Abstract
Background
Multiple drug resistance (MDR) of cancer cells is the main cause of intrinsic or acquired desensitization to chemotherapy in many cancers. A number of studies have found high expression of COX‐2 to be a factor for expression of MDR gene in several cancer. Furthermore, adipose tissue derived mesenchymal stem/stromal cells (ADSC) have been found to increase cyclo‐oxygenase‐2 (COX‐2) expression in some tumour cells. The mechanism for this, however, is not yet clear and needs further study.
Objective
The purpose of this study was to determine whether tumour necrosis factor‐alpha stimulated gene/protein 6 (TSG‐6) secreted from ADSCs is associated with an increase in MDR genes by inducing COX‐2 gene expression in melanoma and osteosarcoma cell lines.
Methods
ADSCs were transfected with TSG‐6 siRNA or Control RNA respected, and cancer cell line were transfected with COX‐2 siRNA or Control RNA respected. Using trans well coculture system, the interactions of ADSCs with tumour cells were investigated.
Results
Increased COX‐2 expression was observed in cancer cell co‐cultured with ADSCs. Additionally, we identified that COX‐2 expression was related to drug resistance genes (P‐glycoprotein, multidrug resistance‐associated protein). Transfecting canine ADSCs with small interfering RNA, TSG‐6 secreted from ADSCs was found to be a major factor in the regulation of COX‐2 expression and drug resistance genes in osteosarcoma and melanoma cell lines.
Conclusion
TSG‐6 mediated COX‐2 up‐regulation is a possible mechanism of chemoresistance development induced by ADSCs. These findings provide better understanding about the mechanism associated with ADSC‐induced chemoresistance in cancer.
Keywords: adipose tissue‐derived stem cell, canine, cyclooxygenase‐2, drug resistance gene, melanoma, osteosarcoma, tumour necrosis factor‐alpha stimulated gene/protein 6
TSG‐6 mediated COX‐2 up‐regulation could be a possible mechanism underlying the development of chemoresistance induced by mesenchymal stem cells.

1. INTRODUCTION
Drug resistance can occur within tumour cells, either due to genetic changes that increase drug outflow (resistance), or as a result (exogenous) of the tumour microenvironment that protects tumour cells from treatment (Meads et al., (2008)). Although cancer cells develop various mechanisms of resistance to chemotherapy agents, the first players implicated in drug resistance are drug transporters from ABC family (Fantappiè et al., 2002). Some members of this family are primary active transporters, which significantly regulate the absorption, metabolism, cellular effects and toxicity of pharmacological agents (Glavinas et al., 2004). P‐glycoprotein (P‐gp) or multidrug resistance‐associated protein (MRP) is the best‐known transporters and several studies showed that overexpression of these genes in cancer cells is associated with poor response to chemotherapy (McKenna and Padua, (1997); Ling, 1997). Therefore, various studies have been conducted not only in veterinary medicine but also in human medicine to elucidate the roles of P‐gp and MRP1 in various tumour cells. Overexpression of P‐gp in cancer was an intrinsic or acquired process. P‐gp confers resistance by preventing sufficient accumulation of anticancer drugs in cells, thereby preventing cytotoxic or apoptotic effects (Callaghan et al., 2014). MRP1 is closely related to drug resistance in many types of cancer. Most of the drugs carried out of the cell by MRP1 are bulky, hydrophobic molecules, and enter the cell by simple diffusion across the lipid bilayer of the extracellular membrane. High expression of MRP1 is known as a negative prognostic marker for early breast cancer, and previous studies show a strong association between MRP1 expression level and survival time (Munoz et al., 2007).
Cyclooxygenase‐2 (COX‐2) is an enzyme that promotes the formation of substances that cause inflammation and pain (Ghosh et al., 2010). It also affects the growth of tumour cells (Pires et al., 2010). In addition, up‐regulation and overexpression of COX‐2 in tumour cells was shown to increase invasiveness of malignant cells, angiogenesis and to reduced apoptosis (Kang et al., 2011). Interestingly, Sui et al (Sui et al., 2011) reported that high COX‐2 expression affects the drug resistance genes. Consistent with the implications of these findings, several studies showed an association between COX‐2 related drug resistance and worse survival in patients with cancers (Januchowski et al., 2016; Kang et al., 2011; Sui et al., 2011; Uchida et al., 2005).
It is increasingly clear that the tumour microenvironment plays an important role in the development of drug resistance (Meads et al., 2009; Roodhart et al., 2011). The fact that certain properties of stromal cells in the tumour microenvironment often have a poor prognosis further demonstrates the important role of the microenvironment in therapeutic response (Meads et al., 2009; Roodhart et al., 2011). Consistent with the implications of these findings, increasing evidence has suggested mesenchymal stem/stromal cells (MSCs) to play an important role in controlling tumour progression and drug sensitivity, along with drug resistance in tumour microenvironment (Houthuijzen et al., 2012). In addition, several studies showed that application of MSCs to human ovarian cancer cell line (Wen et al., 2017), breast cancer cell line (Chen et al., 2014, 2014; Lu et al., 2017; Yeh et al., 2017), and osteosarcoma cell line (Tu et al., 2016) has confirmed the induction of drug resistance genes. Although the precise mechanisms are far from being understood completely, some studies suggest that MSC may help to increase COX‐2 genes expression in tumour cells (Wang et al., 2010). However, further research is needed to determine whether MSC‐induced COX‐2 expression in cancer causes resistance genes. In addition, it should be investigated which factors of MSCs are associated with the increase of COX‐2 in tumour cells.
Tumour necrosis factor alpha‐stimulated gene/protein 6 (TSG‐6) is activated in a variety of cells during inflammation (Milner & Day, 2003). It has tissue‐protective properties, mediating many of the beneficial activities of MSCs (Day and Milner, (2019)). Although the underlying mechanism of TSG‐6 secreted from stem cells is not clear, it is one of the biologically active molecules that regulate the local immune response and repair damaged tissues (Caplan & Correa, 2011; Kusuma et al., 2017; Li et al., 2017). A previous study with macrophage cell line had revealed that TSG‐6 protein up‐regulates the expression of COX‐2 enzyme (Mindrescu et al., 2005). Based on these findings, we hypothesized that TSG‐6 secreted from MSCs might also increase the expression of COX‐2 in cancer cells.
