Abstract
Brucellosis is a zoonotic disease which is endemic to certain regions of the world including Sub‐Saharan Africa. The aim of this article is to provide a recent and rapid review on brucellosis prevalence in East African Community (EAC) countries. Literature was obtained using Google Scholar search engine and screened for relevancy and fulfilment of criteria to 1, 17, 4, 4, 30 and 29 articles retained for brucellosis prevalence in Burundi, Kenya, Rwanda, South Sudan, Tanzania and Uganda. Recent literature (published in the last decade 2010 to 2019) was considered for prevalence results in this review. In EAC, livestock had an animal‐level prevalence of 0.2% to 43.8%, 0.0% to 20.0% and 0.0% to 13.8% for cattle, goats and sheep respectively. In humans, the prevalence varied mostly between 0.0% and 35.8%. In conclusion, brucellosis is quite prevalent in the region. The reported prevalence calls for plans or more efforts from individual member countries and from EAC, as a region, to control brucellosis.
Keywords: Brucella, brucellosis, East Africa, prevalence
Recently published literature shows that brucellosis is quite prevalent in the East African region. Most of the recent reviewed studies focused on specific identified areas or at risk communities such as exposed agro‐pastoral communities or patients attending hospitals with febrile illness symptoms.These studies call for countries and the region to plan or revive efforts towards the control and eradication of brucellosis.

1. INTRODUCTION
Brucellosis is a bacterial zoonotic infection which is among the most widespread and top neglected diseases in the world (World Health Organization, 2012).
Brucellosis is caused by bacteria belonging to the genus Brucella of which the most relevant species to livestock animal health and public health are B. abortus, B. melitensis, B. ovis and B. suis (Corbel, 2006; Godfroid et al., 2014). The infection in livestock animals occurs when the animals come in contact with infected animals, materials from infected animals (aborted materials, fetal membranes, vaginal discharges, milk, manure) or infected environment (Hamdy & Amin, 2002; Kaur et al., 2018; Langoni et al., 2000; Mugizi, Boqvist, et al., 2015; Mugizi, Muradrasoli, et al., 2015; Tekle et al., 2019). In livestock animals such as cattle, goats, sheep and pigs, brucellosis causes abortions and other reproductive disorders such as stillbirths, weak calves at birth, retained placenta and longer calving intervals in female animals (Boukary et al., 2013; McDermott et al., 2013; Schmutz et al., 1996).
Being a zoonotic disease, brucellosis is transmitted from animals to humans. Humans contract brucellosis when their skin (especially skin with cuts) or nasopharyngeal mucous tissues/membrane come in contact with infected animals’ materials such as abortion materials, fetuses, placental materials, vaginal discharges, urine and manure (Corbel, 2006; Pappas et al., 2006). Humans are also infected following the consumption of unpasteurized milk and milk products and improperly cooked meat from infected animals (Casalinuovo et al., 2016; Dadar et al., 2019). In humans, brucellosis causes a febrile illness with intermittent fevers, sweats, chills, weakness, malaise, headache, insomnia, anorexia, joint and muscle pain, constipation, sexual impotence, nervousness and depression (Acha & Szyfres, 2001; Corbel, 2006; Food & Drug Administration, 2012; Pappas et al., 2006). In human females, brucellosis may also be accompanied by abortion (Ali et al., 2016; Khan et al., 2001; Yang et al., 2018).
Although neglected, brucellosis results in animal health burdens for the farmers and is a public health concern. It is, therefore, important for countries to implement necessary measures to control and eradicate brucellosis. For any measures to be implemented, however, brucellosis prevalence and epidemiological data are needed to form the basis for planning and tackling effectively this zoonotic infection. In African countries, in general, brucellosis prevalence and epidemiological data have been presented in a number of reviews (Akakpo & Bornarel, 1987; Boukary et al., 2014; Craighead et al., 2017; Ducrotoy et al., 2015; McDermott & Arimi, 2002; McDermott et al., 2013) and showed the presence and prevalence of brucellosis on the continent in the recent past years. In EAC countries, in particular, a review is yet to be conducted to focus and provide updated data on brucellosis prevalence in the region.
The aim of this article was, therefore, to review and summarize recent literature (2010–2019) on the prevalence of brucellosis in animals and humans, with reference to EAC countries.
2. METHODOLOGY
2.1. Literature search
To write this review, a literature search was conducted using Google Scholar database. Literature search was conducted for all relevant and recent articles (published from 2010 to 2019) on brucellosis prevalence in the six EAC countries, namely, Burundi, Kenya, Rwanda, South Sudan, Tanzania and Uganda (Figure 1). The used search terms were “Brucellosis AND Prevalence AND Country”, “Animal Brucellosis AND country”, “Human Brucellosis AND country”, “Bovine Brucellosis AND country”, “Caprine brucellosis and country”, “Ovine brucellosis AND country” and “Porcine brucellosis AND country”. Following literature search and articles screening, 1, 17, 4, 4, 30 and 29 articles were retained for results presentation on brucellosis prevalence in Burundi, Kenya, Rwanda, South Sudan, Tanzania and Uganda respectively (Figure 2).
FIGURE 1.
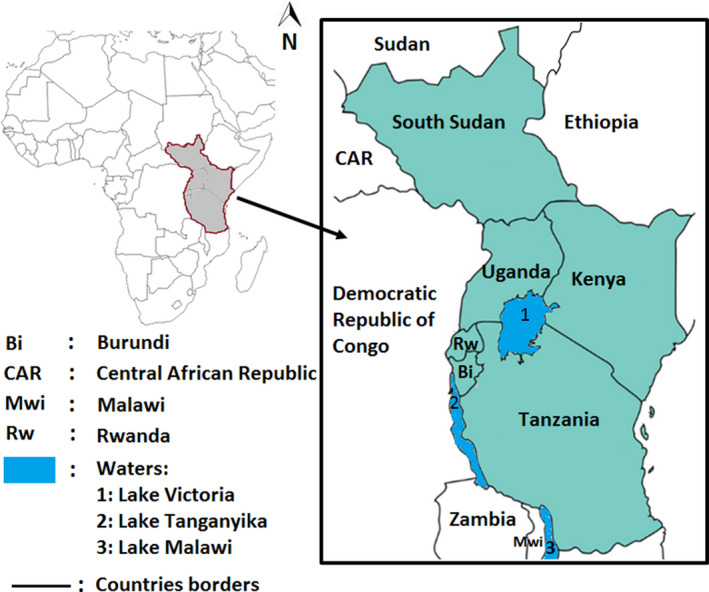
Map showing the location of the six EAC member countries (in grey)
FIGURE 2.

Literature search and screening for articles to be included in results on brucellosis prevalence in EAC countries
In addition to Google Scholar literature search, relevant reports on country's profiles were obtained and consulted from websites of governments’ institutions such as National Bureaus of Statistics and Ministries of Agriculture and from international institutions such as Food and Agriculture Organization of the United Nations (FAO).
2.2. Inclusion and exclusion criteria
To have a recent picture of brucellosis prevalence in EAC countries, articles were included if they reported on or included data on brucellosis (animal and/or human) prevalence in at least one of the EAC countries (Burundi, Kenya, Rwanda, South Sudan, Tanzania and Uganda), if they were published in the last decade (between 2010 and 2019) and if they were articles published in scientific journals. Articles were excluded if they did not report on brucellosis prevalence in EAC countries and if they reported on brucellosis prevalence in EAC countries but were published before 2010. Articles were also excluded if they were review articles or were other scientific work (theses, posters and reports) not published in scientific journals.
3. RESULTS
In EAC and in the last decade (2010 to 2019), a number of studies have been conducted on both animal and human brucellosis prevalence. The number of brucellosis studies in the EAC countries is, however, different from more brucellosis studies conducted in Uganda, Tanzania and Kenya compared with Rwanda, South Sudan and Burundi.
3.1. Brucellosis prevalence in Burundi
Burundi is the second smallest EAC country located at the West of the regional block (Figure 1) and sharing borders with Rwanda at the North, Tanzania at the East and South, Rwanda and Democratic republic of Congo at the West. Burundi has a total area of 27,834 square kilometres and a population estimated at 11.5 million (World Bank, 2019). Burundi's most popular livestock are small ruminants (Desiere et al., 2015) with 40% to 60% of rural households owning goats and/or sheep compared to 10% to 20% of the rural households owning cattle (Jeníček & Grofová, 2015). According to 2018 estimations by FAO, there were 3,249,827 goats, 1,110,936 cattle, 774,689 pigs and 548,608 sheep in Burundi (Food and Agriculture Organization of United Nations, n.d.). Cattle rearing is especially observed in the Imbo plain at the most western part of the country and in the provinces of Bururi, Mwaro and Muramvya (Manirakiza et al., C; USAID, 2009). In Burundi, the traditional extensive agro‐pastoral cattle production system is gradually being replaced by the mixed crops–livestock system in which cattle is kept in closed spaces and fed with cut and carried forage and crop residues. Around cities like Bujumbura where the demand in fresh milk is high, the intensive cattle production system is the most commonly practiced (Manirakiza et al., 2020).
Very few studies on brucellosis prevalence in Burundi exist in literature. Although the detection of the first cases of human brucellosis were reported in what used to be Rwanda–Urundi in 1930s (Pergher & Noel, 1936), a few further studies on brucellosis in Burundi were reported, one in the 1960s and the other in the 1980s (Merker & Schlichting, 1984; Thienpont et al., 1961). Recently and in the last decade, one study on brucellosis prevalence in Burundi was found in searched literature. The study, which was conducted on dairy herds in West and Central Africa by testing farm bulk milks with milk Enzyme Linked Immuno‐Sorbent Assay, reported a sero‐prevalence of 14.7% (95% CI: 9.4–20.8) among dairy cattle herds in peri‐urban Bujumbura (Musallam et al., 2019). For the purpose of this review, recent (2010 to 2019) brucellosis studies on other livestock and on humans in Burundi were not found or were not reported in searched literature.
3.2. Brucellosis prevalence in Kenya
Kenya is the biggest economy in EAC. Kenya is located at the East of the regional block (Figure 1) and sharing borders with Ethiopia and South Sudan at the North, Somalia at the East, Tanzania at the South and Uganda at the West. Kenya is 580,370 square kilometres and has a population of 47.6 million (Kenya National Bureau of Statistics, 2019). Kenya's livestock census of 2009 indicated that cattle population was estimated at 17,467,774 heads and goats were 27,740,153 heads while the sheep population was 17,129,606 (Kenya National Bureau of Statistics, 2019). According to the same census, high populations of livestock were observed in the Rift Valley region. In Kenya, livestock is raised under different livestock production systems including intensive, semi‐intensive and extensive production systems. Dairy cattle, which are an important part of livestock in Kenya, are raised under different production systems. It was estimated that 45% of dairy cattle farms practice the semi‐intensive/semi‐grazing system while 35% of the farms practice the intensive system but on a small scale. The rest of dairy cattle farms practice controlled extensive system (10% of farms), uncontrolled extensive system (5% of farms) and intensive system on a large scale (5% of farms; Food and Agriculture Organization of United Nations, 2018a). Livestock, in general, depends on rangelands resources which support 70% of the livestock in the country (Kenya Ministry of Agriculture & Livestock, 2019).
Brucellosis has been studied in Kenya for a long time as shown by a recent review on the disease frequency in humans and animals and risk factors for human infection from studies conducted as early as 1916 (Njeru, Wareth, et al., 2016). In the last decade (2010–2019), a number of studies have also reported on brucellosis prevalence in Kenya (Table 1).
TABLE 1.
