Abstract
African swine fever (ASF) is a highly contagious fatal infectious disease of pigs and wild suids. The disease has a worldwide occurrence and significant impact on pig production. Two adult intensively raised large white boars from two farms in Jos with a history of sudden death were diagnosed of ASF between July and August 2019. Post‐mortem examination of carcasses grossly showed splenomegaly, haemorrhagic lymphadenitis and hepatomegaly with severe congestion. The kidneys were enlarged and had generalized petechiae and blood clot in the pelvis. The heart was moderately enlarged. Microscopic examination of the spleen and lymph nodes revealed severe lymphocytic depletion, haemorrhage and severe haemosiderosis. The liver was severely congested with focal coagulative necrosis of the hepatocytes. The kidneys were severely congested and showed renal tubular necrosis with few tubular protein casts. Tissue samples were confirmed to be positive for African swine fever virus (ASFV) by polymerase chain reaction (PCR) assay, and phylogenetic analysis revealed that the isolate belonged to genotype I.
Keywords: African swine fever, diagnosis, laboratory, Nigeria, pig, smallholder farms
African swine fever is a highly contagious fatal infectious disease of pigs with a worldwide occurrence and economic importance. Two adult large white boars from two farms in Jos were diagnosed with African swine fever in 2019 through gross and histopathological evaluations, viral isolation and polymerase chain reaction. Sequences obtained were compared and deposited in the GenBank and were accessioned MN888693 and MN888694 and the phylogram showed that the ASFV belongs to genotype I.
1. INTRODUCTION
African swine fever (ASF) is a highly contagious, fatal, economically important, transboundary, viral disease of pigs caused by a DNA virus belonging to the genus Asfivirus and in the family Asfarviridae (Murphy et al., 1999). The first reported ASF outbreak in Nigeria was in 1998 at a farm located in Lagos (FAO, 1998; Odemuyiwa et al., 2000). The disease spread widely within the country and has become endemic resulting in huge economic losses to the pig industry (Fadiga et al., 2013; Igbokwe & Maduka, 2018). ASF is a very fatal disease that can cause up to 100% mortality in a naïve pig population (Costard et al., 2009). Poor biosecurity, bad abattoir practices and extensive or free‐range pig farming systems are known risk factors that facilitate the widespread dissemination of the disease in the country (Owolodun et al., 2010). In West Africa, ASF is reported more in domestic pig population, with humans and other fomites potentiating its spread with a one‐time incidence in wild suids (Dixon et al., 2020; Luther et al., 2007). However, both domestic and wild pigs are susceptible to ASFV, but the wild suids are known to be asymptomatic. Areas with high pig‐related activities, such as marketing, consumption and farming, have higher prevalence compared to areas with less pig‐related activities. Farm‐gate buyers, marketing systems and transport of untested pigs within the country have greatly aided the circulation of the virus (Fasina et al., 2010). ASF is characterized by a febrile syndrome, erythema and cyanosis of the skin, anorexia, bloody diarrhoea, abortion in pregnant sows, meningitis, interstitial pneumonia, high morbidity and mortality (Fasina et al., 2010; Igbokwe & Maduka, 2018). Diagnosis of ASF is based on history, clinical signs, lesions and laboratory confirmation by viral isolation and molecular characterization. This clinical case report represents the investigation of an acute ASF outbreak (July–August, 2019) in pig farms in Jos, Nigeria.
2. MATERIALS AND METHODS
2.1. History and clinical presentations
The two cases reported in this outbreak were presented to the Veterinary Teaching Hospital, University of Jos, 2 weeks apart, that is, last week of July and first week of August, 2019. The chief complaint from both farmers was sudden high mortalities. The first farmer had about 60 pigs of different age groups, and six of the pigs died before he was advised to dispose the rest of the pigs for slaughter, disinfect the farm and fallow it before restocking. The second farmer had four adult pigs and all died of the ASF. He was also advised to disinfect the farm and allow it to fallow before restocking. The clinical signs observed in both farms were sudden deaths of pigs in good body conditions, moderate multifocal areas of hyperaemia on the skin and weakness.
2.1.1. Post‐mortem examination (PM)
The PM was carried out on two submitted carcasses and gross post‐mortem lesions observed were identified and recorded.
2.1.2. Histopathology
Tissue samples of the affected organs (spleen, lymph nodes, liver, kidney and heart) were collected and fixed in 10% neutral buffered formalin. The samples were dehydrated in graded concentrations of alcohol, cleared in xylene and impregnated in paraffin wax. Samples were subsequently incubated in vacuum oven at 60°C, embedded in plastic embedding rings, cut into 5‐µM sections using a microtome, deparaffinized with xylene, rehydrated in graded concentrations of alcohol, stained with haematoxylin and eosin and viewed under light microscope at X40 objective as outlined by Baker et al. (2000).
2.1.3. Virus isolation
African swine fever virus (ASFV) was recovered from pooled tissues cultured on porcine leucocyte primary cell line and presumptively identified by the haemadsorption test, with pig erythrocytes on the inoculated cell culture plate incubated for 1 day at 37℃ in a CO2 incubator as outlined by the standard operating procedure for the isolation of ASFV by the European Union Reference Laboratory for ASF (EURL‐ASF, 2013).
