Abstract
Peripheral primitive neuroectodermal tumours are rare tumours in juveniles. The current patient was a paraplegic 8‐month‐old Scottish deerhound with a suspected pulmonary mass. Radiographically, there was a large extrapleural mass within the mid‐left hemithorax. On MRI, the mass was mainly hyperintense on T2‐weighted images, isointense on T1‐weighted images and was heterogeneously strongly contrast enhancing with a multilobulated appearance, spinal cord compression, paraspinal musculature invasion and intrathoracic extension. Those changes were confirmed on post‐mortem, and the mass diagnosed based on immunohistochemistry.
Keywords: dog, juvenile, MRI, pPNET, spinal tumour
1. INTRODUCTION
Primitive neuroectodermal tumours (PNET) are rare neuroendocrine tumours that arise from primitive cells of the embryonic neural tube. Three categories have been established based on location: central primitive neuroectodermal tumour, neuroblastoma and peripheral primitive neuroectodermal tumour (pPNET) (Meuten, 2008; Sturm et al., 2016). In humans, pPNETs are usually characterized as solid masses arising from the abdominal cavity or the retroperitoneal space, usually occurring in children and young adults with a slight male predominance (Dehner, 1986; Mobley et al., 2006). These tumours have a very high rate of metastasis and a poor outcome with a progression‐free survival time of 5 years when surgery and adjunctive therapy are used (Mobley et al., 2006). pPNETs are defined as embryonal undifferentiated tumours of neural crest origin arising in the peripheral nervous system. From an immunoreactivity's standpoint, depending on the degree of differentiation the tumours cells may be positive for glial and neural markers such as neuron‐specific enolase (NSE), neurofilament, synaptophysin, S100 protein and glial fibrillary acid protein (Junginger et al., 2013; Koestner & Higgins, 2002; Maxie & Youssef, 2007). Most of the described primitive neuroectodermal tumours in veterinary medicine are central primitive neuroectodermal tumours. To the author's knowledge, only three cases of confirmed pPNET have been reported in the veterinary literature and only one of them was evaluated with MRI (De Cock et al., 2004; Hosokawa et al., 1998; Junginger et al., 2013).
An 8‐month‐old, female entire, Scottish deerhound was referred to the emergency service for paraplegia and suspected pulmonary mass. Three days before the presentation, the patient was brought to the referring veterinarian for an acute onset of hindlimb paralysis. At the time the dog received non‐steroidal anti‐inflammatory medication (Metacam, 0.05 mg/lb, Boehringer Ingelheim). Following this treatment, the patient's clinical signs improved mildly; however, subsequent signs of neck pain developed. Twenty‐four hours after the initial presentation, the patient's status deteriorated and thoracic radiographs were acquired by the primary care veterinarian. A pulmonary mass was identified, and the dog was referred for additional diagnostics and treatment.
On referral presentation, the dog was bright, alert and responsive. All the vital parameters were within normal limits. The neurological examination was consistent with a T3‐L3 myelopathy with knuckling on both hind limbs. The remainder of the spinal cord and cranial nerve assessment was normal.
Over the 72 hr of hospitalization, blood samples were taken twice for complete blood cell count as well as biochemistry. The only consistent abnormality noted was the mildly elevated creatinine kinase [±1,000; 53–337] which was most consistent with mild muscle trauma. Urinalysis was performed and was within normal limits. Virology and serology were negative for canine distemper virus, Ehrlichiosis canis, Rocky Mountain spotted fever, neospora and toxoplasma.
On thoracic radiographs, a well‐defined soft tissue mass (88 × 44 × 60 mm) was present within the left hemithorax extending from the fifth to the seventh intercostal spaces. On the lateral projections, the mass was in the dorsal third of the thorax, dorsal to the carina and ventral to the vertebral bodies of T6–T8. On the ventrodorsal projection, the mass was superimposed on the caudal subsegment of the left cranial lung lobe. There were no radiographically evident osseous changes, pulmonary metastasis or intrathoracic lymphadenomegaly. The remainder of the thoracic structures were also considered to be within normal limits (Figure 1a).
FIGURE 1.