Studies on livestock and human brucellosis prevalence in Kenya in the last decade (2010–2019)
| Species and study scope | Sample size | Diagnostic test | Prevalence in % (95% confidence interval) | Reference |
|---|---|---|---|---|
| Cattle | ||||
| Subnational | 356 | CFT | 0.9–4.6 a | Chota et al. (2016) |
| Subnational | 225 | i‐ELISA | 12.4 (7.7–15.4) | Enström et al. (2017) |
| Subnational | 983 | Rapid immuno‐chromatographic flow assay | 0.2 (0.0–0.5) c | Fèvre et al. (2017) |
| Subnational | 441 | c‐ELISA | 6.3 (4.0–8.6) | Kairu‐wanyoike et al. (2019) |
| Subnational | 149 | RBT | 10.7 a | Kosgei et al. (2014) |
| Subnational | 208 | MRT | 7.7 a | Gicheru et al. (2015) |
| Subnational | 250 | RBT & c‐ELISA | 21.9 a | Nakkel et al. (2016) |
| Subnational | 398 | c‐ELISA | 16.8 (13.2–20.4) | Okumu et al. (2019) |
| Subnational | 2,978 | i‐ELISA | 4.1 (3.4–4.8) | Osoro et al. (2015) |
| Goats | ||||
| Subnational | 123 | CFT | 0.0–20.0 a | Chota et al. (2016) |
| Subnational | 961 | c‐ELISA | 3.3 (2.1–4.4) | Kairu‐wanyoike et al. (2019) |
| Subnational | 92 | RBT | 13.0 a | Kosgei et al. (2014) |
| Subnational | 167 | RBT & c‐ELISA | 7.3 a | Nakkel et al. (2016) |
| Subnational | 4,080 | c‐ELISA | 10.7 (9.3–12.3) | Osoro et al. (2015) |
| Sheep | ||||
| Subnational | 30 | CFT | 0.0 to 13.8 a | Chota et al. (2016) |
| Subnational | 623 | c‐ELISA | 1.4 (0.5– 2.3) | Kairu‐wanyoike et al. (2019) |
| Subnational | 73 | RBT | 8.2 a | Kosgei et al. (2014) |
| Subnational | 167 | RBPT & c‐ELISA | 8.6 a | Nakkel et al. (2016) |
| Subnational | 3,088 | c‐ELISA | 7.3 (6.1–8.8) | Osoro et al. (2015) |
| Humans | ||||
| Subnational | 562 | b | 17.1 a | Chota et al. (2016) |
| Subnational | 2,113 | Rapid immuno‐ chromatographic flow assay | 0.6 (0.2–0.9) | Fèvre et al. (2017) |
| Subnational | 1,022 | IgG‐ELISA | 35.8 (32.8–38.8) | Kairu‐wanyoike et al. (2019) |
| Subnational | 317 | RBT & c‐ELISA | 1.3 a | Nakkel et al. (2016) |
| Subnational | 2,811 | IgG‐ELISA | 16.4 (13.5–19.6) | Osoro et al. (2015) |
| Subnational | 825 | Rapid immuno‐chromatography flow assay | 3.4 a | de Glanville et al. (2017) |
| Subnational | 1,043 | b | 32.3 a | Maiyo and Obey (2016) |
| Subnational | 1,067 | RBT, IgM/IgG‐ELISA & q‐PCR | 13.7 (11.7–15.9) | Njeru, Melzer, et al. (2016) |
Abbreviations: c‐ELISA, Competitive Enzyme‐Linked Immuno‐Sorbent Assay; CFT, Complement Fixation Test; i‐ELISA, Indirect Enzyme‐Linked Immuno‐Sorbent Assay; IgG/IgM‐ELISA, Immunoglobulins G and M Enzyme‐Linked Immuno‐Sorbent Assay; IgG‐ELISA, Immunoglobulin G Enzyme‐Linked Immuno‐Sorbent Assay; MRT, Milk Ring Test; q‐PCR, Quantitative Polymerase Chain Reaction; RBT, Rose Bengal Test.
Prevalence confidence interval not provided in the original article.
Diagnostic test not specified (Study based on health centre results’ records).
The computed confidence interval value was <0.0.
Using a variety of diagnostic tests most of which were serological (Table 1), prevalence in cattle varied between 0.2% and 21.9% (Chota et al., 2016; Enström et al., 2017; Fèvre et al., 2017; Gicheru et al., 2015; Kairu‐wanyoike et al., 2019; Kosgei et al., 2014; Nakkel et al., 2016; Okumu et al., 2019; Osoro et al., 2015). For the small ruminants, brucellosis prevalence ranged from 0.0% to 20.0% in goats and 0.0% to 13.8% in sheep (Chota et al., 2016; Kairu‐wanyoike et al., 2019; Kosgei et al., 2014; Nakkel et al., 2016).
Human brucellosis was studied especially for small livestock keeping communities and for patients attending hospitals with brucellosis symptoms. The reported prevalence ranged from 0.6% to 35.8% in humans (Chota et al., 2016; de Glanville et al., 2017; Fèvre et al., 2017; Kairu‐wanyoike et al., 2019; Maiyo & Obey, 2016; Nakkel et al., 2016; Njeru, Melzer, et al., 2016; Osoro et al., 2015). However, a higher county‐level prevalence of 46.5% among humans was reported in the county of Marsabit in a study conducted for a group of three counties (including Marsabit) to determine sero‐prevalence and risk factors for brucellosis among humans and their livestock (Osoro et al., 2015).
Of the two recent studies which covered brucellosis in camels, one reported a prevalence of 11.1% in camels in Marsabit county (Osoro et al., 2015) while the other reported a prevalence of 0% in five camels tested in West Pokot county (Chota et al., 2016).
In the last decade, a few studies also covered or extended their investigations on the prevalence of Brucella antibodies in milk in Kenya. One recent study, which investigated the incidence and knowledge of brucellosis in Kahuro district, Murang'a county, used the Milk Ring Test and reported that 22% of the analysed 150 pooled milk samples were positive to brucellosis (Njuguna et al., 2017). In the same study, 230 individual farm bulk milks were collected from farmers and analysed and 24% were positive to brucellosis. Such prevalence in raw milks, although not directly indicating the individual animal prevalence in cattle, is an indication of brucellosis prevalence in cattle in Kenya and the risk of transmission to consumers when milk is not pasteurized prior to using.
3.3. Brucellosis prevalence in Rwanda
Rwanda is the smallest country in EAC located at the West of the regional block (Figure 1) and sharing borders with Uganda at the North, Tanzania at the East, Burundi at the South and Democratic Republic of Congo at the West. Rwanda has a total area of 26,338 square kilometres. The total population in the country is estimated at 12.0 million (National Institute of Statstics of Rwanda, 2019). Rwanda's important livestock are cattle, goats, sheep and pigs. The reported numbers of different livestock showed that the country had a total of 1,856,490 cattle, 2,283,445 goats, 499,316 sheep and 703,145 pigs. The Eastern province had the highest number of cattle (28.3%) followed closely by the southern province with 27.3% of cattle (National insititute of statistics of Rwanda, 2018). In Rwanda, cattle is raised mainly under the small‐scale zero grazing system which is practiced by 80% of cattle keeping households. Semi‐intensive and extensive/open grazing systems are practiced by 3% and 17% of the cattle keeping households respectively (Land O’ lakes, 2014).
Brucellosis studies have been conducted in Rwanda, although few and scattered over the years (Akayezu, 1984; Chatikoba et al., 2008; Gafirita et al., 2017; Manishimwe et al., 2015; Ndazigaruye et al., 2018; Rujeni & Mbanzamihigo, 2014; Thienpont et al., 1961). In the last decade (2010–2019), a total of four studies were published on animal and human brucellosis in the country (Table 2). For animal brucellosis, a study published in 2015 (Manishimwe et al., 2015) was focusing on comparing Rose Bengal Test (RBT) with competitive Enzyme Linked Immuno‐Sorbent Assay (c‐ELISA) in detecting Brucella antibodies in cattle serum. The study was conducted on a total of 2017 sera previously collected from 157 cattle farms in Kigali and reported a bovine brucellosis prevalence of 2.0% using RBT and 1.7% using c‐ELISA. A second study, published in 2018, was conducted in Nyagatare district with the aim of analysing the risk factors associated with brucellosis in cattle in the district (Ndazigaruye et al., 2018). The overall reported bovine brucellosis prevalence in Nyagatare district was 18.9%. With these studies, brucellosis prevalence in the last decade varied between 1.7% and 18.9% among cattle in Rwanda. No studies on brucellosis in small ruminants were found in searched literature for Rwanda and in the last decade.
TABLE 2.
Studies on livestock and human brucellosis prevalence in Rwanda in the last decade (2010–2019)
| Species and study scope | Sample size | Diagnostic test | Prevalence in % (95% Confidence Interval) | Reference |
|---|---|---|---|---|
| Cattle | ||||
| Subnational | 2,017 | RBT | 2.0 a | Manishimwe et al. (2015) |
| c‐ELISA | 1.7 a | |||
| Subnational | 604 | RBT | 18.9 a | Ndazigaruye et al. (2018) |
| Humans | ||||
| Subnational | 198 | RBT | 6.1 (0.6–7.8) | Gafirita et al. (2017) |
| Subnational | 60 | RBT | 25 a | Rujeni and Mbanzamihigo (2014) |
c‐ELISA: Competitive Enzyme‐Linked Immuno‐Sorbent Assay; RBT: Rose Bengal Test.
Prevalence confidence interval not provided in the original article.
Reported human brucellosis in Rwanda in the last decade varied between 6.1% and 25% according to two studies (Gafirita et al., 2017; Rujeni & Mbanzamihigo, 2014). In the first study conducted on women presenting with abortion or stillbirth of unknown origin at Huye district hospital, a prevalence of 25% among those women was reported (Rujeni & Mbanzamihigo, 2014). The second study covered patients attending Nyagatare district hospital, willing to participate in the study and having any of the following symptoms: intermittent or persistent fever, headache, weakness, profuse sweating, chills, arthralgia, weight loss and joint pain. The study reported a prevalence of 6.1% (Gafirita et al., 2017).
3.4. Brucellosis prevalence in South Sudan
South Sudan is the newest EAC member country located at the north of the regional block (Figure 1) and bordered by Sudan at the North, Ethiopia at the East, Kenya, Uganda and Democratic Republic of Congo at the South and Central African Republic at the West. South Sudan has an area of 644,329 square kilometres. South Sudan population was estimated at 12.2 million (United Nations Economic Commission for Africa, 2017). Livestock, including cattle, goats and sheep, is a very important agricultural subsector in South Sudan where the livestock per capita holding is considered among the first in Africa. Cattle, goats, sheep, camel and pig populations were estimated at 17,729,188; 12,307,686; 11,682,172; 23,583 and 14,406 respectively (Onyango et al., 2015). Livestock is distributed all over South Sudan but more livestock populations are observed in the upper half of the country in the states of Northern Bhar El Ghazal, Warrap, Jonglei, Lakes, Western Bhar El Ghazal and Unit (Onyango et al., 2015). In South Sudan, livestock is reared under agro‐pastoralism and pastoralism mainly with 85% of South Sudanese households involved in livestock being agro‐pastoralists and the remaining 15% being pastoralists (Emmanuel et al., 2018; Food and Agriculture Organization of United Nations, 2016).
As is the case for Burundi and Rwanda, studies on brucellosis in South Sudan are still few. Among the few studies undertaken and published in the period of 2010 to 2019 (Table 3) was a cross‐sectional study conducted in peri‐urban Juba and in Terekeka county on bovine brucellosis (Lita et al., 2016). In this study, an overall individual animal sero‐prevalence of 31.9% by RBT and 29.3% by c‐ELISA was reported. A series of three brucellosis sero‐prevalence studies was also conducted by Madut et al. on febrile patients attending hospital, on cattle and their herders and on slaughter house workers in the region of Bahr el Ghazal (Madut et al., 2019; Madut, Muwonge, et al., 2018; Madut, Nasinyama, et al., 2018). The prevalence among 416 febrile patients attending Wau hospital in Bahr el Ghazal region was 23.3% after tests of blood samples by RBT and Serum Agglutination and confirmation of results by c‐ELISA (Madut, Muwonge, et al., 2018; Madut, Nasinyama, et al., 2018). Of the 893 bovine sera and 87 herders’ sera tested using RBT and confirmation by c‐ELISA, the reported overall prevalence was 31.0% among cattle and 33.3% among cattle herders (Madut, Muwonge, et al., 2018; Madut, Nasinyama, et al., 2018). In the same region of Bahr el Ghazal, a total 234 slaughterhouse workers were screened for brucellosis infection using RBT and the overall prevalence was 32.1% following c‐ELISA confirmation of results (Madut et al., 2019). With these few studies in the last decade, reported brucellosis prevalence in South Sudan varied from 29.3% to 31.9% among cattle and from 23.3% to 33.3% among humans.
TABLE 3.
Studies on livestock and human brucellosis prevalence in South Sudan in the last decade (2010–2019)
| Species and study scope | Sample size | Diagnostic test | Prevalence in % (95% Confidence Interval) | Reference |
|---|---|---|---|---|
| Cattle | ||||
| Subnational | 160 | RBT | 31.9 a | Lita et al. (2016) |
| 147 | c‐ELISA | 29.3 a | ||
| Subnational | 893 | RBT & c‐ELISA | 31.0 (28.0–34.2) | Madut, Muwonge, et al. (2018); Madut, Nasinyama, et al. (2018) |
| Humans | ||||
| Subnational | 416 | RBT, SAT & c‐ELISA | 23.3 a | Madut, Muwonge, et al. (2018); Madut, Nasinyama, et al. (2018) |
| Subnational | 87 | RBT & c‐ELISA | 33.3 (23.9–44.3) | Madut, Muwonge, et al. (2018); Madut, Nasinyama, et al. (2018) |
| Subnational | 234 | RBPT & c‐ELISA | 32.1 a | Madut et al. (2019) |
Abbreviations: c‐ELISA, Competitive Enzyme‐Linked Immuno‐Sorbent Assay; RBT, Rose Bengal Test; SAT, Serum Agglutination Test.
