Abstract
Both adenoid cystic carcinoma (ACC) and thyroid metastasis are quite peculiar clinical presentations. ACC is a malignant salivary gland-type tumour mostly found in the head and neck district, but that can arise from different organs. Due to its rarity, it can pose problems in the diagnostic and therapeutic management.
A 72-year-old woman presented for a persistent non-productive cough. A computed tomography showed a lung mass. She underwent lung surgery, and the lesion was an ACC primitive of the lung. She developed hoarseness and ultrasound and cytology confirmed metastatic involvement of left thyroid lobe from ACC. A total thyroidectomy was performed, followed by radiotherapy.
The present case highlights the need to be aware of possible metastatic thyroid localization of ACC originating in lower airways. This is a very rare event, and clinical and cytological findings must be carefully examined. It represents an opportunity to consider the current knowledge about ACC metastasis to thyroid.
INTRODUCTION
Adenoid cystic carcinoma (ACC) is a rare cancer of the head and neck (H&N) district but that can arise in many other areas. Thyroid metastases are a very uncommon presentation in surgical practice; thyroid involvement is usually due to locally aggressive tumours of H&N district. We present a case of a thyroid metastasis from a pulmonary ACC treated 6 months prior.
CASE REPORT
A 72-year-old Italian woman came for persistent non-productive cough since late 2017. Chest X-ray showed leftvbasilar atelectasia. Previous history of smoking, hypothyroidism, osteoporosis and gastroesophageal reflux disease was noted. A chest computed tomography (CT) displayed subcentimetric cavitated nodules and a 38-mm solid lung mass (Fig. 1a). Enlarged lymph nodes were present in the left hilar region.
Figure 1 .
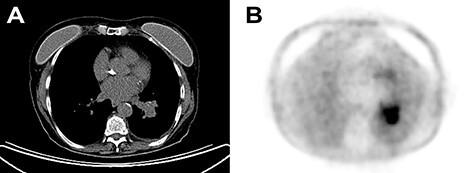
(A) Chest CT with contrast enhancement displaying a solid lung mass of 38 mm characterized by polycyclic margins and dysmorphic calcification. (B) PET/CT with fluorodeoxyglucose revealing high glucose metabolism of the lung mass in the perihilar region of the left inferior lung lobe, near the left inferior lobar bronchus.
A positron emission tomography (PET)/CT revealed a lung mass near the left inferior lobar bronchus (Fig. 1b). There were no other pathologic enhancement on such examination. Scintigraphy excluded bone involvement. A bronchoscopy showed a whitish polilobular mass at emergence of left inferior lobe. Histology revealed neoplastic cells with solid growth pattern. Immunophenotype: CK7+, actin+, vimentin+, CK5/6+, TTF−, chromogranin−, CD56−, synaptophysin−, p63−, GFAP−, CEA−, ki67 15%. Malignant cells were found on the bronchoalveolar lavage. A neck and salivary gland ultrasound (US) excluded lesions to thyroid and to major salivary glands, along with absence of lateral cervical and submandibular pathological lymphadenopathy.
In April 2018 she underwent a left lower lung lobectomy with atrial and bronchial plasty in posterolateral thoracotomy. The histopathological examination revealed the lesion to be an ACC. The visceral pleura was not involved, but there was neoplastic infiltration of the vascular margin. Four out of 11 peribronchial lymph nodes were positive for metastatic cells, whereas paratracheal, mediastinal, hilar and interlobar lymph nodes were negative for neoplasia. The intraoperative histological examination of the left lower lobar bronchus’ section ring resulted clean; further analysis revealed focal nests of infiltration of ACC. No tumour cells were found in the pleural fluid. A multidisciplinary evaluation, considering the clinical picture, preoperative examination and the pathological specimen concluded that it was a primary ACC of the lung pT2b pN1 (TNM VIII ed. 2017) and gave indication for adjuvant radiant treatment without chemotherapy. The patient underwent radiotherapy on the left hilar region, with a total dose of 60 Gy in 30 fractions.