In this study, we investigated the effect of TSG‐6, from canine adipose‐derived stem/stromal cells (cADSC), on the expression of COX‐2‐related drug‐resistance genes (P‐gp, MRP1) in canine melanoma and osteosarcoma cell lines.
2. MATERIALS AND METHODS
2.1. Harvest and isolation of cADSCs
cADSCs were isolated from subcutaneous fat of three healthy dog donors; written informed consent was obtained from the dog owners. Obtained fat samples were finely minced and digested with 0.1% collagenase type IA solution (Gibco/Life Technologies). After digestion, cells were neutralized with Dulbecco's modified eagle's medium (DMEM; PAN‐Biotech, Aidenbach) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin‐streptomycin, and centrifuged. Cell pellet was suspended in DMEM and filtered using 70‐μm Falcon cell strainer (Fisher Scientific). Red blood cell lysis buffer (Sigma‐Aldrich) was used to remove erythrocytes. Cells were washed finally with Dulbecco's phosphate‐buffered saline (DPBS) and centrifuged. Cell pellets were re‐suspended in DMEM and seeded on cell culture plates. cADSC used in this experiment were passaged less than five passages.
2.2. Cell lines
Canine malignant melanoma cell line LMeC (RRID: CVCL_L361) was provided by Professor Nobuo Sasaki, University of Tokyo. (Inoue et al., (2004)) Canine osteosarcoma cell line D17 [American Type Culture Collection (ATCC) CCL‐183] was obtained from the Laboratory of Veterinary Pharmacology, Seoul National University.
2.3. Cell culture
D17 and cADSC cells were cultured in DMEM (PAN‐Biotech) and LMeC cells were cultured in Roswell Park Memorial Institute medium (RPMI; PAN‐Biotech). Both the culture media were supplemented with 10% FBS and 1% penicillin‐streptomycin. Cells were incubated in humidified atmosphere containing 5% CO2, and the temperature was maintained at 37°C. Sub‐culture was performed when 70%–80% confluence was achieved. The culture medium was changed every 2–3 days.
2.4. Characterization of canine adipose tissue‐derived mesenchymal stem cells
We identified cADSC surface markers using flow cytometry. 1 × 106 cADSCs were suspended in DPBS (PAN‐Biotech) and stained with monoclonal antibodies against CD29‐FITC (1:200, BD Biosciences), CD44‐FITC (1:200, Invitrogen), CD45‐FITC (1:200, BD Biosciences), CD34‐PE (1:200, BD Biosciences), CD90‐PE (1:200, Invitrogen) and CD73‐PE (1:200, BD Biosciences). Then, these cells were incubated on ice for 15–20 min in the dark. Unstained cells were used as negative controls. Cells were sorted using FACS AriaⅡ system (BD Biosciences) and analysed using FlowJo 7.6.5 software (Tree Star, Inc.). To confirm their trilineage differentiation capacity, cells were differentiated into adipocytes, chondrocytes, and osteocytes using differentiation medium (Gibco). The medium was changed every 2–3 days for 2 weeks, as per the manufacturer's protocol, and cells were stained with Oil red O, Alizarin Red and Alcian Blue, respectively. These results of characterization are described in supplementary Figure 1.
FIGURE 1.
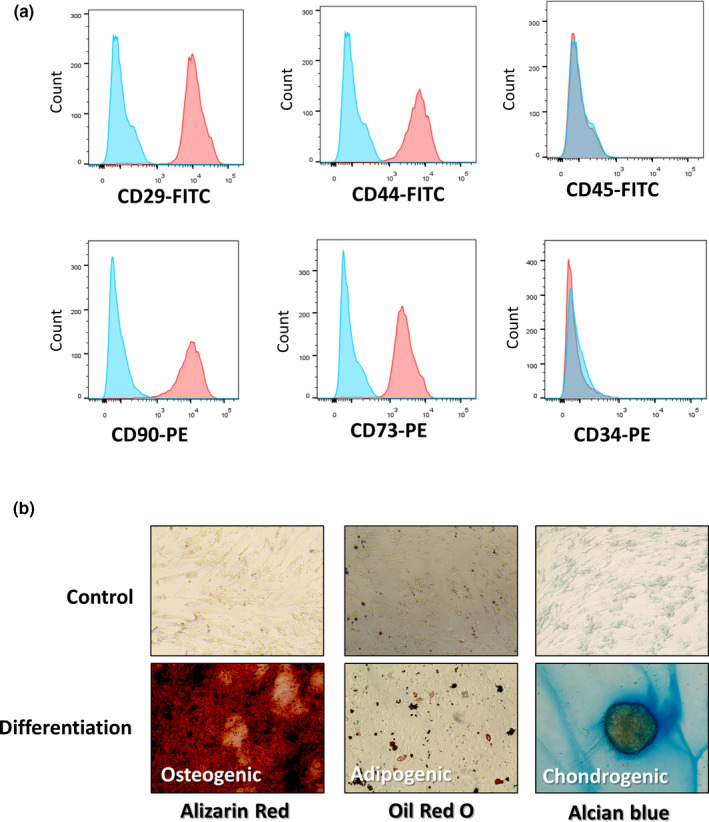
Canine adipose‐derived stem cells (cADSCs) exhibited characteristics of mesenchymal stem cells. (a) Immune phenotypic analysis of cADSC by flow cytometry. CD29, CD44, CD90 and CD73 were highly expressed and CD 45 and CD34 were low. (Red: stained marker, blue: negative (unstained) control) (b) Osteogenic (Alizarin Red S staining), adipogenic (Oil Red O staining), chondrogenic (Alcian Blue staining) differentiation of cADSCs
2.5. Co‐culture experiments
D17 and LMeC cells were seeded in multi‐well plates and cADSC cells were seeded on cell culture inserts with pore size of 0.4 μm (SPL Life Sciences, Pocheon, Gyeonggi‐do, Korea). After 24 hr of pre‐incubation, inserts were transferred. All experiments were performed after 48 hr of co‐culture incubation.