Prevalence confidence interval not provided in the original article.
3.5. Brucellosis prevalence in Tanzania
Tanzania is an EAC country located at the South of the regional block (Figure 1) and sharing borders with Uganda at the North, Kenya at the North‐East, Mozambique at the South, Malawi at the South‐West, Zambia and Democratic Republic of Congo at the West and Burundi and Rwanda at the North‐West. According to Tanzania National Bureau of Statistics, Tanzania has a total area of 947,300 square kilometres and a population of 54.2 million (Tanzania National Bureau of Statistics, 2019). Tanzania's most important livestock is cattle at an estimated population of 30,672,001 heads followed by goats estimated at 19,055,651 heads and sheep estimated at 5,565,986 heads (Tanzania National Bureau of Statistics, 2017). In Tanzania, livestock is especially raised in Shinyanga, Mwanza and Tabora regions (Engida et al., 2015).
Many brucellosis studies have been conducted in Tanzania. Recent brucellosis studies in cattle in Tanzania used serological diagnostic tests, mostly the RBT to screen samples and c‐ELISA to confirm the results (Table 4). They reported an individual cattle brucellosis prevalence varying mostly between 0.2% and 11.7% (Asakura et al., 2018a,2018b; Assenga et al., 2015; Chitupila et al., 2015; Chota et al., 2016; Kayombo et al., 2017; Mathew, 2017; Sagamiko et al., 2018; Shirima et al., 2010; Shirima & John, 2016; Swai & Schoonman, 2010, 2012).
TABLE 4.
Studies on livestock and human brucellosis prevalence in Tanzania in the last decade (2010–2019)
| Species and study scope | Sample size | Diagnostic test | Prevalence in % (95% Confidence Interval) | Reference |
|---|---|---|---|---|
| Cattle | ||||
| Subnational | 667 | RBT & c‐ELISA | 0.2 (0.0–1.1) d | Asakura et al. (2018a) |
| 673 | RBT & c‐ELISA | 7.0 (5.7–8.4) | ||
| Subnational | 1,103 | RBT & c‐ELISA | 6.8 (5.4–8.5) | Assenga et al. (2015) |
| Subnational | 410 | RBT & c‐ELISA | 5.6 (3.8–8.3) | Chitupila et al. (2015) |
| Subnational | 1,376 | RBT & c‐ELISA | 1.0–11.4 a | Chota et al. (2016) |
| Subnational | 192 | RBT & c‐ELISA | 4.2 a | Kayombo et al. (2017) |
| Subnational | 658 | i‐ELISA | 5.4 a | Mathew, 2017) |
| Subnational | 200 | RBT | 21.5 (16–27) | Mathew et al. (2015) |
| i‐ELISA | 48.0 (41–55) | |||
| Subnational | 296 | RBT & c‐ELISA | 7.8 a | Shirima and John (2016) |
| Subnational | 929 | RBT & c‐ELISA | 2.8 (1.4–5.6) | Sagamiko et al. (2018) |
| 282 | RBT & c‐ELISA | 11.3 (9.4–13.5) | ||
| Subnational | 2,723 | c‐ELISA | 4.9 a | Shirima et al. (2010) |
| Subnational | 51 | RBT | 11.7 (9.1–14.9) | Swai and Schoonman (2012) |
| Subnational | 655 | RBT | 5.3 (3.1–7.8) | Swai and Schoonman (2010) |
| Subnational | 483 | RBT & c‐ELISA | 28.9 a | Shirima et al. (2014) |
| Goats | ||||
| Subnational | 248 | RBT & c‐ELISA | 1.6 (0.4–4.1) | Assenga et al. (2015) |
| Subnational | 50 goats | RBT | 0 | Mathew et al. (2015) |
| i‐ELISA | 2.0 (0.0–7.0) d | |||
| Subnational | 75 | RBT & c‐ELISA | 0 | Shirima and John (2016) |
| Subnational | c | RBT & c‐ELISA | 0 | Shirima et al. (2014) |
| Sheep | ||||
| Subnational | 35 | RBT | 0 | Mathew et al. (2015) |
| i‐ELISA | 5.7 (0.0–17.0) d | |||
| Subnational | 42 | RBT & c‐ELISA | 0 | Shirima and John (2016) |
| Humans | ||||
| Subnational | 340 | RBT, BAPA & Riv. T | 0.6 (0.1–2.1) | Assenga et al. (2015) |
| Subnational | 455 | MAT & Blood culture | 3.5 a | Bouley et al. (2012) |
| Subnational | 1,095 | MAT & Blood culture | 2.9 a | Carugati et al. (2018) |
| Subnational | 562 | MAT | 6.9 a | Cash‐Goldwasser et al. (2017) |
| Subnational | 370 | IgM‐ELISA , IgG‐ELISA & MAT | 7.0 a | Chipwaza et al. (2015) |
| Subnational | 578 | b | 28.2 a | Chota et al. (2016) |
| Subnational | 118 | MAT | 13.6 a | Crump et al. (2013) |
| Subnational | 250 | SAT | 48.4 (42–54) | Mirambo et al. (2018) |
| Subnational | 382 | Rapid Brucella serum agglutination | 14.1 (10.6–17.5) | Mngumi et al. (2016) |
| Subnational | 148 | SAT | 58.1 (50–66) | Mujuni et al. (2018) |
| Subnational | 13,642 | b | 5.8 a | Nonga and Mwakapeje (2017) |
| Subnational | 82 | RBT & c‐ELISA | 0 | Shirima and John (2016) |
| Subnational | 120 | c‐ELISA | 10.0 a | Shirima et al. (2014) |
| Subnational | 460 | c‐ELISA | 8.3 a | Shirima et al. (2010) |
Abbreviations: BAPA, Buffered Acidified Plate Antigen Test; c‐ELISA, Competitive Enzyme‐Linked Immuno‐Sorbent Assay; i‐ELISA, Indirect Enzyme‐Linked Immuno‐Sorbent Assay; IgG‐ELISA, Immunoglobulin G Enzyme‐Linked Immuno‐Sorbent Assay; IgM‐ELISA, Immunoglobulin M Enzyme‐Linked Immuno‐Sorbent Assay; MAT, Microscopic Agglutination Test; RBT, Rose Bengal Test; Riv. T, Rivanol precipitation Test; SAT, Slide Agglutination Test.
Prevalence confidence interval not provided in the original article.
Diagnostic test not specified (Study based on health centre results’ records).
Sample size not specified.
The computed confidence interval value was <0.0.
However, in one study which was conducted on animals from a single farm with a total of 350 cattle, a higher individual cattle brucellosis prevalence of 48% was reported (Mathew et al., 2015). The dairy herd from which the high individual cattle brucellosis was reported is located in the southern highlands of Tanzania and had been experiencing abortions (Mathew et al., 2015). Another investigative study following an abortion storm on a research farm reported an individual cattle brucellosis prevalence of 28.9% (Shirima et al., 2014). Brucellosis in this farm was eventually controlled through culling, among other measures, and brought to 0.0% over a period of 5 years.
Human brucellosis studies in Tanzania included mostly hospital patients with symptoms, like fever and spontaneous abortions, and communities in pastoral and agro‐pastoral areas. The reported human brucellosis prevalence in Tanzania from recent studies (2010–2019) varied mostly between 0% and 28.2% (Assenga et al., 2015; Bouley et al., 2012; Carugati et al., 2018; Cash‐goldwasser et al., 2017; Chipwaza et al., 2015; Chota et al., 2016; Crump et al., 2013; Mngumi et al., 2016; Nonga & Mwakapeje, 2017; Shirima et al., 2010; Shirima & John, 2016). It should, however, be mentioned that a prevalence as high as 58.1% was reported from a study which investigated the association of Brucella seropositivity with abortion for a group of 148 women with spontaneous abortions and 250 women with full‐term deliveries. The group of women with spontaneous abortions had a prevalence of 58.1%, while the group with full‐term deliveries had a prevalence of 26% (Mujuni et al., 2018). A different but also focused study on 250 abattoir workers and meat vendors in the city of Mwanza reported that 48.4% of them were Brucella seropositive (Mirambo et al., 2018).
Fewer studies were conducted on small ruminants in Tanzania in the last decade. Brucellosis prevalence in goats was between 0% and 2.0% while brucellosis prevalence in sheep was between 0% and 5.7% (Assenga et al., 2015; Mathew et al., 2015; Shirima & John, 2016).
Apart from common livestock animals, a few recently published studies (2010–2019) in Tanzania covered other animals. A study on the epidemiology of Brucella infection in human, livestock and wildlife interface in the Katavi‐Rukwa ecosystem included 38 buffaloes, two lions and two zebras and reported a brucellosis prevalence of 7.9% in buffaloes while one of the two lions was seropositive and none of the zebras was (Assenga et al., 2015). In a study to investigate a farm which had been experiencing cattle abortions, 6 dogs were included and none of the dogs tested positive for brucellosis (Mathew et al., 2015). Dogs were also covered in another study with a sample of 100 dogs randomly selected in the region of Morogoro and no dog was positive to Brucella canis (Muhairwa et al., 2012). A study conducted on camels in agro‐pastoral communities of Northern Tanzania reported a prevalence of 2.1% at an animal level for a sample of 193 camels selected from 14 traditional herds (Swai et al., 2011). A low prevalence of 0.7% was reported in pigs from five selected pig slaughter facilities in Dar‐Es‐Salam (Simon et al., 2015).
A few recent studies (published from 2010 to 2019) covered also the prevalence of Brucella antibodies in raw marketed milk in Tanzania. One such study which sought to evaluate the microbiological quality and associated health risks for raw milk marketed in Tanga region reported that 56% of 59 raw milk samples collected from selling points and deliverers were Brucella positive (Swai & Schoonman, 2011). This high milk contamination may be due to milk bulking and pooling from different cattle and farms. It presents a risk for transmission of brucellosis to consumers and calls for milk boiling or pasteurization prior to using. Another study which, in addition to animal sera, investigated the milk from dairy animals reported a prevalence of Brucella in 3.7% and 0% of cattle milk and goat milk respectively (Assenga et al., 2015). Herd‐level brucellosis prevalence was, also, investigated in a study using bulk farm milk. The prevalence of Brucella in milk at herd level was 44.4% of 124 agro‐pastoral farms investigated in Morogoro region (Asakura et al., 2018a,2018b).
In addition to determining brucellosis prevalence in animals and humans, a few studies in Tanzania identified and characterized some prevalent Brucella species. In their investigative study on a single farm, Mathew and colleagues (Mathew et al., 2015) cultured, identified and characterized Brucella isolates from cattle to be Brucella abortus biovar 3. In another study published about the same time, molecular methods were used and Brucella abortus biovar 1 was identified and characterized from cattle milk (Assenga et al., 2015).
3.6. Brucellosis prevalence in Uganda
Uganda is an EAC country at the Central‐West of the regional block (Figure 1). Uganda shares borders with South Sudan at the North, Kenya at the East, Tanzania at the South, Rwanda at the South‐West and Democratic Republic of Congo at the West. As indicated by Uganda Bureau of Statistics, Uganda is 241,550.7 square kilometres in total area and has a human population of 41.0 million (Uganda Bureau of Statistics, 2018). Uganda's important livestock are cattle, goats, sheep, pigs and poultry. There were 14,189,000 cattle, 16,034,000 goats, 4,445,000 sheep and 4,109,000 pigs in 2017 (Uganda Bureau of Statistics, 2018). The most important livestock in Uganda is cattle. Cattle is especially found in the “cattle corridor” which extends from the northeast of the country, with the highest concentration of cattle, to the southwest (Egeru et al., 2014). Cattle in Uganda is raised under commercial ranching, pastoral, agro‐pastoral and semi‐intensive production systems, the agro‐pastoral system being the most common one (Food and Agriculture Organization of United Nations, 2019).
In a recent report, FAO estimated brucellosis prevalence in Uganda at a national level to be 10% in cattle and 5.5% in cattle keepers (Food and Agriculture Organization of United Nations, 2018b). In addition to FAO’s national estimations, many other studies on brucellosis have been conducted in Uganda. According to most recent cattle brucellosis studies published from 2010 to 2019 (Table 5), brucellosis was diagnosed using mostly serological tests (Table 5). The reported brucellosis prevalence in cattle (animal level) varied a lot and was between 1.2% and 43.8% (Bugeza et al., 2019; Ezama et al., 2019; Kabi et al., 2015; Kashiwazaki et al., 2012; Lolli et al., 2016; Makita et al., 2011; Miller et al., 2016; Mugizi, Boqvist, et al., 2015; Mugizi, Muradrasoli, et al., 2015; Nanfuka, 2018; Nguna et al., 2019; Nina et al., 2017; Nizeyimana et al., 2013).
