Abstract
Background.
A recent family study of young adult males suggests a shared familial liability between ADHD and high body mass index (BMI), and a genome-wide meta-analysis reported a genetic correlation of 0.26 between ADHD and BMI. To date, it is unclear whether these findings generalize to the relationship between ADHD and clinically diagnosed obesity.
Method.
By linking the Swedish national registers, we identified 2 538 127 individuals born between 1973 and 2000, together with their siblings and cousins. The risk of clinical obesity in individuals with ADHD was compared with the risk in those without ADHD. The relative contributions of genetic and environmental factors to the association between ADHD and clinical obesity were examined via assessment of the familial co-aggregation of the two conditions and quantitative genetic analyses.
Results.
Individuals with ADHD were at an increased risk of clinical obesity compared to those without (risk difference 3.73%, 95% CI 3.55–3.90%, risk ratio 3.05, 95% CI 2.95–3.15). Familial co-aggregation of ADHD and clinical obesity was detected and the strength of the co-aggregation decreased by decreasing genetic relatedness. The correlation between the liabilities to ADHD and clinical obesity can be entirely attributed to their genetic correlation (rg 0.30, 95% CI 0.17–0.44).
Conclusion.
The association between ADHD and clinical obesity in adolescence and young adulthood can be entirely attributed to genetic underpinnings shared by the two conditions. Children with ADHD should be monitored for weight gain so that preventive measures can be taken for those on a suboptimal trajectory.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common childhood-onset neurodevelopmental disorder (Polanczyk et al., 2007, Thomas et al., 2015). The core symptoms and impairments of ADHD persist into adolescence and adulthood in a sizable proportion of childhood patients (Faraone et al., 2006). ADHD tends to coexist with not only psychiatric disorders (McGough et al., 2005) but also somatic diseases (Instanes et al., 2016). One increasingly studied comorbid somatic condition of ADHD over the past few years is obesity. Given the global epidemic of obesity (Cuschieri and Mamo, 2016) and its link with a variety of adverse health outcomes, including but not limited to type 2 diabetes mellitus, cardiovascular disease, and increased mortality (Global BMI Mortality Collaboration, 2016), a better understanding of the relationship between ADHD and obesity may have implications in reducing the public health burden of obesity and obesity-related illness.
Two recent meta-analyses (Cortese et al., 2016, Nigg et al., 2016) support an association between ADHD and obesity in adulthood, without evident sex differences in the strength of the association measured by odds ratio (OR). Life style factors and common genetic alterations have been proposed as plausible underlying mechanisms (Cortese and Tessari, 2017). In most studies entering the meta-analyses, obesity assessment was based on body mass index (BMI) ≥30 kg/m2. Studies involving individuals who underwent bariatric surgery, a treatment option for severe and refractory obesity (Nguyen and Varela, 2017), were not included in the meta-analyses, despite preceding investigations showing overrepresentation of individuals with ADHD symptoms in young adults seeking bariatric surgery (Gruss et al., 2012). Compared to obesity defined by BMI ≥30 kg/m2, clinically diagnosed obesity may better reflect the pathological aspect of body fat deposition. Bariatric surgery can neither confound nor mediate the association between ADHD and obesity diagnosed before the surgery. When examining modifying effects of sex on the association between ADHD and obesity, previous studies focused predominantly on the OR scale (Cortese et al., 2016, Nigg et al., 2016), whereas sex may modify the association on the risk difference (RD) scale which is of great public health importance (Knol and VanderWeele, 2012). Hence, a population-based investigation of the association between ADHD and clinical obesity as well as sex differences in the strength of the association measured by RD is needed.
A register-based family study of 472 735 young adult males suggests shared familial liability between ADHD and high BMI (Chen et al., 2017). The finding resonates with a recent genome-wide meta-analysis of clinically diagnosed ADHD in 20 183 cases and 35 191 controls reported a genetic correlation of 0.26 between ADHD and BMI (Demontis et al., 2017). A large representative study on the association between and familial co-aggregation of ADHD and clinical obesity in individuals of both sexes would add to the existing literature the relative contributions of genetic and environmental factors to the overlap between the two conditions.
In this population-based study, we aimed to (1) investigate the association between ADHD and clinical obesity in adolescence and young adulthood using data from the Swedish national registers, and (2) estimate the extent to which the association could be attributed to genetic underpinnings shared by ADHD and clinical obesity via assessment of familial co-aggregation of the two conditions and quantitative genetic analyses.
Method
Data sources and study population
The Swedish Medical Birth Register contains data on approximately 98% of all births in Sweden since 1973 (Cnattingius et al., 1990). The Swedish Total Population Register provides information on sex, dates of birth, death, and migration for all Swedish residents who were born since 1932 and alive in 1968 (Ludvigsson et al., 2016). By linking these two registers, we identified all individuals delivered in single birth between December 31, 1973 and January 1, 2000. Individuals who had congenital malformation, died, or emigrated before age 13 were excluded, leaving a final study population of 2 538 127 individuals aged 13 to 40 on December 31, 2013 which was defined as the end of the observational period.
We further identified all possible sibling pairs and cousin pairs nested in the study population via the Multi-Generation Register. The register links individuals born in Sweden since 1932 and registered as living in Sweden since 1961 to their biological parents (Ekbom, 2011, Statistics Sweden, 2013), enabling identification of family members of varying degrees of relatedness. In total, we identified 2 703 662 pairs of full siblings, 441 956 pairs of maternal half siblings, 472 108 pairs of paternal half siblings, and 10 158 536 pairs of full cousins (i.e., children of full siblings), for assessment of the family co-aggregation of ADHD and obesity.