In late August 2018 she reported worsening hoarseness and US showed an enlarged left thyroid lobe with pseudonodular formations. Fine needle aspiration cytology showed poorly differentiated basaloid cells, Bcl2+, CD117+, TTF1−, thyreoglobulin−, chromogranin−, consistent with ACC localization. A restaging CT showed an inhomogeneous alteration of the left thyroid lobe without lateral cervical significant lymphadenopathies (Fig. 2). Brain CT was negative for metastatic localization. The upper airway evaluation revealed a fixity of the left hemilarynx.
Figure 2 .
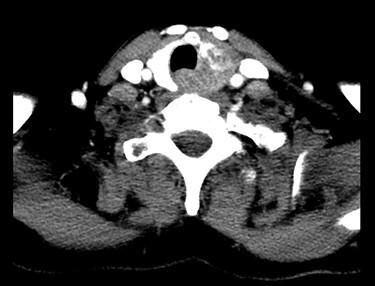
Chest CT showing an inhomogeneous alteration of the left thyroid lobe, corresponding to the metastatic thyroid involvement from the ACC.
In December 2018 a total thyroidectomy was conducted, with finding of extensive infiltration of perithyroidal soft tissue, incorporation of the left inferior laryngeal nerve, infiltration of the trachea, without infiltration of the oesophagus (Fig. 3). The left thyroid lobe resulted subverted by neoplastic proliferation with necrosis, perineural and vascular invasion (PNVI), infiltration of perithyroidal soft tissue, lymph node and surgical margins (Fig. 4). Immunophenotype: smooth muscle actin (SMA)+, CD117+, CK7+, TTF1−, PAX8−, Thyroglobulin−, NapsinA−, CD45−, CK20−, S100−, GFAP−, p63−, GATA3− (Fig. 5). After comparison with the previous examination, the immunomorphological patterns were similar, and the possibility of a primary ACC of thyroid was also considered, but since preoperative radiological and nuclear imaging examination excluded thyroid involvement, the most likely hypothesis was for the thyroid lesion to be a metastatic localization of a primary ACC of the lung.
Figure 3 .
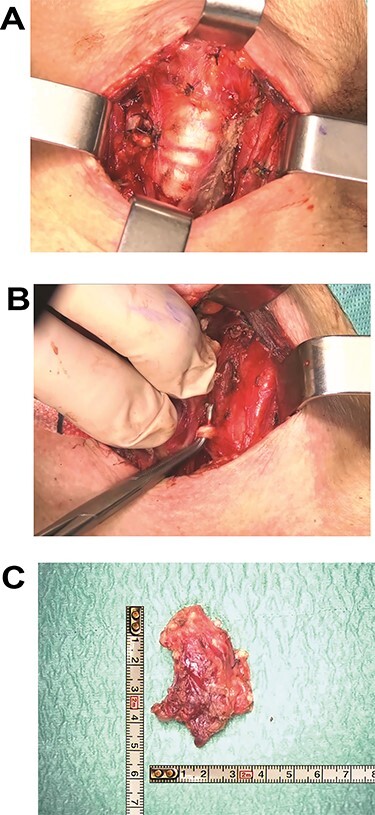
(A) Left thyroid lobe dissection with identification of noble structures. (B) Surgical field at the end of the total thyroidectomy with demonstration of tracheal involvement. (C) Macroscopic specimen of left thyroid lobe with neoplastic mass infiltrating perithyroidal tissues.
Figure 4 .
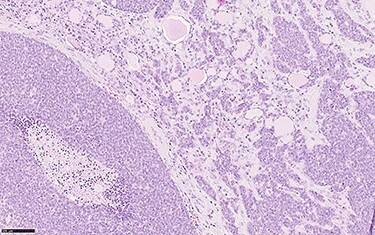
Histological section showing solid sheets, with comedo necrosis, and nests of neoplastic cells infiltrating thyroidal parenchyma (haemotoxylin and eosin, o.m. ×20).
Figure 5 .
