PDH). Fatty acids are transported into the cardiomyocyte, partly via CD36 and FA transport protein-1 (FATP-1), where they are esterified to fatty acyl-CoA. The acyl group are transferred to carnitine by carnitine palmitoyl transferase (CPT-1) and transported into the mitochondria where CPT-2 converts it back to fatty acyl CoA which can then undergo β-oxidation producing acetyl CoA. Ketones (i.e., ß-hydroxybutyrate) are transported into the cell via SLC16A1 where βOHB dehydrogenase 1 (BDH1) catalyses the oxidation of βOHB to acetoacetate (AcAc). AcAc is then activated by succinyl-CoA:3 oxoacid-CoA transferase (SCOT) to acetoacetyl-CoA (AcAc-CoA) which undergoes a thiolysis reaction producing acetyl-CoA. Branched chain amino acids (BCAAs) are transported into the cell by the branched chain amino acid:cation symporter family (LIVCS). In the mitochondria, BCAAs are converted to ketoacids by branched chain aminotransferase (BCATm). Acetyl CoA and succinyl CoA are subsequently formed from branch chain acid alpha-keto acid dehydrogenase (BCKDH). The acetyl CoA generated by fatty acid β-oxidation, glucose oxidation, ketone oxidation and BCAA oxidation enter the tricarboxylic acid (TCA) cycle, generating flavin adenine dinucleotide (FADH2) and nicotinamide adenine dinucleotide (NADH) which then enters the electron transport chain, consuming oxygen (O2) to generate adenosine triphosphate (ATP). Numbers in blue represent the contribution of the individual pathways to overall ATP production.
B: Alterations in ketone oxidation, amino acid oxidation, fatty acid oxidation, glycolysis, glucose oxidation, and lactate oxidation in the failing heart. An arrow facing up indicates an increase and down indicates a decrease. Numbers in blue represent the contribution of the individual pathways to overall ATP production.
Abbreviations: Glucose transporter 1 and 4 (GLUT1, GLUT4), mitochondrial pyruvate carrier (MPC), pyruvate dehydrogenase (PDH), monocarboxylate transporter (MCT), lactate dehydrogenase (LDH), CD36/fatty acid transporter (CD36/FAT), carnitine palmitoyl transferase (CPT-1), nicotinamide adenine dinucleotide (NADH2), adenosine triphosphate (ATP), adenosine diphosphate (ADP), tricarboxylic acid (TCA), β-hydroxybutyrate (βOHβ), monocarboxylate transporter 1 (SLC16A1), β-hydroxybutyrate dehydrogenase 1 (BDH1), succinyl-CoA:3 oxoacid-CoA transferase (SCOT), branched chain amino acid cation symporter (LIVCS), mitochondrial branched chain amino-transaminase (BCATm), branched chain α-keto acid dehydrogenase (BCKDH).
Fatty acids are delivered to the heart either as fatty acids bound to albumin in the blood, or as fatty acids hydrolysed from triacylglycerols (TGs) contained in chylomicrons and very low-density lipoproteins (VLDL). Following uptake into the cardiomyocyte, fatty acids are esterified forming fatty acyl-CoA. The fatty acid moiety is then transferred to carnitine by carnitine palmitoyl transferase 1 (CPT-1) to form long chain acylcarnitine 14, which is then transported into the mitochondria where the fatty acid group is again transferred to CoA to form fatty acyl-CoA. This fatty acyl-CoA then undergoes β-oxidation to produce acetyl-CoA, that enters the TCA cycle, and flavin adenine dinucleotide (FADH2) and nicotinamide adenine dinucleotide (NADH) which enter the ETC to generate ATP. The TCA cycle also generates NADH and FADH2 that feed into the ETC, which in the presence of oxygen results in the conversion of ADP to ATP (Figure 1). Fatty acid oxidation in the heart is highly regulated, including via: 1) fatty acid supply to the heart, 2) fatty acid uptake, 3) malonyl CoA induced CPT-1 inhibition 15 (malonyl CoA is produced via acetyl CoA carboxylase 16 and degraded by malonyl CoA decarboxylase 17, 4) the ratios of FAD/FADH2 and NAD+/NADH (which can influence enzymatic activity of acyl-CoA dehydrogenase and 3-hydroxyacyl-CoA dehydrogenase, respectively), 5) the mitochondrial acetyl-CoA/CoA ratio which can influence the activity of 3-ketoacyl-CoA thiolase, 6) post-translational modification of fatty acid oxidative enzymes, and 7) transcriptional regulation of fatty acid oxidative enzyme expression. This highly coordinated regulation of fatty acid oxidation is central to the ability of the heart to maintain its ability to switch between available substrates. While fatty acids produce the greatest ATP yield per 2 carbon unit of all energy substrates, they also have the highest oxygen requirement to produce this ATP. As a result, fatty acids are the least efficient (ATP produced/O2 consumed) myocardial energy substrates.
Glucose is an important fuel of the heart that can generate ATP both from cytoplasmic glycolysis, and the mitochondrial oxidation of the pyruvate derived from glycolysis (Figure 1A). It is the most efficient of the energy substrates, due primarily to the anaerobic production of ATP generated by the glycolytic conversion of glucose to pyruvate. Glucose is taken up by the cardiomyocyte GLUT1 and GLUT4 transporters. Although GLUT4 is responsible for insulin-dependent uptake of glucose, its translocation is also modulated by myocardial contraction. As such, GLUT4 quantitatively remains the major portal for myocardial glucose uptake (Figure 1A). Once glucose is transported into the cell it is phosphorylated by hexokinase, generating glucose 6-phosphate (G6P). G6P can then be utilised in multiple pathways, which include the generation of pyruvate via glycolysis, the synthesis of glycogen, or being shuttled into the hexosamine biosynthetic or pentose phosphate pathways. The pyruvate generated from glycolysis can either be converted to lactate or be transported into the mitochondria via the mitochondrial pyruvate carrier (MPC). The majority of pyruvate is converted to acetyl-CoA by pyruvate dehydrogenase (PDH) and lesser amounts to oxaloacetate via pyruvate carboxylation (mediated by malic enzyme or pyruvate carboxylase) to generate oxaloacetate contributing importantly to anaplerosis that replenishes TCA intermediates. Acetyl-CoA is then further metabolized in the TCA cycle. PDH is activated via dephosphorylation by PDH phosphatase and inhibited by PDH kinase (PDK), the latter of which is activated by increased acetyl-CoA/CoA and NADH/NAD+ ratios.
Lactate is also an important energy substrate of the heart, especially under conditions in which circulating lactate levels rise 18, 19. Lactate is taken up by the heart via a monocarboxylic anion transporter (MCT4), and then converted to pyruvate via lactate dehydrogenase. This pyruvate then follows a similar fate as the pyruvate derived from glycolysis. Recent studies have suggested that lactate might represent the major source of pyruvate in the heart 20, may have signaling properties 21 and could under some circumstances provide carbons to the TCA in a pyruvate-independent manner 22, 23.
Ketone bodies are increasingly being recognized as an important energy substrate of the heart 24, 25. Ketone bodies are produced in the liver from acetyl-CoA (predominately sourced from fatty acid oxidation). β-hydroxybutyrate (βOHB) is the predominant ketone body oxidised in the heart. Its uptake is facilitated by SLC16A1 after which it is transported into the mitochondria for oxidation (Figure 1). β-hydroxybutyrate dehydrogenase 1 (BDH1) catalyses the oxidation of βOHB to acetoacetate (AcAc). AcAc is then activated by the CoA transferase succinyl-CoA:3 oxoacid-CoA transferase (SCOT) to acetoacetyl-CoA (AcAc CoA). AcAc-CoA then undergoes a thiolysis reaction from which acetyl-CoA is produced, that then enters the TCA cycle (Figure 1A). Ketones are readily metabolized by the heart, and if circulating ketone levels are elevated they can become a major fuel of the heart 26. When considering oxygen consumption for ATP production, ketones are more efficient than fatty acids, but less energy efficient than glucose (discussed in more detail below).
Oxidation of amino acids are also a potential source of ATP production by the heart. Branched chain amino acid (BCAA) oxidation is the best characterized source of amino acid oxidation in the heart 27. The first step of BCAA metabolism in the heart involves their transamination to their corresponding branched chain α-keto-acid (BCKA) by the mitochondrial branched chain amino-transaminase (BCATm) 28. This step is reversible and involves the transfer of the α-amino group to α-ketoglutarate producing glutamate. The second step in BCAA metabolism involves the oxidative decarboxylation of the BCKAs by the mitochondrial branched chain α-keto acid dehydrogenase (BCKDH). BCKDH activity is regulated by phosphorylation of BCKDH by a BCKDH kinase (BDK), which inhibits activity, and dephosphorylation by PPC2m, which activates BCKDH. The products of BCKDH either generates acetyl-CoA for the TCA cycle or succinyl-CoA for anaplerosis (Figure 1). While BCAA oxidation is only a minor source of ATP production in the heart (<2% of ATP production)19, BCAAs do have an important role in modulating signaling pathways in the heart including insulin and mTOR signaling. In particular, increased systemic BCAAs can promote development of insulin resistance 29. Two main mechanisms have been proposed for BCAA induced insulin resistance: 1) persistent mTOR signaling (in particular via leucine), that impairs insulin signal transduction via insulin receptor substrates (IRS) and 2) impaired BCAA metabolism, resulting in accumulation of BCAA metabolites that exert toxic effects.
In summary, the healthy adult heart has a high metabolic flexibility, with fatty acids being the predominant substrate used for ATP production, followed lactate, ketone bodies, glucose, and then branched chain amino acids.
Fuel Preference in the Failing Heart
Dramatic changes in energy metabolism can occur in the failing heart. Of importance, the failing heart loses its metabolic flexibility and can become energy deficient due to a decrease in its ability to produce ATP 2, 3. As a result, the end-stage failing heart can have up to 30% less ATP content than a healthy heart 30, 31. As mentioned, the majority of ATP produced in a healthy heart occurs as a result of mitochondrial oxidative metabolism, and this energy deficit is likely due to the presence of a reduced mitochondrial oxidative capacity in heart failure (Figure 1B) 32, 33. Impaired mitochondrial function in the failing heart can occur due to a number of reasons, including: 1) increased reactive oxygen species (ROS) production and dysregulation of mitochondrial Ca2+ homeostasis, 2) impairments in mitochondrial dynamics, sustained mitophagy, and increased autophagic cell death of cardiomyocytes and 3) alterations in transcriptional regulation of mitochondrial proteins and increases in post-translational protein modification (see 32, 34–41 for reviews).
Heart failure is characterized by changes in myocardial redox regulation, predominantly characterized by oxidative stress, although reductive stress has also been described 42. Increased ROS has been described in failing human hearts and in animal models of heart failure 43–46. Furthermore, circulating ROS levels have been shown to predict cardiovascular outcomes in some patients 47. Mechanistic studies suggest that ROS increases lipid peroxidation, damages mitochondrial DNA, depletes antioxidants and reduces mitochondrial ATP production 47, 48. Mitochondrial Ca2+ is an important contributor to mitochondrial dysfunction in heart failure 49. Insufficient Ca2+ could reduce the activity of metabolic enzymes in the mitochondria while mitochondrial Ca2+ overload may activate cell death pathways 50. Moreover, the dysregulation of Ca2+ homeostasis seen in heart failure, may result in the mitochondria acting as a sink for Ca2+.
Mitochondrial dynamics describes a process by which mitochondrial undergo cycles of fission and fusion, which are essential for maintaining mitochondrial homeostasis 51, 52. This process is regulated by conserved proteins that include the fusion proteins OPA1 and mitofusins (Mfn1 and Mfn2) and the fission proteins Drp1 and Fis1. Mitochondrial fusion contributes to the formation of elongated interconnected mitochondrial networks and fission may lead to mitochondrial fragmentation or disruption of the myocardial mitochondrial network 53. Expression of proteins that regulate mitochondrial dynamics are altered in failing hearts, with altered morphology consistent with increased mitochondrial fission have been described 54. Excessive or unopposed fission can lead to mitochondrial fragmentation, mitophagy, decreased anti-oxidative capacity and increased production of ROS and cell death 55. Mitophagy is critical in maintaining mitochondrial quality control by removing damaged mitochondria. Mitophagy is increased in the failing heart, which could represent an adaptive response to limit mitochondrial damage and to maintain ATP production 35. However, sustained mitophagy in heart failure can induce excessive mitochondrial clearance that reduces the number of mitochondria in the heart. Furthermore, in states in which mitophagy is impaired, dysfunctional mitochondria cannot be adequately degraded. These damaged mitochondria can disrupt energetics in the mitochondrial network or can induce collateral damage by activating cell death pathways such as autophagy 35, 56. Taken together, altered mitochondrial dynamics may be an important contributor to the overall reduction in mitochondrial oxidative capacity and therefore ATP production in heart failure.
Compromised mitochondrial biogenesis in heart failure contributes to impaired mitochondrial function. An important transcriptional regulator of mitochondrial biogenesis is PPARγ coactivator-1α (PGC1-α). PGC1α activates the transcriptional expression of nuclear respiratory factor-1 NRF-1 and NFR2, whose target genes encode proteins that mediate mitochondrial replication, maintenance and to generate components of the electron transport chain 57. In heart failure, PGC1α is repressed, which correlates with decreased mitochondrial biogenesis 58. The transcription factor PPARα, which is the predominant isoform regulating fatty acid oxidation in the heart is also repressed in the failing heart 1. This leads to decreased expression of many genes involved in fatty acid uptake and oxidation. However, complicating this issue is that PPARα can be activated in some forms of HFpEF, particularly when HFpEF is associated with diabetes and obesity, resulting in an up-regulation of fatty acid oxidation 39, 59. Post-translational modification of mitochondrial proteins also contributes to a decrease in mitochondrial oxidative capacity in heart failure. These changes, which include altered protein acetylation, will be discussed later in this review.
Glycolytic compensation in heart failure:
A compensatory response to reduced mitochondrial oxidative metabolism and ATP production in heart failure is an induction of glycolysis 60. Increased myocardial glucose uptake in heart failure has been associated with increased expression of GLUT1 glucose transporter particularly in animal models in conjunction with increased activity of phosphofructokinase 1 (PFK-1, the first enzyme involved in glycolysis), and glycolytic flux 61, 62. However, this increase in glycolysis is insufficient to completely compensate for the energy deficit in heart failure or to restore cardiac function. This is in part due to glycolysis producing only two ATP molecules per glucose molecule, compared to 31 ATP molecules that would have been produced if the pyruvate from glycolysis was oxidized. Although increased glycolysis only marginally increases ATP generation, an important consequence of this is increased flux into metabolic pathways that branch from glycolysis such as the polyol and hexosamine biosynthetic pathways that could independently activate signaling pathways that contribute to myocardial remodelling 11, 63. Furthermore, increased glycolysis is uncoupled from the oxidation of pyruvate and lactate leading to accumulation of H+ in the cytoplasm. Increased Na+/H+ exchange (NHE) activation coupled with increase Na+/Ca2+ exchange may contribute to cytosolic Ca2+ accumulation 64.
Fatty acid oxidation in heart failure:
The energy metabolic changes occurring in heart failure are generally accepted to include reductions in mitochondrial fatty acid oxidation (Figure 1B) 65. The rate of myocardial fatty acid oxidation decreases with the progression of heart failure severity. However, in studies assessing 13C palmitate uptake and clearance during the stage of compensated hypertrophy (normal systolic function) no differences in fatty acid uptake or oxidation were observed in humans, Dahl salt sensitive rats fed a high salt diet, spontaneously hypertensive rats, or Wistar rats 8 weeks post myocardial infarction 66–68. However, as the severity of heart failure progresses (EF < 50%), decreased fatty acid oxidation has been reported in humans with idiopathic dilated cardiomyopathy (IDCM).69–71, Dahl salt sensitive rats fed a high salt diet, spontaneously hypertensive rats, 20-week-Wister rats 6 months post myocardial infarction, and in canine models of cardiac pacing 67, 68, 72–74. This decrease in fatty acid oxidation, however, is not always a consistent finding. Other studies have observed no differences in fatty acid uptake in patients with IDCM, or an actual increase in fatty acid uptake in patients with congestive heart failure 75–77. These divergent patterns of fatty acid utilisation are likely attributable to the differences in disease severity e.g., ejection fractions ranging from 16–48% and the presence of co-morbidities such as obesity and the metabolic syndrome in some subjects who participated in human clinical studies. In those studies that reported reduced fatty acid oxidation a parallel decrease in the expression and/or activity of genes and enzymes involved in transcriptional regulation of fatty acid oxidation (PPARα, retinoid X receptor α (a cofactor of PPAR α and PGC-1α, and estrogen related receptors (ERRα and ERRγ) were observed, as well as a number of enzymes involved in fatty acid oxidation, including CPT-1, MCAD, CD36 and FATP1 67, 68, 72–74, 78, 79. Similarly, in pressure-overload induced heart failure resulting from transverse aortic constriction (TAC), diminished fatty acid oxidation flux is associated with a lower fraction of ATP generation 80. These expressional changes are consistent with a reduction in fatty acid oxidation in heart failure. However, although fatty acid oxidation is reduced, fatty acids still account for a greater proportion of mitochondrial ATP generation than glucose in the failing heart 19.