Next, we randomly selected one pair of siblings from each nuclear family to obtain a sibling sample consisting of 664 721 pairs of full siblings, 68 347 pairs of maternal half siblings, and 69 351 pairs of paternal half siblings. This sample was used for quantitative genetic analysis.
All demographic and health data were collected by the Swedish National Board of Health and Welfare, and pseudonymized by Statistics Sweden, an independent government agency, to mask the identity of individual participants. The study was approved by the regional ethics review board in Stockholm, Sweden.
Ascertainment of ADHD
The Swedish National Patient Register (NPR) and the Prescribed Drug Register (PDR) were used for ascertainment of ADHD. The NPR compiles hospital discharge records of psychiatric inpatient care since 1973, with complete nationwide coverage achieved since 1987 (Ludvigsson et al., 2011). From 2001 onwards, the register also involves approximately 80% of outpatient visits to specialists (Ludvigsson et al., 2011). Diagnoses in the NPR are coded according to International Classification of Diseases, 8th revision (ICD-8) during 1969–1986, ICD-9 during 1987–1996, and ICD-10 from 1997 onwards. The PDR provides information on all drugs prescribed and dispensed to the entire population in Sweden since July 1, 2005, including prescribing dates and active ingredients coded according to the anatomical therapeutic chemical classification system (Wettermark et al., 2007). In the current study, individuals with ADHD were ascertained based on having at least one registered diagnosis of ADHD (ICD-9: 314; ICD-10: F90) in the NPR or at least one registered prescription of methylphenidate (N06BA04), amphetamine (N06BA01), dexamphetamine (N06BA02), lisdexamfetamine (N06BA12), or atomoxetine (N06BA09) in the PDR at any point between their third birthday and 31 December 2013.
Ascertainment of clinical obesity
The NPR was also used to identify individuals diagnosed with obesity (ICD-8: 277.99; ICD9: 278A & 278B; ICD10: E65 & E66) at any point between their 13th birthday and 31 December 2013 (N=48 725). In the current study, data on BMI at obesity diagnosis were not available. Nonetheless, we attempted to gain some understanding about the distribution of BMI among males diagnosed with obesity using data from the Swedish Military Service Conscription Register. The register contains information on directly measured weight and height for Swedish males at conscription (approximately 18 years of age) since 1968 (Gale et al., 2013). The information for females was not available. BMI was calculated as body weight in kilograms divided by height in meters squared. Among individuals with both a diagnosis of obesity and information on BMI (N=4 517), 54.2% had BMI ≥30 kg/m2, 24.7% had BMI ≥35 kg/m2, and 7.5% had BMI ≥40 kg/m2 at conscription. Moreover, of all individuals diagnosed with obesity, 21.0% underwent obesity surgery for management of severe obesity according to the NPR. One validation study reported high accuracy of obesity surgery registration in the NPR in 2011, with an estimated positive predictive value of 97% (Tao et al., 2016). In Sweden, surgical treatment of obesity should be reserved for individuals with BMI ≥40 kg/m2, or BMI ≥35 kg/m2 and obesity-related comorbidities (Boman et al., 2011). Taken together, individuals with clinical obesity in the current study seemed to suffer from relatively severe level of obesity.
Covariates
Several variables were selected as covariates, including age at the end of observation period, sex, birth order (1st, 2nd, 3rd, or ≥4th), maternal age at delivery (<35 years or ≥ 35 years), paternal age at childbirth (<45 years or ≥ 45 years), family education, and psychiatric comorbidity. Age at the end of observational period was treated as a categorical variable with each year as a separate category. It served as a proxy for both the length of observational period and change in register coverage and public awareness of ADHD and clinical obesity. Information on education was retrieved from the Longitudinal Integration Database for Health Insurance and Labor Market (Statistics Sweden, 2011). Family education was defined as the highest level of education achieved by either parent and categorized into elementary & upper secondary education (<13 years) or higher education (≥13 years). Diagnoses of comorbid psychiatric conditions, particularly depression (ICD-8: 296.2, 298.0, 300.4; ICD-9: 296B, 300E; ICD-10: F32 – F34), anxiety (ICD-8: 300 except 300.4; ICD-9: 300 except 300E; ICD-10: F40 – F42, F44 – F45, F48), bipolar disorder (ICD-8: 296.1-3, 296.8; ICD-9: 296A, 296C, 296D, 296E, 296W; ICD-10: F30, F31), and substance use disorder (ICD-8: 303, 304; ICD-9: 303 – 305; ICD-10: F10 – F19) were extracted from the NPR and treated as binary variables (presence or absence).
Statistical Analyses
First, we compared the risk of obesity in individuals with ADHD to the risk in those without ADHD. Logistic regression models and regression standardization approach (Sjolander, 2016) were used to obtain age-adjusted estimates of risk, risk difference (RD), and risk ratio (RR), together with their respective 95% confidence intervals (CIs). The estimates were further adjusted for birth order, maternal age at delivery, paternal age at childbirth, and family education. Since depression, anxiety, bipolar disorder, and substance use disorder frequently co-occur with ADHD and obesity, the models were further adjusted for these conditions to examine the influence of psychiatric comorbidity on the observed associations. We also rerun the model after excluding individuals with other psychiatric disorder than ADHD (ICD-9: 290 – 319 except 314; ICD-10: F00 – F99 except F90). All the aforementioned analyses were performed in the entire study population, and separately for males and females. Sex differences in the RD and RR were examined by testing the statistical significance of an interaction term of ADHD by sex.