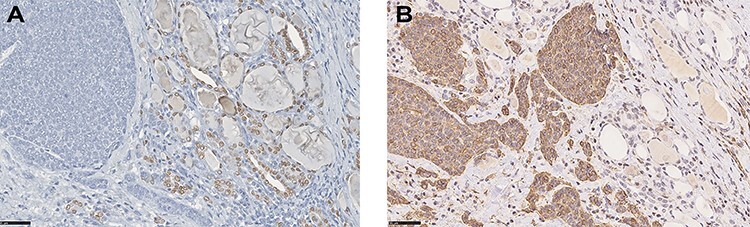
(A) IHC for TTF-1 demonstrating negativity in neoplastic cells, whereas nuclei of normal thyroid cells stain positive (IHC, DAB stain, o.m. ×40). (B) Nests of tumour cells positive for SMA infiltrating thyroid parenchyma between follicles (IHC, DAB stain, o.m. ×40).
After 3 months a CT revealed a 2-cm area near the cervical oesophagus and the first tracheal ring, with annular appearance and peripheric contrast enhancement. Radiotherapy was performed on thyroid lodge, residual disease and lymph nodes. In summer 2019 a brain metastasis was diagnosed and treated with stereotactic radiotherapy. In April 2020 she developed a complex clinical picture comprising a seizure and an acute heart failure. Imaging studies showed absence of oncological relapse. Despite treatment, she died after progressive worsening of her general conditions.
DISCUSSION
ACC is a rare type of cancer, mostly found in the H&N district being part of the salivary gland-type tumours (SGTs) but that can arise in many other areas of the body. Pulmonary SGTs can arise at any level of the tracheobronchial tree. ACC-L is often a central lesion presenting as an endobronchial mass, resulting in cough, dyspnoea, haemoptysis, fever, stridor, chest pain. Patients are usually treated for asthma or bronchitis, with a diagnostic delay of 4 months. Less frequently they arise in the peripheric parenchyma, resulting asymptomatic [1–6]. The most frequent localization of ACC-L is in the trachea and intermedium bronchus in 61.8% of cases, in lobar bronchi in 32.3% and as peripheral lesion in 5.9% [3, 4, 7, 8]. ACC-L is generally less circumscribed than other pulmonary SGTs, and it appears more often in the central area. It can occasionally be confounded with a mucinous adenocarcinoma. Immunohistochemistry (IHC) is essential. ACC-L is immunoreactive to pancytokeratin, CK7 and p63 and variably reactive to SMA, S100 and vimentin [1, 3, 9]. Negative markers are synaptophysin and TTF-1, and also CD56, CK20 and CgA [1, 3]. TTF-1, expressed in thyroid and lung, is present in 60–70% of lung adenocarcinomas, whereas it appears to be absent in ACC-L, although an ACC-L with TTF-1+ has been reported [3, 4, 8].
Despite its low growing behaviour, ACC is relentless and unpredictable. More than 50% of salivary ACC spread to distant sites even decades after surgery. Therefore, while 5-year survival is 60–70%, it drops under 30% after 10 years [10]. Lung metastases are the most common. Its predominant spreading routes are direct invasion and submucosal spreading and PNVI, which prevent obtaining surgical radicality [1, 11, 12]. In ACC-T, direct invasion of thyroid gland manifests similar to primary thyroid tumours. IHC with TTF-1 and thyroglobulin can be helpfuljb [4, 13].
In literature only one article tried to put together different reports of thyroid involvement from ACC-L, but it didn’t report the difference between direct invasion and actual metastatic involvement [2]. We therefore propose that selection again, expanded with latest reports and such distinction (Table 1). Among the 17 cases in literature with thyroid involvement from ACC, including our case only four are actual thyroid metastases, the others all represent direct invasion from ACC-T. Of these four, only our case represents a metastasis from ACC-L [10, 13, 14].
Table 1.
Cases of thyroid involvement from ACC reported in literature.