2.6. Transfection with siRNA
All siRNAs were purchased from Santa Cruz Biotechnology. D17 and the LMeC cell lines were transfected with siCOX‐2 (sc‐29279), while cADSCs were transfected with siTSG‐6 (sc‐39819). The control group was transfected with control siRNA (sc‐37007). Lipofectamine 2000 (Invitrogen)‐siRNA complexes were prepared according to the manufacturer's instructions, and cells were transfected for 48 hr.
2.7. RNA extraction, cDNA synthesis and quantitative real time PCR (qRT‐PCR)
Total RNA for qRT‐PCR analysis was extracted using the easy‐BLUE total RNA extraction kit (iNtRON Biotechnology). cDNA was synthesized from the extracted RNA using the CellScript cDNA Master Mix (CellSafe). Samples were analysed in duplicate using 10 μl of AMPIGENE qPCR Green Mix Hi‐ROX with SYBR Green dye (Enzo Life Sciences), 400 nM each of forward and reverse primers (Table 1; BIONICS) and 1 μl of cDNA. Glyceraldehyde 3‐ phosphate dehydrogenase (GAPDH) was used to normalize the expression of target genes.
TABLE 1.
Sequences of PCR primers used in this study
| Target gene | Primer | Sequence | References |
|---|---|---|---|
| Canine | Forward | TCC GTC TTA ATA GGA GTG AAA GAT G | Song et al. (2018) |
| TSG−6 | Reverse | AGA TTT AAA AAT TCG CTT TGG ATC T | |
| Canine | Forward | GCC TTA CCC AGT TTG TGG AA | Rai et al. (2008) |
| COX−2 | Reverse | AGC CTA AAG CGT TTG CGA TA | |
| Canine | Forward | CTA TGC CAA AGC CAA AGT ATC | Zandvliet et al. (2015) |
| P‐gp | Reverse | GAG GGC TGT AGC TGT CAA TC | |
| Canine MRP1 | Forward | CGT GAC CGT CGA CAA GAA CA | Zandvliet et al., 2014) |
| Reverse | CAC GAT GCT GAT GCT GAC CA | ||
| Canine | Forward | TTA ACT CTG GCA AAG TGG ATA TTG T | Ju‐Hyun et al., 2020) |
| GAPDH | Reverse | GAA TCA TAC TGG AAC ATG TAC ACC A |
2.8. Western blot analysis
Total protein was extracted from the cultured cells using the PRO‐PREP protein extraction solution (iNtRon Biotechnology), and quantified using DC protein assay kit (Bio‐Rad). After separating 20 μg of protein by SDS‐PAGE, they were transferred on to polyvinylidene difluoride membranes (Millipore). The membranes were then blocked with 5% non‐fat dry milk and incubated with primary antibodies against COX‐2 (1:500; Santa Cruz Biotechnology) and β‐actin (1:1,000, Santa Cruz Biotechnology) at 4°C overnight. Next day, the membranes were incubated with secondary antibody for 1 hr at 20°C and then visualized by chemiluminescence (Advansta, Menlo Park).
2.9. Statistical analysis
Statistical significance was determined by one‐way analysis of variance using GraphPad Prism software (v.6.01; GraphPad Software Inc.). All data are expressed as the mean ± SD. A p value of <.05 was considered as statistically significant.
3. RESULTS
3.1. cADSCs exhibited characteristics of mesenchymal stem cells
After three days of seeding, cADSCs showed fibroblastic morphology (spindle‐shaped) and adhered to the culture plate. Stem cell surface markers were identified using flow cytometry, these cells expressed CD29, CD73, CD90 and CD44 and showed low expression of cell markers CD45 and CD34 (Figure 1a). cADSCs cultured in differentiation medium had the capacity to differentiate into adipocytes, osteocytes, and chondrocytes. In the group that did not use the differentiation medium, it was confirmed that differentiation did not occur (Figure 1b).
3.2. cADSCs increased COX‐2 expression in cancer cell lines
Using trans‐well coculture system, as the percentage of cADSCs increased, the degree of COX‐2 expression in tumour cells was confirmed. The total number of tumour cells and cADSCs was cultured at 1 x 106 cells/well, as a result of co‐culturing D17 and LMeC with cADSC at a ratio of 1:4 and 1:1, COX‐2 expression was increased in both cancer cells as the proportion of cADSCs increased (Figure 2a,b).
FIGURE 2.
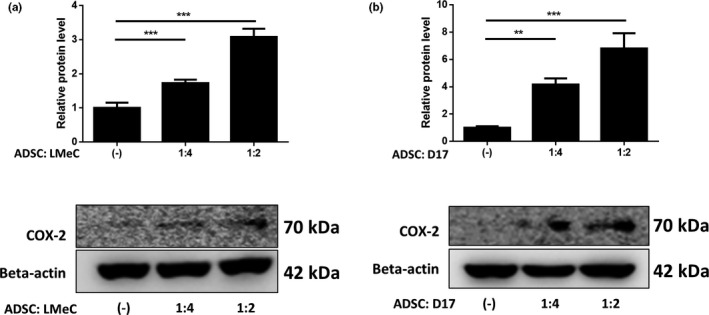
cADSCs increased COX‐2 expression in cancer cell lines. Co‐culture with cADSCs increased COX‐2 protein levels of (a) canine melanoma (LMeC) and (b) osteosarcoma (D17) cell lines. The COX‐2 protein level increased as the proportion of cADSC increased. Experiments were performed in triplicate and repeated three times with similar results. Results are shown as mean ± SD (*p < .05, **p < .01, ***p < .001 by one‐way ANOVA analysis)
3.3. Drug resistance gene expression in cancer cell lines increased in presence of cADSCs, and decreased with interference of COX‐2
Since COX‐2 is associated with multidrug resistance, we assessed the effect of COX‐2 expression on drug resistance genes. When co‐cultured with cADSCs, MRP1 and P‐gp expression was significantly higher in both the cancer cell lines than when the cancer cells were cultured alone. Next, COX‐2 expression in D17 and LMeC was knocked down by transfection of siRNAs. Cancer cell lines transfected with siRNAs retained their cell viability (see Additional file 1). Stem cells and tumour cells were cultured at a ratio of 1:4. While COX‐2 expression was down‐regulated in cancer cell lines transfected with COX‐2 targeted siRNA (siCOX2), no significant change was observed in those transfected with the control siRNA, compared to that in naïve cancer cell lines (see Additional file 1). When cancer cell lines were transfected with siCOX2, we found that COX‐2 expression did not increase even on co‐culturing with cADSCs at the protein level (Figure 3a,b) and gene level (Figure 3c,d). Furthermore, we found the expression of MRP1 and P‐gp genes in both cancer cell lines, transfected with siCOX2, to be significantly lower than that in cells transfected with control siRNA and in naïve cancer cell lines (Figure 3c,d).
