Abstract
Trichinellosis is a major foodborne parasitosis caused by Trichinella spiralis. In the present study, a serine protease gene from an adult T. spiralis (Ts-Adsp) cDNA library was cloned, expressed in Escherichia coli and purified by Ni-affinity chromatography. Previous studies of our laboratory have found that mice vaccinated with recombinant Ts-Adsp protein (rTs-Adsp) exhibited partial protection against T. spiralis infection. In this study, the protective effect of rTs-Adsp against T. spiralis infection in pigs was further explored. The cell-mediated and humoral immune responses induced by rTs-Adsp were measured, including the dynamic trends of specific antibody levels (IgG, IgG1, IgG2a and IgM), as well as the levels of cytokines (IFN-γ, IL-2, IL-4, and IL-10) in the serum. Moreover, the changes in T lymphocytes, B lymphocytes, and neutrophils were measured to evaluate cellular immune responses in pigs vaccinated with rTs-Adsp. The results indicated that a Th1-Th2 mixed immune response with Th1 predominant was induced by rTs-Adsp after vaccination. Flow cytometric analysis showed that the proportions of CD4+ T cells, B cells, and neutrophils in the immunized groups were significantly increased. Furthermore, pigs vaccinated with rTs-Adsp exhibited a 50.9% reduction in the muscle larvae burden, compare with pigs from the PBS group five weeks after challenged. Our results suggested that rTs-Adsp elicited partial protection and it could be a potential target molecule for preventing and controlling Trichinella transmission from pigs to human.
Author summary
Trichinellosis is a global foodborne parasitic disease caused by consuming raw or poorly cooked meat. The porcine products are the most common source. Therefore, it will have a great significance for public health security and human health to prevent and control the trichinellosis. We previously found that mice vaccinated with recombinant Adsp protein (rTs-Adsp) exhibited partial protection against T. spiralis infection. In this study, the protective effect of rTs-Adsp against challenge infections with T. spiralis in pigs was further explored. We found that rTs-Adsp elicited partial protection and it could be an important target molecule for preventing and controlling T. spiralis transmission from pigs to human.
Introduction
Trichinella spiralis (T. spiralis) is a foodborne parasite that can infect a wide range of animals, such as mammals, birds and reptiles [1]. Trichinellosis caused by T. spiralis is a public health hazard and can affect food safety, especially pork-related products [1]. The source of human infection is mainly digestion of raw or poorly cooked meat, and porcine products are the most common source [2–4]. Therefore, the important measure to control trichinellosis should prevent the transmission from pigs to human [5]. The development of vaccines against T. spiralis infection in pigs might be a promising method of parasite control. However, so far, most studies on Trichinella vaccines have been performed in mouse models, and very few anti-Trichinella infection studies have been performed on pigs [6]. The exploitation of vaccines against T. spiralis infection in pigs is an important measure by which to block infection from pigs to humans [6, 7].
Proteases are a type of enzymes that are widely distributed in eukaryotes, prokaryotes, and viruses [8]. Research has shown that serine proteases participate in many different events in the life cycle of a parasite [8]. Serine proteases from parasites are thought to be key factors in the process of establishing infection. Moreover, serine proteases are important enzymes that exist in excretory-secretory (ES) production of T. spiralis. Many studies have shown that proteases exhibited immune protection effects against T. spiralis infection in mice [9–13]. Among these proteases, serine proteases play a crucial role in T. spiralis invasion of host cells and are involved in the processes of immune evasion [14]. There is much evidence showing that serine proteases in parasite ES products perform multiple functions and involve the processes of parasite nutrition and immune evasion [15–17]. In addition, serine proteases have been proven to be involved in parasite survival, and these proteases have been considered as candidate antigens for vaccines against parasite infection [18–20].
The entire life cycle of T. spiralis is divided into three major developmental stages: adult worms (AD), newborn larvae (NBL), and muscle larvae (ML) [21]. Serine protease-like antigens have been identified in adult stages of T. spiralis. Adult stage is a major stage for the reproduction of T. spiralis. If growth from ML to AD is interrupted, the quantity of NBL will be reduced, which will hamper or reduce the production of ML in the muscle [22]. It was reported that a serine protease (Ts-Adsp) was screened from T. spiralis adult and that mice vaccination with recombinant Adsp protein (rTs-Adsp) elicited a 46.5% reduction in ML [9]. Sun et al. screened a serine protease (TsSP) from ES products of T. spiralis and found that mice vaccinated with recombinant TsSP protein (rTsSP) exhibited 52.70% and 52.10% reduction in AD and ML, respectively [7]. Moreover, a previous study of our laboratory reported that serine protease from T. spiralis adult might be participated in the process of capsule formation and protect the NBL in the circulatory system of the host [23]. Therefore, serine proteases from T. spiralis adults have become a promising vaccine targets.
In recent years, a number of vaccines against T. spiralis have been developed to prevent transmission from animals to humans. Most potential vaccine candidates were selected from recombinant proteins and ES products. A previous study found that serine protease from T. spiralis adults exhibited partial protection effects against challenging with T. spiralis larvae [9]. In this study, the serine protease gene of T. spiralis was identified from a cDNA library of T. spiralis adult worms (Ts-Adsp) and purified by Ni-affinity chromatography. To further explore the protective effect of the rTs-Adsp protein, pigs were immunized with the rTs-Adsp protein to evaluate its immune protection effects against T. spiralis infection. Studies have found that high effective vaccine efficacy against trichinellosis are associated with high levels of humoral and cellular immune response in mice [7, 24, 25]. The antibodies can kill the ML, reduce the larval infectivity, and obstruct larval development through an ADCC mechanism [26]. Moreover, the expulsion of T. spiralis adults is mainly regulated by CD4+/Th2 cytokines and depends on the production of IL-4 and IL-13, since inhibition of these cytokines extends parasite survival [27]. In this study, we have provided the possible immune mechanism that occurs after immunization with rTs-Adsp protein in pigs.
Materials and methods
Ethics statement
All animals were raised carefully in accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China. The protocol of the animal experiments was reviewed and approved by the Institutional Animal Care and Use Committee of Jilin University (protocol # 20170318).