TABLE 5.
Studies on livestock and human brucellosis prevalence in Uganda in the last decade (2010–2019)
| Species and study scope | Sample size | Diagnostic test | Prevalence in % (95% Confidence Interval) | Reference |
|---|---|---|---|---|
| Cattle | ||||
| Subnational | 728 | RBT & i‐ELISA | 3.2 (1.9–4.5) | Bugeza et al. (2019) |
| Subnational | 839 | RBT | 34.7 a | Ezama et al. (2019) |
| National | 925 | i‐ELISA & c‐ELISA | 8.64 a | Kabi et al. (2015) |
| Subnational | 1,237 | RBT & i‐ELISA | 21.5 a | Kashiwazaki et al. (2012) |
| 1,033 | RBT & i‐ELISA | 3.4 a | ||
| Subnational | 3,935 | RBT | 9.8 (8.9–10.7) | Lolli et al. (2016) |
| Subnational | 423 | c‐ELISA | 5.0 (2.7–9.3) | Makita et al. (2011) |
| Subnational | 768 | RBT (Abortus antigen) | 12.9 (10.0–16.2) | Miller et al. (2016) |
| RBT (Melitensis antigen) | 15.7 (12.4–19.3) | |||
| Subnational | 1,007 | i‐ELISA & c‐ELISA | 7.5 (6.1–9.4) | Mugizi, Boqvist, et al. (2015); Mugizi, Muradrasoli, et al. (2015) |
| Subnational | 1,503 | SAT & i‐ELISA | 23.0 a | Nanfuka, 2018) |
| Subnational | 345 | i‐ELISA | 1.2 a | Nguna et al. (2019) |
| Subnational | 1,749 | RBT & i‐ELISA | 43.8 a | Nina et al. (2017) |
| Subnational | 149 | i‐ELISA | 3.3 a | Nizeyimana et al. (2013) |
| Goats | ||||
| Subnational | 305 | RBT | 8.8 a | Dubad et al. (2015) |
| Subnational | 729 | RBT | 8.8 (6.9–11.1) | Lolli et al. (2016) |
| Subnational | 315 | RBT (Abortus antigen) | 1.1 (0–6.0) d | Miller et al. (2016) |
| RBT (Melitensis antigen) | 9.8 (3.8–15.7) | |||
| Subnational | 351 | i‐ELISA | 0.3 a | Nguna et al. (2019) |
| Sheep | ||||
| Subnational | 95 | RBT | 10.5 a | Dubad et al. (2015) |
| Subnational | 306 | RBT | 2.6 (1.2–5.3) | Lolli et al. (2016) |
| Humans | ||||
| Subnational | 216 | RBT | 33 (27–39) | Ezama et al. (2019) |
| Subnational | 216 | RBT & IgM‐ELISA | 13.4 a | Ezama et al. (2018) |
| Subnational | 177 | Rapid agglutination test | 10.7 a | Frank et al. (2017) |
| Subnational | 9,177 | PAT | 14.4 a | Kansiime et al. (2015) |
| Subnational | 200 | SAT and TAT | 7.5 a | Majalija et al. (2018) |
| Subnational | 235 | RBT | 14.9 (10.6–20.1) | Migisha et al. (2018) |
| Blood culture | 4.3 a | |||
| Subnational | 236 | IgG/IgM‐LFA | 8.1 (3.2–13.0) | Miller et al. (2016) |
| Subnational | 251 | PAT | 18.7 a | Muloki et al. (2018) |
| Subnational | 232 | MAT & TAT | 10 (6–16) | Nabukenya et al. (2013) |
| Subnational | 113 | i‐ELISA | 4−12 a | Nanfuka (2018) |
| Subnational | 161 | Rapid Agglutination Test, TAT & c‐ELISA | 5.8 (3.3–8.3) | Nasinyama et al. (2014) |
| 168 | Rapid Agglutination Test, TAT & c‐ELISA | 9.0 (4.7–13.3) | ||
| Subnational | 451 | i‐ELISA | 4.4 a | Nguna et al. (2019) |
| Subnational | 235 | SAT & RBT | 17.0 a | Tumwine et al. (2015) |
Abbreviations: c‐ELISA, Competitive Enzyme‐Linked Immuno‐Sorbent Assay; i‐ELISA, Indirect Enzyme‐Linked Immuno‐Sorbent Assay; IgG/IgM‐LFA, Immunoglobulins G and M lateral flow assay; IgM‐ELISA, Immunoglobulin M Enzyme‐Linked Immuno‐Sorbent Assay; MAT, Microplate Agglutination Test; PAT, Plate Agglutination Test; RBT, Rose Bengal Test; SAT, Serum Agglutination Test; TAT, Tube Agglutination Test.
Prevalence confidence interval not provided in the original article.
Diagnostic test not specified (Study based on health centre results’ records).
Sample size not specified.
The computed confidence interval value was <0.0.
Small ruminants were also covered in recent brucellosis studies in Uganda. The reported brucellosis prevalence in goats was lower compared with cattle and varied between 0.3% and 9.8% (Dubad et al., 2015; Lolli et al., 2016; Miller et al., 2016; Nguna et al., 2019). The reported prevalence in sheep varied between 2.6% and 10.5% (Dubad et al., 2015; Lolli et al., 2016).
Among recent studies, one study investigated brucellosis in pigs from three districts in Uganda where pig keeping is commonly practiced. The reported prevalence in the districts varied from 0.0% to 0.2% (Erume et al., 2016).
Human brucellosis was also reported in recent brucellosis studies in Uganda published from 2010 to 2019. The reported human brucellosis prevalence varied between 4.0% and 33.0% (Ezama et al., 2018, 2019; Frank et al., 2017; Kansiime et al., 2015; Majalija et al., 2018; Migisha et al., 2018; Miller et al., 2016; Muloki et al., 2018; Nabukenya et al., 2013; Nanfuka, 2018; Nasinyama et al., 2014; Nguna et al., 2019; Tumwine et al., 2015). These human brucellosis results should, however, be interpreted considering that most of them were conducted on suspected patients attending hospitals with febrile illness or having prolonged fevers and on exposed cattle keepers and farm attendants in pastoralist and agro‐pastoralist communities. Human brucellosis studies used serological diagnosis tests with only one study adding blood culturing to confirm the presence of Brucella spp. (Migisha et al., 2018).
A few recent studies in Uganda were also conducted to determine the prevalence of Brucella antibodies in cattle milk. Raw milk samples collected from dairy farms, milk shops, street vendors, milk deliverers, boiling points, dairies and milk collection centres were tested using serological diagnosis tests and the prevalence varied between 6.5% and 40% (Hoffman et al., 2016; Kamwine et al., 2017; Makita et al., 2010; Rock et al., 2016). While these results are from bulk and pooled raw milks and cannot be related to individual cattle prevalence, they are still an indication of the presence of brucellosis in cattle.
4. DISCUSSION
Brucellosis is considered endemic across the African continent (Franc et al., 2018). The aim of this article was to review recent literature (2010–2019) on the prevalence of brucellosis in animals and humans with reference to EAC countries. This review indicates that brucellosis is prevalent in EAC with prevalence ranges that are quite variable within individual EAC countries and between countries. This variation should, however, be looked at considering that the retained and reviewed studies used different serological diagnostic tests and different sampling techniques from populations of various sizes. The practiced livestock production systems were also different, from the traditional free open grazing system through mixed agro‐pastoral system to zero grazing system.
The cattle brucellosis prevalence range of 0.2% to 43.8% observed over EAC is comparable but higher than the prevalence in Sub‐Saharan Africa (SSA). A review of brucellosis sero‐prevalence studies published from 2003 to 2015 from 12 different SSA countries showed that brucellosis prevalence was between 1.0% and 36.6% among cattle raised under a variety of livestock production systems (Ducrotoy et al., 2015). The cattle brucellosis prevalence ranges of 0. 2% to 43.8% reported in the current review is, also, comparable but higher than previous cattle brucellosis prevalences reported for specific EAC countries (McDermott & Arimi, 2002) or estimated for EAC as a region (International Livestock Research Institute, 2012). In their review on cattle brucellosis in SSA including specific EAC countries (Tanzania, Kenya, Burundi and Uganda), McDermott and Arimi reported a brucellosis prevalence range varying mostly from 1.8% in Tanzania to 25.4% in Burundi (McDermott & Arimi, 2002). Grace and colleagues also estimated a lower prevalence (8.2%) among cattle in the East African region (International Livestock Research Institute, 2012). In the current review and across other reports (Ducrotoy et al., 2015; International Livestock Research Institute, 2012; McDermott & Arimi, 2002), higher cattle brucellosis prevalence is observed in cattle raised in larger pastoral and agro‐pastoral herds and lower prevalence is observed in small holder farms. The pastoral and agro‐pastoral livestock production systems, in which cattle closely interact within herds and between different herds, are practiced by at least some communities or subregions in each of the EAC countries. In such livestock production systems, cattle share grazing areas, watering sources and bulls for natural breeding. Brucellosis is transmitted from animal to animal and has been reported to spread and stay in herds through contaminated grazing areas, contaminated water and through natural breeding by brucellosis‐positive bulls (Aparicio, 2013). In their efforts to control cattle brucellosis, EAC and EAC countries should, therefore, include and adapt brucellosis control strategies recommended for pastoral and agro‐pastoral livestock production systems with high brucellosis prevalence.
Outside Africa, a number of developed countries including New Zealand, Sweden, Denmark, Norway, Finland, Germany, Switzerland, Canada, Japan, UK, Netherlands, Belgium, Luxembourg and Austria have controlled and eradicated brucellosis (Benkirane, 2006; Pappas et al., 2006). Other developing countries in Central and South America, South and South‐East Asia and Middle East are, like Africa, still facing bovine brucellosis, although the reported prevalence is lower. In Central American countries of Guatemala, Belize, Honduras, El Salvador, Nicaragua, Costa Rica and Panama, bovine brucellosis was estimated to be from 4% to 8% and bovine brucellosis was 3% to 4%, 2% to 2.5% and 0.04% to 0.28% among cattle in Paraguay, individual Dairy cattle in Argentina and across federal Brazil (Lopes et al., 2010). Brucellosis estimates from South Asia and South East Asia regions were 16.0% and 2.9% respectively (International Livestock Research Institute, 2012). Cattle individual sero‐prevalence in Middle East was from 0.8% to 12.2% (Musallam et al., 2016).
As observed in the current review, studies on brucellosis in small ruminants are fewer compared with studies on cattle. Indeed, for three of the six covered EAC countries, brucellosis in small ruminants has not yet been studied or is yet to be reported. This is a similar situation across SSA where there is limited information on brucellosis studies on small ruminants (Ducrotoy et al., 2015). For the few reported studies on goats and sheep brucellosis in EAC, the prevalence range was 0% to 20.0% among goats and 0% to 13.8% among sheep. This EAC prevalence range is higher compared with the SSA prevalence which varied between 0% and 4.8% for sheep and between 0% and 5.5% for goats (Ducrotoy et al., 2015). Although studies on brucellosis in small ruminants are still few, there is a need to evaluate the significance of this infection among small ruminants in EAC and across Africa. Indeed, goats and sheep are natural hosts and mainly infected by Brucella melitensis (Bruce, 1887). Among Brucella species, B. melitensis is the most virulent to humans resulting in a more acute infection (Mantur et al., 2007; Moreno, 2014). Outside Africa, brucellosis in small ruminants is still prevalent and remains a major problem in the Mediterranean region, the Middle East, Central Asia, South Asia and South‐East (McDermott et al., 2013; Musallam et al., 2016). The recent brucellosis studies in EAC indicate the prevalence of the infection among livestock and human in the region and call for plans in individual countries to control brucellosis. FAO has given general guidelines in controlling brucellosis, which can be very well adapted by the different individual EAC countries. In regions, like EAC, where brucellosis prevalence is higher than 10%, FAO recommends a mass brucellosis vaccination until the prevalence is reduced to below 2% (Food and Agriculture Organization of United Nations, 2009). Once the prevalence is brought under control, further eradication strategies can, then, be considered. Prior to any brucellosis control strategy, epidemiological studies are needed to determine the prevalence taking into account the different regions within the same country or different countries within a same region (Blasco, 2010).