FIGURE 3.
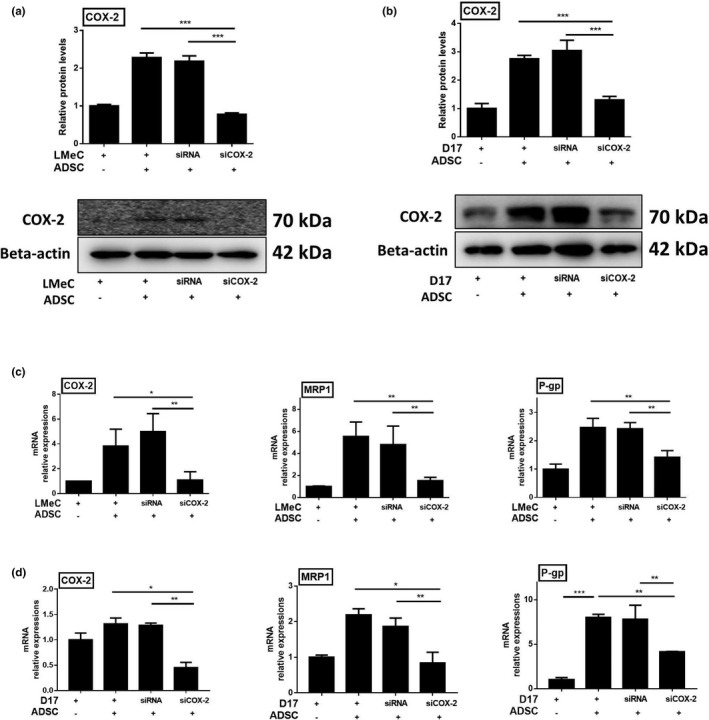
Drug resistance gene expression in cancer cell lines increased in the presence of cADSCs, and decreased with interference of COX‐2. COX‐2 protein levels in LMeC (a) and D17 (b) cancer cell lines. mRNA expression levels of COX‐2, MRP1, P‐gp in LMeC (c) and D17 (d) cancer cell lines. COX‐2 and drug resistance gene MRP1, P‐gp expression of cancer cell lines increased significantly with the presence of cADSCs . When COX‐2 was down regulated with COX‐2 targeted siRNA (siCOX‐2) in cancer cell lines, MRP1 and P‐gp expression in LMeC, D17 cells was decreased even when cultured with cADSCs. Experiments were performed in triplicate and repeated three times with similar results. Results are shown as mean ± standard deviation (*p < .05, **p < .01, ***p < .001 by one‐way ANOVA analysis)
3.4. 3.4.TSG‐6 secreted from cADSCs elevated the expression of COX‐2 in cancer cell lines
In an earlier study, TSG‐6 was shown to up‐regulate COX‐2 synthesis in macrophage cell lines. Therefore, we investigated whether TSG‐6 secreted by cADSCs can influence the expression of COX‐2 in cancer cell lines. We used TSG‐6 targeted siRNA (siTSG‐6) to check if TSG‐6 mediates COX‐2 expression in cancer cell lines. Cell viability of cADSCs transfected with siRNAs remained unchanged, and TSG‐6 expression of cADSCs transfected with siTSG‐6 was significantly lower compared to that of cADSCs transfected with control siRNA and of naïve cADSCs (see Additional file 2). When siTSG‐6‐transfected cADSCs were co‐cultured with D17 and LMeC cell lines, COX‐2 mRNA expression of both the cancer cell lines was significantly lower than that in the cADSCs transfected with control siRNA and in naïve cADSCs (Figure 4a,b). Furthermore, the expression of the COX‐2 protein was lower in the D17 and LMeC cell lines co‐cultured with siTSG‐6‐transfected cADSCs than that in the cells co‐cultured with control siRNA‐transfected and naïve cADSCs (Figure 4c,d).
FIGURE 4.
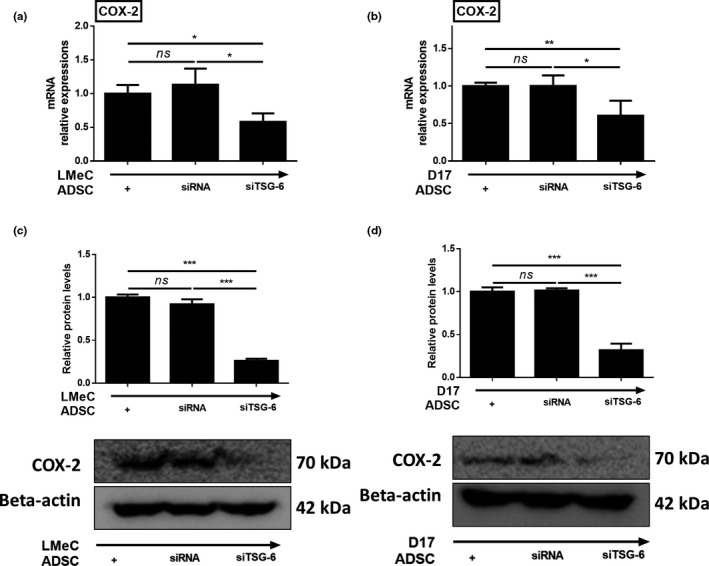
TSG‐6 secreted from cADSC elevated the expression of COX‐2 in cancer cell lines. mRNA expression levels of COX‐2 in LMeC (a), and D17 (b) cell lines. Protein levels of COX‐2 in LMeC (c) and D17 (d) cell lines. COX‐2 mRNA and protein expression in cancer cell lines decreased significantly when they were co‐cultured with TSG‐6 targeted siRNA (siTSG‐6) transfected cADSCs. Experiments were performed in triplicate and repeated three times with similar results. Results are shown as mean ± SD (*p < .05, **p < .01, ***p < .001 by one‐way ANOVA analysis)
3.5. 3.5.TSG‐6 affected drug resistance genes in cancer cell lines
Having established that TSG‐6 secreted by cADSCs induces COX‐2 expression in D17 and LMeC cell lines, we next investigated whether the secreted TSG‐6 affects the expression of drug resistance genes in both cancer cell lines. After transfection with siRNAs, cADSCs were co‐cultured with D17 and LMeC cell lines. In both cancer cell lines co‐cultured with siTSG‐6‐transfected cADSCs, the expression of MRP1 and P‐gp was significantly lower than that in cells co‐cultured with control siRNA‐transfected cADSCs and naïve cADSCs (Figure 5a,b).
