Plants coordinate the behavior of physically distant organs via mobile signals that travel between tissues. Within-plant signaling is important for systemic defense: local pathogen infection triggers a systemic response that leads to enhanced resistance to various pathogens in distal parts of the plant. A critical systemic defense response is known as systemic acquired resistance (SAR) and a major mobile signal that activates SAR in systemic tissues is N-hydroxy-pipecolic acid (NHP, Chen et al., 2018; Hartmann et al., 2018).
The key enzymes required for NHP biosynthesis are AGD2-LIKE DEFENSE RESPONSE PROTEIN 1 (ALD1) that catalyzes the first step from lysine (Návarová et al., 2012), and FLAVIN-DEPENDENT MONOOXYGENASE 1 (FMO1) that forms NHP from pipecolic acid (Pip, Chen et al., 2018; Hartmann et al., 2018). Plants deficient in ALD1 or FMO1 cannot produce NHP nor trigger SAR upon pathogen infection.
In addition to NHP, a glycosylated metabolite of NHP was identified in systemic tissues during SAR (Chen et al., 2018). Whether glycosylated NHP participated in SAR or served as an inactive storage form of NHP, and which glycosyltransferase was responsible for its formation, remained elusive. In the current issue of The Plant Cell, three independent studies report identification of UDP-DEPENDENT GLYCOSYLTRANSFERASE 76B1 (UGT76B1) as an enzyme responsible for formation of NHP-O-β-glycoside from NHP and show that NHP accumulation leads to constitutive local and systemic defense responses in plants deficient in NHP glycosylation.
UGT76B1 glycosylates the immune mediators salicylic acid (SA) and isoleucic acid (ILA) and its transcript was induced by Pip FMO1-dependently (von Saint Paul et al., 2011; Noutoshi et al., 2012; Hartmann et al., 2018). This prompted first authors Sibylle Bauer, Dereje W. Mekonnen and colleagues (Bauer et al., 2021) to analyze the metabolites formed in UGT76B1-deficient and overexpressing plants in response to Pip. They found glycosylated NHP in wild-type and UGT76B1 overexpressing plants but not in the mutant, suggesting that in addition to SA and ILA, the enzyme also glycosylates NHP. Similarly, first authors Lennart Mohnike, Dmitrij Rekhter, Weijie Huang and coworkers (Mohnike et al., 2021) identified glycosylated NHP in pathogen-challenged wild-type, but not ugt76b1-1 mutants by a nontargeted metabolomics approach. Eric C. Holmes and colleagues (Holmes et al., 2021) reached the conclusion that UGT76B1 glycosylates NHP by a different approach: they selected a set of candidate glycosyltransferases and tested them in a heterologous system. They found that expression of UGT76B1 in NHP-producing tomatoes that transiently expressed Arabidopsis ALD1 and FMO1, led to formation of glycosylated NHP.
The authors of all three studies also performed in vitro assays to compare the glycosylation of different substrates—NHP, SA, and ILA—by UGT76B1. While the enzyme glycosylated all molecules and they mutually competed with each other as substrates (Bauer et al., 2021), UGT76B1 appeared to glycosylate NHP faster than SA in experiments by Holmes et al. (2021) but vice versa in experiments by Mohnike et al. (2021). The substrate preference of the enzyme may vary depending on plant physiological status and abundance of NHP, SA, and ILA, allowing adjustment of immune responses in local and systemic tissues during SAR.