Most of the studies considered in the current review were targeting livestock and humans in identified suspected agro‐pastoral communities within individual EAC countries. In designing their approach to control brucellosis, EAC countries could take advantage of such studies to determine the epidemiological units of intervention and design‐appropriate brucellosis control strategies. In designing any brucellosis control strategy, there are, however, important considerations for the strategy to be implemented successfully. In countries where brucellosis control in livestock was, for example, implemented and attained success, adequate veterinary infrastructure was in place or put in place, awareness campaigns conducted to concerned farmers and economic resources availed by government and government donors (Blasco, 2010; Food and Agriculture Organization of United Nations, 2014). It is also critical that the country's or region's political will to control brucellosis is demonstrated and maintained throughout the implementation of any brucellosis control strategy. In developed countries where brucellosis was controlled and eradicated, a strong political support and a legal framework to enforce control measures were important to the success of the control and eradication programs (Food and Agriculture Organization of United Nations, 2014).
With a brucellosis control strategy in place, the invested efforts and economic resources have to be maintained over the course of the strategy implementation which is usually long. In countries where brucellosis is high, FAO recommends to start by long‐term mass vaccination to control the disease. This vaccination can take up to 10 years to sustainably bring brucellosis prevalence to low levels (Food and Agriculture Organization of United Nations, 2014).
Once a brucellosis control strategy is designed and needed resources availed, the implementing country needs to also ensure that good farm management is practiced to enable the control strategy to be effective and successful. The control of livestock movement, screening of replacement livestock prior to their introduction to the farms, hygienic disposal of abortive materials are all good farm management practices that have been reported as additional elements to the success of any brucellosis control strategy (Avila‐Granados et al., 2019; Bamaiyi et al., 2014; Perez‐Sancho et al., 2015; Zamri‐Saad & Kamarudin, 2016).
5. CONCLUSION
The literature presented in this review shows that brucellosis is prevalent in livestock in EAC. For cattle, which was the most studied among livestock, the reported individual cattle brucellosis prevalence varied mostly from 0.2% to 43.8% in the region. In small ruminants, brucellosis prevalence studies were very few and reported a prevalence varying between 0.0% and 20.0% in goats and 0.0 and 13.8% in sheep at EAC level. With most animal studies in EAC having focused on cattle, establishing the prevalence of brucellosis in small ruminants is also needed.
At EAC level, the reported prevalence of human brucellosis among studied patients attending hospitals and exposed pastoral and agro‐pastoral communities varied mostly from 0.0% to 35.8%. The reviewed studies show the continued existence and prevalence of brucellosis in EAC. These studies are a basis for individual EAC countries and the region to plan or revive efforts towards the control and eradication of brucellosis. Any brucellosis control plan will need, however, a well‐designed strategy considering the involved economic resources, adequate veterinary services, time and consistency over the implementation time.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The authors have adhered to ethical policies of the journal. No ethical approval or consent forms were necessary as this is a review article.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Juvenal Djangwani: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Visualization; Writing‐original draft; Writing‐review & editing. George Abong: Conceptualization; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing‐review & editing. Lucy Gicuku Njue: Conceptualization; Methodology; Resources; Supervision; Validation; Visualization; Writing‐review & editing. Dasel Wambua Mulwa Kaindi: Conceptualization; Methodology; Resources; Supervision; Validation; Visualization; Writing‐review & editing.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.425.
ACKNOWLEDGEMENTS
This work was funded by the Borlaug Higher Education for Agricultural Research and Development program based at Michigan State University.
Djangwani J, Ooko Abong’ G, Gicuku Njue L, Kaindi DWM. Brucellosis: Prevalence with reference to East African community countries – A rapid review. Vet Med Sci. 2021;7:851–867. 10.1002/vms3.425
DATA AVAILABILITY STATEMENT
This is a review article and no new data were created or analysed.
REFERENCES
- Acha, P. N. , & Szyfres, B. (2001). Bacterioses. In Acha P. N., & Szyfres B. (Eds.), Zoonoses and communicable diseases common to man and animals (Vol. I, Issue 3, p. 423). Pan American Health Organization. 10.1590/s1135-57272005000300012 [DOI] [Google Scholar]
- Akakpo, A. J. , & Bornarel, P. (1987). Epidémiologie des brucelloses animales en Afrique tropicale: Enquêtes clinique, sérologique et bactériologique. Revue Scientifique et Technique de l’OIE, 6(4), 981–1027. 10.20506/rst.6.4.313 [DOI] [PubMed] [Google Scholar]
- Akayezu, J. M. V. (1984). A propos d ’ une enquete sero‐epizootiologique sure la brucellose bovine au Rwanda. Ecole Inter‐Etats des Sciences et Medecine Veterinaires (EISMV)‐Dakar‐Senegal. [Google Scholar]
- Ali, S. , Akhter, S. , Neubauer, H. , Scherag, A. , Kesselmeier, M. , Melzer, F. , Khan, I. , El‐Adawy, H. , Azam, A. , Qadeer, S. , & Ali, Q. (2016). Brucellosis in pregnant women from Pakistan: An observational study. BMC Infectious Diseases, 16(1), 1–6. 10.1186/s12879-016-1799-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio, E. D. (2013). Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus . Revue Scientifique et Technique de l’OIE, 32(1), 53–60. 10.20506/rst.32.1.2187 [DOI] [PubMed] [Google Scholar]
- Asakura, S. , Makingi, G. , Kazwala, R. , & Makita, K. (2018a). Brucellosis risk in urban and agro‐pastoral areas in Tanzania. EcoHealth, 15, 41–51. 10.1007/s10393-017-1308-z [DOI] [PubMed] [Google Scholar]
- Asakura, S. , Makingi, G. , Kazwala, R. , & Makita, K. (2018b). Herd‐level risk factors associated with Brucella sero‐positivity in cattle, and perception and behaviours on the disease control among agro‐pastoralists in Tanzania. Acta Tropica, 187(August), 99–107. 10.1016/j.actatropica.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Assenga, J. A. , Matemba, L. E. , Muller, S. K. , Malakalinga, J. J. , & Kazwala, R. R. (2015). Epidemiology of Brucella infection in the human, livestock and wildlife interface in the Katavi‐Rukwa ecosystem. Tanzania. BMC Veterinary Research, 11(1), 189. 10.1186/s12917-015-0504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila‐Granados, L. M. , Garcia‐Gonzalez, D. G. , Zambrano‐Varon, J. L. , & Arenas‐Gamboa, A. M. (2019). Brucellosis in Colombia: Current status and challenges in the control of an endemic disease. Frontiers in Veterinary Science, 6(SEP), 1–12. 10.3389/fvets.2019.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamaiyi, P. H. , Hassan, L. , & Zainal, M. A. (2014). Updates on brucellosis in Malaysia and Southeast Asia. Malaysian Journal of Veterinary Research, 71–82. [Google Scholar]
- Bank, W. (2019). World bank‐Burundi population. World Bank. https://data.worldbank.org/indicator/SP.POP.TOTL?locations=BI&most_recent_value_desc=false&view=chart [Google Scholar]
- Benkirane, A. (2006). Ovine and caprine brucellosis: World distribution and control/eradication strategies in West Asia/North Africa region. Small Ruminant Research, 62(1‐2), 19–25. 10.1016/j.smallrumres.2005.07.032 [DOI] [Google Scholar]
- Blasco, J. (2010). Control and eradication strategies for Brucella melitensis infection in sheep and goats. Section of Biological and Medical Sciences of the Macedonian Academy of Sciences and Arts, 165, 145–165. [PubMed] [Google Scholar]
- Boukary, A. R. , Saegerman, C. , Adehossi, E. , Matthys, F. , Vias, G. F. , Yenikoye, A. , & Thys, E. (2014). La brucellose en Afrique subsaharienne. Annales de Medecine Veterinaire, 158(1), 283–289. [Google Scholar]
- Boukary, A. R. , Saegerman, C. , Abatih, E. , Fretin, D. , Bada, R. A. , De Deken, R. , Harouna, H. A. , Yenikoye, A. , & Thys, E. (2013). Seroprevalence and potential risk factors for Brucella Spp. infection in traditional cattle, sheep and goats reared in urban, periurban and rural areas of Niger. PLoS ONE, 8(12), 1–12. 10.1371/journal.pone.0083175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouley, A. J. , Biggs, H. M. , Stoddard, R. A. , Morrissey, A. B. , Bartlett, J. A. , Afwamba, I. A. , Maro, V. P. , Kinabo, G. D. , Saganda, W. , Cleaveland, S. , & Crump, J. A. (2012). Brucellosis among hospitalized febrile patients in northern Tanzania. American Journal of Tropical Medicine and Hygiene, 87(6), 1105–1111. 10.4269/ajtmh.2012.12-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, D. (1887). Note on the discovery of a microorganism in malta fever. John Brigg. [Google Scholar]
- Bugeza, J. , Muwonge, A. , Munyeme, M. , Lasuba, P. , Godfroid, J. , & Kankya, C. (2019). Seroprevalence of bovine brucellosis and associated risk factors in Nakasongola district. Uganda. Tropical Animal Health and Production, 51(7), 2073–2076. 10.1007/s11250-018-1631-6 [DOI] [PubMed] [Google Scholar]
- Carugati, M. , Biggs, H. M. , Maze, M. J. , Stoddard, R. A. , Cash‐Goldwasser, S. , Hertz, J. T. , Halliday, J. E. B. , Saganda, W. , Lwezaula, B. F. , Kazwala, R. R. , Cleaveland, S. , Maro, V. P. , Rubach, M. P. , & Crump, J. A. (2018). Incidence of human brucellosis in the Kilimanjaro Region of Tanzania in the periods 2007–2008 and 2012–2014. Transactions of the Royal Society of Tropical Medicine and Hygiene, 112(3), 136–143. 10.1093/trstmh/try033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalinuovo, F. , Ciambrone, L. , Cacia, A. , & Rippa, P. (2016). Contamination of bovine, sheep and goat meat with Brucella spp. Italian Journal of Food Safety, 5(3). 10.4081/ijfs.2016.5913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash‐Goldwasser, S. , Maze, M. J. , Rubach, M. P. , Biggs, H. M. , Stoddard, R. A. , Sharples, K. J. , Halliday, J. E. B. , Cleaveland, S. , Shand, M. C. , Mmbaga, B. T. , Muiruri, C. , Saganda, W. , Lwezaula, B. F. , Kazwala, R. R. , Maro, V. P. , & Crump, J. A. (2017). Risk factors for human brucellosis in Northern Tanzania. American Journal of Tropical Medicine and Hygiene, 98(2), 598–606. 10.4269/ajtmh.17-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatikoba, P. , Manzi, M. , Kagarama, J. , Rwemarika, J. D. , & Umunezero, O. (2008). The prevalence of bovine brucellosis in milking dairy herds in nyagatare and its implications on dairy productivity and public health. The 3rd International Conference on Appropriate Technology (3rd ICAT), 368–376. http://www.howard.edu/library/scholarship@howard/books/2008/icat2008.pdf
- Chipwaza, B. , Mhamphi, G. G. , Ngatunga, S. D. , Selemani, M. , Amuri, M. , Mugasa, J. P. , & Gwakisa, P. S. (2015). Prevalence of bacterial febrile illnesses in children in Kilosa district, Tanzania. PLOS Neglected Tropical Diseases, 9(5). 10.1371/journal.pntd.0003750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitupila, G. Y. , Komba, E. V. G. , & Mtui‐Malamsha, N. J. (2015). Epidemiological study of bovine brucellosis in indigenous cattle population in Kibondo and Kakonko districts, Western Tanzania. Livestock Research for Rural Development, 27(6). [Google Scholar]