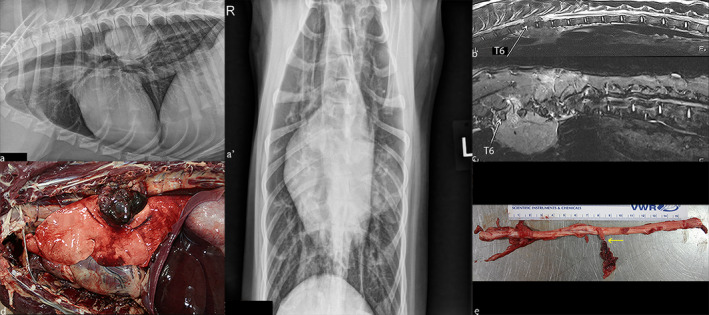
Comparative appearance of the mass on radiographs (a), on MRI (b, c) and on post‐mortem examination (d, e). A, Right lateral (a) and ventrodorsal (a’) projection of the thorax showing a large well‐defined soft tissue mass within the left hemithorax. The mass is located within the dorsal third of the thorax, just above the carina and ventral to the vertebral bodies of T6–T8. There is no appreciable bony involvement. (b) Sagittal T2‐weighted image on midline. A heterogeneous extradural mass, hyperintense compared with the surrounding musculature is seen. The mass extended from the dorsal thoracic cavity towards the vertebral bodies of T6–T8. (c) Sagittal STIR image on the right of midline, there is at the level of T7–T8 invasion of the dorsal paraspinal musculature by some markedly hyperintense tissue extending from the spinal column. This was not appreciated on any of the previous sequences. On the right ventral side of the vertebral bodies of T6–T7, there is a 59 × 17 × 18 mm (length, width, height) mildly hyperintense well‐defined mass compared with the surrounding musculature. (d) Necropsy examination of the left thoracic cavity reveals an 80 × 70 × 40 mm multilobular mass exiting the left sixth and seventh intervertebral space that compresses the left caudal lung lobe. (e) Within the spinal canal, there is a 20 mm in diameter, lobulated, haemorrhagic and necrotic mass involving the left seventh thoracic spinal nerve roots and spinal nerve (arrow) apparently continuous with the thoracic mass outside the foramen (not depicted on the picture above)
Subsequently, MRI was performed with the patient in dorsal recumbency using a 1.5 Tesla magnet and a spine array coil (1.5T Philips Infinion, Philips Healthcare).
The thoracolumbar spine (from T3 to L3) was imaged using the following sequences: T2‐weighted turbo spin echo (TSE) sagittal (TR 4236 ms; TE 114.1 ms; 3 mm contiguous), STIR TSE sagittal (TR 4362 ms; TE 16.3 ms; 3 mm contiguous), Half‐Fourier Acquistion Single‐Shot Turbo Spine Echo pulse sequence (HASTE) sagittal (TR 60,000 ms; TE 613.8 ms; 3 mm contiguous), T2‐weighted TSE transverse from T6 to T10 (TR 4991 ms TE 118.3 ms; 5 mm sp), T1‐weighted SE transverse (TR 828 ms; TE 9.8 ms; 5 mm contiguous), T1‐weighted SE transverse post‐contrast (TR 828 ms; TE 9.8 ms; 5 mm contiguous) and T1‐weighted SE dorsal post‐contrast (TR 926 ms; TE 9.8 ms; 6 mm contiguous) (0.25 ml/kg of gadolinium‐based contrast agent b intravenously (IV) (Prohance, gadoteridol, Bracco).
On T2‐weighted images, there was a large extradural mass measuring 80 × 50 × 50 mm (length, width, height) extending from T6 to T8 (Figure 1b). At this level, it occupied between 66% and 75% of the lumen of the vertebral canal and was lateralized to the left side, thus causing severe spinal cord compression (Figure 2a). The mass was heterogeneously hyperintense compared with the surrounding musculature and extended through the left intervertebral foramina towards the dorsal aspect of the thoracic cavity where it was multilobulated. The left intervertebral foramen at T7–T8 appeared enlarged due to the presence of the expansile mass. The mass also extended along the right ventral side of the vertebral bodies of T6–T7 into the right dorsal thorax.
FIGURE 2.