Increases in plasma concentrations of fatty acids have been associated with increased risk for developing heart failure 81. Myocardial fatty acid oxidation increases in response to conditions such as type 2 diabetes (T2D), obesity and insulin resistance. Obese women with left ventricular hypertrophy and reduced cardiac efficiency show increased myocardial fatty acid uptake and oxidation, with the severity of their insulin resistance correlating with the higher rates of fatty acid oxidation 82. In addition, type 2 diabetic men with T2D cardiomyopathy also exhibit increased fatty acid uptake and oxidation 83. These finding are consistent with animal models of obesity and T2D, such as diet induced obese (DIO), db/db and ob/ob mice. In these animals, hearts predominantly rely on fatty acid oxidation, while exhibiting left ventricular hypertrophy 84, diastolic dysfunction 85, 86 and in severe cases, systolic dysfunction 87–89. In a murine model of HFpEF, involving a high fat diet, aging, and deoxycorticosterone treatment, fatty acid oxidation was also increased 90. Despite the close association, a causal role for increased fatty acid oxidation rates in cardiac dysfunction of obesity or diabetes is less certain. Since cardiac lipid uptake is increased in these models an imbalance between lipid uptake and oxidation likely contributes to lipotoxicity. Increasing mitochondrial fatty acid oxidation in mice with diet induced obesity restores the balance and prevents cardiac dysfunction while it does not affect cardiac function or longevity of normal mice 91, 92. Furthermore, transgenic models in which fatty acid oxidation is inhibited exhibit cardiac hypertrophy and accelerated impairment in ejection fraction in response to pressure overload 93, 94, 95.
Glucose metabolism in heart failure:
While glucose uptake is commonly reported to be increased in heart failure, this is not always accompanied by an increase in glucose oxidation. More commonly, a decrease in glucose oxidation and an increase in glycolysis is observed (Figure 1B). In patients with IDCM (88–98%) 69–71, in canine models of cardiac pacing (~ 150%) 73 and in Dahl salt sensitive rats fed a high salt diet, an increase in glucose uptake is seen 68. GLUT1 expression is also increased, suggesting an increased capacity for glucose uptake that may have a predominant glycolytic fate 67, 72. In the majority of these models, the oxidation of pyruvate derived from glucose (i.e. glucose oxidation), is decreased in mouse models of heart failure 96–98, in pacing-induced heart failure in pigs and in humans with end-stage heart failure 99. In support of this decrease in glucose oxidation, myocardial biopsies from patients with heart failure were found to have reduced expression of MCT1, PDH, MPCs and of pyruvate/alanine aminotransferases, suggesting reduced transport and metabolism of pyruvate 100, 101. However, in a study in pacing-induced heart failure in dogs an increase in glucose oxidation was observed 44. Increased anaplerosis represents an important adaptation in the failing heart mediated by increased carboxylation of pyruvate by malic enzyme 1 (ME1), which is induced in response to pressure overload and in failing human hearts 102–104. Increased pyruvate carboxylation reduces the efficiency of glucose oxidation and may contribute to oxidative stress by consuming NADPH 104. Indeed, lowering ME1 in failing rat hearts increased pyruvate flux into the TCA and restored redox homeostasis by normalizing reduced glutathione (GSH) 104.
The decrease in myocardial glucose oxidation seen in HFrEF is also seen in heart failure associated with obesity and diabetes. Rodent models of T2D and insulin resistance that exhibit left ventricular hypertrophy and diastolic dysfunction 105, 106, also have decreased glucose oxidation rates 107, 108. Decreases in myocardial glucose oxidation are very prominent in obese and diabetic mice that develop LV hypertrophy and diastolic dysfunction 109–111. This decrease in glucose oxidation is also seen in angiotensin II induced heart failure in mice, which is accompanied by increased PDK4 expression and reduced PDH activity 107. This decline in glucose oxidation in angiotensin II treated mice can be blunted in response to PDK4 deletion 108. Intriguingly, angiotensin II treatment has also been shown to ameliorate diastolic dysfunction in diabetic db/db mice 112. In mice that develop LVH due to aortic constriction, a decrease in myocardial glucose oxidation precedes the development of diastolic dysfunction 96, which further supports a potential role of reduction in glucose oxidation in the development of heart failure 108. Notably, in some studies of mouse models of heart failure with reduced rates of FAO, the relative contribution of glucose oxidation to TCA flux might be increased, although net TCA flux is reduced 80. Transgenic mice with mutations that prevent the uptake of glucose or oxidation of pyruvate also develop LVH, diastolic dysfunction (GLUT4 deletion)113 and systolic dysfunction (PDH deletion).114 Upstream of PDH activity, the mitochondrial pyruvate carrier (MPC) provides a new therapeutic point of potential metabolic regulation. Recently, a series of studies, have examined the role of loss-of-function of these proteins in the regulation of cardiac metabolism and function 115. Mice with cardiac specific deletion of the MPC initially develop age-dependent pathological LVH with preserved ejection fraction that is associated with reduced rates of glucose oxidation in concert with increased rates of fatty acid oxidation 116. However, as animals age a dilated cardiomyopathy develops. Taken together, reduced mitochondrial oxidation of pyruvate likely plays an important role in the transition from pathological LVH to heart failure.
Ketone body oxidation in heart failure:
Fasting increases circulating concentrations of ketone bodies 117, 118. Interestingly, in patients with heart failure the fasting-induced increase in circulating ketones is exacerbated. 119. A number of recent studies have shown that myocardial ketone body oxidation in increased in heart failure 120–122. One study quantifying substrate utilization in arterio-venous blood samples reported that ketone body oxidation was increased by ~100% in patients with HFrEF 76. In experimental animals, TAC protocols that induce LVH in mice in the absence of systolic dysfunction, is associated with increased expression of enzymes involved in ketone oxidation, in concert with increased βOHB oxidation 120. The superimposition of myocardial infarction in these mice induces the progression from LVH to HFrEF. This transition is associated with a further increase in oxidation of βOHB that is accompanied by decreased expression of genes involved in fatty acid oxidation and reduced levels of TCA cycle intermediates (with the exception of succinate). In addition, a prolonged 24 hour fast in these mice induced the expression of SLC16A1, which mediates myocardial ketone uptake in the heart. These data suggested that TAC + myocardial infarction-induced HFrEF is associated with an increased capacity for myocardial ketone body uptake and that ketone body oxidation in HFrEF occurs in concert with impaired myocardial fatty acid oxidation and altered anaplerosis 120. In addition, mice with cardiomyocyte specific knockout of SCOT (preventing them from terminally oxidising βOHB), show increased fatty acid oxidation 123. Interestingly, these animals are also more susceptible to TAC induced increases in LV mass 123. These data support the concept that increased ketone body oxidation in heart failure is reciprocally regulated with fatty acid oxidation, which is inhibited. Whether increased ketone metabolism is adaptive or maladaptive in heart failure remains to be established. It is important to consider the efficiency of the substrate and whether ketone body metabolism occurs at the expense of the oxidation of fatty acids or glucose. In regards to efficiency, ketone bodies do indeed produce more energy per 2 carbons than glucose. However, when considering the P/O ratio, ketone bodies are less efficient than glucose. While an increased ketone body metabolism at the expense of fatty acids could represent a desirable shift in substrate preference in the failing heart, it is important to consider that this may also occur at the expense of glucose oxidation, given that ketone bodies are less efficient in regards to their P/O ratio. Studies to rigorously evaluate this possibility remain to be performed.
Changes in ketone body oxidation in HFpEF remain incompletely understood. A recent study reported a 3-Hit murine model of HFpEF (involving age, long-term high-fat diet and deoxycorticosterone pivalate challenge) 90. In contrast to findings in HFrEF, myocardial ketone oxidation was not increased in this HFpEF model. Rather, increasing ketone availability, despite decreased ketone oxidation, reduced proinflammatory cytokine-induced mitochondrial dysfunction and fibrosis in HFpEF. Plasma concentration of ketone bodies are also increased in insulin resistance and T2D, two comorbid conditions associated with increased risk for HFpEF. However, it remains unclear if hyperketonemia in these conditions is associated with increased myocardial uptake and oxidation 117, 118. Serum metabolite analysis detected higher levels of AcAc and β-OHB in patients with HFpEF than in patients with HFrEF, suggesting an increased reliance on ketone bodies as an energy source in HFrEF compared to that in HFpEF 124. However, a role for ketone bodies in the development of HFpEF remains to be definitively established and future work is required to elucidate the contribution of ketone body metabolism in the development of HFpEF.
Branched chain amino acid (BCAA) oxidation in heart failure:
Increases in plasma BCAA levels are seen in heart failure patients and have been suggested to be an early predictor of the future development of cardiovascular disease 125–129. These increases in BCAA levels may be due in part to impaired BCAA oxidation in heart failure 130–132. The accumulation of BCAA’s in heart failure may activate cardiac mTOR signaling, thereby promoting cardiac hypertrophy 133, 134. This is also supported by studies showing that stimulation of BCAA oxidation or inhibition of mTOR (with rapamycin) can improve heart function 132, while BCAA supplementation further increases mTOR signaling and worsens cardiac dysfunction 135.
Modulation of BCKDK activity as an approach to accelerate BCAA metabolism, via dephosphorylation and activation of BCKDH, is shown to lessen contractile dysfunction and cardiac insulin resistance in the settings of HFrEF. For instance, employing the BCKDK inhibitor, BT2, improves BCAA oxidation and reduces BCAA and BCKA accumulation in heart failure 132, 136, 137. This stimulation of BCAA oxidation is accompanied by enhanced cardiac function and glucose oxidation in the failing heart 132, 136, 137. The protein phosphatase PPC2m is also important in BCAA oxidation, as it decreases phosphorylation and inhibition of BCKDH, a key enzyme involved in BCAA oxidation. In mice deficient for PPC2m, myocardial BCAA and BCKA levels are elevated. These mice are more susceptible to HFrEF, following TAC, suggesting that decreased BCAA catabolism can contribute to systolic dysfunction in the stressed heart 132. This same study also reported defects in BCAA metabolism in human heart failure tissue and in mice with TAC induced heart failure 132. The consequences of the accumulation of BCKA were further investigated in vitro, with the authors proposing that products of BCAA oxidation inhibit complex I and increase superoxide production leading to impaired mitochondrial oxidative function 132.
Insulin resistance in the failing heart:
The relationship between insulin resistance and heart failure is complex and involves the cardiac adaptation to the systemic milieu in heart failure that is characterized by generalized insulin resistance in concert with intrinsic changes in insulin signaling within the cardiomyocyte 138. Whole-body insulin resistance, which occurs in diabetes and obesity, is a risk factor for developing heart failure independent of myocardial infarction, hypertension and serum cholesterol levels 36, 97, 107, 110, 112, 139–142. Moreover, diabetes also increases the risk of myocardial infarction by ~2–4 fold 143, and the coexistence of ischemic cardiomyopathy with diabetes accelerates the progression of heart failure 144. Likewise, heart failure itself also aggravates the severity of whole-body insulin resistance36, 145–148. Importantly, an early and critical metabolic alteration in the failing heart is that the heart itself becomes insulin resistant in terms of insulin-mediated glucose uptake and the direct insulin stimulation of glucose oxidation, although increased signaling to cytosolic Akt may persist 138, 142, 149–159. Altered myocardial insulin signaling in heart failure may not only negatively impact cardiac energy metabolism but may also exacerbate the extent of LV remodeling 138,138, 142, 149–157. In addition, cardiac insulin resistance seen in obesity or heart failure is also exacerbated if both co-exist 110, 139–141, 160–163. Insulin signaling in the heart plays an important role in modulating cardiac preference for the primary oxidative substrates, namely glucose and fatty acids, while myocardial utilization of the minor oxidative substrates ketones and amino acids, are not directly regulated by insulin.
Insulin indirectly stimulates cardiac glucose oxidation by enhancing glucose uptake and, as a result, stimulates glycolysis that converts glucose to pyruvate, where the latter is taken up by the mitochondria to be oxidized to acetyl CoA through glucose oxidation. It has also been shown that insulin can directly stimulate mitochondrial glucose oxidation, independent of enhancing glucose uptake or glycolysis 164. While the exact mechanism is not fully understood, direct insulin stimulation of glucose oxidation involves activation of mitochondrial PDH, the main regulatory enzyme of mitochondrial glucose oxidation 164–168. Insulin also directly inhibits cardiac fatty acid oxidation via abrogating the inhibitory effect of 5’AMP-activated protein kinase (AMPK) on acetyl CoA carboxylase (ACC) and increasing malonyl CoA, a potent inhibitor of mitochondrial fatty acid uptake 169. In addition, insulin-stimulated glucose oxidation can also inhibit fatty acid oxidation based on the Randle Cycle phenomena170. While there is still debate about what happens to basal rates of glucose oxidation during the evolution from compensated left ventricular hypertrophy to heart failure, cardiac insulin resistance, which has been generally defined as a marked reduction in cardiac insulin-stimulated glucose oxidation rates, represents one of the early metabolic perturbations that occur in the failing heart 36, 97, 107, 163, 171, 172. As will be discussed below a fuller understanding of myocardial insulin resistance requires evaluation of proximal insulin signaling pathways, the activity of which could be augmented despite reduced myocardial glucose uptake 173. Cardiac insulin resistance negatively impacts cardiac function and energy metabolism 36, 97, 107, 163, 171, 172. In addition, the inhibitory effect of insulin on cardiac fatty acid oxidation becomes less dramatic in the failing heart 36, 163. This is significant since the failing heart is energy-starved 3, 31, which led to the suggestion that the failing heart is “an engine out of fuel” 3, 31. Accordingly, the occurrence of insulin resistance in the failing heart may exacerbate the cardiac energy deficit by reducing cardiac efficiency, thereby contributing to the progression of heart failure 36, 97, 107, 163, 171, 172. In heart failure associated with obesity, restoring systemic and cardiac insulin sensitivity by caloric restriction-induced weight loss correlated with improved contractile function in the failing obese heart 36. Together, these data suggest that cardiac insulin resistance could be a marker of contractile dysfunction. Whether these changes mediate contractile dysfunction remain to be established. Depressed glucose oxidation in the failing heart could potentially contribute to contractile dysfunction via reducing glucose-derived ATP production. Moreover, depressed glucose oxidation also exacerbates uncoupling between glycolysis and glucose oxidation and, as a result, accumulation of ATP hydrolysis-derived protons (i.e., acidosis) in the cytosol. Acidosis can further aggravate contractile dysfunction in the failing heart by desensitizing contractile proteins to Ca2+, inhibiting the slow inward Ca2+ current and redirecting cardiac ATP toward re-establishing ionic homeostasis instead of the contractile machinery 2.
It is noteworthy that MPC expression is downregulated in chronic HF patients 115. Consistent with this, genetic impairment of mitochondrial pyruvate oxidation by deleting MPC expression is sufficient to induce LVH, age-dependent cardiomyopathy and increased susceptibility to pressure-overload induced heart failure 101, 115, 116, 174, 175. Overexpression of MPC enhances coupling between glycolysis and glucose oxidation and reduces cardiac dysfunction and adverse remodeling via increasing glucose oxidation 115. Thus, reduced glucose oxidation that occurs in conjunction with myocardial insulin resistance may have a detrimental effect on contractile dysfunction. As such, cardiac insulin resistance could potentially not only be a marker of heart failure severity, but also mediate contractile dysfunction in the failing heart. Consistent with this hypothesis, strategies to attenuate adverse remodeling in heart failure have included increasing cardiac energy supply and stimulating glucose cardiac oxidation1–3, in both, experimental 36, 68, 97, 140, 162, 163, 176 and human heart failure 177.
The mechanisms leading to cardiac insulin resistance in heart failure are not fully understood, but contributing factors could include an impaired overall mitochondrial oxidative capacity and accelerated fatty acid oxidation rates 1. Moreover, consistent with reduced glucose oxidation in the failing heart, cardiac insulin resistance is also accompanied by changes in cardiac insulin signal transduction, including activation of proximal insulin signaling pathways such as IRS1 and Akt 36, 158, 159, 163, 173. Recent studies have provided strong evidence for divergent effects of insulin receptor substrates (IRS), namely IRS1 and IRS2, in the failing heart 158, 159, 178, 179. Hyperactivation of IRS-1/Akt1 in the failing heart and genetic deletion of IRS-1 is protective in a mouse model of heart failure 159, suggesting a detrimental effect of IRS-1 in the failing heart. Moreover, deletion of IRS-2 further aggravated LV dysfunction in failing mouse hearts, suggesting a protective role of IRS-2/Akt2 in the heart 159. However, it is still not clear how the signaling of IRS-1/Akt1 or IRS-2/Akt2 influences glucose uptake, glycolysis or glucose oxidation in the failing heart. Despite these alterations in cardiac insulin signaling, it has been shown that glycolysis is up-regulated in the failing heart 60, 180, 181, possibly compensating for reducing mitochondrial oxidative metabolism. Therefore, it seems plausible that the reduction in insulin-stimulated glucose oxidation in the failing heart occurs, at least in part, due to decreased direct stimulation of mitochondrial glucose oxidation by insulin 122, 182. However, how insulin signaling is transduced to the mitochondria to stimulate PDH and glucose oxidation is not known 165–168.