- Chota, A. , Magwisha, H. , Stella, B. , Bunuma, E. , Shirima, G. , Mugambi, J. , Omwenga, S. , Wesonga, H. , Mbatha, P. , & Gathogo, S. (2016). Prevalence of brucellosis in livestock and incidences in humans in east Africa. African Crop Science Journal, 24(1), 45. 10.4314/acsj.v24i1.5s [DOI] [Google Scholar]
- Corbel, M. J. (2006). Brucellosis in humans and animals. World Health Organization, 1–88. 10.2105/AJPH.30.3.299 [DOI] [Google Scholar]
- Craighead, L. , Meyer, A. , Chengat Prakashbabu, B. , Musallam, I. , Akakpo, A. J. , Kone, P. , Guitian, J. , & Häsler, B. (2017). Brucellosis in West and Central Africa: A review of the current situation in a changing landscape of dairy cattle systems. Acta Tropica, 179, 96–108. 10.1016/j.actatropica.2017.12.026 [DOI] [PubMed] [Google Scholar]
- Crump, J. A. , Morrissey, A. B. , Nicholson, W. L. , Massung, R. F. , Stoddard, R. A. , Galloway, R. L. , Ooi, E. E. , Maro, V. P. , Saganda, W. , Kinabo, G. D. , Muiruri, C. , & Bartlett, J. A. (2013). Etiology of severe non‐malaria febrile illness in northern Tanzania: A prospective cohort study. PLoS Neglected Tropical Diseases, 7(7). 10.1371/journal.pntd.0002324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar, M. , Shahali, Y. , & Whatmore, A. M. (2019). Human brucellosis caused by raw dairy products: A review on the occurrence, major risk factors and prevention. International Journal of Food Microbiology, 292, 39–47. 10.1016/j.ijfoodmicro.2018.12.009 [DOI] [PubMed] [Google Scholar]
- de Glanville, W. A. , Conde‐Álvarez, R. , Moriyón, I. , Njeru, J. , Díaz, R. , Cook, E. A. J. , Morin, M. , Bronsvoort, B. M. D. C. , Thomas, L. F. , Kariuki, S. , & Fèvre, E. M. (2017). Poor performance of the rapid test for human brucellosis in health facilities in Kenya. PLoS Neglected Tropical Diseases, 11(4), 1–15. 10.1371/journal.pntd.0005508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiere, S. , Niragira, S. , & D’Haese, M. (2015). Cow or goat? Population pressure and livestock keeping in Burundi. Agrekon, 54(3), 23–42. 10.1080/03031853.2015.1084941 [DOI] [Google Scholar]
- Dubad, A. B. , Baluka, S. A. , & Kaneene, J. B. (2015). Sero‐prevalence of brucellosis in small ruminants in Kiruhura District of Uganda. Advance Tropical Medicine and Public Health International, 5(3), 62–76. [Google Scholar]
- Ducrotoy, M. , Bertu, W. J. , Matope, G. , Cadmus, S. , Conde‐Álvarez, R. , Gusi, A. M. , Welburn, S. , Ocholi, R. , Blasco, J. M. , & Moriyón, I. (2015). Brucellosis in Sub‐Saharan Africa: Current challenges for management, diagnosis and control. Acta Tropica, 165, 179–193. 10.1016/j.actatropica.2015.10.023 [DOI] [PubMed] [Google Scholar]
- Egeru, A. , Wasonga, O. , Kyagulanyi, J. , Majaliwa, G. M. , MacOpiyo, L. , & Mburu, J. (2014). Spatio‐temporal dynamics of forage and land cover changes in Karamoja sub‐region, Uganda. Pastoralism: Research, Policy and Practice, 4(1), 1–21. 10.1186/2041-7136-4-6 [DOI] [Google Scholar]
- Emmanuel, T. A. J. , Tijjani, K. I. , & Çakır, A. (2018). Challenges and possible improvement of livestock sector in south Sudan: Review paper. International Journal of Research‐Granthaalayah, 6(2), 214–223. 10.5281/zenodo.1194652 [DOI] [Google Scholar]
- Engida, E. , Guthiga, P. , & Karugia, J. (2015). The Role of Livestock in the Tanzanian Economy: Policy Analysis Using a Dynamic Computable General Equilibrium Model for Tanzania) ** Senior Policy Analyst, The Regional Strategy Analysis and Knowledge Support System for Eastern and Central Africa ** Coor. Agriculture in an Interconnected World, 22. [Google Scholar]
- Enström, S. , Nthiwa, D. , Bett, B. , Karlsson, A. , Alonso, S. , & Lindahl, J. F. (2017). Brucella seroprevalence in cattle near a wildlife reserve in Kenya. BMC Research Notes, 10(1), 1–5. 10.1186/s13104-017-2941-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erume, J. , Roesel, K. , Dione, M. M. , Ejobi, F. , Mboowa, G. , Kungu, J. M. , Akol, J. , Pezo, D. , El‐Adawy, H. , Melzer, F. , Elschner, M. , Neubauer, H. , & Grace, D. (2016). Serological and molecular investigation for brucellosis in swine in selected districts of Uganda. Tropical Animal Health and Production, 48(6), 1147–1155. 10.1007/s11250-016-1067-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezama, A. , Gonzalez, J. P. , Majalija, S. , & Bajunirwe, F. (2018). Assessing short evolution brucellosis in a highly brucella endemic cattle keeping population of Western Uganda: A complementary use of Rose Bengal test and IgM rapid diagnostic test. BMC Public Health, 18(1), 1–5. 10.1186/s12889-018-5228-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezama, A. , Gonzalez, J. , Sebhatu, T. , Gabriel, T. , & Majalija, S. (2019). Presumptive diagnosis of brucellosis and determination of risk factors for seropositivity among members of cattle keeping households in a high cattle traffic area in the South Western region of Uganda. Global Journal of Infectious Diseases and Clinical Research, 5(1), 16–24. 10.17352/2455-5363.000024 [DOI] [Google Scholar]
- Fèvre, E. M. , De Glanville, W. A. , Thomas, L. F. , Cook, E. A. J. , Kariuki, S. , & Wamae, C. N. (2017). An integrated study of human and animal infectious disease in the Lake Victoria crescent small‐holder crop‐livestock production system, Kenya. BMC Infectious Diseases, 17(1), 1–14. 10.1186/s12879-017-2559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of United Nations . (n.d.). FAOSTAT statistical database. FAO, c1997‐. http://www.fao.org/faostat/en/#data/QA [Google Scholar]
- Food and Agriculture Organization of United Nations . (2009). Brucella melitensis in Eurasia and the Middle East. In Animal Production and Health (Issue May). http://www.fao.org/docrep/012/i1402e/i1402e00.pdf
- Food and Agriculture Organization of United Nations . (2014). FAO works to curb the burden of brucellosis in endemic countries Case studies from Eurasia and the Near East. In FAO Empres (Issue 8).
- Food and Agriculture Organization of United Nations . (2016). The Impact of conflict on the livestock sector in south Sudan. http://files/1298/Gebreyes‐CTheImpactofConflictontheLivestockSector.pdf [Google Scholar]
- Food and Agriculture Organization of United Nations (2018a). Livestock production systems spotlight‐Kenya‐Cattle and poultry sectors. FAO. 10.1016/s0301-6226(97)89729-1 [DOI]
- Food and Agriculture Organization of United Nations . (2018b). The monetary impact of zoonotic diseases on society: Uganda. In African Sustainable Livestock 2050.
- Food and Agriculture Organization of United Nations . (2019). The future of livestock in Uganda‐Opportunities and challenges in the face of uncertainty. Food and Agriculture Organization of the United Nations. Retrieved from http://www.fao.org/3/ca5420en/CA5420EN.pdf [Google Scholar]
- Food and Drug Administration . (2012). Bad bug book: Handbook of foodborne pathogenic microorganisms and natural toxins. In U.S. Department of Health and Human Services (2nd ed.). 10.1016/S1872-2040(10)60451-3 [DOI] [Google Scholar]
- Franc, K. A. , Krecek, R. C. , Häsler, B. N. , & Arenas‐Gamboa, A. M. (2018). Brucellosis remains a neglected disease in the developing world: A call for interdisciplinary action. BMC Public Health, 18(1), 1–9. 10.1186/s12889-017-5016-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, S. , Benson, O. , & Ivan, T. (2017). Human Brucellosis in garzeted forest areas: A case of bwindi impenetrable. Journal of Foodborne and Zoonotic Diseases, 5(1), 7–11. [Google Scholar]
- Gafirita, J. , Kiiza, G. , Murekatete, A. , Ndahayo, L. L. , Tuyisenge, J. , Mashengesho, V. , Ruhirwa, R. , Nyandwi, T. , Asiimwe‐Kateera, B. , Ndahindwa, V. , & Njunwa, K. J. (2017). Seroprevalence of brucellosis among patients attending a District Hospital in Rwanda. American Journal of Tropical Medicine and Hygiene, 97(3), 831–835. 10.4269/ajtmh.16-0632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gicheru, M. N. , Mwangi, E. , & Mbaire, M. R. (2015). Prevalence and knowledge of Brucellosis in dairy cattle in Makuyu Division, Murang’a County, Kenya. International Journal of Scientific Engineering and Technology, 4(12), 549–555. 10.17950/ijset/v4s12/1202 [DOI] [Google Scholar]
- Godfroid, J. , Debolle, X. , Roop, R. M. , O'callaghan, D. , Tsolis, R. M. , Baldwin, C. J. , Santos, R. L. , Mcgiven, J. A. , Olsen, S. C. , Nymo, I. H. , Larsen, A. , Al dahouk, S. , & Letesson, J. J. (2014). The quest for a true One Health perspective of brucellosis. OIE Revue Scientifique et Technique, 33(2), 521–538. 10.20506/rst.33.2.2290 [DOI] [PubMed] [Google Scholar]
- Hamdy, M. E. R. , & Amin, A. S. (2002). Detection of Brucella species in the milk of infected cattle, sheep, goats and camels by PCR. The Veterinary Journal, 163(3), 299–305. 10.1053/tvjl.2001.0681 [DOI] [PubMed] [Google Scholar]
- Hoffman, T. , Rock, K. , Mugizi, D. R. , Muradrasoli, S. , Lindahl‐Rajala, E. , Erume, J. , Magnusson, U. , Lundkvist, Å. , & Boqvist, S. (2016). Molecular detection and characterization of Brucella species in raw informally marketed milk from Uganda. Infection Ecology & Epidemiology, 6(1), 32442. 10.3402/iee.v6.32442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Livestock Research Institute . (2012). Mapping of poverty and likely zoonoses hotspots. Zoonoses Report, 4, 1–119. http://www.ilri.org/ilrinews/index.php/archives/9172 [Google Scholar]
- Jeníček, V. , & Grofová, Š. (2015). Least developed countries – The case of Burundi. Agricultural Economics, 61(5), 234–247. 10.17221/48/2014-AGRICECON [DOI] [Google Scholar]
- Kabi, F. , Muwanika, V. , & Masembe, C. (2015). Spatial distribution of Brucella antibodies with reference to indigenous cattle populations among contrasting agro‐ecological zones of Uganda. Preventive Veterinary Medicine, 121(1–2), 56–63. 10.1016/j.prevetmed.2015.06.007 [DOI] [PubMed] [Google Scholar]
- Kairu‐wanyoike, S. , Nyamwaya, D. , Id, M. W. , Lindahl, J. , Ontiri, E. , Bukachi, S. , Njeru, I. , Karanja, J. , Sang, R. , Grace, D. , & Id, B. B. (2019). Positive association between Brucella spp. seroprevalences in livestock and humans from a cross‐sectional study in Garissa and Tana River Counties, Kenya. PLoS Neglected Tropical Diseases, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamwine, M. , Orikiriza, P. , Taseera, K. , Iramiot, J. S. , Ojuka, P. , Ikiriza, S. , Atwebembeire, J. , Otieno, D. , Tweshengyereze, S. , Mwanga‐Amumpaire, J. , Bazira, J. , & Boum, Y. (2017). Prevalence of antibodies to Brucella species in commercial raw bovine milk in Southwestern Uganda. BMC Research Notes, 10(1), 1–5. 10.1186/s13104-017-2537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansiime, C. , Rutebemberwa, E. , Asiimwe, B. B. , Makumbi, F. , Bazira, J. , & Mugisha, A. (2015). Annual trends of human brucellosis in pastoralist communities of south‐western Uganda: A retrospective ten‐year study. Infectious Diseases of Poverty, 4(1), 1–8. 10.1186/s40249-015-0072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwazaki, Y. , Ecewu, E. , Imaligat, J. O. , Mawejje, R. , Kirunda, M. , Kato, M. , Musoke, G. M. , & Ademun, R. A. O. (2012). Epidemiology of bovine brucellosis by a combination of rose Bengal test and indirect ELISA in the five districts of Uganda. Journal of Veterinary Medical Science, 74(11), 1417–1422. 10.1292/jvms.12-0164 [DOI] [PubMed] [Google Scholar]
- Kaur, P. , Sharma, N. S. , Arora, A. K. , & Deepti. (2018). Investigation of brucellosis in cattle and buffaloes by conventional and molecular assays. Indian Journal of Animal Research, 52(10), 1482–1487. 10.18805/ijar.B-3375 [DOI] [Google Scholar]