| Report | Sex | Age | ACC primitive | Symptoms | Metastasis or direct invasion | Other sites of metastases | Survival after thyroid surgery |
|---|---|---|---|---|---|---|---|
| Kashiwagi et al. [20] | F | 33 | Minor salivary glands (Larynx) | Cough and wheezes | Direct | >24 months (alive) | |
| Kukwa et al. [22] | F | 17 | Trachea | Cough, haemoptysis, dyspnoea | Direct | Lung | >12 months (alive) |
| Rocca et al. [13] | M | 66 | Oral minor salivary glands | Palpable mass | Metastasis, thyroid’s isthmus | NA | |
| Lee et al. [14] | F | 60 | Parotid | No | Metastasis, left thyroid lobe | 36 months (deceased) | |
| Khademi et al. [21] | M | 45 | Larynx | Palpable mass | Direct | NA | |
| Idowu et al. [17] | F | 68 | Trachea | Dyspnoea | Direct | 6 months (deceased) | |
| Idowu et al. [17] | M | 60 | Trachea | Cough, haemoptysis | Direct | Lung | NA |
| Zirkin et al. [16] | F | 66 | Trachea | Dyspnoea | Direct | >48 months (alive) | |
| Subramanian et al. [18] | F | 37 | Trachea | Asthma, stridor | Direct | >3 months (alive) | |
| Qi et al. [2] | M | 46 | Trachea | Dysphagia, dyspnoea | Direct | 1 month (deceased) | |
| Varghese et al. [11] | F | 57 | Trachea | Cough, dyspnoea | Direct | Lymph nodes | |
| Huo et al. [4] | / | / | / | / | Direct | NA | |
| Huo et al. [4] | / | / | / | / | Direct | NA | |
| Huo et al. [4] | / | / | / | / | Direct | NA | |
| Aldrees et al. [19] | F | 47 | Trachea | Palpable mass, cough | Direct | / | |
| Van der Wal et al. [10] | / | / | Parotid | / | Metastasis | Lung | |
| Present case | F | 72 | Lung | Dysphonia | Metastasis, left thyroid lobe | Brain | 14 months (deceased) |
In absence of advanced disease or metastases to multiple sites, surgical resection is the best treatment option. In R1, radiotherapy may obtain a local control, with a prognosis comparable between R0 and R1 treated with radiotherapy. Unfortunately, such evidence is based on reviews of case reports. ACC has proven to be chemoresistant. Since evidence is lacking, ACC cases need a multidisciplinary management to ensure the best possible prognosis [1, 12, 15].
CONCLUSIONS
For both ACC and thyroid metastases, literature is composed only of case reports and few reviews. The lack of prospective trials in both conditions determines the absence of evidence on the best management of these cases. Considering thyroid metastases specifically, since prognosis depends on the nature of the primary tumour, the burden of disease and comorbidities, surgery can achieve a better oncological control in the neck. This can prevent the occurrence of lethal respiratory complications and the progression of symptoms, improving long-term quality of life.
Contributor Information
Giulio Montecamozzo, Department of General Surgery, Luigi Sacco University Hospital, 20157 Milan, Italy.
Francesco Cammarata, Department of General Surgery, Luigi Sacco University Hospital, 20157 Milan, Italy.
Luca Pennacchi, Department of General Surgery, Luigi Sacco University Hospital, 20157 Milan, Italy.
Al’ona Yakushkina, Department of General Surgery, Ospedale di Saronno, 21047 Saronno VA, Italy.
Luca Carsana, Department of Pathology, Luigi Sacco University Hospital, 20157 Milan, Italy.
Pietro Zerbi, Department of Pathology, Luigi Sacco University Hospital, 20157 Milan, Italy; Department of Biomedical and Clinical Sciences Luigi Sacco, University of Milan, 20157 Milan, Italy.