The insulin signaling pathway in the mitochondria is not well characterized. Insulin has been shown to increase mitochondrial fusion in concert with increased mitochondrial oxidation by a mechanism involving induction of OPA1 expression. 183 Another component of the insulin signaling pathway, namely protein kinase B (Akt), can translocate to the mitochondria following insulin stimulation 165–168. Interestingly, mitochondrial translocation of Akt has also been implicated in modulating mitochondrial oxidative phosphorylation 165–168. Therefore, it seems plausible to suggest that Akt could be a potential candidate that mediates the direct insulin stimulation of glucose oxidation. Insulin stimulation results in a marked increase in glucose oxidation rates in the normal mouse heart 182, 184. Of importance is that insulin stimulation in these hearts did not cause significant changes in glycolysis, likely due to the high glycolysis rates seen in the mouse heart, confirming that insulin can directly stimulate glucose oxidation, independent of stimulating glycolysis. Interestingly, direct insulin stimulation of glucose oxidation is associated with a stimulation of mitochondrial Akt, an effect that is also accompanied by the activation of PDH 182. Furthermore, we also demonstrated that inhibiting mitochondrial Akt completely abolishes the direct insulin stimulation of glucose oxidation 182. These findings indicate that insulin-stimulated mitochondrial Akt is a sine qua non for mediating the direct insulin stimulation of cardiac glucose oxidation.
NAD(H) alterations in heart failure
Reduction of nicotinamide adenine dinucleotide (NAD) levels or NAD+/NADH redox state has been observed in many chronic diseases including heart failure 185–189. Recent studies demonstrated benefits of supplying NAD precursors in preclinical models and in patients with heart failure 186, 187, 190. These findings have stimulated the investigation of NAD metabolism in heart failure and raise hope for a new therapeutic approach191. However, mechanisms leading to the altered NAD(H) levels in heart failure are not fully understood. Furthermore, despite the remarkable benefits observed in animal models of heart failure, the molecular targets of increasing NAD levels are less clear. Elucidation of these mechanisms are necessary for a better understanding of metabolic regulation of heart failure, and importantly, to translate the findings into therapy.
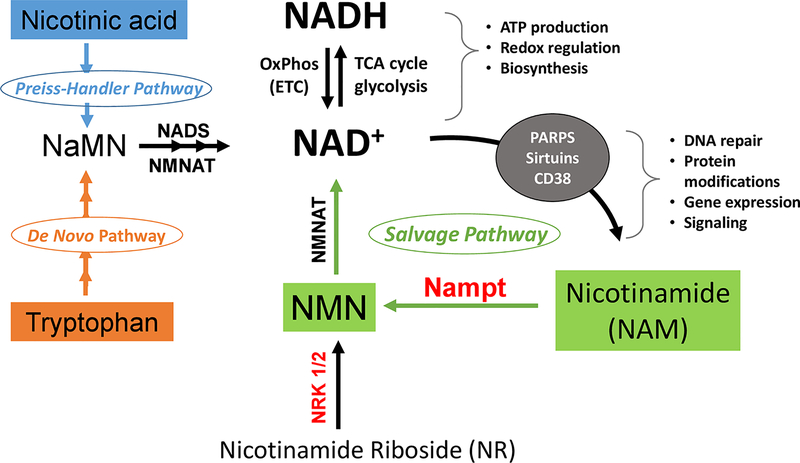
NAD exists in the oxidized (NAD+) and reduced form (NADH); they serve as the major electron carrier coenzyme in substrate metabolism, e.g. glycolysis and TCA cycle, and in oxidative phosphorylation 192. NAD+ is also a required substrate by poly (ADP-ribose) polymerases (PARPs), sirtuins and CD38, thus playing an important role in post-translational modification, DNA damage repair, gene transcription and other signaling mechanisms188, 193, 194. Biosynthesis of NAD in mammalian cells occurs through several routes 195. The de novo biosynthesis pathway starts with tryptophan whereas the Preiss Handler pathway utilizes nicotinic acids. Both converge on nicotinic acid mononucleotide (NaMN) to produce NAD+ through a nicotinamide mononucleotide adenyltransferase (NMNAT) reaction (Figure 2). Important for the maintenance of the NAD pool, nicotinamide (NAM), the product of NAD+ consumption by sirtuins, PARPs or CD38, is converted to nicotinamide mononucleotide (NMN) by nicotinamide phosphoribosyl transferase (NAMPT) for resynthesis of NAD+ (Figure 2).
Figure 2: NAD Metabolism and its biological role in mammalian cells.
NAD carries electrons generated from substrate catabolism, e.g. TCA cycle or glycolysis, to oxidative phosphorylation for ATP production. The NAD+/NADH ratio determines cellular redox and metabolic fluxes. NAD+ is consumed by multiple enzymes, e.g. Sirtuins and PARPs, for protein and nucleotide modification thus regulating signal transduction. These reactions generate nicotinamide (NAM), which, through the salvage pathway, is converted into nicotinamide mononucleotide (NMN) by nicotinamide phosphoribosyl transferase (NAMPT). The NMN is converted to NAD+ by nicotinamide mononucleotide adenyltransferase (NMNAT). Alternatively, nicotinamide riboside (NR) can be phosphorylated by nicotinamide riboside kinase (NRK) 1 or 2 to form NMN. NAD+ can also be synthesized from nicotinic acid (NA) in the Preiss-Handler pathway or from tryptophan in the de novo pathway. Both pathways generate nicotinic acid mononucleotide (NaMN). NMNAT then converts NaMN to nicotinic acid adenine dinucleotide (NaAD) which is further metabolized into NAD+ by NAD synthase (NADS).
Increased consumption, as occurs during increased DNA repair in aging or following activation of CD38 by inflammation, could contribute to the depletion of intracellular NAD levels. Specific mechanisms leading to changes of NAD consumption in heart failure are, however, poorly defined. On the other hand, there is evidence of impaired NAD salvage in heart failure. NAMPT expression is downregulated in human and mouse failing hearts while the expression of NRK2, an enzyme converting nicotinamide riboside (NR) to NMN, is upregulated suggesting a metabolic shift in the salvage pathway (Figure 2) 187, 196, 197. Furthermore, reduced NADH oxidation, due to impaired mitochondrial respiratory function, leads to sequestration of NAD in the NADH form, resulting in a decrease of NAD+ availability and reduced NAD+/NADH ratio 186, 198. Supplementation of NAD precursors NMN or NR are effective in raising NAD levels in mouse failing hearts. Increasing NAD levels in this way has been reported to improve the outcome of heart failure in a variety of mouse models186, 187, 199, 200. The effect of overexpressing NAMPT, however, appears to be model dependent. Cardiac-specific overexpression of NAMPT protects against ischemia-reperfusion injury and isoproterenol induced cardiomyopathy, but worsens the outcome of pressure overload induced heart failure 186, 197, 201.
How does a lower cardiac NAD level contribute to the pathogenesis of heart failure, and why does boosting NAD exert benefit on heart failure? Initial studies focused on the role of NAD+ as a co-substrate for sirtuin deacetylases. Increased protein acetylation has been observed in heart failure or mitochondrial dysfunction induced cardiomyopathy, where decreases in NAD and sirtuins are also found 185, 186. Several studies linked activation of Sirt3, a mitochondrion localized sirtuin, to the cardioprotective effects of increasing NAD levels 186, 199, 200. Sirt3 is involved in deacetylation of a large number of mitochondrial proteins, it remains largely unknown which specific targets downstream of Sirt3 mediate the benefit of increasing NAD in heart failure 32. Moreover, a recent study showed that extreme mitochondrial protein hyperacetylation did not exacerbate heart failure induced by pressure overload in mice 202, calling for the investigation to go beyond Sirt3 and mitochondrial protein acetylation.
NAD levels and NAD+/NADH redox are critical for energy metabolism (Figure 3). Increasing NAD or restoring NAD+/NADH redox improves cardiac energy metabolism in mouse models of heart failure 186, 187. A recent study suggests that hyperacetylation of the muscle form of creatine kinase (CKM) accounts for the long-observed downregulation of CK activity in heart failure 203. The study also showed that acetylation of CKM, cytosolic protein, was NAD+-dependent and mediated by Sirt2. Whether increasing NAD+-dependent deacetylation of CKM improves high energy phosphoryl transfer in the failing heart warrants future study. In addition, supplementation of NAD precursors has recently been found to protect mitochondria of peripheral blood mononucleated cells (PBMC) resulting in reduced inflammatory response in heart failure patients 190. Thus, both cardiac and systemic responses could also contribute to the NAD-dependent salutary effects in heart failure.
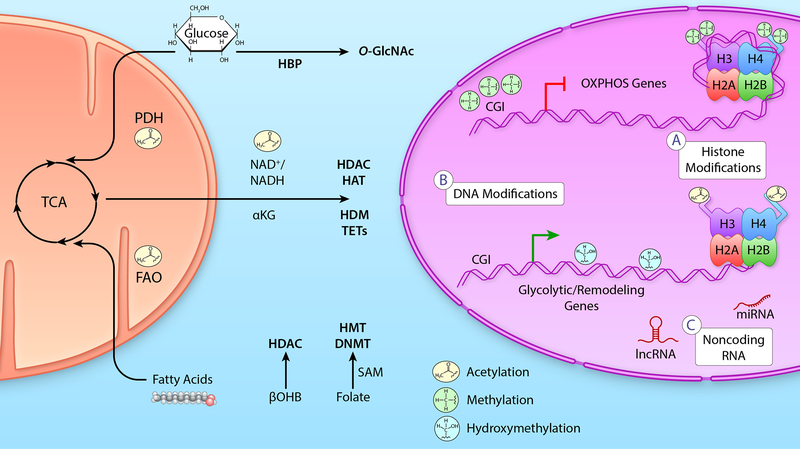
Figure 3: Metabolic signaling to epigenetic transcriptional control in the failing heart.
Left: Mitochondrial metabolic flux of primary cardiac metabolites, glucose and fatty acids, directly alter NAD+/NADH ratios as well as the Acetyl-CoA pool that signal to the nucleus to impact the epigenetic environment. Additionally, glucose that does not enter oxidative metabolism in the mitochondria can signal through the HBP. Additional metabolic intermediates discussed in the text, βOHB, can also regulate activity of epigenetic regulating enzymes as can single carbon metabolism via folate and SAM. Middle: Metabolites and metabolic pathways regulate the activity and/or substrate availability of epigenetic modifiers of histones (e.g., HDAC, HAT, HDM, HMT) and DNA (e.g., DNMT and TET). Right: A) Histone modifications include inhibitory tri-methylation as well as activating acetylation. B) DNA modifications such as DNA methylation (5mC) at CGI in promoters are associated with gene silencing while CGI demethylation and gene body DNA hydroxymethylation (5hmC) modifications are associated with gene activation. C) Although metabolites are not known to be directly regulated by metabolic intermediates, a number of metabolic genes are regulated by miR and lncRNA expression. Please see references included in the main text for additional details. (Illustration credit: Ben Smith).
Abbreviations: α-ketoglutarate (αKG), β-hydroxybutyrate (βOHB), CpG island (CGI), DNA methyltransferase (DNMT), fatty acid oxidation (FAO), hexosamine biosynthetic pathway (HBP), histones (H2A, H2B, H3, H4), histone acetyltransferase (HAT), histone deacetylase (HDAC), histone demethylase (HDM), histone methyltransferase (HMT), long noncoding RNA (lncRNA), microRNA (miR), oxidative phosphorylation (OXPHOS), pyruvate dehydrogenase (PDH) complex, S-adenosylmethionine (SAM), ten-eleven translocation (TET), and tricarboxylic acid (TCA) cycle.
Epigenetic and transcriptional changes in the failing heart
One long held hypothesis surrounding the changes in cardiac metabolic utilization and loss of substrate flexibility is that of transcriptional reprogramming. This was initially described as a return to the foetal gene program,204 to reflect the increased reliance on glucose utilization in heart failure resulting from ischemic injury or pressure-overload. Although this may differ in the context of diabetes where fatty acid utilization is increased but uncoupled,205 the conclusion has remained the same in that many of the changes in cardiac energy metabolism in heart failure described within this review are a result of changes in gene expression. Although decades of research have gone into this area, especially as it relates to the aforementioned PGC-1α and PPARα, over the last 10 years regulation of gene expression in heart failure via the metabolites themselves through epigenetic mechanisms has emerged.206 Epigenetics as a field encompasses long-lasting changes in gene expression without changing the underlying genetic code. Mechanisms of epigenetic regulation include post-translational regulation of histone proteins, DNA modifications, and post-transcriptional regulation via non-coding RNAs. What is particularly enticing is the ability of epigenetic regulation to synthesize changes in intermediary metabolism into changes in gene expression.207
Of the histone modifications explored, protein acetylation has received the most attention for its potential as a therapeutic target in treating heart failure. This in part relates to the fact that a number of the enzymes that control histone acetylation are regulated by NAD(H), as well as the acetyl-CoA used as the substrate generated by enzymes such as PDH, linking the pathways described in previous sections to regulation of transcriptional control (Figure 3). One specific family of enzymes in this role are the sirtuins, a class of protein deacetylases, which are NAD-dependent and have been widely studied for their potential role in cardiovascular disease.208–210 Some of the sirtuins may work through epigenetic regulation while others may directly regulate mitochondrial enzyme function.209 Additionally, a number of histone deacetylase (HDAC) inhibitors have been developed as therapeutics for various cancers, and have been suggested as potential candidates for treatment of heart failure.211 In fact, very recent evidence supports that HDAC inhibition improves cardiopulmonary function in a feline model of HFpEF.212 Although it is important to note that despite the name HDAC, some of the improvements are from non-epigenetic mechanisms of regulation including altered myofibril relaxation,213 while others clearly are mediated by changes in gene expression.214, 215 However, it is clear that additional studies distinguishing direct epigenetic effects of these inhibitors versus indirect changes in enzymatic activity of non-histone targets are required. In addition to remaining questions surrounding HDAC inhibitors, it is important to note that many other metabolites will signal to, or directly modulate, histone post-translational regulation (Figure 3). These mechanisms include ketone body regulation of histone deacetylases,216 TCA metabolic intermediates such as citrate signaling to histone acetyltransferases,217 α-ketoglutarate to histone methyltransferases,218 as well as auxiliary glucose signaling pathways like the hexosamine biosynthesis pathway via direct O-GlcNAcylation of histones219 or indirect O-GlcNAcylation of HDAC4.220 Many of these pathways have not been fully defined in the heart.
In addition to epigenetic regulation of gene expression by histones, direct modification of DNA may also occur via modification of cytosine nucleotides that are followed by a guanine nucleotide (CpG) by methylation (5mC) or hydroxymethylation (5hmC) (Figure 3B). In general, 5mC occurring at the promoter of a gene is inversely related to gene expression, while 5hmC which is relatively understudied occurs in the gene body, the binding of which is directly proportional to changes in gene expression. Early studies in this area found that DNA 5mC was associated with changes in expression of angiogenesis-related genes related to the progression of human heart failure,221 and regulation of heart failure genes in a mouse model of TAC.222 Inhibition of DNA methylation may attenuate cardiac hypertrophy associated gene expression changes in norepinephrine-treated rats.223 More recently it was shown in human heart failure that some of these changes in DNA methylation are highly correlated with coordinate changes in enzymes responsible for the fatty acid to glucose utilization switch, likely through a mechanism involving DNA methyltransferase 3A (DNMT3A).224 Interestingly, this mechanism was further defined in human ischemic heart failure to include parallel and inverse epigenetic regulation via the histone methyltransferase and its interaction with DNMT to supress expression of oxidative phosphorylation genes while concurrently interacting with the transcription factor FOXM1 to upregulate cellular remodeling genes.225 Comparatively much less is known about the role of 5hmC in the regulation of cardiac gene expression with a number of papers emerging the last few years.226–229 However, some evidence for a disparate relationship between DNA methylation and changes in cardiac gene expression in the context of diabetes has recently emerged.230 Therefore, additional studies surrounding the contribution, regulation, and reversal of these pathways remains warranted.
The final area of epigenetic regulation is that of non-coding RNAs (Figure 3C). This, like histone modifications, includes a large number of different pathways encompassing microRNA (miR) to long non-coding RNA (lncRNA). However, given the relative ease of measuring non-coding RNAs in the circulation even though this pathway was one of the last to be studied, there is now a large literature surrounding its role in the regulation of gene expression in heart failure as recently reviewed,231–233 and therefore not described in detail in this review.