2.1.4. Polymerase chain reaction (PCR) confirmation of ASFV
Genomic DNA was extracted from tissue samples (spleen, lymph node and kidney) using QIAamp DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer's specifications. Lyophilized freeze‐dried E70 from the reference laboratory for ASF (CISA‐INIA, Madrid, Spain) was used as control of this study. The presence of ASFV DNA was confirmed by the amplification of 278 bp fragment of the VP72 gene (OIE, 2008) using the following primer pairs: (a) p72 D [GTACTGTAACGCAGCACAG (forward)] and p72‐U [GGCACAAGTTCGGACATGT (reverse)] and (b) CVR‐FL1 [TCG GCC TGA AGC TCA TTA G (forward)] and [CVR‐FL2 CAG GAA ACT AAT GAT GTT CC (reverse)] (OIE, 2008). The conditions for the PCR assay were as follows: 19 PCR buffer (50‐mM KCl and 10‐mM Tris–HCl), 2‐mM MgCl2, 0.4‐µM concentration of primers, 0.2‐mM dinucleotide triphosphates (dNTPs) and 2.5 U Taq polymerase in a total volume of 25 µl. The PCR assay was performed in an Applied BioSystems® thermal cycler 9500 (Applied BioSystems) with an initial denaturation at 94°C for 15 s, followed by 30 cycles of denaturation at 94°C for 15 s, annealing at 62°C for 15 s and extension at 72°C for 15 s; and a final extension step at 72°C for 5 min. The PCR products were resolved by electrophoresis in a 1.5% agarose gel and the ladder used was 100 bp. The DNA amplicons were sequenced using Sanger sequencing by LGC genomics (GmbH). The electropherographs of the sequenced genes were aligned, trimmed and deposited in the Genbank.
3. RESULTS
Post‐mortem examination revealed splenomegaly and severe congestion 2/2 (Figure 1), enlarged and haemorrhagic lymph nodes 2/2 (Figure 2), hepatomegaly and severe congestion ½ (Figure 3a), white multifocal patches on the liver ½ (Figure 3b), globosed, severely congested, generalized cortical petechiae and severe blood clot in the pelvis 2/2 (Figure 4a,b). Thus, the histopathological findings were: spleen: severe lymphocyte depletion (Figure 5). Lymph node: severe lymphocyte depletion and severe haemosiderosis (Figure 6). Liver: severe congestion with focal areas of coagulation necrosis (Figure 7). Kidney: severe congestion and renal tubular and glomerular necrosis with few tubular protein cast (Figure 8a,b).
Figure 1.
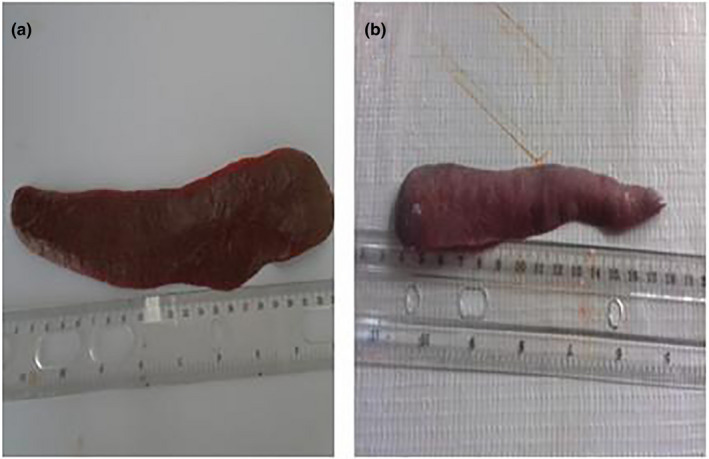
(a) Photograph of normal spleen of a pig showing no observable gross lesion. (b) Photograph of spleen of a pig infected with African swine fever virus showing severe congestion and moderate splenomegaly
Figure 2.
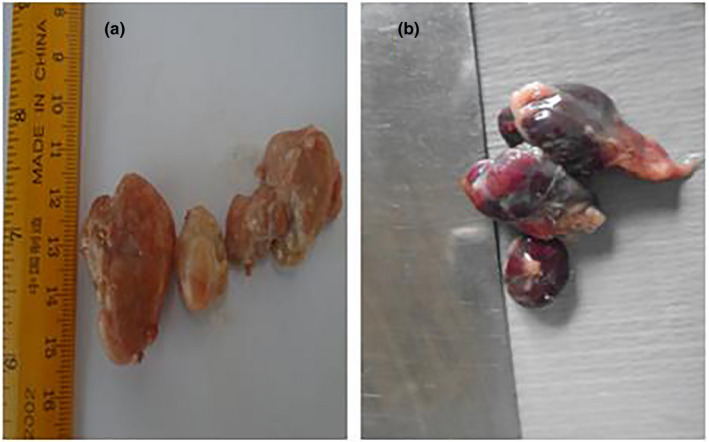
(a) Photograph of normal lymph nodes of a pig showing no observable gross lesion. (b) Photograph of lymph nodes of a pig infected with African swine fever virus showing severe haemorrhagic lymphadenitis
Figure 3.