Transverse T2‐weighted pre‐ (a) and T1‐weighted pre‐contrast (b), T1‐weighted post‐contrast (c) administration at the level of T7, and T1 post‐contrast administration in the dorsal plane (d): The mass is heterogeneously hyperintense on the T2‐weighted pre‐contrast sequences with invasion of the spinal canal, surrounding musculature and dorsal aspect of the thoracic cavity on the right side (a). The mass was isointense to the musculature on the T1 pre‐contrast sequences (b). On the post‐contrast T1 images (c, d), there is heterogeneous contrast enhancement of the mass which is stronger dorsally. On the dorsal plane, the two aspects of the mass can be seen on both sides of the vertebrae
On T1‐weighted images, the mass was similar in shape as previously described but was overall isointense to the surrounding musculature.
At the level of T7–T8 on the STIR sequences, there was invasion of the dorsal paraspinal musculature by some markedly hyperintense tissue (Figure 1c). This was not appreciated on any of the previous sequences and was extending from the previously described mass.
On the HASTE images, there was complete disruption of the hyperintense signal associated with the epidural fat and CSF at the level of T7–T8.
After gadolinium administration, there was heterogeneous contrast enhancement of the mass (Figure 2b,c). The dorsal aspect of the mass, including the area contiguous to the spinal cord and the area invading the paraspinal musculature, was strongly contrast enhancing. The ventral aspect of the mass and the portion of the mass on the contralateral side showed only mild heterogeneous enhancement. The visualized lymph nodes were considered to be within normal limits for size and appearance on MRI.
Based on the MR images, the most likely diagnosis was a neoplastic mass arising from the spinal cord extending into the thoracic cavity and paraspinal musculature. Primary consideration at the time was given to a nerve sheath tumour. Less likely differentials included nephroblastoma or neuroepithelioma.
Due to the extent of the mass, and its complex surgical approach, the owner elected for euthanasia of the dog. A full necropsy was performed in the following hour.
On gross examination, there was a large multilobulated mass in the dorsal aspect of the mid‐thoracic cavity with the following main areas.
In the left thoracic cavity, there was an 80 × 70 × 40 mm (length, width, height) multilobular mass that compressed the left caudal lung lobe (Figure 1d). This mass was tightly adhered to the parietal pleura, covering the ventral aspect of the seventh and eighth thoracic vertebrae and the aortic adventitia. The mass was mottled red to purple, multilobular, haemorrhagic and necrotic. Multifocally, the mass contained firm, tan to creamy‐white, approximately 5–15 mm in diameter nodules and infiltrated the seventh intervertebral space and foramen and the muscle surrounding the transverse processes of the seventh and eighth thoracic vertebrae.
In the right thoracic cavity, there was a 35 × 20 × 20 mm (length, width, height) multilobular mass, which was also tightly adhered to the ventral aspect of the sixth and seventh thoracic vertebrae.
Within the spinal canal, there was a 20 mm in diameter, lobulated, haemorrhagic and necrotic mass continuous with the thoracic mass outside the foramen noted above (Figure 1d). The mass involved the left seventh thoracic spinal nerve roots and spinal nerve abutting and mildly compressing the adjacent spinal cord at T7–T8 (Figure 1e). The mass was in very close contact but did not invade the adventitia of the descending aorta.
Histologically, the neoplastic cells comprising the thoracic masses were round with anisokaryotic, round to oval to carrot‐shaped or indented basophilic hyperchromatic nuclei and scant amounts of amphophilic cytoplasm. The tumour cells were supported by a lightly eosinophilic fibrillar stroma. The cells tended to nest or occasionally formed neuronal‐type rosettes or pseudorosettes. Immunohistochemically, the tumour cells were strongly positive for vimentin and S‐100 and variably positive for NSE and synaptophysin, were negative for CD3, CD79a, MUM1, CD20, CD18 and cytokeratin and did not stain with toluidine blue. Mitotic activity was very high (>9–10 mitotic figures/hpf). In sections of the adjacent spinal cord, there was moderate oedema and multifocal axonal degeneration. Neoplastic cells were identified within mediastinal lymph nodes. The histopathological changes were similar to those described in a case of pPNET in a dromedary (Weiss & Walz, 2009). The final diagnosis based on location, histomorphological and immunohistochemical characteristics was a peripheral PNET such as neuroblastoma or Askin's tumour (peripheral neuroepithelioma of the thoraco‐pulmonary region) with associated mediastinal lymph node metastasis.