Second, we assessed family co-aggregation of ADHD and obesity in full siblings, half siblings, and full cousins. Specifically, we compared the risk of obesity in relatives of individuals with ADHD to the risk in relatives of individuals without ADHD. To assess the relative importance of genetic and shared environmental influences on the familial co-aggregation, we tested the difference in RD between maternal and paternal half siblings. In Sweden, children continued to live predominantly with their mothers following parental separation during the study period (Statistics Sweden, 1994). Maternal half siblings were thus assumed to share more environmental factors than paternal half siblings. Since maternal and paternal half siblings are similar in their genetic sharing (on average 25%), a significantly higher association in maternal half siblings than in paternal half siblings would indicate the importance of shared environmental influence on the familial co-aggregation. On the other hand, non-significant difference in the association between maternal and paternal half siblings, in combination with significant lower RDs compared to full siblings, would highlight the role of genetic factors in the familial co-aggregation. The analyses were adjusted for sex and age of both the index person and the relative, and ADHD status of the relative (Chen et al., 2017).
Finally, we performed quantitative genetic analysis to assess the relative contributions of genetic and environmental factors to the correlation between ADHD and obesity on a liability scale. The joint distribution of the liabilities to ADHD and obesity was assumed to follow a multivariate normal distribution (Neale et al., 1992). Phenotypic correlation (i.e., cross-disorder within-individual correlation) was calculated for the entire sample of sibling pairs. Within-disorder cross-sibling and cross-disorder cross-sibling correlations were calculated separately in full siblings, maternal half siblings, and paternal half siblings. A bivariate Cholesky decomposition model was fitted to decompose the variance in each condition and the covariance of the two conditions into additive genetic (A), dominant genetic (D), shared environmental (C), and non-shared environmental (E) components. The ADCE model was then compared with ACE model and AE model. Likelihood ratio tests were used to determine the best fitting model (i.e., a model with as few parameters as possible and no significant deterioration in fit). Model fitting was based upon the following assumptions: correlation for additive genetic components is 0.50 in full siblings and 0.25 in half siblings; correlation for dominant genetic components is 0.25 in full siblings and zero in half siblings; correlation for shared environmental components is 1.00 in full and maternal half siblings and 0.00 in paternal half siblings.
All statistical hypotheses were two-sided, with a significance level of 5%. SAS software version 9.4 was used for constructing analytic datasets. Logstic regression and regression standardization were performed using stdReg package, and quantitative genetic analysis was carried out using OpenMx package (Neale et al., 2016) in R software version 3.3 (R Development Core Team, 2012).
Results
Descriptive statistics
Among 2 538 127 individuals included in the study population, 80 009 (3.15%) were diagnosed with ADHD and 48 725 (1.92%) were diagnosed with obesity during the observational period. Other descriptive characteristics of the study population are shown in Table 1.
Table 1.
Descriptive characteristics of the study population
| Variable | With ADHD (N = 80 009) | Without ADHD (N = 2 458 118) |
|---|---|---|
| Age at the end of observational period, mean (SD) years | 22.92 (7.20) | 26.64 (7.93) |
| Female, N (%) | 29 829 (37.28) | 1 209 273 (49.20) |
| Birth order, N (%) | ||
| 1st | 34 066 (42.58) | 1 021 815 (41.57) |
| 2nd | 27 444 (34.30) | 907 711 (36.93) |
| 3rd | 12 069 (15.08) | 377 941 (15.38) |
| ≥ 4th | 6 430 (8.04) | 150 651 (6.13) |
| Maternal age at delivery, N (%) | ||
| <35 years | 71 602 (89.49) | 2 196 557 (89.36) |
| ≥35 years | 8 397 (10.50) | 261 344 (10.63) |
| Missing | 10 (0.01) | 217 (0.01) |
| Paternal age at childbirth, N (%) | ||
| <45 years | 76 922 (96.14) | 2 386 888 (97.10) |
| ≥45 years | 2 306 (2.88) | 57 716 (2.35) |
| Missing | 781 (0.98) | 13514 (0.55) |
| Family education, N (%) | ||
| Elementary & upper secondary (≤12 years) | 50 550 (63.18) | 1 315 723 (53.53) |
| Higher (≥13 years) | 29 384 (36.73) | 1 141 066 (46.42) |
| Missing | 75 (0.09) | 1 329 (0.05) |
| Psychiatric comorbidities, N (%) | ||
| Depression | 20 882 (26.10) | 103 233 (4.20) |
| Anxiety | 23 827 (29.78) | 121 608 (4.95) |
| Substance use disorder only | 16 773 (20.96) | 90 187 (3.67) |
| Bipolar disorder | 5 093 (6.37) | 13 452 (0.55) |
| Obesity, N (%) | 3 748 (4.68) | 44 977 (1.83) |
Association between ADHD and clinical obesity
Figure 1 illustrates age-adjusted absolute risks of clinical obesity by ADHD status. Generally, the risk of obesity in individuals with ADHD (5.55%) was three times higher than the risk in individuals without ADHD (1.82%), equivalent to an RD of 3.73% (Table 2). Females were more likely to be diagnosed with obesity than males both in individuals with ADHD and in those without (Figure 1). The RD was higher in females (5.11%) than in males (2.86%), whereas the RR was higher in males (3.74) than in females (2.86). The associations remained statistically significant when the models were additionally adjusted for birth order, parental age at childbirth, and family education (Table 2). So did the associations after further adjustment of depression, anxiety, substance use disorder, and bipolar disorder (Table 2) or excluding individuals with psychiatric disorders other than ADHD (Table 2). Adjustment for each selected comorbid condition at a time attenuated the magnitude of the association to different degrees (Table 2). All RDs and RRs were statistically different between males and females (p<0.001).