- Kayombo, G. , Makingi, G. , Nonga, H. , Misinzo, G. , & Kazwala, R. (2017). Studies of brucellosis in lactating cows in Babati district, Tanzania. Tanzania Veterinary Journal, 35(1), 90–101. [Google Scholar]
- Kenya Ministry of Agriculture, Livestock, F. and I . (2019). Draft national livestock policy. Kenya Ministry of Agriculture, Livestock, Fisheries and Irrigation ‐ State Department for Livestock Draft. Retrieved from https://www.kilimo.go.ke/wp‐content/uploads/2019/02/Draftreviewed‐National‐Livestock‐Policy‐February‐2019.pdf [Google Scholar]
- Kenya National Bureau of Statistics . (2019). 2019 Kenya Population and Housing Census. Volume IV: Distribution of population by social‐economic characteristics. Kenya National Bureau of Statistics. Retrieved from https://africacheck.org/wp‐content/uploads/2020/06/VOLUME‐IVKPHC‐2019.pdf [Google Scholar]
- Khan, M. Y. , Mah, M. W. , & Memish, Z. A. (2001). Brucellosis in Pregnant Women. Clinical Infectious Diseases, 32(8), 1172–1177. 10.1086/319758 [DOI] [PubMed] [Google Scholar]
- Kosgei, P. K. , Bebora, L. , Waiboci, L. , Kitala, P. , & Kiambi, S. (2014). Estimating prevalence of brucellosis in livestock and assessment of knowledge, attitudes and practices of respective communities in Baringo county, Kenya. Fourth RUFORUM Biennial Regional Conference, 21(July), 297–301. http://www.ruforum.org/sites/default/files/Kosgei.pdf [Google Scholar]
- Land O’ lakes, I. (2014). Baseline survey report for Rwanda Dairy Competitiveness Program (RDCP). [Google Scholar]
- Langoni, H. , Ichihara, S. M. , da Silva, A. V. , Pardo, R. B. , Tonin, F. B. , Mendonca, L. J. P. , & Machado, J. A. D. (2000). Isolation of brucella spp from milk of brucellosis positive cows in São Paulo and Minas Gerais states. Brazilian Journal of Veterinary Research and Animal Science, 37(6), 10.1590/s1413-95962000000600004 [DOI] [Google Scholar]
- Lita, E. , Nasinyama, G. W. , Ochi, E. B. , James, B. , & Erume, J. (2016). Seroprevalence and risk factors associated with bovine Brucellosis in Central. 3(7), 454–462. 10.21276/sjavs.2016.3.7.3 [DOI] [Google Scholar]
- Lolli, C. , Marenzoni, M. L. , Strona, P. , Lappo, P. G. , Etiang, P. , & Diverio, S. (2016). Infections and risk factors for livestock with species of Anaplasma, Babesia and Brucella under semi‐nomadic rearing in Karamoja Region, Uganda. Tropical Animal Health and Production, 48(3), 603–611. 10.1007/s11250-016-1005-x [DOI] [PubMed] [Google Scholar]
- Lopes, B. L. , Nicolino, R. , & P.A. Haddad, J. (2010). Brucellosis – Risk factors and prevalence: A review. The Open Veterinary Science Journal, 4(1), 72–84. 10.2174/1874318801004010072 [DOI] [Google Scholar]
- Madut, N. A. , Muleme, J. , Kankya, C. , Nasinyama, G. W. , Muma, J. B. , Godfroid, J. , Jubara, A. S. , & Muwonge, A. (2019). Sero‐prevalence of brucellosis among slaughterhouse workers in Bahr el Ghazal region, South Sudan. BMC Infectious Diseases, 7(JUN), 1–7. 10.3389/fpubh.2019.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madut, N. A. , Muwonge, A. , Nasinyama, G. W. , Muma, J. B. , Godfroid, J. , Jubara, A. S. , Muleme, J. , & Kankya, C. (2018). The sero‐prevalence of brucellosis in cattle and their herders in Bahr el Ghazal region, South Sudan. PLoS Neglected Tropical Diseases, 12(6), 1–14. 10.1371/journal.pntd.0006456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madut, N. A. , Nasinyama, G. W. , Muma, J. B. , Sube, K. L. L. , Ocan, M. , Muwonge, A. , Godfroid, J. , Jubara, A. S. , & Kankya, C. (2018). Prevalence of brucellosis among patients attending Wau Hospital, South Sudan. PLoS One, 13(6), 1–12. 10.1371/journal.pone.0199315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiyo, G. , & Obey, J. K. (2016). Distribution and prevalence of human Brucellosis among patients reporting at Chemundu Dispensary, Nandi County, Kenya. Baraton Interdisciplinary Research Journal, 6, 73–82. http://ueab.ac.ke/BIRJ/wp‐content/uploads/2016/10/Article12.pdf [Google Scholar]
- Majalija, S. , Luyombo, P. , & Tumwine, G. (2018). Sero‐prevalence and associated risk factors of Brucellosis among Malaria negative febrile out‐patients in Wakiso district, Central Uganda. BMC Research Notes, 11(1), 1–6. 10.1186/s13104-018-3907-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita, K. , Fevre, E. M. , Waiswa, C. , Eisler, M. C. , & Welburn, S. C. (2010). How human brucellosis incidence in urban Kampala can be reduced most efficiently? A stochastic risk assessment of informally‐marketed milk. PLoS One, 5(12), 10.1371/journal.pone.0014188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita, K. , Waiswa, C. , & Mthrusfieldedacuk, M. T. (2011). Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri‐urban areas of. BMC Veterinary Research. 10.1186/1746-6148-7-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manirakiza, J. , Hatungumukama, G. , Besbes, B. , & Detilleux, J. (2020). Characteristics of smallholders’ goat production systems and effect of Boer crossbreeding on body measurements of goats in Burundi. Pastoralism, 10(1), 10.1186/s13570-019-0157-5 [DOI] [Google Scholar]
- Manirakiza, J. , Hatungumukama, G. , Thévenon, S. , Gautier, M. , Besbes, B. , Flori, L. , & Detilleux, J. (2017). Effect of genetic European taurine ancestry on milk yield of Ankole‐Holstein crossbred dairy cattle in mixed smallholders system of Burundi highlands. Animal Genetics, 48(5), 544–550. 10.1111/age.12578 [DOI] [PubMed] [Google Scholar]
- Manishimwe, R. , Ntaganda, J. , Habimana, R. , Nishimwe, K. , Byukusenge, M. , Dutuze, F. , Ayabagabo, J. D. , And, U. L. , & Rukundo, J. C. (2015). Comparison between rose Bengal plat test and competitive enzyme linked immunosorbent assay to detect bovine Brucellosis in Kigali City, Rwanda. Journal of Veterinary Science & Technology, 06(01), 2–5. 10.4172/2157-7579.1000211 [DOI] [Google Scholar]
- Mantur, B. , Amarnath, S. , & Shinde, R. (2007). Review of clinical and laboratory features of human brucellosis. Indian Journal of Medical Microbiology, 25(3), 188–202. 10.4103/0255-0857.34758 [DOI] [PubMed] [Google Scholar]
- Mathew, C. (2017). Reproductive Infections in Cattle in Tanzania – Lessons for Control Priorities. SOJ Microbiology & Infectious Diseases, 5(2), 1–9. 10.15226/sojmid/5/2/00169 [DOI] [Google Scholar]
- Mathew, C. , Stokstad, M. , Johansen, T. B. , Klevar, S. , Mdegela, R. H. , Mwamengele, G. , Michel, P. , Escobar, L. , Fretin, D. , & Godfroid, J. (2015). First isolation, identification, phenotypic and genotypic characterization of Brucella abortus biovar 3 from dairy cattle in Tanzania. BMC Veterinary Research, 11(1), 1–9. 10.1186/s12917-015-0476-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, J. J. , & Arimi, S. M. (2002). Brucellosis in sub‐Saharan Africa: Epidemiology, control and impact. Veterinary Microbiology, 90(1–4), 111–134. 10.1016/S0378-1135(02)00249-3 [DOI] [PubMed] [Google Scholar]
- McDermott, J. J. , Grace, D. , & Zinsstag, J. (2013). Economics of brucellosis impact and control in low‐income countries. Scientific and Technical Review of the Office International Des Epizooties, 32(1), 249–261. 10.20506/rst.32.1.2197 [DOI] [PubMed] [Google Scholar]
- Merker, M. , & Schlichting, H. (1984). Note sur la brucellose au Burundi. Revue D’elevage et de Medecine Veterinaire Des Pays Tropicaux, 138–144. [PubMed] [Google Scholar]
- Migisha, R. , Nyehangane, D. , Boum, Y. , Page, A. L. , Zúñiga‐Ripa, A. , Conde‐Álvarez, R. , Bagenda, F. , & Bonnet, M. (2018). Prevalence and risk factors of brucellosis among febrile patients attending a community hospital in south western Uganda. Scientific Reports, 8(1), 1–8. 10.1038/s41598-018-33915-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R. , Nakavuma, J. L. , Ssajjakambwe, P. , Vudriko, P. , Musisi, N. , & Kaneene, J. B. (2016). The prevalence of Brucellosis in cattle, goats and humans in rural Uganda: A comparative study. Transboundary and Emerging Diseases, 63(6), e197–e210. 10.1111/tbed.12332 [DOI] [PubMed] [Google Scholar]
- Mirambo, M. M. , Mgode, G. F. , Malima, Z. O. , John, M. , Mngumi, E. B. , Mhamphi, G. G. , & Mshana, S. E. (2018). Seroposotivity of Brucella spp. and Leptospira spp. antibodies among abattoir workers and meat vendors in the city of Mwanza, Tanzania: A call for one health approach control strategies. PLoS Neglected Tropical Diseases, 12(6), 39–52. 10.1371/journal.pntd.0006600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mngumi, E. B. , Mirambo, M. M. , Wilson, S. , & Mshana, S. E. (2016). Predictors of specific anti‐Brucella antibodies among humans in agro‐pastoral communities in Sengerema district, Mwanza, Tanzania: The need for public awareness. Tropical Medicine and Health, 44(1), 5–10. 10.1186/s41182-016-0034-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, E. (2014). Retrospective and prospective perspectives on zoonotic brucellosis. Frontiers in Microbiology, 5(MAY), 1–18. 10.3389/fmicb.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugizi, D. R. , Boqvist, S. , Nasinyama, G. W. , Waiswa, C. , Ikwap, K. , Rock, K. , Lindahl, E. , Magnusson, U. , & Erume, J. (2015). Prevalence of and factors associated with Brucella sero‐positivity in cattle in urban and peri‐urban Gulu and Soroti towns of Uganda. Journal of Veterinary Medical Science, 77(5), 557–564. 10.1292/jvms.14-0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugizi, D. R. , Muradrasoli, S. , Boqvist, S. , Erume, J. , Nasinyama, G. W. , Waiswa, C. , Mboowa, G. , Klint, M. , & Magnusson, U. (2015). Isolation and molecular characterization of Brucella isolates in cattle milk in Uganda. BioMed Research International, 2015, 10.1155/2015/720413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhairwa, A. P. , Mwakijungu, E. O. , Msoffe, P. L. M. , & Mtambo, M. M. A. (2012). Seroprevalence and factors affecting canine monocytic ehrlichiosis and canine brucellosis in Tanzania. Research Opinions in Animal & Veterinary Sciences, 0343. [Google Scholar]
- Mujuni, F. , Andrew, V. , Mngumi, E. B. , Chibwe, E. , Mshana, S. E. , & Mirambo, M. M. (2018). Predominance of Brucella abortus antibodies among women with spontaneous abortion in the city of Mwanza: Unrecognized link or coincidence ? BMC Research Notes, 1–5, 10.1186/s13104-018-3906-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muloki, H. N. , Erume, J. , Owiny, D. O. , Kungu, J. M. , Nakavuma, J. , Ogeng, D. , & Nasinyama, G. W. (2018). Prevalence and risk factors for brucellosis in prolonged fever patients in post‐conflict Northern Uganda. African Health Sciences, 18(1), 22–28. 10.4314/ahs.v18i1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam, I. I. , Abo‐Shehada, M. N. , Hegazy, Y. M. , Holt, H. R. , & Guitian, F. J. (2016). Systematic review of brucellosis in the Middle East: Disease frequency in ruminants and humans and risk factors for human infection. Epidemiology and Infection, 144(4), 671–685. 10.1017/S0950268815002575 [DOI] [PubMed] [Google Scholar]
- Musallam, I. , Ndour, A. P. , Yempabou, D. , Ngong, C.‐A. , Dzousse, M. F. , Mouiche‐Mouliom, M.‐M. , Feussom, J. M. K. , Ntirandekura, J. B. , Ntakirutimana, D. , Fane, A. , Dembele, E. , Doumbia, A. , Ayih‐Akakpo, A.‐H. , Pato, P. , Pali, M. , Tapsoba, A. S. R. , Compaore, G. M. , Gagara, H. , Garba, A. I. , … Guitian, J. (2019). Brucellosis in dairy herds: A public health concern in the milk supply chains of West and Central Africa. Acta Tropica, 197(March), 105042. 10.1016/j.actatropica.2019.105042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabukenya, I. , Kaddu‐Mulindwa, D. , & Nasinyama, G. W. (2013). Survey of Brucella infection and malaria among Abattoir workers in Kampala and Mbarara Districts, Uganda. BMC Public Health, 13(1), 2–7. 10.1186/1471-2458-13-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakkel, M. J. , Arimi, S. M. , Kitala, P. K. , Nduhiu, G. , Njenga, J. M. , & Wabacha, J. (2016). A sero‐epidemiological survey of Brucellosis, Q‐fever and Leptospirosis in livestock and humans and associated risk factors in Kajiado County‐ Kenya. Journal of Tropical Diseases, 4(3), 10.4172/2329-891X.1000215 [DOI] [Google Scholar]