Primitive neuroectodermal tumours are rare tumours that arise from primitive cells of the embryonic neural tube. As PNETs exhibit great diversity in both their clinical and histopathologic appearances, this has made the classifying challenging and controversial (Batsakis et al., 1996). Tumours classified as PNET are composed of small, round and undifferentiated cells that have a histologic appearance similar to the germinal or matrix cells of the embryonic neural tube (Kim et al., 2006). They are derived from a germinal neuroepithelial cell that has the potential to differentiate (Meuten, 2008) and their subclassification is based on the tissue of origin: if the tumour arises from either the brain or spinal cord it is considered as being a central primitive neuroectodermal tumour; if the tumour arises from the autonomic nervous system, it is classified as neuroblastoma; and if the tumour arises from any other tissue (i.e. soft tissue, bone), it is classified as pPNET (Sturm et al., 2016). In humans, three major types of pPNET exist: intraosseous (Ewing's sarcoma), extraosseous and the thoraco‐pulmonary Askin's tumour (Facemire et al., 2012; Kim et al., 2006; Rizzo et al., 2008; Weiss & Walz, 2009). pPNETs in human medicine are usually characterized as a solid mass arising from in the abdominal cavity or retroperitoneal space in children and young adults with a slight male predominance (Dehner, 1986; Kim et al., 2006; Mobley et al., 2006). These tumours are associated with a high rate of metastasis and a rather poor outcome (Kim et al., 2006; Mobley et al., 2006).
In veterinary medicine, most cases of PNET reported are comprised of central primitive neuroectodermal tumours arising from the brain parenchyma (Gains et al., 2011; Headley et al., 2009; Mobley et al., 2006; Snyder et al., 2008). Based on a relatively recent review article, primitive neuroectodermal tumours represent 2.8% of all the dogs diagnosed with primary intracranial neoplasia (Snyder et al., 2008). The most common description for central primitive neuroectodermal tumours is an ill‐defined intra‐axial mass hypo to isointense on T1‐weighted sequences, and hyperintense on T2 weighted with heterogeneous contrast enhancement (Gains et al., 2011; Snyder et al., 2008) when compared with normal surrounding musculature.
Peripheral primitive neuroectodermal tumours have been reported uncommonly in the dog, (Abedi et al., 2013; De Cock et al., 2004; Hosokawa et al., 1998; Junginger et al., 2013) twice in bovines (Argenta et al., 2018; Berrocal et al., 2005) with a single case reported in equine,( Facemire et al., 2012) camelid (Weiss & Walz, 2009) and in a formosan serow (Hsieh et al., 2019).
Neuroblastomas also called neuroepitheliomas (Forrest et al., 1997; Kelly, 1975; Matsushima et al., 1998) are more common and have been described as commonly occurring at the level of the thoraco‐lumbar spine of young large breed dogs (Tamke & Foley, 1987). Despite a relatively slow rate of growth of these tumours, most patients are presented for acute onset of hindlimbs paresis or paralysis (Tamke & Foley, 1987).
To the author's knowledge, the only cases classified as a PNET for which an MRI description is available is an olfactory neuroblastoma in a dog (Kitagawa et al., 2006) and a pPNET at the level of the 11th thoracic to 3rd lumbar vertebrae in a dog (Junginger et al., 2013). The central PNET extended from the nasal cavity to the olfactory bulb was isointense on both T1‐ and T2‐weighted images and enhanced after contrast administration when compared with the surrounding musculature. The limited information regarding the pPNET’s MRI appearance was that the tumour was heterogeneous on T2, FLAIR and T1 with slight non‐homogeneous contrast enhancement.
To the authors' knowledge, this is the first MRI description in the veterinary literature of a dog with a pPNET seemingly arising from the spinal canal, which was strongly contrast enhancing, extended through a vertebral foramen into the thoracic cavity, hence mimicking other tumour types.