Fig. 1.
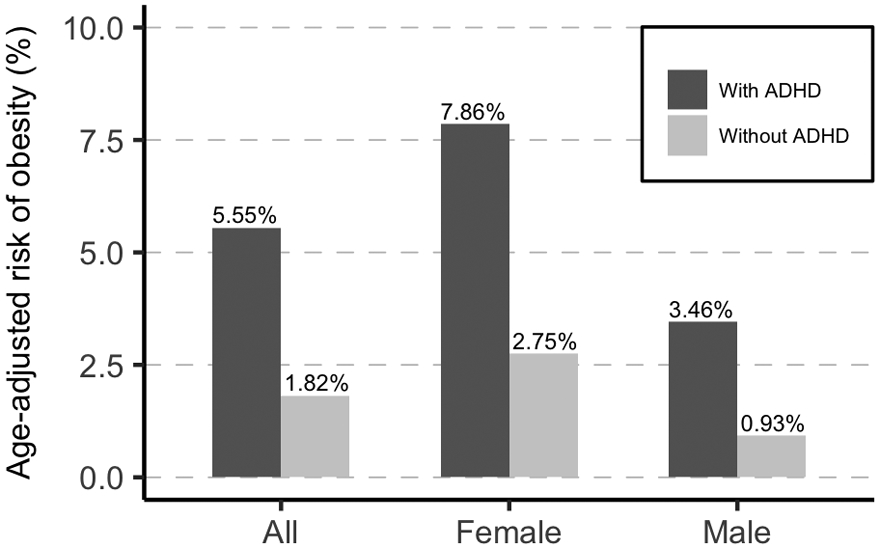
Risk of obesity in individuals with and without ADHD adjusted for age at the end of observational period
Table 2.
Association between ADHD and obesity in the entire study population
| RD (95% CI), % | RR (95% CI) | |
|---|---|---|
| Model 1 | ||
| All | 3.73 (3.55 - 3.90) | 3.05 (2.95 - 3.15) |
| Females | 5.11 (4.77 - 5.44) | 2.86 (2.73 - 2.98) |
| Males | 2.53 (2.37 - 2.70) | 3.74 (3.55 - 3.93) |
| Model 2 | ||
| All | 3.30 (3.14 - 3.47) | 2.82 (2.72 - 2.91) |
| Females | 4.55 (4.24 - 4.86) | 2.66 (2.54 - 2.77) |
| Males | 2.27 (2.12 - 2.43) | 3.46 (3.28 - 3.63) |
| Model 3: model 2 additionally adjusted for all selected comorbid conditions | ||
| All | 1.81 (1.67 - 1.95) | 1.98 (1.91 - 2.06) |
| Females | 2.24 (1.99 - 2.50) | 1.81 (1.71 - 1.90) |
| Males | 1.61 (1.48 - 1.76) | 2.73 (2.57 - 2.89) |
| Model 4: model 2 additionally adjusted for depression only | ||
| All | 2.13 (1.99 - 2.27) | 2.16 (2.08 - 2.24) |
| Females | 2.85 (2.58 - 3.12) | 2.03 (1.93 - 2.13) |
| Males | 1.76 (1.62 - 1.90) | 2.89 (2.73 - 3.05) |
| Model 5: mode 2 additionally adjusted for anxiety only | ||
| All | 2.18 (2.03 - 2.32) | 2.19 (2.11 - 2.27) |
| Females | 2.89 (2.62 - 3.16) | 2.04 (1.94 - 2.14) |
| Males | 1.84 (1.69 - 1.98) | 2.97 (2.81 - 3.13) |
| Model 6: model 2 additionally adjusted for substance use disorder only | ||
| All | 3.13 (2.96 - 3.30) | 2.72 (2.62 - 2.82) |
| Females | 4.08 (3.77 - 4.39) | 2.48 (2.37 - 2.60) |
| Males | 2.17 (2.01 - 2.32) | 3.34 (3.16 - 3.52) |
| Model 7: model 2 additionally adjusted for bipolar disorder only | ||
| All | 2.98 (2.82 - 3.14) | 2.64 (2.54 - 2.73) |
| Females | 4.02 (3.71 - 4.33) | 2.46 (2.34 - 2.57) |
| Males | 2.19 (2.04 - 2.35) | 3.37 (3.19 - 3.54) |
| Model 8: model2 in individuals without other psychiatric disorders than ADHD | ||
| All | 3.54 (3.37 - 3.71) | 3.31 (3.19 - 3.42) |
| Females | 4.82 (4.50 - 5.15) | 3.05 (2.91 - 3.19) |
| Males | 2.44 (2.28 - 2.60) | 4.11 (3.89 - 4.33) |
RD: risk difference; RR: risk ratio; CI: confidence interval
Model 1: Adjusted for age at the end observational period
Model 2: Adjusted for age at the end observational period, birth order, maternal age at delivery, paternal age at childbirth, and family education
Familial co-aggregation of ADHD and clinical obesity
Full siblings of individuals with ADHD showed excess risk for clinical obesity compared to full siblings of individuals without ADHD (RD 1.11%, 95% CI 0.98–1.24%). Significant associations were also observed in half siblings (RD 0.88%, 95% CI 0.71–1.04%) and full cousins (RD 0.67%, 95% CI 0.61–0.74%), with the magnitude being lower than that in full siblings (p<0.001). Nevertheless, the RDs did not differ significantly (p=0.556) between maternal and paternal half siblings (Table 5). The corresponding RRs are shown in Table 5.