Parasite and animals
In this study, T. spiralis (ISS534) was obtained from Wistar rats by pepsin–HCl digestion. Eighteen two-month-old large white pigs were obtained from a standardized pig farm (Jilin Province, China). All pigs had not been vaccinated with a T. spiralis vaccine. Moreover, all pigs were fed an antibiotic-free energy diet. Before the experiment, blood from all pigs were collected to analyze the blood routine examination. The fecal sample were collected to test other parasite eggs by flotation and sedimentation method. All pigs were kept under standard pig houses in our laboratory and underwent a week of health observation.
Preparation of plasmid pET28a-Ts-Adsp
The coding sequence of the Ts-Adsp gene (GenBank EU263332.1) was amplified from a cDNA library of T. spiralis adult worms by PCR (forward primer 5′-GAATCCGAATTATGAATGTG-3′, containing the EcoRI restriction site, and reverse primer 5′-CTCGAGACGGAAAAAAGT-3′, containing the XhoI restriction site). The PCR product was cloned into prokaryotic expression vector pET28a and transformed into Escherichia coli (E. coli) BL21 (DE3) cells.
Preparation and identification of recombinant Ts-Adsp (rTs-Adsp)
The complete sequence of the Ts-Adsp gene was cloned into the pET28a expression vector using T4 DNA ligase. Then, the plasmid pET28a-Ts-Adsp was transformed into E. coli BL21 (DE3) cells. The expression of rTs-Adsp was induced with 1mM IPTG (isopro-pyl-β-D-thioga-lactopyranoside) for 6 h at 20°C. Then, purification of the rTs-Adsp was performed as previously described [28]. Finally, the rTs-Adsp protein was identified by 12% SDS–PAGE and western blot analysis, as previously described [13]. Briefly, the rTs-Adsp protein was electro-transferred to a nitrocellulose membrane. After blocking with 5% skimmed milk, the membrane was incubated with serum from pig infected with 20000 T. spiralis ML at a 1:200 dilution in TBST overnight. The serum from Trichinella–negative pig served as a negative control. Then the membrane was incubated with HRP-conjugated goat anti-swine IgG (Bio-Rad) at a 1:4000 dilution in TBST for 2 h. Finally, the membrane was incubated with the enhanced chemiluminescence (ECL) reagents and subsequently photographed on BioMax film. Contaminating endotoxin was removed from the rTs-Adsp protein by a ToxOut High-Capacity Endotoxin Removal Kit (Biovision, USA) according to the manufacturer’s instructions. Briefly, at least four endotoxin standard solutions were prepared to generate a standard curve. The absorbance of each reaction was read at 545 nm. Endotoxin concentrations of samples were calculated from the standard curve. The residual endotoxin in the rTs-Adsp protein was approximately 0.1431 EU/mL.
Immunization procedure and challenge
Montanide IMS 1313 N VG PR (IMS1313) was obtained from SEPPIC Corporation (Paris, France). Eighteen piglets were randomly divided into 3 groups, namely the PBS group, IMS1313 group (with a 1:1 dilution in PBS) and rTs-Adsp protein group (with a 1:1 dilution in IMS1313). In total, six pigs in each group were immunized twice at a four-week interval. In the rTs-Adsp protein group, each pig was immunized with 1 mg rTs-Adsp (1 mg/mL). In the group of PBS and IMS1313, each pig was immunized with 2 mL PBS, IMS1313, respectively. Three weeks after the last immunization, each group of pigs was inoculated with 5000 T. spiralis muscle larvae by oral administration.
Specific antibody response
Blood from pigs in each group was collected to evaluate the changes in specific antibodies (IgG, IgG1, IgG2a and IgM) by an indirect enzyme-linked immunosorbent assay. Briefly, microtiter plates were coated with 100 μL purified protein at 4°C overnight. The titers of specific IgG and IgG subtypes were detected as described previously [28]. For IgM detection, the titers of specific IgM were detected as described previously [28].
Cytokine assays
Serum samples of pigs were obtained two weeks after the last immunization. Cytokines (IFN-γ, IL-2, IL-4, and IL-10) in the serum samples were detected using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The levels of cytokines in the serum were extrapolated according to the standard curve provided by the ELISA kit.
Flow cytometry
Blood from six pigs in each group was collected for flow cytometry analysis. The relative ratios of T cells, B cells, and neutrophils were evaluated by flow cytometry. Briefly, the cells were obtained from blood lysed by red blood cell lysis buffer (Solarbio, Beijing, China). For antibody labeling of T cells, the cells were stained with 2 μL mouse anti-porcine CD3e-FITC (SouthernBiotech, Birmingham, AL, USA), 2 μL mouse anti-porcine CD4-PE (SouthernBiotech) and 2 μL mouse anti-porcine CD8a-PE-Cy5 antibodies (Abcam, Cambridge, MA, USA). For antibody labeling of B cells, the cells were stained with 6 μL mouse anti-porcine CD3e-FITC (SouthernBiotech) and 2 μL mouse anti-porcine CD21-PE antibodies (Abcam). For antibody labeling of neutrophils, the cells were stained with 10 μL mouse anti-porcine CD14-PE (Abcam), 5 μL mouse anti-porcine SWC8 (Abcam) and 5 μL goat anti-mouse IgM-FITC antibodies (SouthernBiotech). Then, the cells and antibodies were incubated on ice for 30 min in the dark. After washing twice with PBS, the stained cells were resuspended in 300 μL of PBS. Finally, the stained cells were measured by a flow cytometer (BD Biosciences). The percentages of T cells, B cells, and neut.rophils were analyzed using FlowJo.
Evaluation of larval burden
Six pigs in each group were sacrificed five weeks after challenge. Six different types of muscles were obtained from each pig, respectively, including the diaphragm, tongue, gastrocnemius, trapezius, gluteus maximus, and flexor tendon. Fifty grams of muscle of each type were collected to evaluate immune protection. Finally, the number of muscle larvae per gram of muscle (LPG) and the worm reduction rate were evaluated by comparison with those of the PBS group.
Statistical analysis
GraphPad Prism 8.0 software was used to perform statistical analysis. Two experimental groups were compared using Student’s t-test for nonparametric data. Three groups were compared using ANOVA with Dunnett’s multiple comparison test as indicated. Normality (Shapiro-Wilk test) and homogeneity of variance (Levene’s test) were performed in each case. The data are displayed as the means ± standard deviations (SD). The significance of the differences between the groups was expressed as *p< 0.05, **p< 0.01, and ***p< 0.001.