Glucose metabolic changes in heart failure: Beyond ATP Production
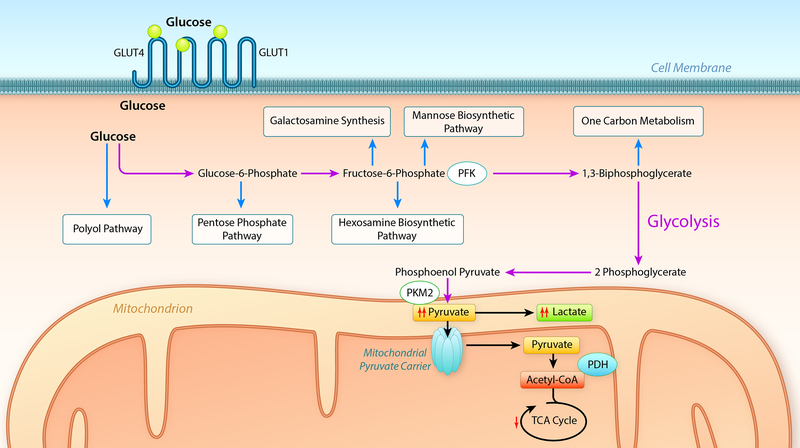
Fatty acids represent the major substrate for normal hearts. Although in heart failure fatty acid oxidation is reduced relative to non-failing hearts, it remains the predominant metabolic substrate even for the failing heart. A recent study in humans, achieved by measuring arteriovenous differences in multiple metabolites 19 revealed that in the fasting state the healthy human heart generates 85% of its ATP from fatty acid sources, 6.4% from ketones, 4.6% from amino acids and 2.8% from lactate. Whereas in patients with HFrEF substrate contributions to ATP generation is 71% from fatty acids, 16.4% from ketones, 5% from lactate and 6.4% from amino acids. Importantly, both normal or failing hearts under these conditions utilized minimal amounts of glucose. Interestingly there was a significant rate of amino acid turnover of ~ 2% per day. In studies from independent groups in which myocardial biopsies were obtained in patients from end-stage heart failure and analyzed by metabolomics, a common theme was accumulation of pyruvate and other glycolytic intermediates 61, 78, 115, 174. In all of these studies, expression levels of the mitochondrial pyruvate carrier were reduced and in one study the phosphorylation of PDH was increased. Two recent independent reports confirmed the repression of MPC expression in human heart failure and also in animal models 101, 175. Thus impaired mitochondrial pyruvate utilization has emerged as a reproducible signature of the failing heart. The reduction in pyruvate utilization is not likely to contribute to “energy starvation” in the heart failure, but rather may contribute to adverse LV remodeling by non-oxidative metabolism of glycolytic metabolites into pathways such as the polyol pathway, the hexosamine biosynthetic pathway (HBP), the pentose phosphate pathway (PPP) and one carbon cycle pathways (Figure 4) 101, 116, 174, 175. Unloading of failing hearts following LVAD implantation was associated with reduced flux through the HBP and polyol pathway, but increased PPP flux leading to generation of reduced NADPH that improves redox homeostasis and ribitol, which would mediate changes in alpha-dystroglycan, which would lead to improved extracellular matrix homeostasis 174. Although flux through these pathways might be small under physiological conditions, a persistent increase over time could lead to the accumulation of metabolic intermediates that could impact cellular function. For example, the cellular consequence of altered flux through the hexosamine biosynthetic pathway is accumulation of O-GlcNAc modifications, which have been described in multiple models of heart failure and which might be sufficient to induce LV remodeling 234, 235.
Figure 4: Pathways of non-oxidative glucose metabolism whose by-products contribute to cardiac remodeling.
Schematic summary of glucose uptake via GLUT1 and GLUT4 transporters and entry of glucose into the glycolytic pathway (purple arrows) leading to the generation of pyruvate. In heart failure, impaired entry of pyruvate into mitochondria or decreased mitochondrial pyruvate metabolism leads to accumulation of glycolytic intermediates and increased flux into accessory pathways (blue arrows) such as the polyol pathway, pentose phosphate pathway, hexosamine biosynthetic pathway, mannose and galactosamine synthetic pathways and one carbon metabolism pathways, products of which have been linked to the activation of signaling pathways that may contribute to left ventricular modelling. Regulatory steps in glycolysis that have been implicated in heart failure include phosphofructokinase (PFK), pyruvate kinase (PKM1), the mitochondrial pyruvate carrier (MPC) and pyruvate dehydrogenase (PDH). (Illustration credit: Ben Smith).
Additional mechanistic insights have been obtained from animal models and studies of cultured myocytes in which MPC expression or function are altered 101, 115, 116, 175. These studies all reveal that inhibiting mitochondrial pyruvate utilization by genetically deleting expression of MPC subunits (Mpc1 or Mpc2) or by pharmacologic inhibitors is sufficient to induce pathologic cardiac hypertrophy and heart failure 101, 115, 116, 175. Metabolomics analysis reveals increased flux into non-oxidative pathways such as the HBP, glycogen synthesis, the PPP and amino acid biosynthetic pathways (Figure 5). Overexpression of MPC in hearts subjected to TAC, or treating isoproterenol-treated mice with an inhibitor of the lactate transporter MCT4, which increased the generation of pyruvate and flux through the MPC limited adverse LV remodeling and reversed these metabolomics changes 101, 115. In this regard, lactate itself was also shown to exhibit a potent anti-hypertrophic effect after TAC via a mechanism that involves NDRG3 and ERK 21.
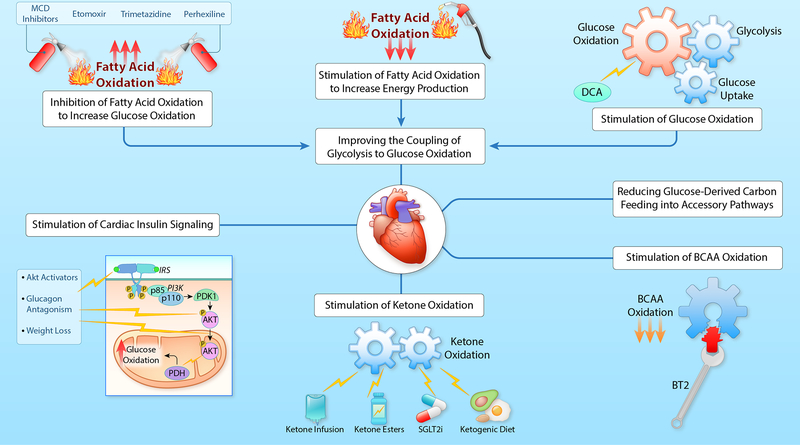
Figure 5: Targeting cardiac metabolism to protect the failing heart.
MCD, malonyl CoA decarboxylase; DCA, dichloroacetate; IRS, insulin receptor substrate; PI3K, phosphatidylinositol 3-kinase; PDK1, 3-phosphoinositide-dependent protein kinase-1; Akt, protein kinase B; PDH, pyruvate dehydrogenase; BCAA, branched chain amino acids; BT2, branched chain keto acid dehydrogenase kinase inhibitor; SGLT2i, sodium/glucose cotransporter-2 inhibitors. (Illustration credit: Ben Smith).
Treatment of mice with myocardial Mpc deficiency with a ketogenic or high-fat diet completely reversed the pathological LV remodeling and heart failure 116, 175. Mechanistically, these hearts at baseline exhibited increased rates of fatty acid oxidation and the ketogenic diet suppressed glycolysis, lactate and pyruvate accumulation and flux of glucose carbons into non-oxidative metabolic pathways such as the HBP and glycogen 116. MPC deficient hearts fail more rapidly when they are subjected to TAC. Introducing a ketogenic diet at the onset of TAC does not rescue this phenotype suggesting that simply switching substrates will not substitute for the acute increase in hemodynamic requirements 116. Intriguingly, pre-treating these animals with a ketogenic diet prior to TAC prevents accelerated heart failure. This suggests a temporal relationship between reducing the accumulation of glycolytic intermediates and reversing the negative consequences of accumulation of these intermediates of non-oxidative glucose metabolism, on ventricular remodeling (Figure 4)
Accumulation of pyruvate and amino acid derived acyl carnitines are noted in hearts of spontaneously hypertensive rats early in their development of pathological LVH and treatment with metformin reverses these metabolic abnormalities in concert with LVH regression despite persistent hypertension 236, 237. Taken together, these findings reveal a strong relationship between non-oxidative metabolism of glucose and glycolytic intermediates and the development of pathological LV remodeling.
Observations in other animal models support a role for non-oxidative glucose metabolism in pathological cardiomyocyte hypertrophy and have provided additional mechanistic insights. Studies in cultured cardiomyocytes reveal that glucose-derived metabolites provide building blocks for increasing cardiomyocyte hypertrophy via the generation of aspartate through the TCA cycle, which supplies nitrogen for nucleotide biosynthesis 238. Interestingly, MPC deletion which reduces pyruvate import into mitochondria also causes hypertrophy. However, glucose-derived carbon could enter the mitochondria via alternative metabolic pathways, such as alanine, in the absence of MPC 116, the contribution to aspartate or nucleotide synthesis remains to be determined. Moreover, studies of the MPC mutants raise the possibility that glucose-derived carbons could be mediating ventricular remodeling by activating signaling pathways that promote hypertrophy. Glucose suppresses BCAA catabolism by inhibiting CREB-mediated KLF transcription that suppresses expression of genes that encode BCAA catabolic genes. BCAA accumulation promotes cardiac hypertrophy by activating mTOR signaling 239. Glucose 6 phosphate (G6P) has also been shown to a potent activator of mTOR signaling 240. Accumulation of G6P has been noted in animal models and humans with heart failure and levels fall when hearts are unloaded 241. Other regulators of glycolysis have been shown to play regulatory roles in hypertrophic adaptations in the heart. Glycolytic regulation at the level of phosphofructokinase 2 (PFK2) has been suggested to be part of a transcriptional pathway that regulate signal transduction nodes that mediate physiological versus pathological LVH (Figure 5). Specifically, repression of PFK2, as occurs following exercise leads to activation of transcription factors associated with physiological cardiac hypertrophy, whereas activation of PFK2 is associated with signaling pathways that promote pathological LV remodeling 242. Heart failure in animals and humans is associated with increased expression of the fetal isoform of pyruvate kinase (PKM2), which in contrast to the adult isoform PKM1 is less efficient in converting phosphoenolpyruvate (PEP) to pyruvate + ADP 243. The consequence of increased PKM2 expression would be accumulation of glycolytic intermediates, which would increase flux into non-oxidative pathways of glucose metabolism (Figure 4).
Mice with cardiomyocyte KO of the GLUT4 glucose transporter develop a compensatory increase in GLUT1 and a two-fold increase in basal myocardial glucose uptake 113. These animals develop pathologic LVH that is associated with evidence of increased non-oxidative glucose metabolism such as glycogen accumulation 244. One potential mechanism for LVH in these animals is oxidative stress arising in the cytosol and not the mitochondria, supporting the concept that increased availability of glycolytic intermediates could be maladaptive 245. Similar mechanism may contribute to metabolic maladaptation in the context of heart failure and diabetes. As previously discussed, heart failure is associated with increased myocardial utilization of ketones. However in heart samples obtained from humans with heart failure and diabetes, genes encoding proteins responsible for ketone body catabolism are repressed 246. Moreover in a transgenic model of inducible GLUT4 expression in which diabetes could be superimposed, one of the most significantly repressed pathway are those encoding ketone catabolism 246, 247. In addition, increased glucose entry leads to O-GlcNAc modifications of transcriptional regulators of mitochondrial electron transport genes leading to reduced expression. Furthermore, mitochondrial proteins are also subjected to increased O-GlcNAc modifications, which could independently reduce their activity247. Together, these findings suggest that heart failure in combination with diabetes limits the ability of the failing heart to utilize ketones that in concert with exacerbated mitochondrial dysfunction increases toxicity from glycolytic intermediates while aggravating energy deficiency.
Given the relatively low utilization of glucose by the heart in the fasting state the question arises as to what are the major physiological roles for glucose utilization in the heart. Studies in GLUT4 deficient hearts have revealed that the ability of the heart to increase glucose utilization via GLUT4 is essential for myocardial adaptations following diverse stressors such as acute ischemia, swim training to induce physiological hypertrophy or TAC-induced pathological hypertrophy 244, 248. Similar conclusions can be drawn from mice with high-level transgenic overexpression of GLUT1 which are more resilient to I/R injury and pressure overload hypertrophy 249, 250. Also, reducing glucose availability in hearts of mice with BCAA accumulation arising from impaired BCAA catabolism leads to impaired recovery from I/R injury 251. Inducible GLUT1 expression at the time of TAC, preserves mitochondrial function and attenuates early remodeling, but does not ultimately prevent heart failure 252. Importantly, GLUT1 is dispensable for response to TAC, suggesting that the maladaptation resulting from pressure overload is not primarily driven by defects in glucose entry, but rather from the fate of glycolytic intermediates, which is regulated at the level of pyruvate utilization by mitochondria 253. It is important to note though, that the ability to increase fatty acid oxidation during post-ischemic reperfusion is critically important for myocardial recovery post-ischemia even when glucose supply is adequate 254–257.
Lipotoxicity in heart failure: Role of fatty acid oxidation
Lipotoxicity describes the consequences of accumulation of lipid moieties in the heart that activate signaling pathways that impair myocardial function. It is important to note that increasing fatty acid oxidation per se does not inexorably lead to lipotoxicity. This has been elegantly demonstrated in many studies performed in Acc−/− hearts that have high rates of fatty acid oxidation on the basis of reduced levels of malonyl CoA 91, 92. Lipotoxicity represents an important mechanism that augments heart failure risk in diabetes, as extensively reviewed 258, 259. Lipotoxicity occurs when mitochondrial oxidative capacity is unable to adapt to myocardial lipid overload as occurs in obesity or diabetes, or in animal models with overexpression of fatty acid transporters or transcriptional activators that regulate fatty acid oxidative pathways such as PPARα or PPARγ 260. Lipid accumulation has been long described in end-stage failing hearts and is exacerbated when heart failure is superimposed on diabetes 261, 262. The mechanisms responsible for lipotoxicity, particularly in the context of diabetes have been extensively reviewed 258–260. Mechanisms linking lipotoxicity and heart failure include oxidative stress 53, 263, 264, suppressed autophagy 265, mitochondrial uncoupling 266–268 altered mitochondrial dynamics 53, 189, accumulation of toxic lipid intermediates such as ceramide or diacylglycerol 265, 269 ER stress 270, 271 and inflammation 272, 273.
Therapeutic strategies targeting cardiac metabolism to treat heart failure
Given the close relationship between altered myocardial metabolism and heart failure, metabolic modulation remains a promising approach to treating heart failure or reducing ventricular remodelling. Pathways, which have been subjected to therapeutic manipulation in animal and human studies are summarized in Figure 5 and detailed in the following sections.
Stimulating ketone oxidation:
Recent studies have suggested that increasing cardiac ketone oxidation is adaptive in the failing heart and can improve heart function (see 25, 274, 275 for reviews) (Figure 5). This can be achieved by increasing circulating ketone body levels, primarily by one of four approaches: 1) ketone infusions, 2) ketone ester administration, 3) SGLT2 inhibitors, and 4) administration of a ketogenic diet. A study by Nielsen R et al 276 showed that acute infusions of ß-hydroxybutyrate (ßOHB) into patients with HFrEF improves contractile performance. Chronic administration of ßOHB in dogs with pacing-induced heart failure also decreased adverse remodelling 277. Acute administration of a ketone ester (KE, (R-3-hydroxybutyral(R)-3-hydroxybutyrate) to HFrEF patients, resulted in an increase in circulating ßOHB by 12.9-fold and an improvement in contractile function 278. Ketone ester administration was also shown to improve cardiac function and to reduce cardiac remodeling in mouse and rat models of heart failure 279. Unfortunately, it is difficult to chronically maintain elevated circulating ketone levels with either ketone or ketone ester infusions. SGLT2 inhibitors are one approach to overcoming this problem. Although originally developed as anti-hyperglycaemic agents to treat diabetes, SGLT2 inhibitors have recently been shown to have dramatic cardioprotective effects in heart failure patients 280–282. One of the proposed methods by which SGLT2 inhibitors may improve cardiac function in heart failure is by increasing circulating ketone bodies and increasing energy supply to the failing heart 283. Alternatively, increased circulating ketones following SGLT2 inhibitor administration have been proposed to decrease inflammation in the failing heart by modulating the NLRP3 inflammasome 284. Ketogenic diets are another approach to raising circulating ketones. However, feeding mice with heart failure a ketogenic diet results in very modest improvements in end-diastolic volume and end-systolic volume in a pressure-overload mouse model of heart failure 277.
Stimulating glucose oxidation and improving insulin sensitivity:
Recognising the negative impact of cardiac insulin resistance on glucose oxidation rates and cardiac function in the failing heart, several studies have shown that stimulating cardiac glucose oxidation enhances cardiac function and limits the impact of cardiac insulin resistance in the failing heart. Employing dichloroacetate (DCA), a direct inhibitor of pyruvate dehydrogenase kinase, to stimulate PDH complex activity has been an effective approach to stimulate glucose oxidation in different settings of heart failure. DCA improves post-ischemic recovery and cardiac efficiency following ischemia and reperfusion via stimulating glucose oxidation and enhancing the coupling between glycolysis and glucose oxidation 285. Similar improvement in the coupling between glycolysis and glucose oxidation by DCA treatment is also observed in hypertrophied rat hearts 286. In the Dahl-salt sensitive rats, DCA supplementation attenuates the transition from compensated heart failure to heart failure, and improves survival by limiting oxidative stress and improving cardiac reserve 68. Using magnetic resonance spectroscopy with hyperpolarized [13C]pyruvate and magnetic resonance imaging, a recent study showed that PDH flux is decreased by ~50% in a porcine model of heart failure 287. However, DCA treatment improved the contractile reserve and abrogated hypertrophy by enhancing PDH flux in this large animal heart failure model 287. Although not all clinical data are consistent 288, these promising experimental data were recapitulated in small clinical trials. For instance, DCA administration in patients with angina and coronary artery disease augments left ventricle stroke volume and enhances myocardial efficiency (cardiac work/myocardial oxygen consumption) 289, consistent with glucose being an oxygen-efficient substrate for the heart compared to fatty acid 2, 182. In line with that, DCA stimulates glucose oxidation and improved left ventricle mechanical efficiency by reducing myocardial oxygen consumption and enhancing cardiac work in patients with New York Heart Association class III and IV congestive heart failure 177. Another effective approach to enhance insulin sensitivity and glucose oxidation in the failing heart involves enhancing cardiac insulin signaling. For instance, antagonizing glucagon action, using a human monoclonal antibody (mAb A) against glucagon receptor (GCGR), a G-protein coupled receptor, can improve cardiac insulin sensitivity, glucose oxidation and cardiac function post-myocardial infarction 163. Cardiac insulin sensitivity is further impaired when heart failure is associated with diabetes and obesity; both are accompanied by cardiac insulin resistance. Recent studies have shown that weight loss due to different dietary interventions improves cardiac function by enhancing insulin signaling and insulin-stimulated glucose oxidation rates in heart failure associated with obesity 36, 110.