Table 5.
Estimated variance explained by genetic and environmental factors from bivariate ACE model
| Estimated Variance (95% CI) |
||||||
|---|---|---|---|---|---|---|
| Additive genetic (A) | Shared environmental (C) | Non-shared environmental (E) | ||||
| Disorder | Unique | Shared | Unique | Shared | Unique | Shared |
| ADHD | 0.70 (0.60 - 0.80) | 0.07 (0.01 - 0.13) | 0.04 (0.00 - 0.08) | 0.00 (−0.01 - 0.01) | 0.19 (0.15 - 0.23) | 0.00 (0.00 - 0.00) |
| Obesity | 0.43 (0.30 - 0.55) | 0.04 (0.01 - 0.08) | 0.11 (0.05 - 0.17) | 0.00 (−0.02 - 0.03) | 0.42 (0.35 - 0.49) | 0.00 (0.00 - 0.00) |
| Genetic correlation (rg) | Shared environmental correlation (rc) | Non-shared environmental correlation (re) | ||||
| ADHD and Obesity | 0.30 (0.17 - 0.44) | 0.19 (−0.37 - 0.75) | −0.03 (−0.19 - 0.13) | |||
Proportion of phenotypic correlation explained by shared genetic influence: 0.98 (0.55 - 1.00)
r: correlation; CI: confidence interval
Quantitative genetic analysis
The correlation between the liabilities to ADHD and clinical obesity was estimated to be 0.19 (95% CI 0.18–0.21). Full siblings showed higher within-disorder cross-sibling correlations and cross-disorder cross-sibling correlation than maternal half siblings, indicating genetic influence on not only the variance in each condition but also the covariance between the two conditions (Table 4). Cross-sibling correlations on ADHD did not differ between maternal and paternal half siblings, suggesting that shared environmental influence is of limited importance for the variance in ADHD (Table 4). Contrarily, higher cross-sibling correlation on clinical obesity in maternal half siblings than in paternal half siblings points to significant shared-environmental influence on the variance in clinical obesity (Table 4). Finally, cross-disorder cross-sibling correlation was similar in maternal and paternal half siblings, suggesting shared environmental factors playing only a limited role in the covariance between ADHD and clinical obesity (Table 4). According to the results of likelihood ratio tests, the bivariate ACE model was the best fitting model (supplement Table S1). The heritability of ADHD was estimated to be 77% (95% CI 69–85%) and the heritability of obesity was 47% (95% CI 34–59%). The bivariate analysis showed that 7% of the total variance (9% the of genetic variance) in ADHD was in common with clinical obesity (Table 5 & supplement Figure S1), and 4% of the total variance (9% of the genetic variance) in clinical obesity was in common with ADHD. The genetic correlation between the two conditions was 0.30 (95% CI 0.17–0.44). The environmental overlap between ADHD and clinical obesity was not statistically significantly (Table 5).
Table 4.
Within-disorder cross-sibling correlations and cross-disorder cross-sibling correlations between ADHD and obesity
| Within-disorder cross-sibling |
Cross-disorder cross-sibling | |||
|---|---|---|---|---|
| No. of pairs | rADHD (95% CI) | robesity (95% CI) | r (95% CI) | |
| Full Siblings | 664 721 | 0.42 (0.41 - 0.43) | 0.34 (0.33 - 0.36) | 0.10 (0.09 - 0.12) |
| Maternal Half Sibling | 68 347 | 0.25 (0.22 - 0.27) | 0.24 (0.20 - 0.28) | 0.07 (0.03 - 0.10) |
| Paternal Half Sibling | 69 351 | 0.21 (0.18 - 0.24) | 0.13 (0.09 - 0.18) | 0.06 (0.02 - 0.10) |
r: correlation; CI: confidence interval
Discussion
In this population-based register study, we found a statistically significant association between ADHD and clinical obesity in adolescence and young adulthood, while taking into account several selected covariates. Familial co-aggregation of ADHD and clinical obesity was detected in full siblings, half siblings, and cousins, with the strength of the co-aggregation decreasing with decreasing genetic relatedness. Quantitative genetic analysis suggests the association between ADHD and clinical obesity could be entirely attributed to their genetic overlap.