Results
Analysis of rTs-Adsp protein
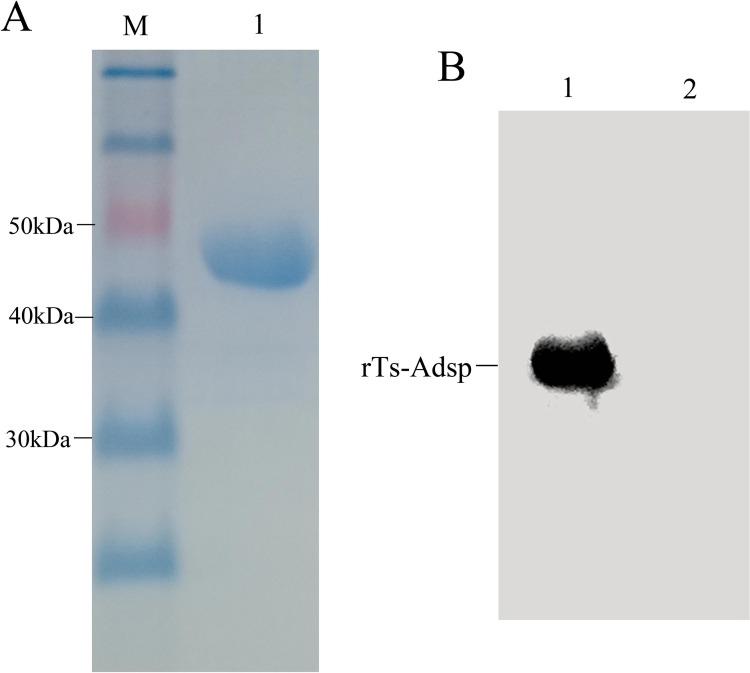
SDS-PAGE results indicated that the molecular mass of the rTs-Adsp protein was approximately 47.5 kDa, and the purified protein was visualized as a single band (Fig 1A). Western blotting results indicated that the rTs-Adsp protein was recognized by the serum of pig infected with T. spiralis for 60 days (Fig 1B). The results suggested that the rTs-Adsp protein was purified successfully and had good reactogenicity.
Fig 1. Purification and identification of recombinant rTs-Adsp by SDS-PAGE and western blot.
(A) Purified rTs-Adsp was analyzed by SDS-PAGE. Lane M: protein molecular weight marker, Lane 1: purified rTs-Adsp by Ni-affinity chromatograph. (B) The antigenicity of rTs-Adsp was identified by western blot. Lane 1: rTs-Adsp incubated with serum from pig infected with T. spiralis at 60 dpi, Lane 2: rTs-Adsp incubated with serum from Trichinella–negative pig.
Immune responses in vaccinated pigs
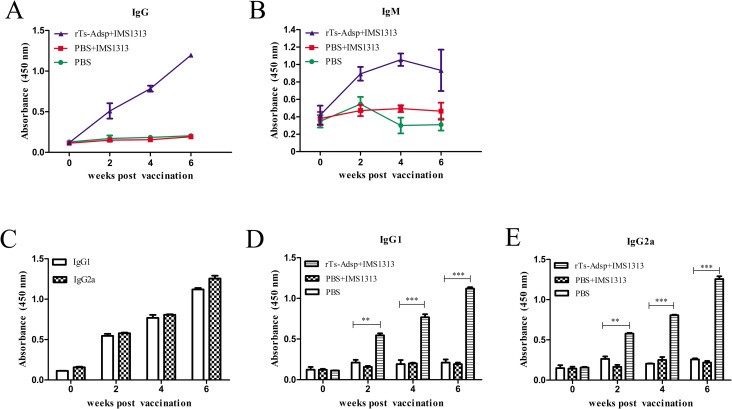
To determine the antibody response to rTs-Adsp in vaccinated pigs, serum was collected before and after vaccination. In IgG and IgM detection, compared with those of pigs immunized with PBS, the levels of specific IgG and IgM in pigs immunized with the rTs-Adsp protein were significantly elevated (Fig 2A and 2B). IgG subtypes detection results showed that the levels of IgG subtype in pigs immunized with the rTs-Adsp protein were very significantly elevated (Fig 2D and 2E). Moreover, the titers of IgG2a were higher than those of IgG1 (Fig 2C), suggesting that a Th1-dominant Th1/Th2 mixed immune response was induced by the rTs-Adsp protein.
Fig 2. Immune responses from the vaccinated pigs.
(A) The levels of IgG against rTs-Adsp were measured by ELISA. (B) The levels of IgM in the sera of immunized pigs were measured by ELISA. (C) IgG subclass responses to the rTs-Adsp were measured by ELISA. (D) The IgG1 subclass responses against rTs-Adsp were evaluated at different time points. (E) The IgG2a subclass responses against rTs-Adsp were evaluated at different time points. The values shown for each group are the mean ± SD of the antibody levels. Significant differences were as follows: *p< 0.05; **p< 0.01; ***p< 0.001.
Evaluation of cytokine production
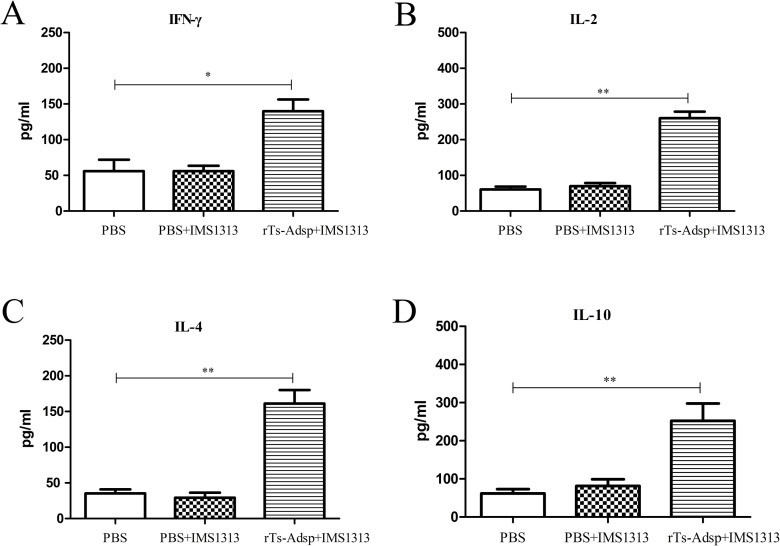
To evaluate the cell-mediated immune response of mice vaccinated with rTs-Adsp, the concentration of cytokines (IL-2, IFN-γ, IL-4, and IL-10) associated with Th1and Th2 were examined by ELISA. The results showed that the titers of cytokines in pigs immunized with rTs-Adsp protein were significantly elevated compared with those of the PBS group; however, there was no significant change between the PBS group and the adjuvant group (Fig 3). The results confirmed that a Th1 and Th2 mixed immune response was induced after immunization with rTs-Adsp.