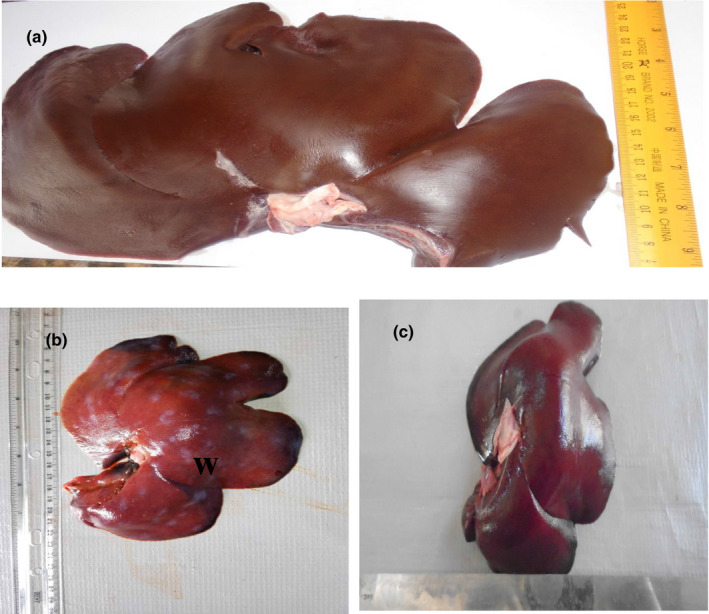
(a) Photograph of a normal liver of a pig showing no observable gross lesion. (b) Photograph of liver of a pig infected with African swine fever virus showing severe multifocal white patchy areas (W). (c) Photograph of liver of a pig infected with African swine fever virus showing severe congestion
Figure 4.
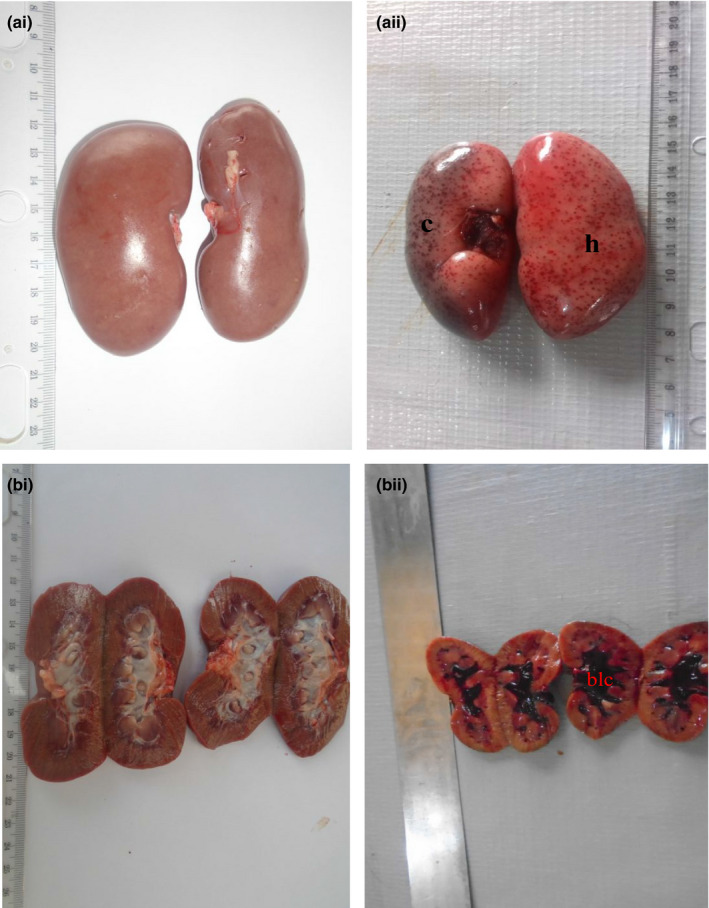
(ai) Photograph of normal kidneys of a pig showing no observable gross lesion. (aii) Photograph of kidneys of a pig infected with African swine fever virus showing moderate congestion (c) and severe generalized petechial haemorrhages (h). (bi) Photograph of transverse section of normal kidneys showing no observable gross lesion. (bii) Photograph of transverse section of kidneys of pig infected with African swine fever virus showing severe blood clot in the medulla (blc)
Figure 5.