The age-adjusted risk of clinical obesity was estimated to be 5.6% among individuals with ADHD, much lower than the risks for high BMI reported by the meta-analysis (28.2% in adults with ADHD and 10.8% in children with ADHD) (Cortese et al., 2016). We speculate that the discrepancy is primarily due to the differences in assessment approach for obesity. In most studies entering the meta-analysis, obesity was defined by BMI ≥ 30 for adults, and BMI > 95 percentile for children and adolescents. Individuals undergoing bariatric surgery were excluded from the meta-analyses but included in our study. Accordingly, in the current study, individuals whose obesity condition captured the attention of health professionals were likely to have high extreme BMI and/or suffered from other medical complications (Fruh, 2017); they mainly represented those actively seeking treatment for obesity or obesity-related medical conditions.
The sex differences in absolute risk and RD might to some extent be explained by difference in health care seeking behavior between males and females. Females visit health care providers more frequently than males for both mental and physical concerns (Thompson et al., 2016). Further, higher RDs in females suggest that proper management of ADHD as well as its comorbid psychiatric disorders, if proven effective in preventing or mitigating clinical obesity, might likely benefit more females with ADHD than males. The estimated RRs appeared to be higher in males than in females because males without ADHD (reference for RR in males) had a relatively lower risk of clinical obesity than females without ADHD (reference for RR in females). It is not entirely clear whether the observed sex differences reflect sex-specific pathophysiological mechanisms underlying the association between ADHD and clinical obesity. Given a lack of evidence for sex-specific effects of common genetic variants on either ADHD or BMI (Locke et al., 2015, Martin et al., 2017) and that sex-limitation model in quantitative genetic analysis using siblings is not yet a standard method, we did not explore sex differences in the subsequent analyses.
Consistent with earlier research, adjustment for comorbid psychiatric disorders including depression, anxiety, bipolar disorder, and substance use disorder somewhat attenuated the association between ADHD and obesity (Cortese and Tessari, 2017). Genetic overlap across these psychiatric disorders, ADHD, and obesity may, in part, account for this attenuation (Lee et al., 2013, Demontis et al., 2017, van Hulzen et al., 2017). It is also possible that ADHD, if left untreated or treated improperly, might give rise to psychiatric disorders, which are in turn associated with clinical obesity. Nevertheless, the statistically significant association between ADHD and clinical obesity in individuals without any other psychiatric disorders indicates that alternative explanations for the association should be considered.
In the current study, relatives of individuals with ADHD showed an excess risk for clinical obesity compared to relatives of individuals without ADHD, after adjustment for ADHD status in the relative. The RDs decreased with decreasing relatedness between the index person and the relative, indicating a shared familial liability to ADHD and clinical obesity. Furthermore, there was no significant difference in RD between maternal and paternal half siblings, suggesting that shared environmental factors play a very limited role in the familial co-aggregation of ADHD and clinical obesity. Hence, the shared familial liability was mainly attributable to the genetic overlap between the two conditions. It is noteworthy that among relatives of individuals without ADHD, half siblings appeared to be at a more elevated risk of clinical obesity than full siblings and cousins. This is not surprising given that having a half sibling might serve as an index of exposure to family dysfunction and that the latter was found to be associated with obesity (Halliday et al., 2014). Half siblings therefore seem to be a high-risk group that may open new opportunities for research in influence of gene-environment interplay on clinical obesity and its association with other conditions.
The genetic overlap between ADHD and clinical obesity was further supported by the results from quantitative genetic analyses showing that the correlation between the two conditions could be entirely attributed to the shared genetic risk factors. The finding, together with the findings from individual-level and familial liability analyses, confirm the critical role of genetic effects in occurrence ADHD and clinical obesity and have implications in treatment planning. Since patients with clinical obesity are likely to be genetically predisposed to ADHD, early detection and proper management of ADHD symptoms may help improve patient adherence to treatment of obesity and obesity related conditions. The moderate genetic correlation estimated in the current study was similar to the common genetic variant correlation between ADHD and BMI reported in a recent genome-wide meta-analysis of ADHD (Demontis et al., 2017). Future studies in independent samples and using different measurements may eventually advance our knowledge in the precise etiology of both ADHD and obesity.
Our study was subject to several limitations. First, the validity of ADHD diagnosis in the NPR has not yet been evaluated. Nevertheless, a prior study of 19 150 twins in Sweden found a high consistency between ADHD diagnosis and parent-rated level of ADHD symptoms. Specifically, 70% twins with an ADHD diagnosis also screened positive for ADHD by their parents (Skoglund et al., 2014). Further evidence for the validity of register-based diagnoses of ADHD comes from a recent GWAS (Demontis et al., 2017), which found a very high genetic correlation (rg 1.17, SE 0.20) between ADHD diagnosed with research interviews and ADHD defined by diagnoses recorded in the Danish national health-care registers, which are similar to the registers in Sweden. In the current study, individuals with ADHD were ascertained via the presence of at least one registered diagnosis of ADHD in the NPR or prescription of ADHD medications in the PDR. According to the treatment recommendations from the Swedish Medical Products Agency, pharmacotherapy should be reserved for individuals with severe ADHD, or those with less severe ADHD who failed respond to non-pharmacological options (Swedish Medical Products Agency, 2016). Thus, the identified individuals with ADHD most likely represented relatively severe ADHD cases. Second, we did not examine to what degree different symptom patterns of ADHD relate to clinical obesity due to lack of measurements on quantitative traits of inattention, hyperactivity, and impulsivity. Third, no prior study assessed the validity of obesity diagnosis in the NPR, and information on BMI at obesity diagnosis was not available. The identified individuals diagnosed with obesity mainly represented those who were referred for obesity treatment due to other reasons, such as infertility, cardiovascular diseases, and surgery, and motivated to change their weight problems. However, the largely overlapping samples of individuals with obesity diagnosis, BMI ≥30 kg/m2 at conscription, and obesity surgery indicate that the obesity diagnosis in the NPR seemed to capture a large number of individuals with very high BMI. Fourth, individuals failing to seek medical care would not be included in the NPR or PDR. Meanwhile, hospital visits due to either ADHD or obesity could increase the chance of detecting the other condition in the same patient, leading to an overestimated association between ADHD and clinical obesity. Finally, in the quantitative genetic analysis, the assumptions for shared environmental effects (i.e., correlation for shared environmental components is 1.00 in full and maternal half siblings and 0.00 in paternal half siblings) were not tested but partially based on the fact that children live predominantly with their mothers after parental separation during the study period in Sweden (Statistics Sweden, 1994). Nonetheless, since previous twin studies consistently reported limited role of shared environmental effects in the variance of ADHD, a slight violation of these assumptions should not dramatically influence the estimated shared environmental correlation.