Fig 3. Cytokine production from sera were evaluated by ELISA.
The levels of (A) IFN-γ (B) IL-2 (C) IL-4 (D) IL-10 are presented as the mean ± SD (n = 6). Asterisks indicate that the production of cytokines from immunized group are significantly different from (*p<0.05, **p<0.01, ***p< 0.001) that of PBS control group.
Evaluation of the changes in T lymphocytes
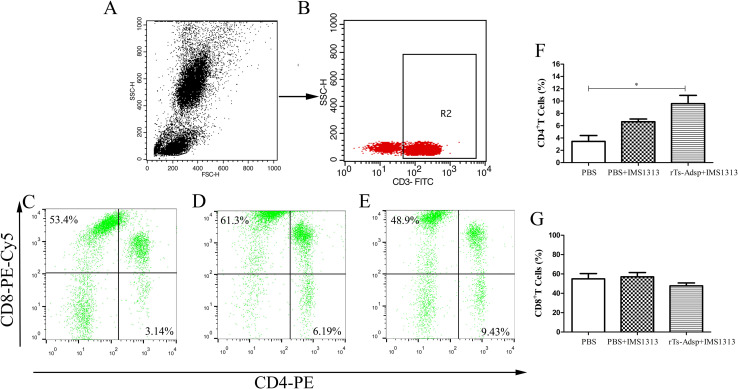
Changes in T cell subsets (CD4+ T and CD8+ T) are the indicators of the immune function status. It is well known that CD4+ T cells play a key role in regulating immune response. Two weeks after the last vaccination, compared with that of pigs immunized with PBS, the proportion of CD4+ T cells in pigs immunized with the rTs-Adsp protein was elevated, and the proportion of CD8+ T cells did not change significantly (Fig 4). The results suggested that the cellular immune response in pigs was induced after vaccination with rTs-Adsp.
Fig 4. Flow cytometry analysis of T lymphocytes in peripheral blood.
The gating strategy are shown in (A) and (B). The percentage change of CD4+ and CD8+ T cells in peripheral blood lymphocytes from (C) PBS-control group (D) IMS1313 adjuvant group (E) rTs-Adsp group. Statistical analysis of (F) the percentage of CD4+ T lymphocytes (G) the percentage of CD8+ T lymphocytes. The values are shown as mean ± SD. Significant differences are as follows: *p<0.05, **p<0.01, ***p<0.001.
Evaluation of the change in B lymphocytes
The humoral immune response ability in a host is related to the changes of B lymphocytes. The proliferation of B cells is required for antibody production, which plays an important role in anti-Trichinella immunity. Two weeks after the last vaccination, compared with that of pigs immunized with PBS, the proportion of B cells in pigs immunized with the rTs-Adsp protein was significantly elevated (Fig 5). The results suggested that the humoral immune response in pigs was enhanced after immunization with rTs-Adsp.
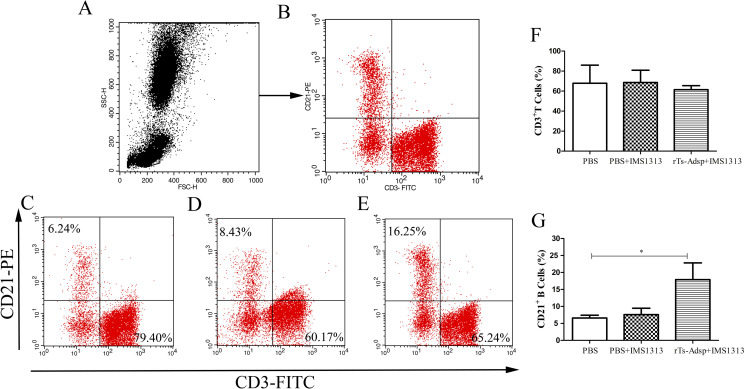
Fig 5. Flow cytometry analysis of B lymphocytes in peripheral blood.
The gating strategy are shown in (A) and (B). The populations change of B lymphocytes in peripheral blood from (C) PBS-control group (D) IMS1313 adjuvant group (E) rTs-Adsp group. Statistical analysis of (F) the percentage of CD3+ T lymphocytes (G) the percentage of CD21+ B lymphocytes. The values are shown as mean ± SD. Significant differences are as follows: *p<0.05, **p<0.01, ***p<0.001.
Analysis of the changes in neutrophils
It is well known that neutrophils play crucial roles in nonspecific immune defense by phagocytizing foreign bodies. Two weeks after the last vaccination, the percentage of neutrophils in the peripheral blood was analyzed using flow cytometry. The results showed that the proportion of neutrophils in the immunized group was significantly elevated compared with the control group (Fig 6). The results indicated that the immune defense ability of pigs was enhanced after vaccination with rTs-Adsp.
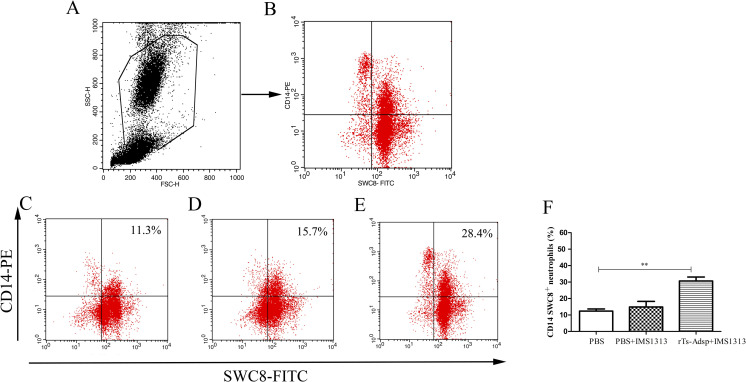
Fig 6. Flow cytometry analysis of neutrophils in peripheral blood.