Piergiorgio Danelli, Department of General Surgery, Luigi Sacco University Hospital, 20157 Milan, Italy; Department of Biomedical and Clinical Sciences Luigi Sacco, University of Milan, 20157 Milan, Italy.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- 1. Falk N, Weissferdt A, Kalhor N, Moran CA. Primary pulmonary salivary gland-type tumors: a review and update. Adv Anat Pathol 2016;23:13–23. [DOI] [PubMed] [Google Scholar]
- 2. Qi D, Feng L, Li J, Liu B, Zhang Q. Primary adenoid cystic carcinoma of the trachea with thyroid invasion: a case report and literature review. Onco Targets Ther 2016;9:6291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu MM, Hu Y, He JB, Li BL. Primary adenoid cystic carcinoma of the lung: clinicopathological features, treatment and results. Oncol Lett 2015;9:1475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huo Z, Meng Y, Wu H, Shen J, Bi Y, Luo Y, et al. . Adenoid cystic carcinoma of the tracheobronchial tree: clinicopathologic and immunohistochemical studies of 21 cases. Int J Clin Exp Pathol 2014;7:7527–35. [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar NS, Iype EM, Thomas S, Sankar UV. Adenoid cystic carcinoma of the trachea. Indian J Surg Oncol 2016;7:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang HL, Xu L, Li FJ. Subglottic adenoid cystic carcinoma mistaken for asthma. J Zhejiang Univ Sci B 2009;10:707–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inoue H, Iwashita A, Kanegae H, Higuchi K, Fujinaga Y, Matsumoto I. Peripheral pulmonary adenoid cystic carcinoma with substantial submucosal extension to the proximal bronchus. Thorax 1991;46:147–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kitada M, Ozawa K, Sato K, Hayashi S, Tokusashi Y, Miyokawa N, et al. . Adenoid cystic carcinoma of the peripheral lung: a case report. World J Surg Oncol BioMed Central 2010;8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cesar A, Moran MD, Michael N, Koss MD, Saul Suster MD. Primary adenoid cystic carcinoma of the lung. A clinicopathologic and immunohistochemical study of 16 cases. Cancer 1994;73:1390–7. [DOI] [PubMed] [Google Scholar]
- 10. Van Der Wal JE, Becking AG, Snow GB, Van Der Waal I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck 2002;24:779–83. [DOI] [PubMed] [Google Scholar]
- 11. Varghese A, Suneha S, Watkinson JC. Adenoid cystic carcinoma of trachea. Indian J Surg Springer India 2017;79:67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molina JR, Aubry MC, Lewis JE, Wampfler JA, Williams BA, Midthun DE, et al. . Primary salivary gland-type lung cancer: Spectrum of clinical presentation, histopathologic and prognostic factors. Cancer Cancer 2007;110:2253–9. [DOI] [PubMed] [Google Scholar]
- 13. Rocca BJ, Barone A, Ginori A, Ambrosio MR, Disanto A. Unusual presentation of metastatic adenoid cystic carcinoma: a challenge in aspiration cytology of the thyroid. Pathologica 2014;106:342–4. [PubMed] [Google Scholar]
- 14. Lee MW, Batoroev YK, Odashiro AN, Nguyen GK. Solitary metastatic cancer to the thyroid: a report of five cases with fine-needle aspiration cytology. Cytojournal 2007;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nixon IJ, Coca-Pelaz A, Kaleva AI, Triantafyllou A, Angelos P, Owen RP, et al. . Metastasis to the thyroid gland: a critical review. Ann Surg Oncol 2017;24:1533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zirkin HJ, Tovi F.. Tracheal carcinoma presenting as a thyroid tumor. J Surg Oncol. 1942;26:268–71. [DOI] [PubMed] [Google Scholar]
- 17. Idowu MO, Reiter ER, Powers CL.. Adenoid cystic carcinoma: a pitfall in aspiration cytology of the thyroid. Am J Clin Pathol. 2004;121:551–6. [DOI] [PubMed] [Google Scholar]
- 18. Subramaniam T, Lennon P, Kinsella J, O'Neill JP. . Laryngeal preservation in managing advanced tracheal adenoid cystic carcinoma. Case Rep Otolaryngol 2015;2015:404586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aldrees T, Alanazi A, Fatani HA, Samman A, Aldhahri SF.. Adenoid cystic carcinoma of the upper airway mimicking a thyroid tumor: a case report. Mol Clin Oncol. 2016;5:367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kashiwagi T, Kanaya H, Konno W, Goto K, Hirabayashi H, Haruna S.. Adenoid cystic carcinoma of the larynx presenting with unusual subglottic mass: Case report. Auris Nasus Larynx 2016;43:562–5. [DOI] [PubMed] [Google Scholar]
- 21. Khademi B, Negahban S, Aledavood A, Bagheri M, Daneshbod K, Mirfazaelian H, et al. . An unusual thyroid mass. Am J Med. 2015;128:e29–30. [DOI] [PubMed] [Google Scholar]
- 22. Kukwa W, Korzen P, Wojtowicz P, Sobczyk G, Kiprian D, Kawecki A, et al. . Tracheal adenoid cystic carcinoma mimicking a thyroid tumor: A case report. Oncol Lett. 2014;8:1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