In conclusion, our findings support an association between ADHD and clinical obesity in adolescence and young adulthood, which is predominantly attributable to the genetic overlap between the two conditions. Children with ADHD should be monitored for weight gain so that preventive measures can be taken for those on a suboptimal trajectory.
Supplementary Material
Table 3.
Familial co-aggregation of ADHD and obesity
| Risk of obesity in relatives of Individuals with ADHD |
Risk of obesity in relatives of Individuals without ADHD |
||||
|---|---|---|---|---|---|
| Type of siblings | Pairs, No. | % (95% CI) | % (95% CI) | RD (95% CI), % | RR (95% CI) |
| Full siblings | 2 703 662 | 2.91 (2.78 - 3.04) | 1.80 (1.78 - 1.81) | 1.11 (0.98 - 1.24) | 1.62 (1.55 - 1.69) |
| Half siblings | 914 064 | 3.68 (3.51 - 3.84) | 2.80 (2.76 - 2.84) | 0.88 (0.71 - 1.04) | 1.31 (1.25 - 1.37) |
| Maternal half siblings | 441 956 | 3.79 (3.56 - 4.02) | 2.94 (2.88 - 2.99) | 0.86 (0.62 - 1.09) | 1.29 (1.21 - 1.37) |
| Paternal half siblings | 472 108 | 3.56 (3.33 - 3.79) | 2.67 (2.63 - 2.72) | 0.88 (0.65 - 1.12) | 1.33 (1.24 - 1.42) |
| Full Cousins | 10 158 536 | 2.70 (2.63 - 2.76) | 2.03 (2.02 - 2.04) | 0.67 (0.61 - 0.74) | 1.33 (1.30 - 1.36) |
RD: risk difference; RR: risk ratio; CI: confidence interval;
All models were adjusted for age at the end of observational period and sex of both the index person and the relative, and ADHD status of the relative
Acknowledgements
This work was supported by Shire International GmbH. Although employees of the Sponsor were involved in the editing and fact checking of information, the content of this manuscript, the design of the work, the interpretation of the data, and the decision to submit the manuscript for publication in Psychological Medicine was made by the authors independently. This work was also supported by the Swedish Research Council (grant #2014-3831), the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework (grant #340-2013-5867), the European Union’s Horizon 2020 research and innovation programme (grant #667302). Dr. Faraone is supported by the K.G. Jebsen Centre for Research on Neuropsychiatric Disorders, University of Bergen, Bergen, Norway, the European Union’s Seventh Framework Programme for research, technological development and demonstration (grant #602805), the European Union’s Horizon 2020 research and innovation programme under grant agreements (grant #667302 and 728018), and National Institute of Mental Health (grant #5R01MH101519 and U01 MH109536-01).
Footnotes
Declaration of interest
In the past year, Dr. Faraone received income, potential income, travel expenses continuing education support and/or research support from Otsuka, Lundbeck, KenPharm, Rhodes, Arbor, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA, Sunovion, Genomind and NeuroLifeSciences. With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. Dr. Larsson has served as a speaker for Eli-Lilly and has received research grants from Shire.