The gating strategy are shown in (A) and (B). The percent change of neutrophils in peripheral blood from (C) PBS-control group (D) IMS1313 adjuvant group (E) rTs-Adsp group. Statistical analysis of (F) the percentage of neutrophils. The values are shown as mean ± SD. Significant differences are as follows: *p<0.05, **p<0.01, ***p<0.001.
Immune protection
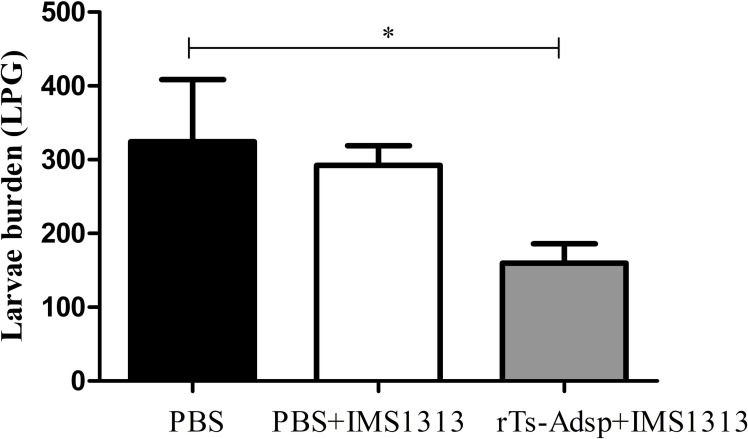
The immune protection of rTs-Adsp against T. spirails infection was evaluated in immunized pigs. Compared with pigs in the PBS-immunized group, pigs in the rTs-Adsp-immunized group showed a 50.9% reduction in the muscle larvae burden (Fig 7). The muscle larvae burden in the immunized group was significantly lower than that of the PBS group (p< 0.05). However, compared with the PBS group, the muscle larvae burden in the adjuvant group did not change significantly (Fig 7). The results demonstrated that pigs immunized with rTs-Adsp exhibited partial protection against T. spiralis infection.
Fig 7. Protective immunity of rTs-Adsp-vaccinated pigs after being challenged with 5000 Trichinella spiralis larvae.
The results are shown as the mean ± SD (n = 6). Asterisks indicate that muscle larvae burden of immunized group is significantly different from (*p<0.05) that of PBS control group.
Discussion
Over the past decades, many efforts have been made to study T. spiralis vaccines, which have contributed to the development of novel vaccines. A number of vaccine strategies have been evaluated, including recombinant protein vaccines, DNA vaccines, and synthesized epitope vaccines [5]. Among them, protein vaccines are a major strategy used to control T. spiralis infection. Moreover, ES products, proteases, whole worms, and surface proteins are mainly selected as candidate immunogens. Serine proteases have been identified in ES production and are thought to be involved in the process of T. spiralis moluting and invasion of host cells [14]. The host will produce specific antibodies and cellular immune responses after vaccination with serine protease-like antigens. Inhibition of the activities of proteases by specific antibodies may have an adverse impact on parasite survival [7, 10, 29, 30]. Sun et al. reported that antibodies induced by a serine protease-like antigen can inhibit larval invasion of the host enterocytes [7]. Moreover, the antibodies can kill the ML, reduce the larval infectivity, and obstruct larval development through an ADCC mechanism [26]. A previous study of our laboratory found that a serine protease from T. spiralis adults exhibited partial protection against challenge with T. spiralis larvae [9]. Therefore, serine proteases could be a potential target molecule for preventing and controlling T. spiralis infection. In this study, the serine protease from T. spiralis adults was expressed and identified to investigate its protective immunity effect against T. spiralis in pigs.
It is well known that the protective effect of most vaccines is related to the antibody responses [31, 32]. Antigens are often screened to develop anti-parasite vaccines based on the degree of antibody responses; thus, vaccines that show high-level protection are usually related to effective antibody responses [33, 34]. Previous studies have found that mice immunized with serine protease-like antigens exhibited high-level cell-mediated and humoral immune responses, eliciting partial protection against challenge with T. spiralis larvae [9, 13, 35]. Most studies have demonstrated that host infection with T. spiralis induced a Th2-based immune response, which is probably related to the immunosuppressive effect of T. spiralis on the host [36–38]. It is well known that the IgG1 isotype represents a Th2 immune response, whereas the IgG2a isotype represents a Th1 immune response [39]. In this study, the levels of antibody responses (IgG, IgG1, IgG2a, and IgM) induced by rTs-Adsp in pigs were analyzed to investigate the protective immunity effect of rTs-Adsp. The results showed that the levels of antibodies in the immunized group were significantly elevated compared to those in the control group. The results showed that a mixed IgG1 and IgG2a antibody response, with IgG2a antibody response being predominant, was induced by the rTs-Adsp protein. Although antibody response is necessary for protective immunity, cell-mediated immunity is also important in eliminating parasites [40]. Furthermore, the titers of cytokines (IL-2, IFN-γ, IL-4, and IL-10) in the serum of pigs were measured. The results showed that the levels of cytokines in the immunized group were significantly elevated compared to the control group. A study found that the expulsion of T. spiralis adults is mainly regulated by CD4+/Th2 cytokines and depends on the production of IL-4 and IL-13, since inhibition of these cytokines extends parasite survival [27]. IL-10 plays a major role in regulating intestinal mast cell responses and is important in protecting against T. spiralis adults [41]. Furthermore, IFN-γ is involved in killing the newborn larvae of T. spiralis by activating macrophages and enhancing the cytotoxic killing of eosinophils and granulocytes [40]. IL-2 is crucially involved in resisting acute acquired infection [42]. The above results demonstrated that rTs-Adsp induced humoral and cell-mediated immune responses.