The MRI characteristics of the described mass were as follows when compared with the surrounding musculature: heterogeneously hyperintense on T2‐weighted sequences, isointense on T1‐weighted sequences and enhancing heterogeneously on the post‐contrast sequences. The mass was characterized further as being multilobulated, extradural, pedunculated, with invasion of the surrounding musculature and extending to and abutting the pulmonary pleura. Unfortunately, the pPNETs’ appearance does not have any specific MRI characteristic which would allow them to be easily differentiated (Mai, 2018). In cases where the mass would match the above description, the list of differentials include extradural tumour such as paraspinal paraganglioma, fibrosarcoma, rhabdomyosarcoma, as well as some of the intradural–extramedullary tumours such as peripheral nerve sheath tumour. A similar lesion localized between T9 and L3 (Mai, 2018) or between T10 and L2 (Withrow, 2007) could also represent a nephroblastoma.
Differential diagnosis of the mass in this case included peripheral nerve sheath tumour, nephroblastoma and neuroepithelioma/neuroblastoma.
Because of the apparent involvement of the nerve roots at T7–T8, a peripheral nerve sheath tumour was considered plausible as these tumours are usually hyperintense to the surrounding musculature on T2‐weighted images (Kraft et al., 2007).
A nephroblastoma, tumour arising from ectopic renal tissue, was another possible differential diagnosis based on the location of the tumour, age of the patient and breed. However, nephroblastomas tend to occur more caudally, usually from T10 to L2, (Fraser McConnell et al., 2003; Meuten, 2008; Sale et al., 2004) are either intramedullary or intradural/extramedullary, (Fraser McConnell et al., 2003) and tend to be solitary (Fraser McConnell et al., 2003) and non‐infiltrative in nature. Furthermore, on MRI they have been described, when compared with the musculature and/or normal spinal cord, as isointense on T1‐weighted sequences (Fraser McConnell et al., 2003; Sale et al., 2004) heterogeneous in intensity on T2‐weighted sequences (Fraser McConnell et al., 2003; Sale et al., 2004) and they may contain small haemorrhagic cysts (Fraser McConnell et al., 2003). None of those cases had an extension of the masses into the abdominal or thoracic cavity (Fraser McConnell et al., 2003; Sale et al., 2004).
In this case, euthanasia of the dog was elected by the owner due to the extent of the mass and its complex surgical implications. In human medicine, treatment includes surgical removal/debulking of the mass and radiotherapy/chemotherapy, which results in an average of 5 years progression‐free survival for 50%–66% of the patients with localized pPNET (Mobley et al., 2006).
In conclusion, a diagnosis of pPNET should be considered in young patients exhibiting signs of hindlimbs neurological dysfunction in which an extrapleural thoracic mass is seen. The extradural spinal mass in our patient extended locally into the thoracic cavity via the intervertebral foramina resulting in one large contiguous mass and a smaller distinct one on the other side. On MRI, the tumour was characterized as an extradural lesion occupying the majority of the vertebral canal, heterogeneously hyperintensity on T2 and STIR and isointensity on T1 with heterogeneous contrast‐enhancement post‐gadolinium administration.
CONFLICT OF INTEREST
N/A.
AUTHOR CONTRIBUTION
Marie de Swarte: Investigation; Writing‐original draft; Writing‐review & editing. Kimberly Anderson: Conceptualization; Validation; Writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.449.
ACKNOWLEDGEMENT
The authors would like to acknowledge posthumously Dr. Richard Weiss for his contribution to this case.
Hespel A‐M, de Swarte M, Anderson K, Weiss R, Hathcock J. Features of a rare peripheral primitive neuroectodermal tumour arising from the thoracic spine in a juvenile canine patient. Vet Med Sci. 2021;7:680–685. 10.1002/vms3.449
†Deceased.