Altering fatty acid oxidation:
As discussed, alterations in fatty acid oxidation in the failing heart are complex, with decreases, no changes and increases in fatty acid oxidation all being reported in the literature (see 1 for review). Despite this, inhibition of fatty acid oxidation has been shown to improve heart function in the failing heart. Inhibitors of fatty acid oxidation such as trimetazidine, etomoxir, and perhexiline have been shown to be cardioprotective in humans with heart failure 290–295. These beneficial effects are thought to be due to an increase in glucose oxidation, secondary to fatty acid oxidation inhibition, resulting in an increase in cardiac efficiency 1.
Malonyl CoA is an endogenous inhibitor of fatty acid oxidation in the heart that acts by inhibiting mitochondrial fatty acid uptake. Malonyl CoA levels are regulated in the heart by malonyl CoA decarboxylase (MCD), which degrades malonyl CoA, and by acetyl CoA carboxylase (ACC), which synthesizes malonyl CoA 17. Inhibition of MCD in a rat model of heart failure results in an increase in myocardial malonyl CoA levels, a decrease in cardiac fatty acid oxidation rates and the prevention of heart failure development 296. In contrast, inhibition of ACC and stimulation of fatty acid oxidation has also been shown to be cardioprotective the failing mouse heart 91, 92. While these beneficial effects of MCD inhibition and ACC inhibition may seem contradictory, interestingly both approaches result in an improved coupling of glycolysis to glucose oxidation, suggesting a decrease in proton production from uncoupled glucose metabolism 91, 296. Inhibition of ACC is also associated with an improved mitochondrial function 92. Additional mechanisms linking altered malonyl CoA levels with amelioration of heart failure could include changes in metabolic intermediates such as acetyl CoA, which could mediate post translational modifications such as protein acetylation, which could independently modulate myocardial metabolism and activity of other regulatory proteins. However recent studies have not found a correlation between mitochondrial protein acetylation and susceptibility of mouse hearts to pressure overload induced heart failure 185, 202.
Stimulating branched-chain amino acid (BCAA) oxidation:
High levels of circulating BCAA and its metabolites, namely branched-chain keto acids (BCKAs) (α-ketovalerate (produced from valine), α-keto-ß-methylvalerate (produced from isoleucine), and α-ketoisocaproate (produced from leucine), have also been positivity correlated with increased cardiovascular disease risk 127, 297, 298. Of interest is that plasma as well as cardiac levels of BCAA and BCKAs increase in the rodent models of myocardial ischemia/reperfusion injury 135, 163, 251 and heart failure 132, 299. Disruption of cardiac BCAA oxidation aggravates cardiac insulin resistance and contractile dysfunction in murine models of myocardial ischemia/reperfusion 251 and aortic constriction 132, while BCAA supplementation further deteriorates cardiac dysfunction and increases infarct size in a mouse model of coronary artery ligation-induced myocardial infarction135. Restriction of BCAA availability also enhances, through an unknown mechanism, fatty acid contribution to ATP production and reduced triglyceride accumulation in hearts of Zucker fatty rats 300. In contrast, pharmacological interventions to enhance BCAA oxidation and/or decrease circulating BCAA levels are cardioprotective in the ischemic and the failing heart 132, 135, 251, 299. Taken together, these studies show that alterations in BCAA oxidation are strongly linked to the development cardiac insulin resistance and contractile dysfunction in different forms of heart failure.
Direct measurement of the BCAA oxidation rates in the heart showed only 1–2% of the total cardiac ATP is produced from BCAA 299, 301. This suggests that it is highly unlikely that interventions to increase BCAA catabolism would materially impact its contribution to cardiac ATP generation or alter the relative utilisation of fatty acids or glucose 302. However, accumulation of BCAA163, 299 and/or BCKA303–305 as a result of impaired BCAA oxidation may increase mTOR activity and impair insulin signaling in the failing heart. Furthermore, high levels of BCAA 251 and/or BCKA306 will impair cardiac PDH activity, to further reduce glucose oxidation in the failing heart163. Impaired protein expression of BCAA catabolic enzymes is evident in the human heart with dilated cardiomyopathy, which is also associated with increased levels of myocardial BCAAs 136. In line with this, treatment with the BCKDK inhibitor, BT2, increases BCAA oxidation in conjunction with reducing BCAA accumulation136 and BCKAs132, 137 in failing murine hearts. However, the exact mechanism through which impaired BCAA oxidation contributes to the development and/or severity of decreased oxidative metabolism of glucose remains to be elucidated. Whether there are divergent roles of BCAA and BCKA in triggering mTOR signaling and mediating myocardial insulin resistance is also yet to be characterized. Recent studies by our group suggested that BCAA, not BCKA, have a dominant role in aggravating cardiac hypertrophy in the failing heart via activating mTOR, while BCKA, not BCAA, induces cardiac insulin resistance via inhibiting cardiac insulin signaling 307, 308.
Conclusions
The failing heart is energy deficient, primarily due to a decrease in mitochondrial oxidative capacity, increased glycolysis uncoupled from glucose oxidation, and either a decrease or no change in fatty acid oxidation. In contrast, in times of increased fatty acid availability, as occurs in obesity, an increase in cardiac fatty acid oxidation may occur. These energy metabolic changes result in the failing heart becoming less efficient. Alterations in mitochondrial oxidative metabolism in the failing heart are due to both transcriptional changes in key enzymes involved in these metabolic pathways, as well as alterations in redox state and metabolite signaling that contribute to post-translational epigenetic changes in the control of expression of genes encoding energy metabolic enzymes. Alterations in the fate of glucose also contribute to the pathology of heart failure. Of importance, pharmacological targeting of mitochondrial oxidative metabolism has emerged as a novel therapeutic approach to improving cardiac efficiency, decreasing the energy deficit and improving cardiac function in the failing heart.
Acknowledgments
Funding:
GDL is funded by a Canadian Institute for Health Research Foundation Grant and a Heart and Stroke Foundation of Canada Grant. QGK is supported by an Alberta Innovates Postgraduate Fellowship in Health Innovation. RT is funded by grants from the National Institute of Health HL129510, HL110349, HL142628, and HL144937. AW is funded by NIH grants HL133011 and HL152354. EDA is funded by grants from the National Institutes of Health HL141783, HL127764, and HL112413 and the American Heart Association grants 20SFRN35120123 and 16SFRN31810000.
Abbreviations:
- ATP
Adenosine triphosphate
- ADP
adenosine diphosphate
- AcAc
acetoacetate
- ACC
acetyl CoA carboxylase
- Akt
protein kinase B
- AMPK
5’ AMP-activated protein kinase
- βOHβ
β-hydroxybutyrate
- BDH1
β-hydroxybutyrate dehydrogenase
- BCAA
branched chain amino acid
- BCAT
branched chain aminotransferase
- BCATm
mitochondrial branched chain
- BCKA
branched chain α-keto-acids
- BCKDH
branched chain α-keto acid
- BT2
BCKDH inhibitor
- BDK
BCKDH kinase
- CPT-1
carnitine palmitoyl transferase
- CoA
coenzyme
- CD36
cluster of differentiation 36
- CD38
cluster of differentiation 38
- CK
creatine kinase
- CKM
muscle form of CK
- DCA
dichloroacetate
- DNMT3A
DNA methyltransferase 3A
- Drp1
dynamin-related protein 1
- ETC
electron transport chain
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- ERRα
estrogen related receptors α
- ERRγ
estrogen related receptors γ
- FAO
fatty acid oxidation
- FATP-1
fatty acid transport protein-1
- FADH2
flavin adenine dinucleotide
- Fis1
mitochondrial fission 1 protein
- FOXM1
forkhead box protein M1
- GCGR
glucagon receptor
- G6P
glucose 6-phosphate
- GLUT1
glucose transporter 1
- GLUT4
glucose transporter 4
- GSH
reduced glutathione
- CpG
guanine nucleotide
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HBP
hexosamine biosynthetic pathway
- H2A, H2B, H3 and H4
histones 2A, 2B, 3 and 4
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HDM
histone demethylase
- 5hmC
hydroxymethylation
- IDCM
idiopathic dilated cardiomyopathy
- IRS
insulin receptor substrate
- KO
knock out
- LDH
lactate dehydrogenase
- LIVCS
branched chain amino acid\cation symporter family
- LV
left ventricle
- LVH
LV hypertrophy
- LVAD
LV assist device
- lncRNA
long non-coding RNA
- ME1
malic enzyme 1
- MCD
malonyl CoA decarboxylase
- mTOR
mammalian target of rapamycin
- MCAD
medium chain acyl CoA dehydrogenase
- 5mc
methylation
- miR
microRNA
- MPC
mitochondrial pyruvate carrier
- Mfn1
mitofusin 1
- Mfn2
mitofusin 2
- MCT1
monocarboxylate transporter 1
- SLC16A1
monocarboxylate ketone transporter
- NAD
nicotinamide adenine dinucleotide oxidized
- NADH
nicotinamide adenine dinucleotide reduced
- NADPH
nicotinamide adenine dinucleotide phosphate
- NADS
NAD synthase
- NAM
nicotinamide
- NDRG3
N-myc downstream-regulated gene 3
- NHE
sodium/hydrogen exchanger
- NMN
nicotinamide mononucleotide
- NMNAT
nicotinic acid mononucleotide adenyltransferase
- NR
nicotinamide riboside
- NRF-1
nuclear respiratory factor-1
- NRF-2
nuclear respiratory factor-2
- NRK
nicotinamide riboside kinase
- OPA1
mitochondrial dynamin like GTPase
- OXPHOS
oxidative phosphorylation
- PARPs
poly (ADP-ribose) polymerases
- PDK1
3-phoshoinositide-dependent protein kinase 1
- PEP
phosphoenolpyruvate
- PFK-1
phosphofructokinase 1
- PFK-2
phosphofructokinase 2
- PPARα
peroxisome proliferator activator receptor α
- PPARγ
peroxisomal proliferator activator receptor γ
- PGC1-α
PPARγ coactivator 1-α
- PPC2m
protein phosphatase C2m
- PDH
pyruvate dehydrogenase
- PDK4
PDH kinase 4
- PKM
pyruvate kinase
- PP2Cm
mitochondrial-target 2C-type ser/thr protein phosphatase
- PPP
pentose phosphate pathway
- ROS
reactive oxygen species
- SAM
S-adenosylmethionine
- SCOT
succinyl-CoA:3 oxoacid-CoA transferase
- SGLT2
sodium/ glucose co-transporter 2
- SGLT2i
SGLT2 inhibitor
- Sirt3
sirtuin 3
- T2D
type 2 diabetes
- TAC
transverse aortic constriction
- TET
ten-eleven translocation
- TCA
tricarboxylic acid
- TG
triacylglycerol
- VLDL
very low-density lipoproteins
Footnotes
Conflict of interest: None declared
References
- 1.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010;90:207–258 [DOI] [PubMed] [Google Scholar]
- 2.Karwi QG, Uddin GM, Ho KL, Lopaschuk GD. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med. 2018;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neubauer S The failing heart — an engine out of fuel. N. Engl. J. Med 2007;356:1140–1151 [DOI] [PubMed] [Google Scholar]
- 4.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: Final data for 2009. Natl. Vital Stat. Rep 2011;60:1–116 [PubMed] [Google Scholar]
- 5.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circ. 2016;133:e38–360 [DOI] [PubMed] [Google Scholar]
- 6.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med 2006;355:251–259 [DOI] [PubMed] [Google Scholar]
- 7.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N. Engl. J. Med 2006;355:260–269 [DOI] [PubMed] [Google Scholar]
- 8.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circ. 2012;126:65–75 [DOI] [PubMed] [Google Scholar]
- 9.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005;85:1093–1129 [DOI] [PubMed] [Google Scholar]
- 10.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, et al. Myocardial phosphocreatine-to-atp ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circ. 1997;96:2190–2196 [DOI] [PubMed] [Google Scholar]
- 11.Ritterhoff J, Tian R. Metabolism in cardiomyopathy: Every substrate matters. Cardiovasc. Res 2017;113:411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J. Biol. Chem 1991;266:8162–8170 [PubMed] [Google Scholar]
- 13.Wisneski JA, Stanley WC, Neese RA, Gertz EW. Effects of acute hyperglycemia on myocardial glycolytic activity in humans. J. Clin. Invest 1990;85:1648–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy MS, Pande SV. Malonyl-coa binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc. Natl. Acad. Sci. U. S. A 1987;84:378–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulson DJ, Ward KM, Shug AL. Malonyl coa inhibition of carnitine palmityltransferase in rat heart mitochondria. FEBS Lett. 1984;176:381–384 [DOI] [PubMed] [Google Scholar]
- 16.Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-coa carboxylase regulation of fatty acid oxidation in the heart. J. Biol. Chem 1993;268:25836–25845 [PubMed] [Google Scholar]
- 17.Dyck JR, Lopaschuk GD. Malonyl coa control of fatty acid oxidation in the ischemic heart. J. Mol. Cell. Cardiol 2002;34:1099–1109 [DOI] [PubMed] [Google Scholar]
- 18.van Hall G Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. (Oxf.). 2010;199:499–508 [DOI] [PubMed] [Google Scholar]
- 19.Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang Guo J, et al. Glucose feeds the tca cycle via circulating lactate. Nature. 2017;551:115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai C, Li Q, May HI, Li C, Zhang G, Sharma G, Sherry AD, Malloy CR, Khemtong C, Zhang Y, et al. Lactate dehydrogenase a governs cardiac hypertrophic growth in response to hemodynamic stress. Cell Rep 2020;32:108087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. 2018;27:757–785 [DOI] [PubMed] [Google Scholar]
- 23.Chen YJ, Mahieu NG, Huang X, Singh M, Crawford PA, Johnson SL, Gross RW, Schaefer J, Patti GJ. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol 2016;12:937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol 2013;304:H1060–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karwi QG, Biswas D, Pulinilkunnil T, Lopaschuk GD. Myocardial ketones metabolism in heart failure. J. Card. Fail 2020;26:998–1005 [DOI] [PubMed] [Google Scholar]
- 26.Ho KL, Karwi QG, Wagg C, Zhang L, Vo K, Altamimi T, Uddin GM, Ussher JR, Lopaschuk GD. Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc. Res 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fillmore N, Wagg CS, Zhang L, Fukushima A, Lopaschuk GD. Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am J Physiol Endocrinol Metab. 2018;315:E1046–E1052 [DOI] [PubMed] [Google Scholar]
- 28.Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: Unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab. 2004;286:E64–76 [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. Metabolite profiles and the risk of developing diabetes. Nat. Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bottomley PA, Panjrath GS, Lai S, Hirsch GA, Wu K, Najjar SS, Steinberg A, Gerstenblith G, Weiss RG. Metabolic rates of atp transfer through creatine kinase (ck flux) predict clinical heart failure events and death. Sci. Transl. Med 2013;5:215re213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ. Res 2004;95:135–145 [DOI] [PubMed] [Google Scholar]
- 32.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Invest 2018;128:3716–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circ. 2019;139:1435–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowlton AA, Chen L, Malik ZA. Heart failure and mitochondrial dysfunction: The role of mitochondrial fission/fusion abnormalities and new therapeutic strategies. J. Cardiovasc. Pharmacol 2014;63:196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karwi QG, Zhang L, Altamimi TR, Wagg CS, Patel V, Uddin GM, Joerg AR, Padwal RS, Johnstone DE, Sharma A, et al. Weight loss enhances cardiac energy metabolism and function in heart failure associated with obesity. Diabetes Obes. Metab 2019;21:1944–1955 [DOI] [PubMed] [Google Scholar]
- 37.Smith GL, Eisner DA. Calcium buffering in the heart in health and disease. Circ. 2019;139:2358–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong M, Zablocki D, Sadoshima J. The role of drp1 in mitophagy and cell death in the heart. J. Mol. Cell. Cardiol 2020;142:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ. Res. 2019;124:121–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian R, Colucci WS, Arany Z, Bachschmid MM, Ballinger SW, Boudina S, Bruce JE, Busija DW, Dikalov S, Dorn GW, II, et al. Unlocking the secrets of mitochondria in the cardiovascular system: Path to a cure in heart failure-a report from the 2018 national heart, lung, and blood institute workshop. Circ. 2019;140:1205–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukushima A, Lopaschuk GD. Acetylation control of cardiac fatty acid beta-oxidation and energy metabolism in obesity, diabetes, and heart failure. Biochim Biophys Acta. 2016;1862:2211–2220 [DOI] [PubMed] [Google Scholar]
- 42.Shanmugam G, Wang D, Gounder SS, Fernandes J, Litovsky SH, Whitehead K, Radhakrishnan RK, Franklin S, Hoidal JR, Kensler TW, et al. Reductive stress causes pathological cardiac remodeling and diastolic dysfunction. Antioxid. Redox Signal. 2020;32:1293–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. Br. Heart J. 1991;65:245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Recchia FA, McConnell PI, Bernstein RD, Vogel TR, Xu X, Hintze TH. Reduced nitric oxide production and altered myocardial metabolism during the decompensation of pacing-induced heart failure in the conscious dog. Circ. Res. 1998;83:969–979 [DOI] [PubMed] [Google Scholar]
- 45.Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circ. 1997;96:2414–2420 [DOI] [PubMed] [Google Scholar]
- 46.Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin f2α in pericardial fluid of patients with heart failure. A Potential Role for In Vivo Oxidant Stress in Ventricular Dilatation and Progression to Heart Failure. 1998;97:1536–1539 [DOI] [PubMed] [Google Scholar]