A study found that T. spiralis has an immunosuppressive effect on the natural immune system, and the inhibitory effect is most significant for the adult stage and newborn larval migration stage of T. spiralis [43]. Eliminating the immunosuppression caused by T. spiralis infection plays a major role in killing the parasites. Neutrophils are crucially components of the innate immune response and can regulate adaptive immune responses [44–46]. It was reported that neutrophils can kill worms, depending on antibody dependent cell-mediated cytotoxicity (ADCC) [47]. This effect has been confirmed through in vitro experiments and the development of a T. spiralis vaccine [48, 49]. Moreover, neutrophils have the function of promoting inflammation and fighting pathogen infection [38, 44]. A previous study of our laboratory found that the level of neutrophils was increased after T. spiralis infection [38]. In this study, compared to that of the PBS group, the proportion of neutrophils in the immunized group was increased. The change may contribute to expelling worms and eliminating the immunosuppression caused by T. spiralis infection. Furthermore, the results of T and B lymphocytes indicated that the numbers of CD4+ T lymphocytes and B lymphocytes in the rTs-Adsp group were significantly elevated compared to those of the PBS group. The numbers of CD8+ T lymphocytes was decreased, but not statistically significant compared to those of the PBS group. It was reported that the expulsion of T. spiralis adults is dependent on CD4+ T cells [50]. The resistance of infective muscle larvae is partially dependent on related cytokines regulated by CD4+ T cells [36, 51]. Study found that CD8+ T cells play no significant role in worm expulsion, but that CD4+ T cells may make a significant contribution [52]. Meanwhile, the ratio of CD4+/CD8+ T cells may reflect the ratio of helper T cells to suppressor T cells, and the immune status of the host may be determined by the balance of these cells [53]. In the present study, the ratio of CD4+/CD8+ T cells were elevated. The result indicated that the cellular immune response in pigs was induced after vaccination with rTs-Adsp. B cells can elicit different functions; they can secrete immunoglobulin, produce cytokines, and induce Treg cell production [54, 55]. It is well known that antibodies produced by B cells play a key role during T. spiralis infection. One study reported that IgG antibodies produced by B cells can kill NBL by induction of eosinophils and ADCC [53]. The above results suggested that rTs-Adsp induced a cell-mediated immune response, which is likely to play a major role in protecting the host against T. spiralis infection.
Until now, most studies on Trichinella vaccines have been performed in mouse models, and very few anti-Trichinella infection studies have been performed on pigs. In our previous study, pig vaccinated with DNase II-7/FCA exhibited a 45.7% reduction in the muscle larvae burden [28]. However, due to the adverse reaction of Freund’s adjuvant to experimental animals, its application is gradually limited. Montanide IMS 1313 N VGPR adjuvant used in the present study is comparatively nontoxic and has been applied to animal vaccine research. Previous study found that compared to FCA/FIA-formulated vaccines, an IMS1313/rTs-serpin vaccine displayed higher levels of immune response and protective efficacy [56]. Obviously, the protective effect is crucially associated with the reduction in the muscle larvae burden. A previous study of our laboratory found that mice vaccinated with rTs-Adsp exhibited a 46.5% reduction in muscle larvae [9]. Moreover, mice immunized with pcDNA3.1(+)-Ts-Adsp showed a 50.2% ML reduction, and a combination of pcDNA3.1(+)-Ts-Adsp and rTs-Adsp elicited a 69.5% ML reduction [35]. In the present study, the protective immunity effect of rTs-Adsp against T. spiralis infection in pigs was further explored. The results showed that rTs-Adsp elicited a 50.9% ML reduction in pigs.
In conclusion, rTs-Adsp induced humoral and cell-mediated immune responses in vaccinated pigs. Furthermore, pigs immunized with rTs-Adsp exhibited partial protection against challenge with T. spiralis larvae. Our results indicated that rTs-Adsp could be a potential target molecule for preventing and controlling Trichinella transmission from pigs to humans.
Acknowledgments
We thank Xuejin Su, Xinrui Wang and Li Yang for the technical assistance. Our thanks are also extended to express our gratitude to all the people who made this work.
Data Availability
All relevant data are within the manuscript.
Funding Statement
MYL was supported by the National Key Research and Development Program of China (2018YFC1602500); National Natural Science Foundation of China (NSFC 31872467, 31520103916); Jilin Provincial Science and Technology Development Project (20180520042JH); and Program for JLU Science and Technology Innovative Research Team(2017TD-32). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gottstein B, Pozio E, Nockler K. Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis. Clinical Microbiology Reviews. 2009;22(1):127–45. 10.1128/CMR.00026-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui J, Jiang P, Liu LN, Wang ZQ. Survey of Trichinella infections in domestic pigs from northern and eastern Henan, China. Vet Parasitol. 2013;194(2–4):133–5. 10.1016/j.vetpar.2013.01.038 [DOI] [PubMed] [Google Scholar]
- 3.Jiang P, Zhang X, Wang LA, Han LH, Yang M, Duan JY, et al. Survey of Trichinella infection from domestic pigs in the historical endemic areas of Henan province, central China. Parasitol Res. 2016;115(12):4707–9. 10.1007/s00436-016-5240-x [DOI] [PubMed] [Google Scholar]
- 4.Rostami A, Gamble HR, Dupouy-Camet J, Khazan H, Bruschi F. Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol. 2017;64:65–71. 10.1016/j.fm.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 5.Ortega-Pierres G, Vaquero-Vera A, Fonseca-Linan R, Bermudez-Cruz RM, Argueello-Garcia R. Induction of protection in murine experimental models against Trichinella spiralis: an up-to-date review. Journal of Helminthology. 2015;89(5):526–39. 10.1017/S0022149X15000140 [DOI] [PubMed] [Google Scholar]