REFERENCES
- Abedi, G. , Hesaraki, S. , & Yadegar, O. (2013). Mandibular primitive neuroectodermal tumor in an adult dog. Iranian Journal of Veterinary Research, 14, 261–263. [Google Scholar]
- Argenta, F. F. , Hammerschmitt, M. E. , de Oliveira Reis, M. , da Cruz, R. A. S. , De Lorenzo, C. , Sonne, L. , Driemeier, D. , & Pavarini, S. P. (2018). Nasal peripheral primitive neuroectodermal tumor in a heifer: Case report. Semina: Ciências Agrárias, 39(4), 1843–1848. 10.5433/1679-0359.2018v39n4p1843. [DOI] [Google Scholar]
- Batsakis, J. G. , MacKay, B. , & El‐Naggar, A. K. (1996). Ewing's sarcoma and peripheral primitive neuroectodermal tumor: An interim report. Annals of Otology, Rhinology & Laryngology, 105, 838–843. 10.1177/000348949610501014. [DOI] [PubMed] [Google Scholar]
- Berrocal, A. , Montgomery, D. , Mackie, J. , & Storts, R. W. (2005). Primitive neuroectodermal tumor in the spinal cord of a Brahman crossbred calf. Veterinary Pathology, 42, 834–836. 10.1354/vp.42-6-834. [DOI] [PubMed] [Google Scholar]
- De Cock, H. , Busch, M. , Fry, M. M. , Mehl, M. , Bollen, A. W. , & Higgins, R. J. (2004). A peripheral primitive neuroectodermal tumor with generalized bone metastases in a puppy. Veterinary Pathology, 41, 437–441. 10.1354/vp.41-4-437. [DOI] [PubMed] [Google Scholar]
- Dehner, L. P. (1986). Peripheral and central primitive neuroectodermal tumors. A nosologic concept seeking a consensus. Archives of Pathology & Laboratory Medicine, 110, 997. [PubMed] [Google Scholar]
- Facemire, P. R. , Facemire, L. M. , & Honnold, S. P. (2012). Peripheral primitive neuroectodermal tumor in a two‐year‐old paint horse. Journal of Veterinary Diagnostic Investigation, 24, 794–796. 10.1177/1040638712446505. [DOI] [PubMed] [Google Scholar]
- Forrest, L. J. , Galbreath, E. J. , Dubielzig, R. R. , & Macewen, E. G. (1997). Peripheral neuroblastoma in a dog. Veterinary Radiology & Ultrasound, 38, 457–460. 10.1111/j.1740-8261.1997.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Fraser McConnell, J. , Garosi, L. S. , Dennis, R. , & Smith, K. C. (2003). Imaging of a spinal nephroblastoma in a dog. Veterinary Radiology & Ultrasound, 44, 537–541. 10.1111/j.1740-8261.2003.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Gains, M. J. , Leclerc, M.‐K. , & Bédard, C. (2011). A primitive neuroectodermal tumor with extension into the cranial vault in a dog. The Canadian Veterinary Journal, 52, 1232. [PMC free article] [PubMed] [Google Scholar]
- Headley, S. , Koljonen, M. , Gomes, L. , & Sukura, A. (2009). Central primitive neuroectodermal tumour with ependymal differentiation in a dog. Journal of Comparative Pathology, 140, 80–83. 10.1016/j.jcpa.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Hosokawa, S. , Suzuki, S. , Hibino, N. , Fukuta, T. , Imai, T. , Hayakawa, K. , Nakanowatari, J. , & Sagami, F. (1998). Peripheral primitive neuroectodermal tumor (peripheral neuroepithelioma) in a dog. Journal of the American Association for Laboratory Animal Science, 37, 66–69. [PubMed] [Google Scholar]
- Hsieh, Y.‐H. , Hsu, Y.‐H. , Lien, C.‐Y. , Liu, C.‐H. , & Li, W.‐T. (2019). Retroperitoneal extraosseous peripheral primitive neuroectodermal tumor in a Formosan serow: Case report and literature review. Journal of Veterinary Diagnostic Investigation, 31, 883–888. 10.1177/1040638719879198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junginger, J. , Röthlisberger, A. , Lehmbecker, A. , Stein, V. M. , Ludwig, D. C. , Baumgärtner, W. , & Seehusen, F. (2013). Peripheral primitive neuroectodermal tumour in a dog. Journal of Comparative Pathology, 149, 424–428. [DOI] [PubMed] [Google Scholar]
- Kelly, D. (1975). Neuroblastoma in the dog. The Journal of Pathology, 116, 209–212. 10.1002/path.1711160404. [DOI] [PubMed] [Google Scholar]