- 47.Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med 2011;51:978–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. American Journal of Physiology - Heart and Circulatory Physiology. 2011;301:H2181–H2190 [DOI] [PubMed] [Google Scholar]
- 49.O’Rourke B, Ashok D, Liu T. Mitochondrial ca(2+) in heart failure: Not enough or too much? J. Mol. Cell. Cardiol. 2020;151:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauer TM, Murphy E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ. Res 2020;126:280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GW 2nd. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science. 2015;350:aad2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song M, Dorn GW, 2nd. Mitoconfusion: Noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab. 2015;21:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsushima K, Bugger H, Wende AR, Soto J, Jenson GA, Tor AR, McGlauflin R, Kenny HC, Zhang Y, Souvenir R, et al. Mitochondrial reactive oxygen species in lipotoxic hearts induce post-translational modifications of akap121, drp1, and opa1 that promote mitochondrial fission. Circ. Res. 2018;122:58–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaanine AH, Joyce LD, Stulak JM, Maltais S, Joyce DL, Dearani JA, Klaus K, Nair KS, Hajjar RJ, Redfield MM. Mitochondrial morphology, dynamics, and function in human pressure overload or ischemic heart disease with preserved or reduced ejection fraction. Circ. Heart Fail. 2019;12:e005131. [DOI] [PubMed] [Google Scholar]
- 55.Dirks-Naylor AJ, Kouzi SA, Bero JD, Phan DT, Taylor HN, Whitt SD, Mabolo R. Doxorubicin alters the mitochondrial dynamics machinery and mitophagy in the liver of treated animals. Fundam. Clin. Pharmacol. 2014;28:633–642 [DOI] [PubMed] [Google Scholar]
- 56.Shires SE, Gustafsson ÅB. Mitophagy and heart failure. J. Mol. Med. (Berl.). 2015;93:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finck BN, Kelly DP. Pgc-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 2006;116:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finck BN, Kelly DP. Pgc-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circ. 2004;109:2191–2196 [DOI] [PubMed] [Google Scholar]
- 60.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to atp production in hypertrophied hearts. Am. J. Physiol. 1994;267:H742–750 [DOI] [PubMed] [Google Scholar]
- 61.Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS, et al. Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart: Implications for cardiac reloading and conditioning. JACC Basic Transl Sci. 2016;1:432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei B, Lionetti V, Young ME, Chandler MP, d’Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J. Mol. Cell. Cardiol. 2004;36:567–576 [DOI] [PubMed] [Google Scholar]
- 63.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: Implications beyond atp production. Circ. Res 2013;113:709–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Despa S, Bers DM. Na(+) transport in the normal and failing heart - remember the balance. J. Mol. Cell. Cardiol 2013;61:2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: Molecular regulatory mechanisms. Am. J. Med. Sci 1999;318:36–42 [DOI] [PubMed] [Google Scholar]
- 66.Grover-McKay M, Schwaiger M, Krivokapich J, Perloff JK, Phelps ME, Schelbert HR. Regional myocardial blood flow and metabolism at rest in mildly symptomatic patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol 1989;13:317–324 [DOI] [PubMed] [Google Scholar]
- 67.Rosenblatt-Velin N, Montessuit C, Papageorgiou I, Terrand J, Lerch R. Postinfarction heart failure in rats is associated with upregulation of glut-1 and downregulation of genes of fatty acid metabolism. Cardiovasc. Res 2001;52:407–416 [DOI] [PubMed] [Google Scholar]
- 68.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ. Heart Fail. 2010;3:420–430 [DOI] [PubMed] [Google Scholar]
- 69.Dávila-Román VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2002;40:271–277 [DOI] [PubMed] [Google Scholar]
- 70.Tuunanen H, Engblom E, Naum A, Någren K, Hesse B, Airaksinen KEJ, Nuutila P, Iozzo P, Ukkonen H, Opie LH, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circ. 2006;114:2130–2137 [DOI] [PubMed] [Google Scholar]
- 71.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, et al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H3270–3278 [DOI] [PubMed] [Google Scholar]
- 72.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circ. 1996;94:2837–2842 [DOI] [PubMed] [Google Scholar]
- 73.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid x receptor-alpha in pacing-induced heart failure. Circ. 2002;106:606–612 [DOI] [PubMed] [Google Scholar]
- 74.Heather LC, Cole MA, Lygate CA, Evans RD, Stuckey DJ, Murray AJ, Neubauer S, Clarke K. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc. Res 2006;72:430–437 [DOI] [PubMed] [Google Scholar]
- 75.Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. An evaluation of myocardial fatty acid and glucose uptake using pet with [18f]fluoro-6-thia-heptadecanoic acid and [18f]fdg in patients with congestive heart failure. J. Nucl. Med 2001;42:55–62 [PubMed] [Google Scholar]
- 76.Funada J, Betts TR, Hodson L, Humphreys SM, Timperley J, Frayn KN, Karpe F. Substrate utilization by the failing human heart by direct quantification using arterio-venous blood sampling. PLoS One. 2009;4:e7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Voros G, Ector J, Garweg C, Droogne W, Van Cleemput J, Peersman N, Vermeersch P, Janssens S. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ. Heart Fail. 2018;11:e004953. [DOI] [PubMed] [Google Scholar]
- 78.Gupte AA, Hamilton DJ, Cordero-Reyes AM, Youker KA, Yin Z, Estep JD, Stevens RD, Wenner B, Ilkayeva O, Loebe M, Pet al. Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ. Cardiovasc. Genet 2014;7:266–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karbowska J, Kochan Z, Smolenski RT. Peroxisome proliferator-activated receptor alpha is downregulated in the failing human heart. Cell. Mol. Biol. Lett 2003;8:49–53 [PubMed] [Google Scholar]
- 80.Lahey R, Wang X, Carley AN, Lewandowski ED. Dietary fat supply to failing hearts determines dynamic lipid signaling for nuclear receptor activation and oxidation of stored triglyceride. Circ. 2014;130:1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Djousse L, Benkeser D, Arnold A, Kizer JR, Zieman SJ, Lemaitre RN, Tracy RP, Gottdiener JS, Mozaffarian D, Siscovick DS, et al. Plasma free fatty acids and risk of heart failure: The cardiovascular health study. Circ. Heart Fail 2013;6:964–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196 [DOI] [PubMed] [Google Scholar]
- 83.Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: Studies with cardiac positron emission tomography and magnetic resonance imaging. J. Am. Coll. Cardiol 2009;54:1524–1532 [DOI] [PubMed] [Google Scholar]
- 84.Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circ. 2003;108:754–759 [DOI] [PubMed] [Google Scholar]
- 85.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinol. 2003;144:3483–3490 [DOI] [PubMed] [Google Scholar]
- 86.Hamdani N, Hervent A-S, Vandekerckhove L, Matheeussen V, Demolder M, Baerts L, De Meester I, Linke WA, Paulus WJ, De Keulenaer GW. Left ventricular diastolic dysfunction and myocardial stiffness in diabetic mice is attenuated by inhibition of dipeptidyl peptidase 4. Cardiovasc. Res 2014;104:423–431 [DOI] [PubMed] [Google Scholar]
- 87.Venardos K, De Jong KA, Elkamie M, Connor T, McGee SL. The pkd inhibitor cid755673 enhances cardiac function in diabetic db/db mice. PLoS One. 2015;10:e0120934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li R-j, Yang J, Yang Y, Ma N, Jiang B, Sun Q-w, Li Y-j. Speckle tracking echocardiography in the diagnosis of early left ventricular systolic dysfunction in type ii diabetic mice. BMC Cardiovasc. Disord 2014;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hglut4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–982 [DOI] [PubMed] [Google Scholar]
- 90.Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, Xiao H, Yu H, Zheng Y, Liang Y, et al. Targeting mitochondria-inflammation circuit by beta-hydroxybutyrate mitigates hfpef. Circ. Res 2020 [DOI] [PubMed] [Google Scholar]
- 91.Kolwicz SC Jr., Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl coa carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ. Res 2012;111:728–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shao D, Kolwicz SC Jr., Wang P, Roe ND, Villet O, Nishi K, Hsu YA, Flint GV, Caudal A, Wang W, et al. Increasing fatty acid oxidation prevents high-fat diet-induced cardiomyopathy through regulating parkin-mediated mitophagy. Circ. 2020;142:983–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ. Res 2005;96:225–233 [DOI] [PubMed] [Google Scholar]
- 94.Lewandowski ED, Fischer SK, Fasano M, Banke NH, Walker LA, Huqi A, Wang X, Lopaschuk GD, O’Donnell JM. Acute l-cpt1 overexpression recapitulates reduced palmitate oxidation of cardiac hypertrophy. Circ. Res 2013;112:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, Ding Y, Prasain J, Wood PA, Yang Q. Carnitine palmitoyltransferase-1b (cpt1b) deficiency aggravates pressure-overload-induced cardiac hypertrophy due to lipotoxicity. Circ. 2012;126:1705–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ. Heart Fail 2013;6:1039–1048 [DOI] [PubMed] [Google Scholar]
- 97.Zhabyeyev P, Gandhi M, Mori J, Basu R, Kassiri Z, Clanachan A, Lopaschuk GD, Oudit GY. Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc. Res 2013;97:676–685 [DOI] [PubMed] [Google Scholar]
- 98.Moravec J, El Alaoui-Talibi Z, Moravec M, Guendouz A. Control of oxidative metabolism in volume-overloaded rat hearts. In: Ince C, Kesecioglu J, Telci L, Akpir K, eds. Oxygen transport to tissue xvii. Boston, MA: Springer US; 1996:205–212. [Google Scholar]
- 99.Schroeder MA, Lau AZ, Chen AP, Gu Y, Nagendran J, Barry J, Hu X, Dyck JRB, Tyler DJ, Clarke K, et al. Hyperpolarized (13)c magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur. J. Heart Fail. 2013;15:130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee SH, Hadipour-Lakmehsari S, Kim DH, Di Paola M, Kuzmanov U, Shah S, Lee JJ, Kislinger T, Sharma P, Oudit GY, et al. Bioinformatic analysis of membrane and associated proteins in murine cardiomyocytes and human myocardium. Sci Data. 2020;7:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fernandez-Caggiano M, Kamynina A, Francois AA, Prysyazhna O, Eykyn TR, Krasemann S, Crespo-Leiro MG, Vieites MG, Bianchi K, Morales V, et al. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat Metab 2020;2:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase i activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circ. 2007;115:2033–2041 [DOI] [PubMed] [Google Scholar]
- 103.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: Attenuating upregulated anaplerosis in hypertrophy. Circ. Res 2009;104:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lahey R, Carley AN, Wang X, Glass CE, Accola KD, Silvestry S, O’Donnell JM, Lewandowski ED. Enhanced redox state and efficiency of glucose oxidation with mir based suppression of maladaptive nadph-dependent malic enzyme 1 expression in hypertrophied hearts. Circ. Res 2018;122:836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thakker GD, Frangogiannis NG, Zymek PT, Sharma S, Raya JL, Barger PM, Taegtmeyer H, Entman ML, Ballantyne CM. Increased myocardial susceptibility to repetitive ischemia with high-fat diet-induced obesit. Obesity. 2008;16:2593–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holzem KM, Marmerstein JT, Madden EJ, Efimov IR. Diet-induced obesity promotes altered remodeling and exacerbated cardiac hypertrophy following pressure overload. Physiol Rep 2015;3:e12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mori J, Basu R, McLean BA, Das SK, Zhang L, Patel VB, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: A metabolic contribution to heart failure with normal ejection fraction. Circ. Heart Fail. 2012;5:493–503 [DOI] [PubMed] [Google Scholar]
- 108.Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY. Ang ii causes insulin resistance and induces cardiac metabolic switch and inefficiency: A critical role of pdk4. American Journal of Physiology: Heart and Circulatory Physiology. 2013;304:H1103–H1113 [DOI] [PubMed] [Google Scholar]
- 109.Christe ME, Rodgers RL. Cardiac glucose and fatty acid oxidation in the streptozotocin-induced diabetic spontaneously hypertensive rat. Hypertension. 1995;25:235–241 [DOI] [PubMed] [Google Scholar]
- 110.Sankaralingam S, Abo Alrob O, Zhang L, Jaswal JS, Wagg CS, Fukushima A, Padwal RS, Johnstone DE, Sharma AM, Lopaschuk GD. Lowering body weight in obese mice with diastolic heart failure improves cardiac insulin sensitivity and function: Implications for the obesity paradox. Diabetes. 2015;64:1643–1657 [DOI] [PubMed] [Google Scholar]
- 111.Lopaschuk GD, Folmes CDL, Stanley WC. Cardiac energy metabolism in obesity. Circ. Res 2007;101:335–347 [DOI] [PubMed] [Google Scholar]
- 112.Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, DesAulniers J, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Angiotensin 1–7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ. Heart Fail. 2014;7:327–339 [DOI] [PubMed] [Google Scholar]
- 113.Abel ED, Kaulbach HC, Tian R, Hopkins JCA, Duffy J, Doetschman T, Minnemann T, Boers M-E, Hadro E, Oberste-Berghaus C, Quist W, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of glut4 from the heart. J. Clin. Invest 1999;104:1703–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun W, Quan N, Wang L, Yang H, Chu D, Liu Q, Zhao X, Leng J, Li J. Cardiac-specific deletion of the pdha1 gene sensitizes heart to toxicological actions of ischemic stress. Toxicol. Sci 2016;151:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cluntun AA, Badolia R, Lettlova S, Parnell KM, Shankar TS, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, et al. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y, Taufalele PV, Cochran JD, Robillard-Frayne I, Marx JM, Soto J, Rauckhorst AJ, Tayyari F, Pewa AD, Gray LR, et al. Mitochondrial pyruvate carriers are required for myocardial stress adaptation. Nat Metab. 2020;2:1248–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Du Z, Shen A, Huang Y, Su L, Lai W, Wang P, Xie Z, Xie Z, Zeng Q, Ren H, et al. 1h-nmr-based metabolic analysis of human serum reveals novel markers of myocardial energy expenditure in heart failure patients. PLoS One. 2014;9:e88102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, Härkönen M. Blood ketone bodies in congestive heart failure. J. Am. Coll. Cardiol 1996;28:665–672 [DOI] [PubMed] [Google Scholar]
- 119.Lommi J, Koskinen P, Naveri H, Harkonen M, Kupari M. Heart failure ketosis. J. Intern. Med 1997;242:231–238 [DOI] [PubMed] [Google Scholar]
- 120.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circ. 2016;133:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bedi KC Jr., Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circ. 2016;133:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc. Res. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schugar RC, Moll AR, André d’Avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Molecular Metabolism. 2014;3:754–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, Dyck JRB, Alberta H. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One. 2015;10:e0124844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvadó J, Razquin C, Corella D, Estruch R, Ros E, Fitó M, et al. Plasma branched-chain amino acids and incident crdiovascular disease in the predimed trial. Clin. Chem 2016;62:582–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engstrom G, Ostling G, Clish C, Wang TJ, Gerszten RE, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur. Heart J 2013;34:1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bhattacharya S, Granger CB, Craig D, Haynes C, Bain J, Stevens RD, Hauser ER, Newgard CB, Kraus WE, Newby LK, et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. 2014;232:191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peterson MB, Mead RJ, Welty JD. Free amino acids in congestive heart failure. J. Mol. Cell. Cardiol 1973;5:139–147 [DOI] [PubMed] [Google Scholar]
- 129.Venturini A, Ascione R, Lin H, Polesel E, Angelini GD, Suleiman MS. The importance of myocardial amino acids during ischemia and reperfusion in dilated left ventricle of patients with degenerative mitral valve disease. Mol Cell Biochem 2009;330:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lai L, Leone TC, Keller MP, Martin OJ, Broman AT, Nigro J, Kapoor K, Koves TR, Stevens R, Ilkayeva OR, et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: A multisystems approach. Circ. Heart Fail 2014;7:1022–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, et al. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ. Heart Fail. 2014;7:634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, Rau CD, et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circ. 2016;133:2038–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Neishabouri SH, Hutson SM, Davoodi J. Chronic activation of mtor complex 1 by branched chain amino acids and organ hypertrophy. Amino Acids. 2015;47:1167–1182 [DOI] [PubMed] [Google Scholar]
- 134.Davoodi J, Hutson S. Constitutive activation of mtor pathway by leucine causes heart hypertrophy which can be blocked by rapamycin. The FASEB Journal. 2012;26:1013–1016 [Google Scholar]
- 135.Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, Lee Y, Li C, Zhang L, Lian K,et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 2016;311:H1160–H1169 [DOI] [PubMed] [Google Scholar]