- 6.Zhang N, Li W, Fu B. Vaccines against Trichinella spiralis: Progress, challenges and future prospects. Transbound Emerg Dis. 2018;65(6):1447–58. 10.1111/tbed.12917 [DOI] [PubMed] [Google Scholar]
- 7.Sun GG, Ren HN, Liu RD, Song YY, Qi X, Hu CX, et al. Molecular characterization of a putative serine protease from Trichinella spiralis and its elicited immune protection. Vet Res. 2018;49(1):59. 10.1186/s13567-018-0555-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Wen YJ, Cai YN, Vallee I, Boireau P, Liu MY, et al. Serine Proteases of Parasitic Helminths. Korean J Parasitol. 2015;53(1):1–11. 10.3347/kjp.2015.53.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng S, Wu X, Wang X, Bai X, Shi H, Tang B, et al. Vaccination of mice with an antigenic serine protease-like protein elicits a protective immune response against Trichinella spiralis infection. J Parasitol. 2013;99(3):426–32. 10.1645/12-46.1 [DOI] [PubMed] [Google Scholar]
- 10.Li X, Yao JP, Pan AH, Liu W, Hu XC, Wu ZD, et al. An antigenic recombinant serine protease from Trichinella spiralis induces protective immunity in BALB/c mice. Parasitology Research. 2013;112(9):3229–38. 10.1007/s00436-013-3500-6 [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Wang ZQ, Jin J, Ren HJ, Liu LN, Cui J. Cloning, expression and characterization of a Trichinella spiralis serine protease gene encoding a 35.5 kDa protein. Experimental Parasitology. 2013;134(2):148–54. 10.1016/j.exppara.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Wang ZQ, Hu DD, Cui J. Proteomic Analysis of Trichinella spiralis Muscle Larval Excretory-Secretory Proteins Recognized by Early Infection Sera. Biomed Research International. 2013;2013. 10.1155/2013/139745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Bai X, Wang LB, Shi HN, Van Der Giessen JWB, Boireau P, et al. Immune responses in mice vaccinated with a DNA vaccine expressing serine protease-like protein from the new-born larval stage of Trichinella spiralis. Parasitology. 2017;144(6):712–9. 10.1017/S0031182016002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzik JM. Molecules released by helminth parasites involved in host colonization. Acta Biochimica Polonica. 2006;53(1):33–64. [PubMed] [Google Scholar]
- 15.McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. Proteases in parasitic diseases. Annu Rev Pathol. 2006;1:497–536. 10.1146/annurev.pathol.1.110304.100151 [DOI] [PubMed] [Google Scholar]
- 16.Trap C, Boireau P. [Proteases in helminthic parasites]. Vet Res. 2000;31(5):461–71. 10.1051/vetres:2000132 [DOI] [PubMed] [Google Scholar]
- 17.Saboia-Vahia L, Borges-Veloso A, Mesquita-Rodrigues C, Cuervo P, Dias-Lopes G, Britto C, et al. Trypsin-like serine peptidase profiles in the egg, larval, and pupal stages of Aedes albopictus. Parasit Vectors. 2013;6:50. 10.1186/1756-3305-6-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coombs GH, Mottram JC. Parasite proteinases and amino acid metabolism: possibilities for chemotherapeutic exploitation. Parasitology. 1997;114 Suppl:S61–80. [PubMed] [Google Scholar]
- 19.Dalton JP, Brindley PJ, Knox DP, Brady CP, Hotez PJ, Donnelly S, et al. Helminth vaccines: from mining genomic information for vaccine targets to systems used for protein expression. International Journal for Parasitology. 2003;33(5–6):621–40. 10.1016/s0020-7519(03)00057-2 [DOI] [PubMed] [Google Scholar]
- 20.Borges-Veloso A, Saboia-Vahia L, Cuervo P, Pires RC, Britto C, Fernandes N, et al. Proteolytic profiling and comparative analyses of active trypsin-like serine peptidases in preimaginal stages of Culex quinquefasciatus. Parasit Vectors. 2012;5:123. 10.1186/1756-3305-5-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlan JV, Vongxay K, Khamlome B, Gomez-Morales MA, Pozio E, Blacksell SD, et al. Patterns and Risks of Trichinella Infection in Humans and Pigs in Northern Laos. Plos Neglected Tropical Diseases. 2014;8(7). 10.1371/journal.pntd.0003034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi X, Yue X, Han Y, Jiang P, Yang F, Lei JJ, et al. Characterization of Two Trichinella spiralis Adult-Specific DNase II and Their Capacity to Induce Protective Immunity. Front Microbiol. 2018;9:2504. 10.3389/fmicb.2018.02504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao H, Tang B, Bai X, Wang L, Wu X, Shi H, et al. Characterization of an antigenic serine protease in the Trichinella spiralis adult. Exp Parasitol. 2018;195:8–18. 10.1016/j.exppara.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, Zhan B, Yang Y, Yang X, Zhao X, Wang L, et al. Protective Effect of a Prime-Boost Strategy with the Ts87 Vaccine against Trichinella spiralis Infection in Mice. Biomed Research International. 2014. 10.1155/2014/326860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song YY, Zhang Y, Yang D, Ren HN, Sun GG, Jiang P, et al. The Immune Protection Induced by a Serine Protease Inhibitor From the Foodborne Parasite Trichinella spiralis. Frontiers in Microbiology. 2018;9. 10.3389/fmicb.2018.01544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long SR, Wang ZQ, Jiang P, Liu RD, Qi X, Liu P, et al. Characterization and functional analysis of Trichinella spiralis Nudix hydrolase. Experimental Parasitology. 2015;159:264–73. 10.1016/j.exppara.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 27.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, et al. Interleukin-4-and interleukin-13-mediated host protection against intestinal nematode parasites. Immunological Reviews. 2004;201:139–55. 10.1111/j.0105-2896.2004.00192.x [DOI] [PubMed] [Google Scholar]
- 28.Xu D, Tang B, Yang Y, Cai X, Jia W, Luo X, et al. Vaccination with a DNase II recombinant protein against Trichinella spiralis infection in pigs. Veterinary parasitology. 2020:109069–. 10.1016/j.vetpar.2020.109069 [DOI] [PubMed] [Google Scholar]
- 29.Zhang ZX, Mao YX, Li D, Zhang Y, Li W, Jia HL, et al. High-level expression and characterization of two serine protease inhibitors from Trichinella spiralis. Veterinary Parasitology. 2016;219:34–9. 10.1016/j.vetpar.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 30.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, et al. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69(4):597–604. 10.1016/0092-8674(92)90223-y [DOI] [PubMed] [Google Scholar]