FIGURE 5.
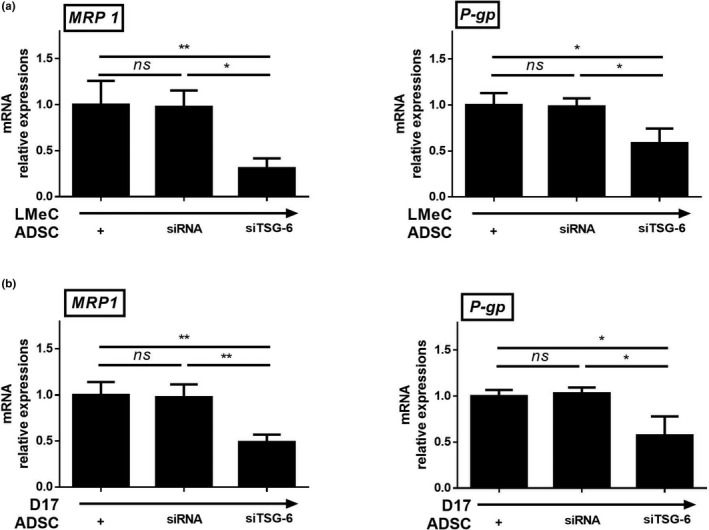
TSG‐6 secreted from cADSCs affected drug resistance genes in cancer cell lines. mRNA expression levels of MRP1 and P‐gp in LMeC (a) and D17 (b) cell lines. Experiments were performed in triplicate and repeated three times with similar results. Results are shown as mean ± standard deviation (*p < .05, **p < .01, ***p < .001 by one‐way ANOVA analysis)
4. DISCUSSION
Our study reveals an important mechanism of drug resistance gene regulation medicated by MSCs. We found a significant association between TSG‐6 secreted from MSC and COX‐2 expression in canine melanoma and osteosarcoma cell lines. In addition, we showed that these association was related with multiple drug resistance expressed in tumours.
COX‐2 is known to have pro‐oncogenic effects, including angiogenesis and tissue invasion (Tsujii et al., 1997, 1998), and its expression is known to be increased in many cancer cells. Activation of the COX‐2 pathway can up‐regulate the expression of ABC transporters (P‐gp and MRP1), which encode efflux pumps and contribute to the development of multidrug resistance (Liu et al., 2010). Since the expression of multi‐drug resistance gene has been associated with poor prognosis of anti‐cancer treatment, COX‐2 inhibitors are commonly used to improve therapeutic outcome of patients with cancer (Ghosh et al., 2010). In addition, the expression of COX‐2 in melanoma (Pires et al., (2010); Martínez et al., 2011) and osteosarcoma (Millanta et al., 2012) has been shown not only to increase tumour resistance genes but also to affect anticancer drug responses. However, there has been no study yet revealing the increase of COX‐2 expression due to the influence of mesenchymal stem cells.
In Figure 3, it was confirmed that a double band of COX‐2 protein was generated by Western blot analysis. Previous study demonstrated that unpurified COX‐2 proteins derived from cell lysates or tissues typically produced a double or triple band in western blot (Maihöfner et al., 2001). Nevertheless, we found that the expression of COX‐2 protein in the two canine cancer cell lines significantly increased in presence of cADSCs. Additionally, we demonstrated that the expression of drug resistance gene (such as P‐gp and MRP) is closely related to the expression of COX‐2. However, it is still unclear which factors in stem cells increase COX‐2‐related genes in tumour cells, and further studies are needed to investigate the relationship between tumour cells and tumour surrounding stromal cells.
Previous studies showed that TSG‐6 is key secretory factor regulating stemness and biological properties of MSCs (Romano et al., (2019)). In addition, Mindrescu et al. had earlier shown that COX‐2 expression in macrophage cell line increased after TSG‐6 protein treatment (Mindrescu et al., 2005). Based on these results, we hypothesized that COX‐2 expression was induced in cancer cell lines from TSG‐6 secreted by cADSC. We used TSG‐6‐targeted siRNAs to check the effect of TSG‐6 on COX‐2 expression of cancer cell lines (Song et al., 2017). When two canine cancer cell lines were co‐cultured with TSG‐6 knock‐down cADSCs, the expression of COX‐2 did not increase, thereby indicating that TSG‐6 is one of the soluble factors of cADSCs that up‐regulate COX‐2 expression in canine melanoma and osteosarcoma cell lines. Moreover P‐gp and MRP1 genes of cancer cell lines were also down‐regulated when they were co‐cultured with TSG‐6 knock‐down cADSCs.
Although TSG‐6 might not be the only factor regulating COX‐2 expression in cancer cell lines, our findings suggested it to play a key role in the development of MSC‐induced chemoresistance. Increasing number of studies have reported about the recruitment of MSCs to the tumour microenvironment (Guan & Chen, 2013), and they are regarded as primary contributors to environment‐mediated drug resistance (Meads et al., 2009). As MSCs might influence the development of canine chemoresistance, further investigation about the safety of mesenchymal stem cell therapy in patients with cancer is needed. Also, to take advantage of stem cell therapy to apply to cancer patients, TSG‐6 might serve as a target to prevent the development of chemoresistance.