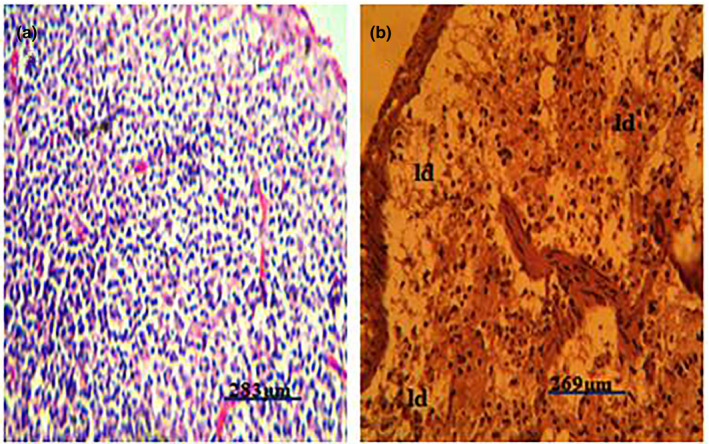
(a) Photomicrograph of a normal spleen of a pig showing no observable microscopic lesion. (b) Photomicrograph of a spleen of a pig infected with African swine fever virus showing severe lymphocyte depletions (ld) in both the red and the white pulps (H&E)
Figure 6.
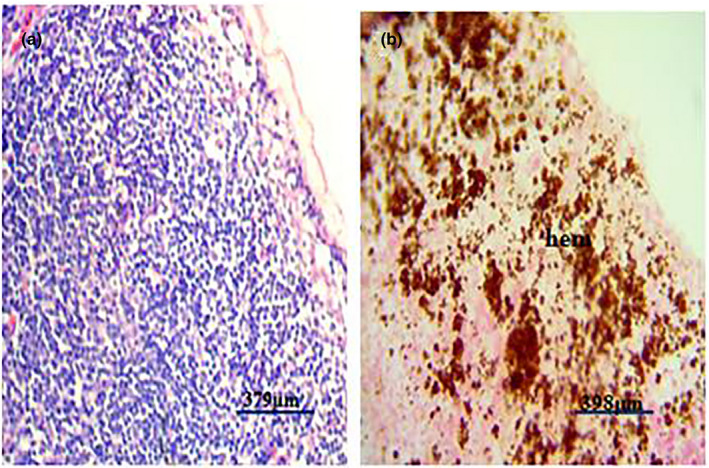
(a) Photomicrograph of a normal lymph node of a pig showing no observable microscopic lesion. (b) Photomicrograph of lymph node of a pig infected with African swine fever virus showing severe haemosiderosis (Hem) (H&E)
Figure 7.
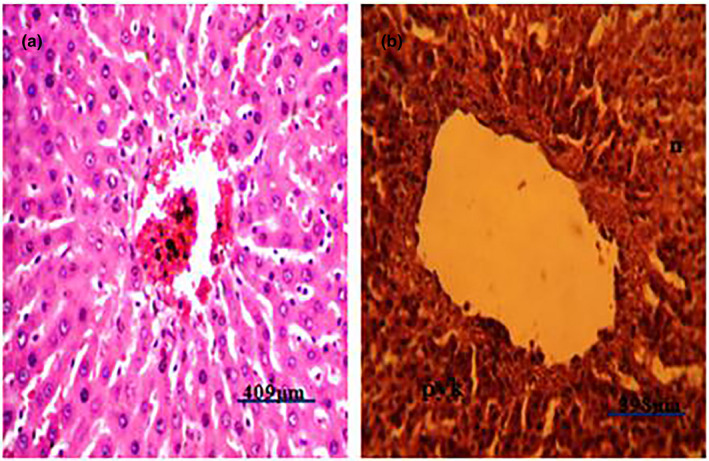
Photomicrograph of liver of a pig infected with African swine fever showing severe coagulation necrosis (N) and pyknosis (pyk) (H&E). (a) Photomicrograph of a normal liver of a pig showing no observable microscopic lesion. (b) Photomicrograph of liver of a pig infected with African swine fever virus showing severe hepatocellular coagulation necrosis (n) and pyknosis (pyk) (H&E)
Figure 8.
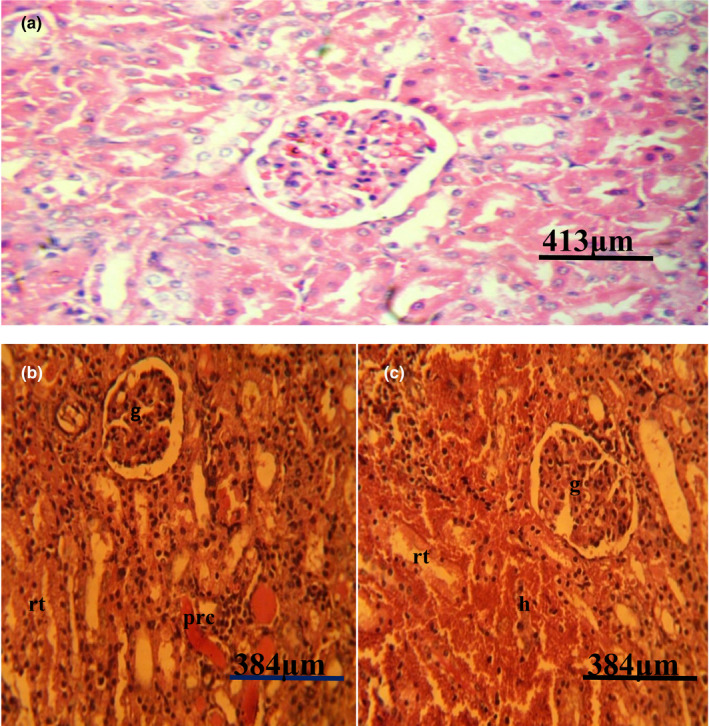
(a) Photomicrograph of a normal kidney of a pig showing no observable microscopic lesion. (b) Photomicrograph of kidney of a pig infected with African swine fever virus showing severe glomerular (g) and renal tubular (rt) necrosis with severe renal protein cast (Prc). (c) Photomicrograph of kidney of a pig infected with African swine fever virus showing severe glomerular (g), and renal tubular (rt) necrosis and severe haemorrhages (H&E)
Viral isolation on the culture plate was revealed by haemadsorption. The presence of the virus was typified by the attachment of large numbers of pig erythrocytes to the surface of infected cells thereby forming the rosette appearance (Figure 9) as opposed to the negative wells which were not inoculated with the infected tissues (Figure 10).