- Kim, M. S. , Kim, B. , Park, C. S. , Song, S. Y. , Lee, E. J. , Park, N. H. , Kim, H.‐S. , Kim, S. H. , & Cho, K. S. (2006). Radiologic findings of peripheral primitive neuroectodermal tumor arising in the retroperitoneum. American Journal of Roentgenology, 186, 1125–1132. 10.2214/AJR.04.1688. [DOI] [PubMed] [Google Scholar]
- Kitagawa, M. , Okada, M. , Yamamura, H. , Kanayama, K. , & Sakai, T. (2006). Diagnosis of olfactory neuroblastoma in a dog by magnetic resonance imaging. Veterinary Record, 159, 288–289. 10.1136/vr.159.9.288. [DOI] [PubMed] [Google Scholar]
- Koestner, A. , & Higgins, R. (2002). Tumors of the nervous system. Tumors in Domestic Animals, 697–738. [Google Scholar]
- Kraft, S. , Ehrhart, E. , Gall, D. , Klopp, L. , Gavin, P. , Tucker, R. , Bagley, R. , Kippenes, H. , Dehaan, C. , Pedroia, V. , Partington, B. , & Olby, N. (2007). Magnetic resonance imaging characteristics of peripheral nerve sheath tumors of the canine brachial plexus in 18 dogs. Veterinary Radiology & Ultrasound, 48, 1–7. 10.1111/j.1740-8261.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- Mai, W. (2018). Magnetic resonance imaging and computed tomography features of canine and feline spinal cord disease. Textbook of veterinary diagnostic radiology (pp. 271–304). Elsevier. [Google Scholar]
- Matsushima, S. , Maruyama, T. , & Tor, M. (1998). Peripheral neuroblastoma in a young beagle dog. Toxicologic Pathology, 26, 806–809. 10.1177/019262339802600614. [DOI] [PubMed] [Google Scholar]
- Maxie, M. , & Youssef, S. (2007). Neoplastic diseases of the nervous system. In Jubb K. V. F., Kennedy P. C. & Palmer N. (Eds.), Pathology of domestic animals (pp. 281–287). Elsevier. [Google Scholar]
- Meuten, D. J. (2008). Tumors in domestic animals. Wiley‐Blackwell. [Google Scholar]
- Mobley, B. C. , Roulston, D. , Shah, G. V. , Bijwaard, K. E. , & McKeever, P. E. (2006). Peripheral primitive neuroectodermal tumor/Ewing's sarcoma of the craniospinal vault: Case reports and review. Human Pathology, 37, 845–853. 10.1016/j.humpath.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Rizzo, S. A. , Newman, S. J. , Hecht, S. , & Thomas, W. B. (2008). Malignant mediastinal extra‐adrenal paraganglioma with spinal cord invasion in a dog. Journal of Veterinary Diagnostic Investigation, 20, 372–375. 10.1177/104063870802000322. [DOI] [PubMed] [Google Scholar]
- Sale, C. , Skerritt, G. , & Smith, K. (2004). Spinal nephroblastoma in a crossbreed dog. Journal of Small Animal Practice, 45, 267–271. 10.1111/j.1748-5827.2004.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Snyder, J. M. , Shofer, F. S. , Winkle, T. J. , Massicotte, C. (2008). Canine intracranial primary neoplasia: 173 cases (1986–2003). Journal of Veterinary Internal Medicine, 20, 669–675. [DOI] [PubMed] [Google Scholar]
- Sturm, D. , Orr, B. A. , Toprak, U. H. , Hovestadt, V. , Jones, D. T. W. , Capper, D. , Sill, M. , Buchhalter, I. , Northcott, P. A. , Leis, I. , Ryzhova, M. , Koelsche, C. , Pfaff, E. , Allen, S. J. , Balasubramanian, G. , Worst, B. C. , Pajtler, K. W. , Brabetz, S. , Johann, P. D. , … Kool, M. (2016). New brain tumor entities emerge from molecular classification of CNS‐PNETs. Cell, 164, 1060–1072. 10.1016/j.cell.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamke, P. G. , & Foley, G. L. (1987). Neuroepithelioma in a dog. The Canadian Veterinary Journal, 28, 606. [PMC free article] [PubMed] [Google Scholar]
- Weiss, R. , & Walz, P. (2009). Peripheral primitive neuroectodermal tumour in a lumbar vertebra and the liver of a dromedary camel (Camelus dromedarius). Journal of Comparative Pathology, 141, 182. 10.1016/j.jcpa.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Withrow, S. J. (2007). Withrow and MacEwen's small animal clinical oncology. Elsevier Health Sciences. [Google Scholar]