Human and canine oral melanoma share a variety of morphological, cytogenetic, molecular and signalling pathway similarities. In fact, dogs share the same environment as humans, so they are exposed to the same carcinogens that can be involved in tumour formation. Therefore, clinical studies of dogs with naturally occurring melanoma to develop new, less toxic and effective treatments are expected to be key data for humans as well as veterinary medicine (Segaoula et al., 2018). Canine and human osteosarcoma are biologically and genetically similar, making canine a valuable representative model. Similarities include pronounced male prevalence, large body size, location of the primary tumour, unknown causes of the disease process and almost always fatal lung metastases. The skeleton is most often affected by both dogs and humans. Genome analysis confirms broad similarities in the mechanisms of progression and metastasis between species (Wilson‐Robles et al., 2019). Therefore, Although this study was conducted in a canine tumour cell line, this study is considered to be important basic data in future human tumour studies.
P‐gp plays an important role in intestinal transport and outflow, altering the bioavailability and pharmaceutical effects of orally administered drugs. Recently gathered evidence shows that overexpression of P‐gp is associated with the occurrence of MDR events in cancer. P‐gp expression is one of the major defence mechanisms adopted by cancer cells when exposed to cytotoxic agents. The efflux pump has been found to play a role in inhibiting the intake of conventional genotoxic anticancer drugs such as vinblastine, paclitaxel and doxorubicin (Sui et al., 2011). MRP1 has a function similar to P‐gp, which releases toxic substances in an ATP‐dependent manner. MRP1 significantly contributes to the outflow of organic anionic substrates, including compounds bound to glucuronide, sulphate and glutathione, as well as several anticancer agents including anthracyclines, vinca alkaloids and methotrexate (Munoz et al., 2007). Through this experiment, it was confirmed that cADSCs significantly increased these resistance genes in tumour cells. Although this study did not confirm that tumour cells with increased resistance genes due to stem cells actually decreased their drug‐sensitivity under anticancer drug conditions, this will be a valuable basis for conducting these experiments.
The present study has a limitation that we did not perform gene expression microarray to figure out which factor is the most up regulated. Also, further research to clarify the detailed mechanism and in vivo experiments are warranted. However, we have demonstrated that TSG‐6 secreted from stromal cells may be involved in the upregulation of tumour cell COX‐2, and this study will be a major basis for the application of stem cells to tumour patients. In addition, it will be a valuable data to study the interaction of tumour cells with tumour microenvironment related to stromal cells around tumours.
5. CONCLUSIONS
In conclusion, the study demonstrated that TSG‐6, secreted by cADSCs, enhances COX‐2 expression and subsequently up‐regulates the drug resistance genes P‐gp and MRP1 in canine melanoma and osteosarcoma cell lines. These findings provide better understanding about the mechanism associated with mesenchymal stem/stromal cell‐induced chemoresistance in cancer.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS' CONTRIBUTIONS
SJY and JHA designed the study, collected, analysed, and interpreted data and wrote the article. SMP, JHL, HKC and KML reviewed the article. WJS and HYY contributed to the conception and design of the study, data analysis and interpretation, and granted final approval of the manuscript. All authors have read and approved the final manuscript.
Se‐Jin YANG: Conceptualization; Data curation; Formal analysis; Investigation; Resources; Visualization; Writing‐original draft. Ju‐Hyun AN: Conceptualization; Data curation; Formal analysis; Investigation; Resources; Validation; Visualization; Writing‐original draft. SUMIN PARK: Writing‐review & editing. Jeong‐Hwa Lee: Writing‐review & editing. Hyung‐Kyu Chae: Writing‐review & editing. Kyung‐Mi LEE: Writing‐review & editing. Woo‐Jin Song: Conceptualization; Formal analysis; Funding acquisition; Supervision; Validation; Writing‐review & editing. Hwa‐Young Youn: Conceptualization; Formal analysis; Funding acquisition; Supervision; Validation; Writing‐review & editing.
ETHICS STATEMENT
All animal experiment procedures were approved by the Institutional Animal Care and Use Committee of SNU (protocol no. SNU‐190908–1), Republic of Korea, and all protocols were in accordance with approved guidelines. The clients agreed to provide adipose tissue of the dog to the laboratory of Veterinary Internal Medicine, Seoul National University and to fully assume the future management. All informed consents are documented.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.442.
Supporting information
Fig S1
Fig S2
Yang S‐J, An J‐H, Park S‐M, et al. Enhanced expression of cyclooxygenase‐2 related multi‐drug resistance gene in melanoma and osteosarcoma cell lines by TSG‐6 secreted from canine adipose‐derived mesenchymal stem/stromal cells. Vet Med Sci. 2021;7:968–978. 10.1002/vms3.442
Se‐Jin Yang and Ju‐Hyun An contributed equally to this work.
Contributor Information
Woo‐Jin Song, Email: ssong@jejunu.ac.kr.
Hwa‐Young Youn, Email: hyyoun@snu.ac.kr.