- 136.Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, Al Batran R, Pherwani S, Ho KL, Boisvenue J, et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc. Diabetol 2019;18:019–0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM, Chuang DT, Wang Y, Sun H. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. Journal of the American Heart Association. 2019;8:e011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Riehle C, Abel ED. Insulin signaling and heart failure. Circ. Res 2016;118:1151–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED, et al. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc. Res 2014;103:485–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Masoud WG, Ussher JR, Wang W, Jaswal JS, Wagg CS, Dyck JR, Lygate CA, Neubauer S, Clanachan AS, Lopaschuk GD. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc. Res 2014;101:30–38 [DOI] [PubMed] [Google Scholar]
- 141.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinol. 2005;146:5341–5349 [DOI] [PubMed] [Google Scholar]
- 142.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol 2009;53:1925–1932 [DOI] [PubMed] [Google Scholar]
- 143.Miettinen H, Lehto S, Salomaa V, Mähönen M, Niemelä M, Haffner SM, Pyörälä K, Tuomilehto J. Impact of diabetes on mortality after the first myocardial infarction. Diabetes Care. 1998;21:69–75 [DOI] [PubMed] [Google Scholar]
- 144.Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J. Am. Coll. Cardiol 2001;38:421–428 [DOI] [PubMed] [Google Scholar]
- 145.Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, Rouleau JL, Sigouin C, Solymoss CB, Tsuyuki R, et al. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur. Heart J 2000;21:1368–1375 [DOI] [PubMed] [Google Scholar]
- 146.Paolisso G, Tagliamonte MR, Rizzo MR, Gambardella A, Gualdiero P, Lama D, Varricchio G, Gentile S, Varricchio M. Prognostic importance of insulin-mediated glucose uptake in aged patients with congestive heart failure secondary to mitral and/or aortic valve disease. Am. J. Cardiol 1999;83:1338–1344 [DOI] [PubMed] [Google Scholar]
- 147.Amato L, Paolisso G, Cacciatore F, Ferrara N, Ferrara P, Canonico S, Varricchio M, Rengo F. Congestive heart failure predicts the development of non-insulin-dependent diabetes mellitus in the elderly. The osservatorio geriatrico regione campania group. Diabetes Metab 1997;23:213–218 [PubMed] [Google Scholar]
- 148.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circ. 2007;116:434–448 [DOI] [PubMed] [Google Scholar]
- 149.Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: Relation to severity and etiology of heart failure. J. Am. Coll. Cardiol 1997;30:527–532 [DOI] [PubMed] [Google Scholar]
- 150.Vardeny O, Gupta DK, Claggett B, Burke S, Shah A, Loehr L, Rasmussen-Torvik L, Selvin E, Chang PP, Aguilar D, et al. Insulin resistance and incident heart failure the aric study (atherosclerosis risk in communities). JACC Heart Fail. 2013;1:531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (ukpds 35): Prospective observational study. BMJ. 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102 [DOI] [PubMed] [Google Scholar]
- 153.Alpert MA. Obesity cardiomyopathy: Pathophysiology and evolution of the clinical syndrome. Am. J. Med. Sci 2001;321:225–236 [DOI] [PubMed] [Google Scholar]
- 154.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The framingham heart study. JAMA. 1991;266:231–236 [PubMed] [Google Scholar]
- 155.Fu F, Zhao K, Li J, Xu J, Zhang Y, Liu C, Yang W, Gao C, Li J, Zhang H, et al. Direct evidence that myocardial insulin resistance following myocardial ischemia contributes to post-ischemic heart failure. Sci. Rep. 2015;5:17927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei Z, Peroutka RJ, Gold JI, Gumpert A, Chen M, Otis NJ,et al. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circ. 2011;123:1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: Molecular mechanisms. Heart Fail. Clin 2012;8:609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, Tateno K, Moriya J, Yokoyama M, Nojima A, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J. Clin. Invest 2010;120:1506–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Riehle C, Weatherford ET, Wende AR, Jaishy BP, Seei AW, McCarty NS, Rech M, Shi Q, Reddy GR, Kutschke WJ, et al. Insulin receptor substrates differentially exacerbate insulin-mediated left ventricular remodeling. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, Muoio DM, Lopaschuk GD. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl coa decarboxylase. Diabetes. 2009;58:1766–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim Biophys Acta. 2005;1734:112–126 [DOI] [PubMed] [Google Scholar]
- 162.Sung MM, Das SK, Levasseur J, Byrne NJ, Fung D, Kim T, Masson G, Boisvenue J, Soltys CL, Oudit GY, et al. Resveratrol treatment of mice with pressure overload-induced heart failure improves diastolic function and cardiac energy metabolism. Circ. Heart Fail. 2014 [DOI] [PubMed] [Google Scholar]
- 163.Karwi QG, Zhang L, Wagg CS, Wang W, Ghandi M, Thai D, Yan H, Ussher JR, Oudit GY, Lopaschuk GD. Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction. Cardiovasc. Diabetol. 2019;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Olefsky JM. Insulin’s effect on glucose oxidation independent of glucose transport. Biochem Biophys Res Commun. 1976;71:106–113 [DOI] [PubMed] [Google Scholar]
- 165.Leonards JR, Landau BR. A study of the equivalence of metabolic patterns in rat adipose tissue: Insulin versus glucose concentration. Arch. Biochem. Biophys 1960;91:194–200 [DOI] [PubMed] [Google Scholar]
- 166.Song HP, Chu ZG, Zhang DX, Dang YM, Zhang Q. Pi3k-akt pathway protects cardiomyocytes against hypoxia-induced apoptosis by mitokatp-mediated mitochondrial translocation of pakt. Cell. Physiol. Biochem 2018;49:717–727 [DOI] [PubMed] [Google Scholar]
- 167.Yang JY, Yeh HY, Lin K, Wang PH. Insulin stimulates akt translocation to mitochondria: Implications on dysregulation of mitochondrial oxidative phosphorylation in diabetic myocardium. J. Mol. Cell. Cardiol 2009;46:919–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li C, Li Y, He L, Agarwal AR, Zeng N, Cadenas E, Stiles BL. Pi3k/akt signaling regulates bioenergetics in immortalized hepatocytes. Free Radic. Biol. Med 2013;60:29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dyck JR, Kudo N, Barr AJ, Davies SP, Hardie DG, Lopaschuk GD. Phosphorylation control of cardiac acetyl-coa carboxylase by camp-dependent protein kinase and 5’-amp activated protein kinase. Eur. J. Biochem 1999;262:184–190 [DOI] [PubMed] [Google Scholar]
- 170.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 171.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail. 2013;6:1039–1048 [DOI] [PubMed] [Google Scholar]
- 172.Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY. Ang ii causes insulin resistance and induces cardiac metabolic switch and inefficiency: A critical role of pdk4. Am J Physiol Heart Circ Physiol. 2013;304:H1103–1113 [DOI] [PubMed] [Google Scholar]
- 173.Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur. Heart J 2010;31:100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Badolia R, Ramadurai DKA, Abel ED, Ferrin P, Taleb I, Shankar TS, Krokidi AT, Navankasattusas S, McKellar SH, Yin M, et al. The role of nonglycolytic glucose metabolism in myocardial recovery upon mechanical unloading and circulatory support in chronic heart failure. Circ 2020;142:259–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McCommis KS, Kovacs A, Weinheimer CJ, Shew TM, Koves TR, Ilkayeva OR, Kamm DR, Pyles KD, King MT, Veech RL, et al. Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice. Nat Metab 2020;2:1232–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Morii I, Kihara Y, Inoko M, Sasayama S. Myocardial contractile efficiency and oxygen cost of contractility are preserved during transition from compensated hypertrophy to failure in rats with salt-sensitive hypertension. Hypertension. 1998;31:949–960 [DOI] [PubMed] [Google Scholar]
- 177.Bersin RM, Wolfe C, Kwasman M, Lau D, Klinski C, Tanaka K, Khorrami P, Henderson GN, de Marco T, Chatterjee K. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J. Am. Coll. Cardiol 1994;23:1617–1624 [DOI] [PubMed] [Google Scholar]
- 178.Fu Q, Xu B, Liu Y, Parikh D, Li J, Li Y, Zhang Y, Riehle C, Zhu Y, et al. Insulin inhibits cardiac contractility by inducing a gi-biased beta2-adrenergic signaling in hearts. Diabetes. 2014;63:2676–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Q, Liu Y, Fu Q, Xu B, Zhang Y, Kim S, Tan R, Barbagallo F, West T, Anderson E, et al. Inhibiting insulin-mediated beta2-adrenergic receptor activation prevents diabetes-associated cardiac dysfunction. Circ. 2017;135:73–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schonekess BO, Allard MF, Lopaschuk GD. Propionyl l-carnitine improvement of hypertrophied heart function is accompanied by an increase in carbohydrate oxidation. Circ. Res. 1995;77:726–734 [DOI] [PubMed] [Google Scholar]
- 181.Schonekess BO, Allard MF, Henning SL, Wambolt RB, Lopaschuk GD. Contribution of glycogen and exogenous glucose to glucose metabolism during ischemia in the hypertrophied rat heart. Circ. Res 1997;81:540–549 [DOI] [PubMed] [Google Scholar]
- 182.Karwi QG, Wagg CS, Altamimi TR, Uddin GM, Ho KL, Darwesh AM, Seubert JM, Lopaschuk GD. Insulin directly stimulates mitochondrial glucose oxidation in the heart. Cardiovasc. Diabetol 2020;19:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Parra V, Verdejo HE, Iglewski M, Del Campo A, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C, Lopez-Crisosto C, et al. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the akt-mtor-nfkappab-opa-1 signaling pathway. Diabetes. 2014;63:75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374 [DOI] [PubMed] [Google Scholar]
- 185.Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC Jr., et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE, et al. Normalization of nad+ redox balance as a therapy for heart failure. Circ. 2016;134:883–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, Gouge A, Gressette M, Manoury B, Blanc J, et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circ. 2018;137:2256–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yoshino J, Baur JA, Imai SI. Nad(+) intermediates: The biology and therapeutic potential of nmn and nr. Cell Metab. 2018;27:513–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hu Q, Zhang H, Gutierrez Cortes N, Wu D, Wang P, Zhang J, Mattison JA, Smith E, Bettcher LF, Wang M, et al. Increased drp1 acetylation by lipid overload induces cardiomyocyte death and heart dysfunction. Circ. Res 2020;126:456–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou B, Wang DD, Qiu Y, Airhart S, Liu Y, Stempien-Otero A, O’Brien KD, Tian R. Boosting nad level suppresses inflammatory activation of pbmcs in heart failure. J. Clin. Invest. 2020;130:6054–6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Walker MA, Tian R. Raising nad in heart failure: Time to translate? Circ. 2018;137:2274–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Canto C, Menzies KJ, Auwerx J. Nad(+) metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Katsyuba E, Auwerx J. Modulating nad(+) metabolism, from bench to bedside. EMBO J 2017;36:2670–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Verdin E Nad(+) in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213 [DOI] [PubMed] [Google Scholar]
- 195.Walker MA, Tian R. Nad(h) in mitochondrial energy transduction: Implications for health and disease. Curr Opin Physiol. 2018;3:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hsu CP, Yamamoto T, Oka S, Sadoshima J. The function of nicotinamide phosphoribosyltransferase in the heart. DNA Repair (Amst). 2014;23:64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Byun J, Oka SI, Imai N, Huang CY, Ralda G, Zhai P, Ikeda Y, Ikeda S, Sadoshima J. Both gain and loss of nampt function promote pressure overload-induced heart failure. Am J Physiol Heart Circ Physiol. 2019;317:H711–H725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex i deficiency increases protein acetylation and accelerates heart failure Cell Metab. 2013;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous nad blocks cardiac hypertrophic response via activation of the sirt3-lkb1-amp-activated kinase pathway. J. Biol. Chem 2010;285:3133–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Martin AS, Abraham DM, Hershberger KA, Bhatt DP, Mao L, Cui H, Liu J, Liu X, Muehlbauer MJ, Grimsrud PA, et al. Nicotinamide mononucleotide requires sirt3 to improve cardiac function and bioenergetics in a friedreich’s ataxia cardiomyopathy model. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through nad+ synthesis in cardiac myocytes. Circ. Res 2009;105:481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Davidson MT, Grimsrud PA, Lai L, Draper JA, Fisher-Wellman KH, Narowski TM, Abraham DM, Koves TR, Kelly DP, Muoio DM. Extreme acetylation of the cardiac mitochondrial proteome does not promote heart failure. Circ. Res 2020;127:1094–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Walker MA, Chavez J, Villet O, Tang X, Keller A, Bruce JE, Tian R. Acetylation of muscle creatine kinase negatively impacts high energy phosphotransfer in heart failure. JCI Insights. 2021:In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: A strong hypothesis. Heart Fail. Rev. 2007;12:331–343 [DOI] [PubMed] [Google Scholar]
- 205.Finck BN. The role of the peroxisome proliferator-activated receptor alpha pathway in pathological remodeling of the diabetic heart. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:391–396 [DOI] [PubMed] [Google Scholar]
- 206.Warren JS, Oka SI, Zablocki D, Sadoshima J. Metabolic reprogramming via pparα signaling in cardiac hypertrophy and failure: From metabolomics to epigenetics. Am J Physiol Heart Circ Physiol. 2017;313:H584–h596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gut P, Verdin E. The nexus of chromatin regulation and intermediary metabolism. Nature. 2013;502:489–498 [DOI] [PubMed] [Google Scholar]
- 208.D’Onofrio N, Servillo L, Balestrieri ML. Sirt1 and sirt6 signaling pathways in cardiovascular disease protection. Antioxid. Redox Signal. 2018;28:711–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Koentges C, Bode C, Bugger H. Sirt3 in cardiac physiology and disease. Front Cardiovasc Med. 2016;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 2015;309:H1375–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.McKinsey TA. Therapeutic potential for hdac inhibitors in the heart. Annu Rev Pharmacol Toxicol. 2012;52:303–319 [DOI] [PubMed] [Google Scholar]
- 212.Wallner M, Eaton DM, Berretta RM, Liesinger L, Schittmayer M, Gindlhuber J, Wu J, Jeong MY, Lin YH, Borghetti G, et al. Hdac inhibition improves cardiopulmonary function in a feline model of diastolic dysfunction. Sci. Transl. Med 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jeong MY, Lin YH, Wennersten SA, Demos-Davies KM, Cavasin MA, Mahaffey JH, Monzani V, Saripalli C, Mascagni P, Reece TB, et al. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci. Transl. Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Evans LW, Bender A, Burnett L, Godoy L, Shen Y, Staten D, Zhou T, Angermann JE, Ferguson BS. Emodin and emodin-rich rhubarb inhibits histone deacetylase (hdac) activity and cardiac myocyte hypertrophy. J. Nutr. Biochem 2020;79:108339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Khan D, Sarikhani M, Dasgupta S, Maniyadath B, Pandit AS, Mishra S, Ahamed F, Dubey A, Fathma N, Atreya HS, et al. Sirt6 deacetylase transcriptionally regulates glucose metabolism in heart. J. Cell. Physiol 2018;233:5478–5489 [DOI] [PubMed] [Google Scholar]
- 216.Abdul Kadir A, Clarke K, Evans RD. Cardiac ketone body metabolism. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165739. [DOI] [PubMed] [Google Scholar]
- 217.Deb DK, Chen Y, Sun J, Wang Y, Li YC. Atp-citrate lyase is essential for high glucose-induced histone hyperacetylation and fibrogenic gene upregulation in mesangial cells. Am. J. Physiol. Renal Physiol 2017;313:F423–F429 [DOI] [PubMed] [Google Scholar]
- 218.Karlstaedt A, Zhang X, Vitrac H, Harmancey R, Vasquez H, Wang JH, Goodell MA, Taegtmeyer H. Oncometabolite d-2-hydroxyglutarate impairs alpha-ketoglutarate dehydrogenase and contractile function in rodent heart. Proc. Natl. Acad. Sci. U. S. A 2016;113:10436–10441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Medford HM, Porter K, Marsh SA. Immediate effects of a single exercise bout on protein o-glcnacylation and chromatin regulation of cardiac hypertrophy. Am J Physiol Heart Circ Physiol 2013;305:H114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kronlage M, Dewenter M, Grosso J, Fleming T, Oehl Ulrike Y, Lehmann Lorenz H, Falcão-Pires I, Leite-Moreira Adelino F, Volk N, Gröne H-J, et al. O-glcnacylation of histone deacetylase 4 protects the diabetic heart from failure. Circ. 2019;0. [DOI] [PubMed] [Google Scholar]