Figure 9.
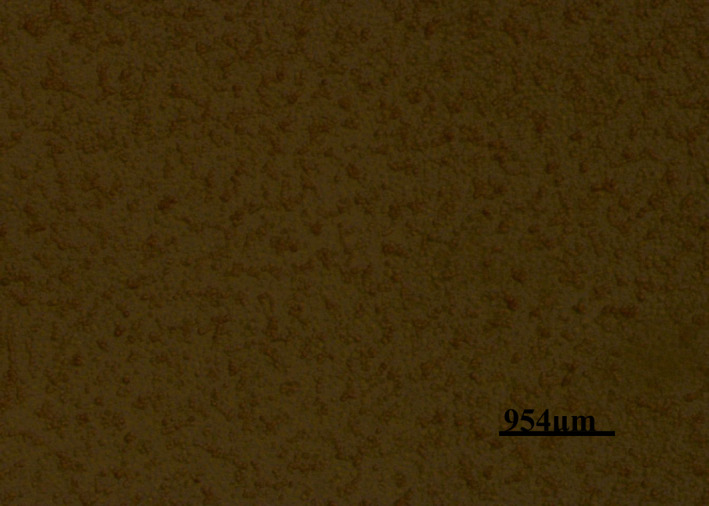
Photomicrograph of a cell culture plate well inoculated with African swine fever‐infected tissue showing haemadsorption (Had) of the pig erythrocytes to the surface of the infected cells
Figure 10.
Photomicrograph of a cell culture plate well not inoculated with African swine fever‐infected tissue showing no haemadsorption (Had) of the pig erythrocytes to the surface of the infected cells
Gel picture (Figure 11) shows that DNA fragments of 278 bp (confirming the presence of ASFV) were successfully extracted and amplified from the spleen, lymph node and kidney of the infected animals. Note that the size of the PCR products in lanes 1, 2 and 3, which were ASFV‐positive amplicons, presented similar DNA fragment base pairs to the product of the positive control of ASFV in lane 4. The nucleotide sequences of ASFV generated from this study were deposited in the Genbank with accession numbers MN888963 and MN888964.
Figure 11.
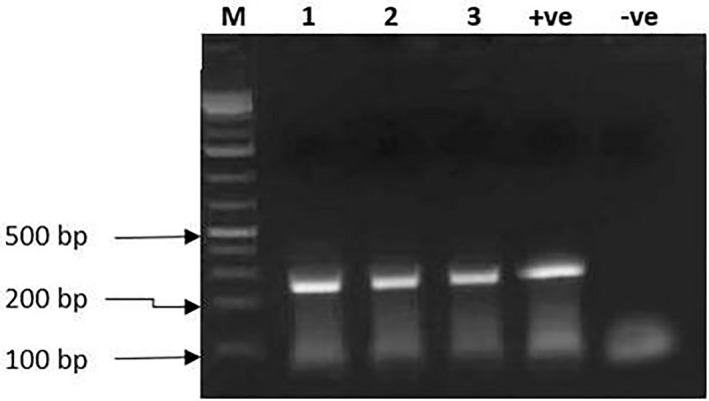
Gel electrophoresis result showing DNA fragments of 278 bp produced from the DNA extracted from the spleen, lymph node and kidney in lanes 1, 2 and 3 and the positive (+ve) control in lane 4, respectively
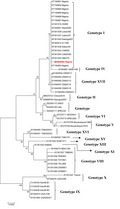
The result of phylogenetic analysis based on the p72 (B646L) gene is presented in Figure 12. It shows the relationship of the sequence from this study to other genotype I reference sequences from the GenBank database. The sequences obtained clustered with other sequences in genotype I from Nigeria and other African countries.
Figure 12.