- Nanfuka, M. (2018). Sero‐prevalence of bovine and human brucellosis on selected farms in South‐western Uganda. Online Journal of Public Health Informatics, 10(1), 2579. 10.5210/ojphi.v10i1.8979 [DOI] [Google Scholar]
- Nasinyama, G. , Ssekawojwa, E. , Opuda, J. , Grimaud, P. , Etter, E. , & Bellinguez, A. (2014). Brucella sero‐prevalence and modifiable risk factors among predisposed cattle keepers and consumers of un‐pasteurized milk in Mbarara and Kampala districts, Uganda. African Health Sciences, 14(4), 790–796. 10.4314/ahs.v14i4.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of statistics of Rwanda . (2018). Agricultural household survey 2016/2017. http://www.statistics.gov.lk/agriculture/Publications/AHS/AHS2016‐17Report.pdf [Google Scholar]
- National Institute of Statistics of Rwanda . (2019). Statistical YearBook. [Google Scholar]
- Ndazigaruye, G. , Mushonga, B. , Kandiwa, E. , Samkange, A. , Segwagwe, B. E. , Segwagwe, B. , & Province, E. (2018). Prevalence and risk factors for brucellosis seropositivity in cattle in Nyagatare District, Eastern Province, Rwanda. Journal of the South African Veterinary Association, 89, 1–8. 10.4102/jsava.v89i0.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguna, J. , Dione, M. , Apamaku, M. , Majalija, S. , Mugizi, D. R. , Odoch, T. , Kato, C. D. , Tumwine, G. , Kabaasa, J. D. , Curtis, K. , Graham, M. , Ejobi, F. , & Graham, T. (2019). Seroprevalence of brucellosis and risk factors associated with its seropositivity in cattle, goats and humans in Iganga district, Uganda. Pan African Medical Journal, 33, 1–10. 10.11604/pamj.2019.33.99.16960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nina, P. M. , Mugisha, S. , Leirs, H. , Basuta, G. I. , & Van Damme, P. (2017). Brucellosis in cattle and micro‐scale spatial variability of pastoral household income from dairy production in south western Uganda. Acta Tropica, 175, 130–137. 10.1016/j.actatropica.2016.11.030 [DOI] [PubMed] [Google Scholar]
- Nizeyimana, G. , Mwiine, F. N. , & Ayebazibwe, C. (2013). Comparative Brucella abortus antibody prevalence in cattle under contrasting husbandry practices in Uganda. Journal of the South African Veterinary Association, 84(1), 1–5. 10.4102/jsava.v84i1.943 [DOI] [PubMed] [Google Scholar]
- Njeru, J. , Melzer, F. , Wareth, G. , El‐Adawy, H. , Henning, K. , Pletz, M. W. , Heller, R. , Kariuki, S. , Fèvre, E. , & Neubauer, H. (2016). Human brucellosis in febrile patients seeking treatment at remote hospitals, northeastern Kenya, 2014–2015. Emerging Infectious Diseases, 22(12), 2160–2164. 10.3201/eid2212.160285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njeru, J. , Wareth, G. , Melzer, F. , Henning, K. , Pletz, M. W. , Heller, R. , & Neubauer, H. (2016). Systematic review of brucellosis in Kenya: Disease frequency in humans and animals and risk factors for human infection. BMC Public Health, 16(1), 1–15. 10.1186/s12889-016-3532-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njuguna, J. N. , Gicheru, M. M. , Kamau, L. M. , & Mbatha, P. M. (2017). Incidence and knowledge of bovine brucellosis in Kahuro district, Murang’a County, Kenya. Tropical Animal Health and Production, 49(5), 1035–1040. 10.1007/s11250-017-1296-6 [DOI] [PubMed] [Google Scholar]
- Nonga, H. E. , & Mwakapeje, E. R. (2017). Trends of human brucellosis in pastoralist communities based on hospital records during 2013–2016 in Ngorongoro District, Tanzania. Tanzania Veterinary Association Proceedings, 34, 34–40. http://www.suaire.sua.ac.tz:8080/xmlui/bitstream/handle/123456789/2536/NongaandMwakapeje.pdf?sequence=1&isAllowed=y [Google Scholar]
- Okumu, T. A. , John, N. M. , Wabacha, J. K. , Tsuma, V. , & Vanleeuwen, J. (2019). Seroprevalence of antibodies for bovine viral diarrhoea virus, Brucella abortus and Neospora caninum, and their roles in the incidence of abortion/foetal loss in dairy cattle herds in Nakuru District, Kenya. BMC Veterinary Research, 15(1), 1–6. 10.1186/s12917-019-1842-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango, D. , Oyoko, G. , Too, R. , & Masake, R. (2015). The contribution of livestock to the south Sudan economy (Issue December). [Google Scholar]
- Osoro, E. M. , Munyua, P. , Omulo, S. , Ogola, E. , Ade, F. , Mbatha, P. , Mbabu, M. , Ng'ang'a, Z. , Kairu, S. , Maritim, M. , Thumbi, S. M. , Bitek, A. , Gaichugi, S. , Rubin, C. , Njenga, K. , & Guerra, M. (2015). Strong association between human and animal brucella seropositivity in a linked study in Kenya, 2012–2013. American Journal of Tropical Medicine and Hygiene, 93(2), 224–231. 10.4269/ajtmh.15-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas, G. , Papadimitriou, P. , Akritidis, N. , Christou, L. , & Tsianos, E. (2006). The new global map of human Brucellosis. The Lancet Infectious Diseases, 6, 91–99. 10.1016/S1473-3099(06)70382-6 [DOI] [PubMed] [Google Scholar]
- Perez‐Sancho, M. , Garci‐Seco, T. , Domininguez, L. , & Alvarez, J. (2015). Control of animal Brucellosis — The most effective tool to prevent human Brucellosis. Intech, Tourism, 13. 10.5772/57353 [DOI] [Google Scholar]
- Pergher, G. , & Noel, G. (1936). Note sur la Fievre Ondulante au Ruanda‐Urundi. Annales de La Societe Belge de Medecine Tropicale.
- Rock, K. T. , Mugizi, D. R. , Ståhl, K. , Magnusson, U. , & Boqvist, S. (2016). The milk delivery chain and presence of Brucella spp. antibodies in bulk milk in Uganda. Tropical Animal Health and Production, 48(5), 985–994. 10.1007/s11250-016-1052-3 [DOI] [PubMed] [Google Scholar]
- Rujeni, N. , & Mbanzamihigo, L. (2014). Prevalence of brucellosis among women presenting with abortion/stillbirth in Huye, Rwanda. Journal of Tropical Medicine, 2014, 3–5. 10.1155/2014/740479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagamiko, F. D. , Muma, J. B. , Karimuribo, E. D. , Mwanza, A. M. , Sindato, C. , & Hang’ombe, B. M. (2018). Sero‐prevalence of Bovine Brucellosis and associated risk factors in mbeya region, Southern highlands of Tanzania. Acta Tropica, 178, 169–175. 10.1016/j.actatropica.2017.11.022 [DOI] [PubMed] [Google Scholar]
- Schmutz, S. M. , Moker, J. S. , Clark, E. G. , & Orr, J. P. (1996). Chromosomal aneuploidy associated with spontaneous abortions and neonatal losses in cattle. Journal of Veterinary Diagnostic Investigation, 8(1), 91–95. 10.1177/104063879600800114 [DOI] [PubMed] [Google Scholar]
- Shirima, G. , Fitzpatrick, J. , Kunda, J. , Mfinanga, S. , Kazwala, R. , Kambarage, D. , & Cleaveland, S. (2010). The role of livestock keeping in human brucellosis trends in livestock keeping communities in Tanzania. Tanzania Journal of Health Research, 12. 10.4314/thrb.v12i3.51261 [DOI] [Google Scholar]
- Shirima, G. , & John, S. K. (2016). Prevalence of brucellosis in the human, livestock and wildlife interface areas of Serengeti. Onderstepoort Journal of Veterinary Research, 83(1). 10.4102/ojvr.v83i1.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirima, G. M. , Masola, S. N. , Malangu, O. N. , & Schumaker, B. A. (2014). Outbreak investigation and control case report of brucellosis: Experience from livestock research centre, Mpwapwa, Tanzania. The Onderstepoort Journal of Veterinary Research, 81(1). 10.4102/ojvr.v81i1.818 [DOI] [PubMed] [Google Scholar]
- Simon, C. , Karimuribo, E. , & Kessy, B. M. (2015). Seroprevalence study of swine brucellosis and knowledge of pig traders and dressers at slaughter facilities in temeke municipality of dar es salaam, Tanzania. Livestock Research for Rural Development, 27. [Google Scholar]
- Swai, E. S. , Moshy, W. , Mbise, E. , Lutatina, J. , & Bwanga, S. (2011). Disease and health conditions affecting camel production in pastoral and agro‐pastoral communities of northern Tanzania. Research Opinions in Animal and Veterinary Sciences, 1(2), 83–88. [Google Scholar]
- Swai, E. S. , & Schoonman, L. (2010). The use of rose Bengal plate test to asses cattle exposure to Brucella infection in traditional and smallholder dairy production systems of Tanga Region of Tanzania. Veterinary Medicine International, 2010, 10.4061/2010/837950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swai, E. S. , & Schoonman, L. (2011). Microbial quality and associated health risks of raw milk marketed in the Tanga region of Tanzania. Asian Pacific Journal of Tropical Biomedicine, 1(3), 217–222. 10.1016/S2221-1691(11)60030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swai, E. S. , & Schoonman, L. (2012). A survey of zoonotic diseases in trade cattle slaughtered at Tanga city abattoir: A cause of public health concern. Asian Pacific Journal of Tropical Biomedicine, 2(1), 55–60. 10.1016/S2221-1691(11)60190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzania National Bureau of Statistics . (2017). Annual agriculture sample survey crop and livestock report, 2016/17. [Google Scholar]
- Tanzania National Bureau of Statistics . (2019). Tanzania in figures 2018. [Google Scholar]
- Tekle, M. , Legesse, M. , Edao, B. M. , Ameni, G. , & Mamo, G. (2019). Isolation and identification of Brucella melitensis using bacteriological and molecular tools from aborted goats in the Afar region of north‐eastern Ethiopia. BMC Microbiology, 19(1), 1–6. 10.1186/s12866-019-1474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thienpont, D. , Vandervelden, M. , Fagard, P. , & Mortelmans, J. (1961). L ’ hygroma brucellique : l ’ aspect clinique caractéristique de la brucellose bovine au Rwanda‐Burundi. Revue d’elevage et de Medecine Veterinaire Des Pays Tropicaux, 1956.
- Tumwine, G. , Matovu, E. , Kabasa, J. D. , Owiny, D. O. , & Majalija, S. (2015). Human brucellosis: Sero‐prevalence and associated risk factors in agro‐pastoral communities of Kiboga District, Central Uganda. BMC Public Health, 15(1), 1–8. 10.1186/s12889-015-2242-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda Bureau Of Statistics . (2018). Statistical abstract. [Google Scholar]
- United Nations Economic Commission for Africa . (2013). South Sudan‐country profile 2017. Africa yearbook (Vol. 9). Author. 10.1007/978-3-319-01384-8_672 [DOI] [Google Scholar]
- United States Agency for International Development (USAID). (2009). Livelihoods Zoning “Plus” Activity in Burundi, A special Report by The Famine Early Warning System Network (FEWS NET), November 2009. https://fews.net/sites/default/files/documents/reports/bi_zonedescriptions_en.pdf
- World Health Organization . (2012). Research priorities for zoonoses and marginalized infections. World Health Organization Technical Report Series, 971. [PubMed] [Google Scholar]
- Yang, H. X. , Feng, J. J. , Zhang, Q. X. , Hao, R. E. , Yao, S. X. , Zhao, R. , Piao, D. R. , Cui, B. Y. , & Jiang, H. (2018). A case report of spontaneous abortion caused by Brucella melitensis biovar 3. Infectious Diseases of Poverty, 7(1), 1–4. 10.1186/s40249-018-0411-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamri‐Saad, M. , & Kamarudin, M. I. (2016). Control of animal brucellosis: The Malaysian experience. Asian Pacific Journal of Tropical Medicine, 9(12), 1136–1140. 10.1016/j.apjtm.2016.11.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article and no new data were created or analysed.