- 221.Movassagh M, Choy MK, Goddard M, Bennett MR, Down TA, Foo RS. Differential DNA methylation correlates with differential expression of angiogenic factors in human heart failure. PLoS One. 2010;5:e8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Angrisano T, Schiattarella GG, Keller S, Pironti G, Florio E, Magliulo F, Bottino R, Pero R, Lembo F, Avvedimento EV, et al. Epigenetic switch at atp2a2 and myh7 gene promoters in pressure overload-induced heart failure. PLoS One. 2014;9:e106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xiao D, Dasgupta C, Chen M, Zhang K, Buchholz J, Xu Z, Zhang L. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc. Res 2014;101:373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pepin ME, Drakos S, Ha C-M, Tristani-Firouzi M, Selzman CH, Fang JC, Wende AR, Wever-Pinzon O. DNA methylation reprograms cardiac metabolic gene expression in end-stage human heart failure. American Journal of Physiology-Heart and Circulatory Physiology. 2019;317:H674–H684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pepin ME, Ha CM, Crossman DK, Litovsky SH, Varambally S, Barchue JP, Pamboukian SV, Diakos NA, Drakos SG, Pogwizd SM, et al. Genome-wide DNA methylation encodes cardiac transcriptional reprogramming in human ischemic heart failure. Lab. Invest 2019;99:371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Greco CM, Kunderfranco P, Rubino M, Larcher V, Carullo P, Anselmo A, Kurz K, Carell T, Angius A, Latronico MVG, et al. DNA hydroxymethylation controls cardiomyocyte gene expression in development and hypertrophy. Nat Commun 2016;7:12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Oda M, Wakabayashi S, Ari Wijetunga N, Yuasa S, Enomoto H, Kaneda R, Yoon SH, Mittal N, Jing Q, Suzuki M, et al. Selective modulation of local linkages between active transcription and oxidative demethylation activity shapes cardiomyocyte-specific gene-body epigenetic status in mice. BMC Genomics. 2018;19:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ciccarone F, Castelli S, Ioannilli L, Ciriolo MR. High dietary fat intake affects DNA methylation/hydroxymethylation in mouse heart: Epigenetic hints for obesity-related cardiac dysfunction. Mol. Nutr. Food Res. 2019;63:e1800970. [DOI] [PubMed] [Google Scholar]
- 229.Tabish AM, Arif M, Song T, Elbeck Z, Becker RC, Knöll R, Sadayappan S. Association of intronic DNA methylation and hydroxymethylation alterations in the epigenetic etiology of dilated cardiomyopathy. American Journal of Physiology: Heart and Circulatory Physiology. 2019;317:H168–H180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lother A, Bondareva O, Saadatmand AR, Pollmeier L, Hardtner C, Hilgendorf I, Weichenhan D, Eckstein V, Plass C, Bode C, et al. Diabetes changes gene expression but not DNA methylation in cardiac cells. J. Mol. Cell. Cardiol 2020;151:74–87 [DOI] [PubMed] [Google Scholar]
- 231.Napoli C, Benincasa G, Donatelli F, Ambrosio G. Precision medicine in distinct heart failure phenotypes: Focus on clinical epigenetics. Am. Heart J 2020;224:113–128 [DOI] [PubMed] [Google Scholar]
- 232.Fang Y, Xu Y, Wang R, Hu L, Guo D, Xue F, Guo W, Zhang D, Hu J, Li Y, et al. Recent advances on the roles of lncrnas in cardiovascular disease. J Cell Mol Med. 2020;24:12246–12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gomes CPC, Schroen B, Kuster GM, Robinson EL, Ford K, Squire IB, Heymans S, Martelli F, Emanueli C, Devaux Y, Action EU-CC. Regulatory rnas in heart failure. Circ. 2020;141:313–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, Christensen G, Gronning-Wang LM, Carlson CR. Cardiac o-glcnac signaling is increased in hypertrophy and heart failure. Physiol. Genomics. 2012;44:162–172 [DOI] [PubMed] [Google Scholar]
- 235.Tran DH, May HI, Li Q, Luo X, Huang J, Zhang G, Niewold E, Wang X, Gillette TG, Deng Y, et al. Chronic activation of hexosamine biosynthesis in the heart triggers pathological cardiac remodeling. Nat Commun 2020;11:1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li J, Minczuk K, Massey JC, Howell NL, Roy RJ, Paul S, Patrie JT, Kramer CM, Epstein FH, Carey RM, et al. Metformin improves cardiac metabolism and function, and prevents left ventricular hypertrophy in spontaneously hypertensive rats. J Am Heart Assoc 2020;9:e015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li J, Kemp BA, Howell NL, Massey J, Minczuk K, Huang Q, Chordia MD, Roy RJ, Patrie JT, Davogustto GE, et al. Metabolic changes in spontaneously hypertensive rat hearts precede cardiac dysfunction and left ventricular hypertrophy. J Am Heart Assoc 2019;8:e010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ritterhoff J, Young S, Villet O, Shao D, Neto FC, Bettcher LF, Hsu YA, Kolwicz SC, Jr., Raftery D, Tian R. Metabolic remodeling promotes cardiac hypertrophy by directing glucose to aspartate biosynthesis. Circ. Res 2020;126:182–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shao D, Villet O, Zhang Z, Choi SW, Yan J, Ritterhoff J, Gu H, Djukovic D, Christodoulou D, Kolwicz SC Jr., et al. Glucose promotes cell growth by suppressing branched-chain amino acid degradation. Nat Commun 2018;9:2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sharma S, Guthrie PH, Chan SS, Haq S, Taegtmeyer H. Glucose phosphorylation is required for insulin-dependent mtor signalling in the heart. Cardiovasc. Res 2007;76:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sen S, Kundu BK, Wu HC, Hashmi SS, Guthrie P, Locke LW, Roy RJ, Matherne GP, Berr SS, Terwelp M, et al. Glucose regulation of load-induced mtor signaling and er stress in mammalian heart. J Am Heart Assoc 2013;2:e004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gibb AA, Epstein PN, Uchida S, Zheng Y, McNally LA, Obal D, Katragadda K, Trainor P, Conklin DJ, Brittian KR, Tseng MT, Wang J, Jones SP, Bhatnagar A, Hill BG. Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circ 2017;136:2144–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rees ML, Subramaniam J, Li Y, Hamilton DJ, Frazier OH, Taegtmeyer H. A pkm2 signature in the failing heart. Biochem Biophys Res Commun 2015;459:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tian R, Abel ED. Responses of glut4-deficient hearts to ischemia underscore the importance of glycolysis. Circ. 2001;103:2961–2966 [DOI] [PubMed] [Google Scholar]
- 245.Li Y, Wende AR, Nunthakungwan O, Huang Y, Hu E, Jin H, Boudina S, Abel ED, et al. Cytosolic, but not mitochondrial, oxidative stress is a likely contributor to cardiac hypertrophy resulting from cardiac specific glut4 deletion in mice. FEBS J. 2012;279:599–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Brahma MK, Ha CM, Pepin ME, Mia S, Sun Z, Chatham JC, Habegger KM, Abel ED, Paterson AJ, Young ME, et al. Increased glucose availability attenuates myocardial ketone body utilization. J Am Heart Assoc. 2020;9:e013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wende AR, Schell JC, Ha CM, Pepin ME, Khalimonchuk O, Schwertz H, Pereira RO, Brahma MK, Tuinei J, Contreras-Ferrat A, et al. Maintaining myocardial glucose utilization in diabetic cardiomyopathy accelerates mitochondrial dysfunction. Diabetes. 2020;69:2094–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wende AR, Kim J, Holland WL, Wayment BE, O’Neill BT, Tuinei J, Brahma MK, Pepin ME, McCrory MA, Luptak I, et al. Glucose transporter 4-deficient hearts develop maladaptive hypertrophy in response to physiological or pathological stresses. Am J Physiol Heart Circ Physiol. 2017;313:H1098–H1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liao R, Jain M, Cui L, D’Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of glut1 prevents the development of heart failure attributable to pressure overload in mice. Circ. 2002;106:2125–2131 [DOI] [PubMed] [Google Scholar]
- 250.Luptak I, Yan J, Cui L, Jain M, Liao R, Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circ. 2007;116:901–909 [DOI] [PubMed] [Google Scholar]
- 251.Li T, Zhang Z, Kolwicz SC Jr., Abell L, Roe ND, Kim M, Zhou B, Cao Y, Ritterhoff J, Gu H,R et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 2017;25:374–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Anderson SM, Abel ED. Inducible overexpression of glut1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. J Am Heart Assoc 2013;2:e000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Riehle C, Abel ED. Glut1 deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. J. Mol. Cell. Cardiol 2014;72:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Harmancey R, Vasquez HG, Guthrie PH, Taegtmeyer H. Decreased long-chain fatty acid oxidation impairs postischemic recovery of the insulin-resistant rat heart. FASEB J. 2013;27:3966–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kolwicz SC Jr., Liu L, Goldberg IJ, Tian R Enhancing cardiac triacylglycerol metabolism improves recovery from ischemic stress. Diabetes. 2015;64:2817–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lam VH, Zhang L, Huqi A, Fukushima A, Tanner BA, Onay-Besikci A, Keung W, Kantor PF, Jaswal JS, Rebeyka IM, et al. Activating pparalpha prevents post-ischemic contractile dysfunction in hypertrophied neonatal hearts. Circ. Res 2015;117:41–51 [DOI] [PubMed] [Google Scholar]
- 257.Yue TL, Bao W, Jucker BM, Gu JL, Romanic AM, Brown PJ, Cui J, Thudium DT, Boyce R, Burns-Kurtis CL, et al. Activation of peroxisome proliferator-activated receptor-alpha protects the heart from ischemia/reperfusion injury. Circ. 2003;108:2393–2399 [DOI] [PubMed] [Google Scholar]
- 258.Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ. Res 2020;126:1501–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nakamura M, Sadoshima J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol 2020;598:2977–2993 [DOI] [PubMed] [Google Scholar]
- 260.Schulze PC, Drosatos K, Goldberg IJ. Lipid use and misuse by the heart. Circ. Res 2016;118:1736–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 2004;18:1692–1700 [DOI] [PubMed] [Google Scholar]
- 262.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knoll R, et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circ. 2012;125:2844–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Michel CI, Holley CL, Scruggs BS, Sidhu R, Brookheart RT, Listenberger LL, Behlke MA, Ory DS, Schaffer JE. Small nucleolar rnas u32a, u33, and u35a are critical mediators of metabolic stress. Cell Metab. 2011;14:33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ilkun O, Wilde N, Tuinei J, Pires KM, Zhu Y, Bugger H, Soto J, Wayment B, Olsen C, Litwin SE, et al. Antioxidant treatment normalizes mitochondrial energetics and myocardial insulin sensitivity independently of changes in systemic metabolic homeostasis in a mouse model of the metabolic syndrome. J. Mol. Cell. Cardiol 2015;85:104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jaishy B, Zhang Q, Chung HS, Riehle C, Soto J, Jenkins S, Abel P, Cowart LA, Van Eyk JE, Abel ED. Lipid-induced nox2 activation inhibits autophagic flux by impairing lysosomal enzyme activity. J. Lipid Res 2015;56:546–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circ. 2005;112:2686–2695 [DOI] [PubMed] [Google Scholar]
- 267.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, et al. Mitochondrial energetics in the heart in obesity-related diabetes: Direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466 [DOI] [PubMed] [Google Scholar]
- 268.Boudina S, Han YH, Pei S, Tidwell TJ, Henrie B, Tuinei J, Olsen C, Sena S, Abel ED. Ucp3 regulates cardiac efficiency and mitochondrial coupling in high fat-fed mice but not in leptin-deficient mice. Diabetes. 2012;61:3260–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 2008;49:2101–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bosma M, Dapito DH, Drosatos-Tampakaki Z, Huiping-Son N, Huang LS, Kersten S, Drosatos K, Goldberg IJ. Sequestration of fatty acids in triglycerides prevents endoplasmic reticulum stress in an in vitro model of cardiomyocyte lipotoxicity. Biochim Biophys Acta. 2014;1841:1648–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res 2006;47:2726–2737 [DOI] [PubMed] [Google Scholar]
- 272.Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: Novel roles of immunometabolism in macrophage activation and inflammation. Circ. Res 2020;126:789–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schilling JD, Machkovech HM, Kim AH, Schwendener R, Schaffer JE. Macrophages modulate cardiac function in lipotoxic cardiomyopathy. Am J Physiol Heart Circ Physiol 2012;303:H1366–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lopaschuk GD, Karwi QG, Ho KL, Pherwani S, Ketema EB. Ketone metabolism in the failing heart. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158813. [DOI] [PubMed] [Google Scholar]
- 275.Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circ. 2020;141:1800–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nielsen R, Moller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frokiaer J, Eiskjaer H, Jespersen NR, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circ. 2019;139:2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Monzo L, Sedlacek K, Hromanikova K, Tomanova L, Borlaug BA, Jabor A, Kautzner J, Melenovsky V. Myocardial ketone body utilization in patients with heart failure: The impact of oral ketone ester. Metabolism. 2020;115:154452. [DOI] [PubMed] [Google Scholar]
- 279.Yurista SR, Matsuura TR, Sillje HHW, Nijholt KT, McDaid KS, Shewale SV, Leone TC, Newman JC, Verdin E, van Veldhuisen DJ, et al. Ketone ester treatment improves cardiac function and reduces pathologic remodeling in preclinical models of heart failure. Circ. Heart Fail. 2020:CIRCHEARTFAILURE120007684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 281.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 282.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 2020;383:1413–1424 [DOI] [PubMed] [Google Scholar]
- 283.Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (sglt2) inhibitors: A state-of-the-art review. JACC Basic Transl Sci 2020;5:632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, Rim JH, Hwang I, Lee CJ, Lee M, et al. Sglt2 inhibition modulates nlrp3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun 2020;11:2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ. Res 1996;79:940–948 [DOI] [PubMed] [Google Scholar]
- 286.Lydell CP, Chan A, Wambolt RB, Sambandam N, Parsons H, Bondy GP, Rodrigues B, Popov KM, Harris RA, Brownsey RW, et al. Pyruvate dehydrogenase and the regulation of glucose oxidation in hypertrophied rat hearts. Cardiovasc. Res 2002;53:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Bogh N, Hansen ESS, Omann C, Lindhardt J, Nielsen PM, Stephenson RS, Laustsen C, Hjortdal VE, Agger P. Increasing carbohydrate oxidation improves contractile reserves and prevents hypertrophy in porcine right heart failure. Sci. Rep 2020;10:8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lewis JF, DaCosta M, Wargowich T, Stacpoole P. Effects of dichloroacetate in patients with congestive heart failure. Clin. Cardiol 1998;21:888–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wargovich TJ, MacDonald RG, Hill JA, Feldman RL, Stacpoole PW, Pepine CJ. Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am. J. Cardiol 1988;61:65–70 [DOI] [PubMed] [Google Scholar]
- 290.Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, Calori G, Alfieri O, Margonato A. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J. Am. Coll. Cardiol 2006;48:992–998 [DOI] [PubMed] [Google Scholar]
- 291.Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, et al. Metabolic modulation with perhexiline in chronic heart failure: A randomized, controlled trial of short-term use of a novel treatment. Circ. 2005;112:3280–3288 [DOI] [PubMed] [Google Scholar]
- 292.Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: A meta-analysis of randomised controlled trials in heart failure. Heart. 2011;97:278–286 [DOI] [PubMed] [Google Scholar]
- 293.Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin. Sci. (Lond.). 2000;99:27–35 [PubMed] [Google Scholar]
- 294.Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: The ergo (etomoxir for the recovery of glucose oxidation) study. Clin. Sci. (Lond.). 2007;113:205–212 [DOI] [PubMed] [Google Scholar]
- 295.Peng S, Zhao M, Wan J, Fang Q, Fang D, Li K. The efficacy of trimetazidine on stable angina pectoris: A meta-analysis of randomized clinical trials. Int. J. Cardiol 2014;177:780–785 [DOI] [PubMed] [Google Scholar]
- 296.Wang W, Zhang L, Battiprolu PK, Fukushima A, Nguyen K, Milner K, Gupta A, Altamimi T, Byrne N, Mori J, Alrob OA, et al. Malonyl coa decarboxylase inhibition improves cardiac function post-myocardial infarction. JACC Basic Transl Sci 2019;4:385–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet 2010;3:207–214 [DOI] [PubMed] [Google Scholar]
- 298.Shah SH, Sun JL, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, Haynes C, Hauser ER, Kraus WE, Granger CB, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J 2012;163:844–850 e841 [DOI] [PubMed] [Google Scholar]
- 299.Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, Al Batran R, Pherwani S, Ho KL, Boisvenue J, et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol. 2019;18:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.McGarrah RW, Zhang GF, Christopher BA, Deleye Y, Walejko JM, Page S, Ilkayeva O, White PJ, Newgard CB. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of zucker fatty rats. Am J Physiol Endocrinol Metab. 2020;318:E216–E223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Fillmore N, Wagg CS, Zhang L, Fukushima A, Lopaschuk GD. Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am J Physiol-Endocrinolo Metabolism. 2018 [DOI] [PubMed] [Google Scholar]
- 302.Newgard Christopher B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Olson KC, Chen G, Xu Y, Hajnal A, Lynch CJ. Alloisoleucine differentiates the branched-chain aminoacidemia of zucker and dietary obese rats. Obesity (Silver Spring). 2014;22:1212–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid {beta}-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic african-american women. J. Nutr 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Redfield MM, Jacobsen SJ, Burnett JC Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202 [DOI] [PubMed] [Google Scholar]
- 306.Jackson RH, Singer TP. Inactivation of the 2-ketoglutarate and pyruvate dehydrogenase complexes of beef heart by branched chain keto acids. J. Biol. Chem 1983;258:1857–1865 [PubMed] [Google Scholar]
- 307.Karwi Q, Uddin GM, Wagg CS, Lopaschuk GD. Abstract mp125: Branched-chain keto acids, not branched-chain amino acids, impairs cardiac insulin sensitivity by disrupting insulin signaling in the mitochondria. Circ. Res 2020;127:AMP125-AMP125 [Google Scholar]
- 308.Uddin GM, Pherwani S, Wagg CS, Gopal K, Batran RA, Zhang L, Wu Y, Hussaini N, Rawat S, Ussher JR, et al. Abstract 868: A cardiac specific branched chain aminotransferase deletion increases insulin stimulated glucose oxidation in the mouse heart. Circ. Res 2019;125:A868–A868 [Google Scholar]