- 31.Diemert DJ, Bethony JM, Pinto AG, Freire J, Santiago H, Correa-Oliveira R, et al. Clinical Development of the Na-Asp-2 Hookworm Vaccine in Previously-Infected Brazilian Adults. Am J Trop Med Hyg. 2008;79(6):345–. [Google Scholar]
- 32.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21(1):225–42. 10.1128/CMR.00046-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diemert DJ, Bethony JM, Hotez PJ. Hookworm vaccines. Clin Infect Dis. 2008;46(2):282–8. 10.1086/524070 [DOI] [PubMed] [Google Scholar]
- 34.Maizels RM, Holland MJ, Falcone FH, Zang XX, Yazdanbakhsh M. Vaccination against helminth parasites—the ultimate challenge for vaccinologists? Immunol Rev. 1999;171:125–47. 10.1111/j.1600-065x.1999.tb01345.x [DOI] [PubMed] [Google Scholar]
- 35.Xu D, Tang B, Wang Y, Zhang L, Qu Z, Shi W, et al. The immune protection induced by a serine protease from the Trichinella spiralis adult administered as DNA and protein vaccine. Acta tropica. 2020;211:105622–. 10.1016/j.actatropica.2020.105622 [DOI] [PubMed] [Google Scholar]
- 36.Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta(1). Journal of Immunology. 2007;178(2):1039–47. [DOI] [PubMed] [Google Scholar]
- 37.Li CK, Ko RC. Inflammatory response during the muscle phase of Trichinella spiralis and T. pseudospiralis infections. Parasitol Res. 2001;87(9):708–14. 10.1007/s004360100420 [DOI] [PubMed] [Google Scholar]
- 38.Wang N, Bai X, Tang B, Yang Y, Wang XL, Zhu HF, et al. Primary characterization of the immune response in pigs infected with Trichinella spiralis. Veterinary Research. 2020;51(1). 10.1186/s13567-020-0741-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawley A, Raymond C, Wilkie BN. Control of immunoglobulin isotype production by porcine B-cells cultured with cytokines. Vet Immunol Immunopathol. 2003;91(2):141–54. 10.1016/s0165-2427(02)00293-3 [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Bai X, Li CY, Tong MW, Zhang PH, Cai W, et al. Molecular Characterization of Fructose-1,6-bisphosphate Aldolase From Trichinella spiralis and Its Potential in Inducing Immune Protection. Front Cell Infect Mi. 2019;9. 10.3389/fcimb.2019.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helmby H, Grencis RK. Contrasting roles for IL-10 in protective immunity to different life cycle stages of intestinal nematode parasites. Eur J Immunol. 2003;33(9):2382–90. 10.1002/eji.200324082 [DOI] [PubMed] [Google Scholar]
- 42.Sa Q, Woodward J, Suzuki Y. IL-2 Produced by CD8(+) Immune T Cells Can Augment Their IFN-gamma Production Independently from Their Proliferation in the Secondary Response to an Intracellular Pathogen. Journal of Immunology. 2013;190(5):2199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai X, Wu X, Wang X, Guan Z, Gao F, Yu J, et al. Regulation of cytokine expression in murine macrophages stimulated by excretory/secretory products from Trichinella spiralis in vitro. Molecular and Cellular Biochemistry. 2012;360(1–2):79–88. 10.1007/s11010-011-1046-4 [DOI] [PubMed] [Google Scholar]
- 44.Jablonska J, Granot Z. Neutrophil, quo vadis? J Leukocyte Biol. 2017;102(3):685–8. 10.1189/jlb.3MR0117-015R [DOI] [PubMed] [Google Scholar]
- 45.Tillack K, Breiden P, Martin R, Sospedra M. T Lymphocyte Priming by Neutrophil Extracellular Traps Links Innate and Adaptive Immune Responses. Journal of Immunology. 2012;188(7):3150–9. 10.4049/jimmunol.1103414 [DOI] [PubMed] [Google Scholar]
- 46.Thewissen M, Damoiseaux J, van de Gaar J, Tervaer JWC. Neutrophils and T cells: Bidirectional effects and functional interferences. Molecular Immunology. 2011;48(15–16):2094–101. 10.1016/j.molimm.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 47.Ren HN, Guo KX, Zhang Y, Sun GG, Liu RD, Jiang P, et al. Molecular characterization of a 31 kDa protein from Trichinella spiralis and its induced immune protection in BALB/c mice. Parasites & Vectors. 2018;11. 10.1186/s13071-018-3198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falduto G, Vila C, Saracino M, Calcagno M, Venturiello S. Trichinella spiralis: killing of newborn larvae by lung cells. Parasitology Research. 2015;114(2):679–85. 10.1007/s00436-014-4233-x [DOI] [PubMed] [Google Scholar]
- 49.Venturiello SM, Giambartolomei GH, Costantino SN. Immune killing of newborn Trichinella larvae by human leucocytes. Parasite immunology. 1993;15(10):559–64. 10.1111/pim.1993.15.10.559 [DOI] [PubMed] [Google Scholar]
- 50.Riedlinger J, Grencis RK, Wakelin D. Antigen-specific T-cell lines transfer protective immunity against Trichinella spiralis in vivo. Immunology. 1986;58(1):57–61. [PMC free article] [PubMed] [Google Scholar]
- 51.Patel N, Kreider T, Urban JF, Gause WC. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. International Journal for Parasitology. 2009;39(1):13–21. 10.1016/j.ijpara.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vallance BA, Galeazzi F, Collins SM, Snider DP. CD4 T cells and major histocompatibility complex class II expression influence worm expulsion and increased intestinal muscle contraction during Trichinella spiralis infection. Infection and immunity. 1999;67(11):6090–7. 10.1128/IAI.67.11.6090-6097.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding J, Bai X, Wang XL, Wang YF, Shi HN, Rosenthal B, et al. Developmental profile of select immune cells in mice infected with Trichinella spiralis during the intestinal phase. Vet Parasitol. 2016;231:77–82. 10.1016/j.vetpar.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 54.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26(4):491–502. 10.1016/j.immuni.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 55.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. CD19(+)CD24(hi)CD38(hi) B Cells Maintain Regulatory T Cells While Limiting T(H)1 and T(H)17 Differentiation. Science Translational Medicine. 2013;5(173). [DOI] [PubMed] [Google Scholar]
- 56.Xu J, Bai X, Wang LB, Shi HN, van der Giessen JWB, Boireau P, et al. Influence of adjuvant formulation on inducing immune response in mice immunized with a recombinant serpin from Trichinella spiralis. Parasite Immunology. 2017;39(7). 10.1111/pim.12437 [DOI] [PubMed] [Google Scholar]