REFERENCES
- An, J. H. , Li, Q. , Bhang, D. H. , Song, W. J. , & Youn, H. Y. (2020). TNF‐α and INF‐γ primed canine stem cell‐derived extracellular vesicles alleviate experimental murine colitis. Scientific Reports, 10(1), 2115. 10.1038/s41598-020-58909-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan, R. , Luk, F. , & Bebawy, M. (2014). Inhibition of the multidrug resistance P‐glycoprotein: Time for a change of strategy? Drug Metabolism and Disposition, 42(4), 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan, A. I. , & Correa, D. (2011). The MSC: An injury drugstore. Cell Stem Cell, 9(1), 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D.‐R. , Lu, D.‐Y. , Lin, H.‐Y. , & Yeh, W.‐L. (2014). Mesenchymal stem cell‐induced doxorubicin resistance in triple negative breast cancer. BioMed Research International, 2014, 532161. 10.1155/2014/532161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, A. J. , & Milner, C. M. (2019). TSG‐6: A multifunctional protein with anti‐inflammatory and tissue‐protective properties. Matrix Biology, 78, 60–83. [DOI] [PubMed] [Google Scholar]
- Fantappiè, O. , Masini, E. , Sardi, I. , Raimondi, L. , Bani, D. , Solazzo, M. , Vannacci, A. , & Mazzanti, R. (2002). The MDR phenotype is associated with the expression of COX‐2 and iNOS in a human hepatocellular carcinoma cell line. Hepatology, 35(4), 843–852. [DOI] [PubMed] [Google Scholar]
- Ghosh, N. , Chaki, R. , Mandal, V. , & Mandal, S. C. (2010). COX‐2 as a target for cancer chemotherapy. Pharmacological Reports, 62(2), 233–244. [DOI] [PubMed] [Google Scholar]
- Glavinas, H. , Krajcsi, P. , Cserepes, J. , & Sarkadi, B. (2004). The role of ABC transporters in drug resistance, metabolism and toxicity. Current Drug Delivery, 1(1), 27–42. [DOI] [PubMed] [Google Scholar]
- Guan, J. , & Chen, J. (2013). Mesenchymal stem cells in the tumor microenvironment. Biomedical Reports, 1(4), 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthuijzen, J. , Daenen, L. , Roodhart, J. , & Voest, E. (2012). The role of mesenchymal stem cells in anti‐cancer drug resistance and tumour progression. British Journal of Cancer, 106(12), 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, K. , Ohashi, E. , Kadosawa, T. , Hong, S.‐H. , Matsunaga, S. , Mochizuki, M. , Nishimura, R. , & Sasaki, N. (2004). Establishment and characterization of four canine melanoma cell lines. Journal of Veterinary Medical Science, 66(11), 1437–1440. [DOI] [PubMed] [Google Scholar]
- Januchowski, R. , Sterzyńska, K. , Zaorska, K. , Sosińska, P. , Klejewski, A. , Brązert, M. , Nowicki, M. , & Zabel, M. (2016). Analysis of MDR genes expression and cross‐resistance in eight drug resistant ovarian cancer cell lines. Journal of Ovarian Research, 9(1), 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J.‐H. , Song, K.‐H. , Jeong, K.‐C. , Kim, S. , Choi, C. , Lee, C. H. , & Oh, S. H. (2011). Involvement of Cox‐2 in the metastatic potential of chemotherapy‐resistant breast cancer cells. BMC Cancer, 11(1), 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma, G. D. , Carthew, J. , Lim, R. , & Frith, J. E. (2017). Effect of the microenvironment on mesenchymal stem cell paracrine signaling: Opportunities to engineer the therapeutic effect. Stem Cells and Development, 26(9), 617–631.28186467 [Google Scholar]
- Li, X. , Yue, S. , & Luo, Z. (2017). Mesenchymal stem cells in idiopathic pulmonary fibrosis. Oncotarget, 8(60), 102600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, V. (1997). Multidrug resistance: Molecular mechanisms and clinical relevance. Cancer Chemotherapy and Pharmacology, 40(1), S3–S8. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Qu, L. , & Tao, H. (2010). Cyclo‐oxygenase 2 up‐regulates the effect of multidrug resistance. Cell Biology International, 34(1), 21–25. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Yang, Y. , Liu, Y. , Hao, Y. , Zhang, Y. , Hu, Y. , Jiang, L. , Gong, Y. , Wu, K. , & Liu, Y. (2017). Upregulation of PAG1/Cbp contributes to adipose‐derived mesenchymal stem cells promoted tumor progression and chemoresistance in breast cancer. Biochemical and Biophysical Research Communications, 494(3–4), 719–727. [DOI] [PubMed] [Google Scholar]
- Maihöfner, C. , Schlötzer‐Schrehardt, U. , Gühring, H. , Zeilhofer, H. U. , Naumann, G. O. , Pahl, A. , Mardin, C. , & Brune, K. (2001). Expression of cyclooxygenase‐1 and‐2 in normal and glaucomatous human eyes. Investigative Ophthalmology & Visual Science. 42(11), 2616–2624. [PubMed] [Google Scholar]
- Martínez, C. , Peñafiel‐Verdú, C. , Vilafranca, M. , Ramírez, G. , Méndez‐Gallego, M. , Buendía, A. , & Sánchez, J. (2011). Cyclooxygenase‐2 expression is related with localization, proliferation, and overall survival in canine melanocytic neoplasms. Veterinary Pathology, 48(6), 1204–1211. [DOI] [PubMed] [Google Scholar]
- McKenna, S. L. , & Padua, R. A. (1997). Multidrug resistance in leukaemia. British Journal of Haematology, 96(4), 659–674. [DOI] [PubMed] [Google Scholar]
- Meads, M. B. , Gatenby, R. A. , & Dalton, W. S. (2009). Environment‐mediated drug resistance: A major contributor to minimal residual disease. Nature Reviews Cancer, 9(9), 665–674. [DOI] [PubMed] [Google Scholar]
- Meads, M. B. , Hazlehurst, L. A. , & Dalton, W. S. (2008). The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clinical Cancer Research, 14(9), 2519–2526. [DOI] [PubMed] [Google Scholar]
- Millanta, F. , Asproni, P. , Cancedda, S. , Vignoli, M. , Bacci, B. , & Poli, A. (2012). Immunohistochemical expression of COX‐2, mPGES and EP2 receptor in normal and reactive canine bone and in canine osteosarcoma. Journal of Comparative Pathology, 147(2–3), 153–160. [DOI] [PubMed] [Google Scholar]