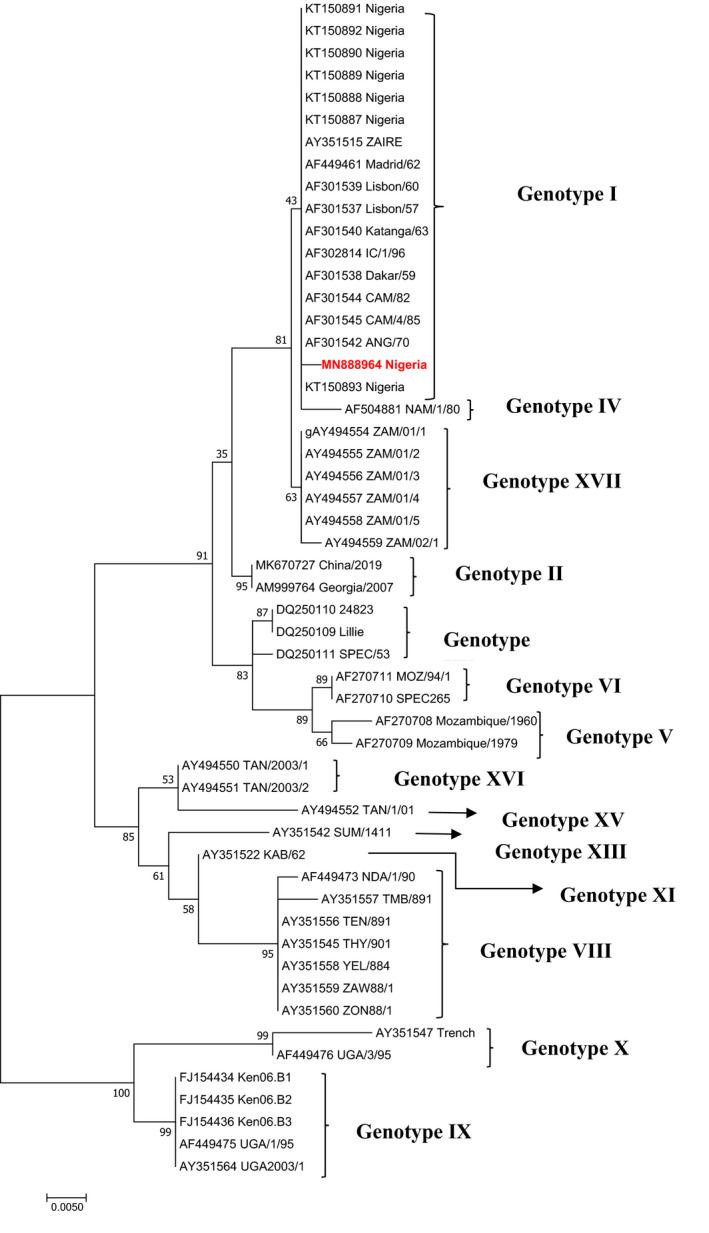
Phylogram showing the genetic relatedness of p72 (B646L) gene nucleotide sequences of African swine fever virus. The tree was constructed in MEGA 6 using maximum likelihood method with 1,000 bootstrap replicates. The sequence for the study is labelled with black triangle in genotype I
4. DISCUSSION
Gross pathological lesions observed in the spleen may be because of leucopoenia and lymphopenia that were said to be occasioned by apoptosis, necrosis of the lymphocytes or due to activation and mobilization of the lymphocytes to fight the virus (Ganowiak, 2012; Jubb et al., 2016; Liao & Padera, 2013).
Similarly, the gross lesions observed on the lymph nodes were likely due to severe haemorrhages and necrosis as reported previously by Jubb et al. (2016). The histopathological lesions recorded in the lymph nodes may be due to apoptosis, necrosis or severe erythrolysis (Ganowiak, 2012; Jubb et al., 2016). Although moderate multifocal white patches on the liver have not been previously reported in ASF, other gross and histopathological lesions observed were previously established and adduced to marked thrombocytopenia or disruption in blood clotting factors. (Jubb et al., 2016). In the kidneys of pigs, the gross and histopathological findings in this study were consistent with earlier reports on ASF and were attributed to be likely due to severe thrombocytopenia or disruption in blood clotting factors (Ganowiak, 2012; Jubb et al., 2016). The gross and histomorphological lesions observed on the heart on the other hand were at variance with earlier reports that established cardiopathology ranging from hydropericardium, haemopericardium and fibrinous pericarditis, which were said to be associated with the chronic form of the disease. The lesions so recorded in the heart were said to be likely caused by oxidative stress and ischaemia (Ganowiak, 2012; Jubb et al., 2016; Semerjyan et al., 2018).
The aetiological diagnosis of the disease was by virus culture and identification via haemadsorption test characterized by erythrocyte attachment to the surface of the infected cells several hours post‐inoculation. This has been documented to be a reliable and effective method of ASFV isolation (Jubb et al., 2016). Furthermore, the aetiological diagnosis was achieved by amplification of viral glycoprotein VP72 from the tissues of the infected pigs which, among others, plays an important role in attaching to and entering target cells. Successful amplification of a 278 bp of the glycoprotein by PCR has earlier been reported to be confirmatory of ASFV in a given clinical sample (Fernandez‐pinero et al., 2012; Jubb et al., 2016; Mwiine et al., 2019).