Plants deficient in UGT76B1 function have constitutively active defense responses, previously attributed to high SA levels of these mutants (von Saint Paul et al., 2011). Experiments by Bauer et al. (2021) and Mohnike et al. (2021) indicate that the primary cause of the enhanced resistance phenotype of ugt76b1 mutants is accumulation of NHP, as the phenotype was lost in the ugt76b1 fmo1 double mutants that cannot produce NHP. Failure to glycosylate NHP in ugt76b1 mutants promotes its accumulation that in turn induces production of SA and leads to constitutive SAR and reduced growth. Thus, UGT76B1 is needed to prevent the growth–immunity balance from tilting too much toward immune activation. At the other end of the scale, UGT76B1-overexpressing Arabidopsis plants were unable to mount SAR (Bauer et al., 2021), indicating that glycosylation of NHP by UGT76B1 causes loss of its biological activity as a SAR-inducer. In line with this, transiently transformed NHP-producing tomato plants lost their ability to elicit SAR in distal leaves when Arabidopsis UGT76B1 was also expressed in the system (Holmes et al., 2021).
Together, the evidence presented in the three studies by Bauer et al. (2021), Mohnike et al. (2021), and Holmes et al. (2021) on the role of UGT76B1 indicates that NHP is the active mobile signal that triggers SA production and SAR in systemic tissues upon infection, whereas glycosylation of NHP by UGT76B1 leads to inactivation of the metabolite, keeping immune responses in check (see figure). The findings open up new questions on the role of glycosylated NHP—is it an inactive intermediate to be sent to degradation, or can it be hydrolyzed to form biologically active NHP again? Analyzing dynamics of NHP and its glycosylated form in locally infected and systemic tissues during the progression of infection and establishment and maintenance of SAR could give further information on the biological role of glycosylated NHP.
Figure.
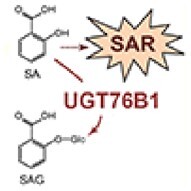
UGT76B1 glycosylates NHP to suppress immune responses. Pathogen infection triggers production of the mobile messenger NHP that promotes SA accumulation and leads to SAR in distal tissues. The glycosyltransferase UGT76B1 inactivates both NHP and SA by glycosylation and formation of NHP-OGlc and SAG, respectively. Adapted from Bauer et al. (2021), Figure 9 and Mohnike et al. (2021), Figure 1.
References
- Bauer S, Mekonnen DW, Hartmann M, Yildiz I, Janowski R, Lange B, Geist B, Zeier J, Schäffner AR (2021) UGT76B1, a promiscuous hub of small molecule-based immune signaling, glycosylates N-hydroxypipecolic acid and balances plant immunity. Plant Cell 33: 714--734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Holmes EC, Rajniak J, Kim J-G, Tang S, Fischer CR, Mudgett MB, Sattely ES (2018) N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc Natl Acad Sci USA 115: E4920–E4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Zeier T, Bernsdorff F, Reichel-Deland V, Kim D, Hohmann M, Scholten N, Schuck S, Bräutigam A, Hölzel T, et al. (2018) Flavin monooxygenase-generated N-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173: 456–469.e16 [DOI] [PubMed] [Google Scholar]
- Holmes EC, Chen Y-C, Mudgett MB, Sattely ES (2021) Arabidopsis UGT76B1 glycosylates N-hydroxy-pipecolic acid and inactivates systemic acquired resistance in tomato. Plant Cell 33: 750--765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnike L, Rekhter D, Huang W, Feussner K, Tian H, Herrfurth C, Zhang Y, Feussner I (2021) The glycosyltransferase UGT76B1 modulates N-hydroxy-pipecolic acid homeostasis and plant immunity. Plant Cell 33: 735--749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Návarová H, Bernsdorff F, Döring A-C, Zeier J (2012) Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24: 5123–5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutoshi Y, Okazaki M, Kida T, Nishina Y, Morishita Y, Ogawa T, Suzuki H, Shibata D, Jikumaru Y, Hanada A, et al. (2012) Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis. Plant Cell 24:3795–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Saint Paul V, Zhang W, Kanawati B, Geist B, Faus-Keßler T, Schmitt-Kopplin P, Schäffner AR (2011) The Arabidopsis lucosyltransferase UGT76B1 conjugates isoleucic acid and modulates plant defense and senescence. Plant Cell 23: 4124–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]