- Milner, C. M. , & Day, A. J. (2003). TSG‐6: A multifunctional protein associated with inflammation. Journal of Cell Science, 116(10), 1863–1873. [DOI] [PubMed] [Google Scholar]
- Mindrescu, C. , Le, J. , Wisniewski, H.‐G. , & Vilcek, J. (2005). Up‐regulation of cyclooxygenase‐2 expression by TSG‐6 protein in macrophage cell line. Biochemical and Biophysical Research Communications, 330(3), 737–745. [DOI] [PubMed] [Google Scholar]
- Munoz, M. , Henderson, M. , Haber, M. , & Norris, M. (2007). Role of the MRP1/ABCC1 multidrug transporter protein in cancer. IUBMB Life, 59(12), 752–757. [DOI] [PubMed] [Google Scholar]
- Pires, I. , Garcia, A. , Prada, J. , & Queiroga, F. (2010). COX‐1 and COX‐2 expression in canine cutaneous, oral and ocular melanocytic tumours. Journal of Comparative Pathology, 143(2–3), 142–149. [DOI] [PubMed] [Google Scholar]
- Rai, M. F. , Rachakonda, P. S. , Manning, K. , Vorwerk, B. , Brunnberg, L. , Kohn, B. , & Schmidt, M. F. (2008). Quantification of cytokines and inflammatory mediators in a three‐dimensional model of inflammatory arthritis. Cytokine, 42(1), 8–17. [DOI] [PubMed] [Google Scholar]
- Romano, B. , Elangovan, S. , Erreni, M. , Sala, E. , Petti, L. , Kunderfranco, P. , Massimino, L. , Restelli, S. , Sinha, S. , & Lucchetti, D. (2019). TNF‐stimulated gene‐6 is a key regulator in switching stemness and biological properties of mesenchymal stem cells. Stem Cells, 37(7), 973–987. [DOI] [PubMed] [Google Scholar]
- Roodhart, J. M. , Daenen, L. G. , Stigter, E. C. , Prins, H.‐J. , Gerrits, J. , Houthuijzen, J. M. , Gerritsen, M. G. , Schipper, H. S. , Backer, M. J. , & van Amersfoort, M. (2011). Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum‐induced fatty acids. Cancer Cell, 20(3), 370–383. [DOI] [PubMed] [Google Scholar]
- Segaoula, Z. , Primot, A. , Lepretre, F. , Hedan, B. , Bouchaert, E. , Minier, K. , Marescaux, L. , Serres, F. , Galiègue‐Zouitina, S. , André, C. , Quesnel, B. , Thuru, X. , & Tierny, D. (2018). Isolation and characterization of two canine melanoma cell lines: New models for comparative oncology. BMC Cancer, 18(1), 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.‐J. , Li, Q. , Ryu, M.‐O. , Ahn, J.‐O. , Bhang, D. H. , Jung, Y. C. , & Youn, H.‐Y. (2017). TSG‐6 secreted by human adipose tissue‐derived mesenchymal stem cells ameliorates DSS‐induced colitis by inducing M2 macrophage polarization in mice. Scientific Reports, 7(1), 5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.‐J. , Li, Q. , Ryu, M.‐O. , Ahn, J.‐O. , Bhang, D. H. , Jung, Y. C. , & Youn, H.‐Y. (2018). TSG‐6 released from intraperitoneally injected canine adipose tissue‐derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Research & Therapy, 9(1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, H. , Zhou, S. , Wang, Y. , Liu, X. , Zhou, L. , Yin, P. , Fan, Z. , & Li, Q. (2011). COX‐2 contributes to P‐glycoprotein‐mediated multidrug resistance via phosphorylation of c‐Jun at Ser63/73 in colorectal cancer. Carcinogenesis, 32(5), 667–675. [DOI] [PubMed] [Google Scholar]
- Tsujii, M. , Kawano, S. , & DuBois, R. N. (1997). Cyclooxygenase‐2 expression in human colon cancer cells increases metastatic potential. Proceedings of the National Academy of Sciences, 94(7), 3336–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii, M. , Kawano, S. , Tsuji, S. , Sawaoka, H. , Hori, M. , & DuBois, R. N. (1998). Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell, 93(5), 705–716. [DOI] [PubMed] [Google Scholar]
- Tu, B. , Zhu, J. , Liu, S. , Wang, L. , Fan, Q. , Hao, Y. , Fan, C. , & Tang, T.‐T. (2016). Mesenchymal stem cells promote osteosarcoma cell survival and drug resistance through activation of STAT3. Oncotarget, 7(30), 48296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, K. , Schneider, S. , Yochim, J. M. , Kuramochi, H. , Hayashi, K. , Takasaki, K. , Yang, D. , Danenberg, K. D. , & Danenberg, P. V. (2005). Intratumoral COX‐2 gene expression is a predictive factor for colorectal cancer response to fluoropyrimidine‐based chemotherapy. Clinical Cancer Research, 11(9), 3363–3368. [DOI] [PubMed] [Google Scholar]
- Wang, K.‐H. , Kao, A.‐P. , Chang, C.‐C. , Lee, J.‐N. , Hou, M.‐F. , Long, C.‐Y. , Chen, H.‐S. , & Tsai, E.‐M. (2010). Increasing CD44+/CD24‐tumor stem cells, and upregulation of COX‐2 and HDAC6, as major functions of HER2 in breast tumorigenesis. Molecular Cancer, 9(1), 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, Y. , Guo, Y. , Huang, Z. , Cai, J. , & Wang, Z. (2017). Adipose‐derived mesenchymal stem cells attenuate cisplatin‐induced apoptosis in epithelial ovarian cancer cells. Molecular Medicine Reports, 16(6), 9587–9592. [DOI] [PubMed] [Google Scholar]
- Wilson‐Robles, H. , Franks, K. , Pool, R. , & Miller, T. (2019). Characterization of five newly derived canine osteosarcoma cell lines. BMC Veterinary Research, 15(1), 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, W.‐L. , Tsai, C.‐F. , & Chen, D.‐R. (2017). Peri‐foci adipose‐derived stem cells promote chemoresistance in breast cancer. Stem Cell Research & Therapy, 8(1), 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandvliet, M. , Teske, E. , & Schrickx, J. (2014). Multi‐drug resistance in a canine lymphoid cell line due to increased P‐glycoprotein expression, a potential model for drug‐resistant canine lymphoma. Toxicology in Vitro, 28(8), 1498–1506. [DOI] [PubMed] [Google Scholar]
- Zandvliet, M. , Teske, E. , Schrickx, J. , & Mol, J. (2015). A longitudinal study of ABC transporter expression in canine multicentric lymphoma. The Veterinary Journal, 205(2), 263–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2