Phylogenetic analysis of the virus obtained revealed that it belongs to genotype I. This is consistent with previous findings of Luka et al. (2017) that only one genotype of the virus is circulating in Nigeria. However, the lack of genetic diversity of the circulating virus in Nigeria suggests that control strategy may not be complicated. Nevertheless, there may be a continued evolution of the virus due to pressure and unreported strains of the virus circulating in the country, and this, therefore, highlights the need for continues epidemiological surveillance to characterize the circulating strains of ASFV in the country as previously recommended (Luka et al., 2017). This, if done, would serve as baseline for formulating adequate and appropriate control strategies against ASF outbreaks, including vaccine production. Furthermore, the phylogenetic analysis revealed that the virus associated with the disease reported in this study belonged to genotype I. This further corroborates the previous reports (Luka et al., 2017; Owolodun et al., 2020) on circulating viruses in Nigeria.
CONFLICT OF INTEREST
The authors declare that there is no competing interest.
AUTHOR CONTRIBUTIONS
Emmanuel Vandi TIZHE: Conceptualization; Methodology; Writing‐original draft. Pam Dachung Luka: Conceptualization; Data curation; Funding acquisition; Supervision; Writing‐review and editing. Adeyinka Jeremy Adedeji: Writing‐review and editing. Polycarp Tanko: Writing‐review and editing. George Yilzem Gurumyen: Writing‐review and editing. Deborah Maigawu Buba: Resources; Writing‐review and editing. Ussa Delia Tizhe: Resources; Writing‐review and editing. Asinamai Athliamai Bitrus: Data curation; Resources; Writing‐review and editing. Arthur Obinna Oragwa: Data curation; Resources; Writing‐review and editing. Samson Shaibu: Writing‐review and editing. Essieniffiok Saturday Unanam: Writing‐review and editing. I. O. Igbokwe: Data curation; Supervision; Writing‐review and editing. Stephen O. Akpavie: Data curation; Supervision; Writing‐review and editing. Celestine Onwu‐Ibe Njoku: Data curation; Supervision; Writing‐review and editing.
ETHICAL STATEMENT
Ethical clearance is not applicable in this study as the data were generated from the swine carcasses submitted to the Veterinary Teaching hospital, University of Jos, 2019 for diagnostic purpose.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.403.
ACKNOWLEDGEMENTS
The author's hereby acknowledge that the PCR and sequencing were sponsored by the ASF research grant of Dr. P.D. Luka and his team to whom the authors remain eternally grateful.
Tizhe EV, Luka PD, Adedeji AJ, et al. Laboratory diagnosis of a new outbreak of acute African swine fever in smallholder pig farms in Jos, Nigeria. Vet Med Sci.2021;7:705–713. 10.1002/vms3.403
DATA AVAILABILITY STATEMENT
The data that support the findings of this study as presented in the manuscript are available from the corresponding author, Dr. Tizhe E.V., upon reasonable and/or genuine request.
REFERENCES
- Baker, J. , Silverton, R. E. , & Pillister, C. J. (2000). Dehydration, impregnation, embedding technique and section preparation. In Butterworth‐Heinemann (Ed.), Introduction to medical laboratory technology (7th ed., pp. 199–242). Elsevier. [Google Scholar]
- Costard, S. , Wieland, B. , de Glanville, W. , Jori, F. , Rowlands, R. , Vosloo, W. , Rooger, F. , Pfoffer, D. U. , & Dixon, L. K. (2009). African swine fever: How can global spread be prevented? Philosophical Transactions of the Royal Society B, 364, 2683–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, L. K. , Stahl, K. , Jori, F. , Vial, L. , & Pfeiffer, D. U. (2020). African swine fever epidemiology and control. Annual Reviews of Animal Biosciences, 8, 221–246. 10.1146/annurev-animal-021419-083741 [DOI] [PubMed] [Google Scholar]
- European Union Reference Laboratory for African Swine Fever . (2013). Procedure for African swine fever virus (ASFV) isolation on Porcine leucocytes and hemadsorption test. SOP/CISA/ASF/VI, 2013, 1, 1–8.
- Fadiga, M. , Jost, C. , & Ihedioha, J. (2013). Financial cost of disease burden, morbidity, and mortality from priority livestock diseases in Nigeria. Disease burden and cost‐benefit analysis of targeted interventions. In International Livestock Research Institute Research report 33, ILRI Nairobi, Kenya. Retrieved from http://cgspace.cgiar.org/bitstream/handle/10568/331418/ResearchReport/_33pdfsequence=2 [Google Scholar]
- FAO . (1998). Food Agricultural Organization of the United Nations: African swine fever in Nigeria hits rural poor. Retrieved from http://www.fao.org/News&Highlights/1998/981201‐e.htm [Google Scholar]
- Fasina, F. O. , Shamaki, D. , Makinde, A. A. , Lombin, L. H. , Lazarus, D. D. , Rufai, S. A. , Adamu, D. , Agwom, S. S. , Pelayo, V. , Soler, A. , Simon, A. , Adedeji, A. J. , Yakubu, M. B. , Mantip, S. , Benshak, A. J. , Okeke, I. , Anago, P. , Mandeng, D. C. , Akanbi, B. O. , … Garllado, C. (2010). Surveillance for African swine fever in Nigeria, 2006–2009. Transboundary and Emerging Diseases, 57, 244–253. 10.1111/j.1865-1682.2010.01142.x [DOI] [PubMed] [Google Scholar]
- Fernandez‐Pinero, J. , Gallardo, C. , Elizalde, M. , Robles, A. , Gomez, C. , Bishop, R. , Heath, L. , Couacy‐Hymann, E. , Fasina, F. O. , Palayo, V. , Soler, A. , & Arias, M. (2012). Molecular diagnosis of African swine fever by a new real‐time PCR using universal probe library. Transboundary and Emerging Diseases, 1–11. 10.1111/j.1865-1682.2012.01317.x [DOI] [PubMed] [Google Scholar]
- Ganowiak, J. (2012). Patho‐anatomical studies of African swine fever in Uganda. In Sveriges lantbruksuniversitet Fakulteten for Veterinarmedicin Och husdjusvetenskap Institutionen for biomedicine Och Veterinas folkhalsovetenskap, sektionen for patologi (pp.652–8607). CGSpace A Repository of Agricultural Research Outputs. [Google Scholar]
- Igbokwe, I. O. , & Maduka, C. V. (2018). Disease burden affecting pig production in Nigeria: Review of current issues and challenges. Revue D’élevage Et De Médecine Vétérinaire Des Pays Tropicaux, 71(1‐2), 1–9. 10.19182/remvt.31290 [DOI] [Google Scholar]
- Jubb, K. V. F. , Kennedy, P. C. , & Palmer, N. C. (2016). African swine fever. In Grant Maxie M. (Ed.), Jubb, Kennedy and Palmer’s pathology of domest animal (Vol. 3, pp. 73–79). Elsevier Health Sciences. [Google Scholar]
- Liao, S. , & Padera, T. P. (2013). Lymphatic function and immune regulation in health and disease. Lymphatic Research and Biology, 11(3), 136–143. 10.1089/lrb.2013.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka, P. D. , Achenbach, J. E. , Mwiine, F. N. , Lamien, C. E. , Shamaki, D. , Unger, H. , & Erume, J. (2017). Genetic characterization of circulating African swine fever viruses in Nigeria (2007–2015). Transboundary and Emerging Diseases, 64(5), 1598–1609. 10.1111/tbed.12553 [DOI] [PubMed] [Google Scholar]
- Luther, N. J. , Majiyagbe, K. A. , Shamaki, D. , Lombin, L. H. , Antiabong, J. F. , Bitrus, Y. , & Owolodun, O. (2007). Detection of African swine fever virus genomic DNA in a Nigerian red river hog (Potamochoerus porcus). Veterinary Records, 160, 59–60. 10.1136/vr.160.2.58 [DOI] [PubMed] [Google Scholar]
- Murphy, F. A. , Gibs, E. P. J. , Horzinek, M. A. , & Studdert, M. J. (1999). Asfarviridae and Iridoviridae. In Veterinary virology (3rd ed., pp. 1–629). Academic Press. [Google Scholar]
- Mwiine, F. N. , Nkamwesiga, J. , Ndekezi, C. , & Ochwo, S. (2019). Molecular characterization of African swine fever virus from outbreaks in Peri‐urban Kampala, Uganda. Advanced Virology, 1–8. 10.1155/2019/1463245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odemuyiwa, S. O. , Isaac, A. A. , Ammeran, M. W. , Adebowale, T. P. , Alaka, O. O. , Oyedele, I. O. , Soyelu, K. O. , Olaleye, D. O. , Otesile, E. B. , & Muller, C. P. (2000). An outbreak swine fever in Nigeria: Virus isolation and molecular characterization of VP72 gene of the first isolate from West Africa. Virus Genes, 20(20), 139–142. [DOI] [PubMed] [Google Scholar]
- OIE . (2008). Office of International Epizootics, 2008: African Swine Fever 2008. Retrieved from https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/AFRICAN_SWINE_FEVER.pdf
- Owolodun, O. A. , Yakubu, B. , Antiabong, J. F. , Ogedengbe, M. E. , Luka, P. D. , John Audu, B. , Ekong, P. S. , & Shamaki, D. (2010). Spatio‐temporal dynamics of African swine fever outbreaks in Nigeria, 2002–2007. Transboundary and Emerging Diseases, 57, 330–339. 10.1111/j.1865-1682.2010.01153.x [DOI] [PubMed] [Google Scholar]
- Semergyan, A. B. , Tatoyan, M. R. , Karalyan, N. Y. , Nersisyan, N. H. , Hakobyan, L. H. , Arzumanyan, H. H. , & Karalyan, Z. A. (2018). Cardiopathology in acute African swine fever. Annals of Parasitology, 64(3), 253–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study as presented in the manuscript are available from the corresponding author, Dr. Tizhe E.V., upon reasonable and/or genuine request.