References
- Boman L, Lestner E, Norström F, Näslund E, Näslund I & Samuelsson O (2011). Nationella medicinska indikationer för primär fetmakirurgi och kvalitetskrav på producenter av primär fetmakirurgi. Sweden. [Google Scholar]
- Chen Q, Kuja-Halkola R, Sjolander A, Serlachius E, Cortese S, Faraone SV, Almqvist C & Larsson H (2017). Shared familial risk factors between attention-deficit/hyperactivity disorder and overweight/obesity - a population-based familial coaggregation study in Sweden. Journal of Child Psychology and Psychiatry and Allied Disciplines 58, 711–718. [DOI] [PubMed] [Google Scholar]
- Cnattingius S, Ericson A, Gunnarskog J & Kallen B (1990). A quality study of a medical birth registry. Scandinavian Journal of Social Medicine 18, 143–8. [DOI] [PubMed] [Google Scholar]
- Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Penalver C, Rohde LA & Faraone SV (2016). Association Between ADHD and Obesity: A Systematic Review and Meta-Analysis. American Journal of Psychiatry 173, 34–43. [DOI] [PubMed] [Google Scholar]
- Cortese S & Tessari L (2017). Attention-Deficit/Hyperactivity Disorder (ADHD) and Obesity: Update 2016. Curr Psychiatry Rep 19, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri S & Mamo J (2016). Getting to grips with the obesity epidemic in Europe. SAGE Open Med 4, 2050312116670406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, Belliveau R, Bybjerg-Grauholm J, Bækved-Hansen M, Cerrato F, Chambert K, Churchhouse C, Dumont A, Eriksson N, Gandal M, Goldstein J, Grove J, Hansen CS, Hauberg M, Hollegaard M, Howrigan DP, Huang H, Maller J, Martin AR, Moran J, Pallesen J, Palmer DS, Pedersen CB, Pedersen MG, Poterba T, Poulsen JB, Ripke S, Robinson EB, Satterstrom FK, Stevens C, Turley P, Won H, , , , Andreassen OA, Burton C, Boomsma D, Cormand B, Dalsgaard S, Franke B, Gelernter J, Geschwind D, Hakonarson H, Haavik J, Kranzler H, Kuntsi J, Langley K, Lesch K-P, Middeldorp C, Reif A, Rohde LA, Roussos P, Schachar R, Sklar P, Sonuga-Barke E, Sullivan PF, Thapar A, Tung J, Waldman I, Nordentoft M, Hougaard DM, Werge T, Mors O, Mortensen PB, Daly MJ, Faraone SV, Børglum AD & Neale BM (2017). Discovery Of The First Genome-Wide Significant Risk Loci For ADHD. bioRxiv. [Google Scholar]
- Ekbom A (2011). The Swedish Multi-generation Register. Methods in Molecular Biology 675, 215–20. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J & Mick E (2006). The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychological Medicine 36, 159–65. [DOI] [PubMed] [Google Scholar]
- Fruh SM (2017). Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract 29, S3–s14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global BMI Mortality Collaboration (2016). Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388, 776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss B, Mueller A, Horbach T, Martin A & de Zwaan M (2012). Attention-deficit/hyperactivity disorder in a prebariatric surgery sample. Eur Eat Disord Rev 20, e103–7. [DOI] [PubMed] [Google Scholar]
- Halliday JA, Palma CL, Mellor D, Green J & Renzaho AM (2014). The relationship between family functioning and child and adolescent overweight and obesity: a systematic review. International Journal of Obesity (2005) 38, 480–93. [DOI] [PubMed] [Google Scholar]
- Instanes JT, Klungsoyr K, Halmoy A, Fasmer OB & Haavik J (2016). Adult ADHD and Comorbid Somatic Disease: A Systematic Literature Review. J Atten Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol MJ & VanderWeele TJ (2012). Recommendations for presenting analyses of effect modification and interaction. International Journal of Epidemiology 41, 514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Mowry BJ, Thapar A, Goddard ME, Witte JS, Absher D, Agartz I, Akil H, Amin F, Andreassen OA, Anjorin A, Anney R, Anttila V, Arking DE, Asherson P, Azevedo MH, Backlund L, Badner JA, Bailey AJ, Banaschewski T, Barchas JD, Barnes MR, Barrett TB, Bass N, Battaglia A, Bauer M, Bayes M, Bellivier F, Bergen SE, Berrettini W, Betancur C, Bettecken T, Biederman J, Binder EB, Black DW, Blackwood DH, Bloss CS, Boehnke M, Boomsma DI, Breen G, Breuer R, Bruggeman R, Cormican P, Buccola NG, Buitelaar JK, Bunney WE, Buxbaum JD, Byerley WF, Byrne EM, Caesar S, Cahn W, Cantor RM, Casas M, Chakravarti A, Chambert K, Choudhury K, Cichon S, Cloninger CR, Collier DA, Cook EH, Coon H, Cormand B, Corvin A, Coryell WH, Craig DW, Craig IW, Crosbie J, Cuccaro ML, Curtis D, Czamara D, Datta S, Dawson G, Day R, De Geus EJ, Degenhardt F, Djurovic S, Donohoe GJ, Doyle AE, Duan J, Dudbridge F, Duketis E, Ebstein RP, Edenberg HJ, Elia J, Ennis S, Etain B, Fanous A, Farmer AE, Ferrier IN, Flickinger M, Fombonne E, Foroud T, Frank J, Franke B, Fraser C, Freedman R, Freimer NB, Freitag CM, Friedl M, Frisen L, Gallagher L, Gejman PV, Georgieva L, Gershon ES, Geschwind DH, Giegling I, Gill M, Gordon SD, Gordon-Smith K, Green EK, Greenwood TA, Grice DE, Gross M, Grozeva D, Guan W, Gurling H, De Haan L, Haines JL, Hakonarson H, Hallmayer J, Hamilton SP, Hamshere ML, Hansen TF, Hartmann AM, Hautzinger M, Heath AC, Henders AK, Herms S, Hickie IB, Hipolito M, Hoefels S, Holmans PA, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hultman CM, Hus V, Ingason A, Ising M, Jamain S, Jones EG, Jones I, Jones L, Tzeng JY, Kahler AK, Kahn RS, Kandaswamy R, Keller MC, Kennedy JL, Kenny E, Kent L, Kim Y, Kirov GK, Klauck SM, Klei L, Knowles JA, Kohli MA, Koller DL, Konte B, Korszun A, Krabbendam L, Krasucki R, Kuntsi J, Kwan P, Landen M, Langstrom N, Lathrop M, Lawrence J, Lawson WB, Leboyer M, Ledbetter DH, Lee PH, Lencz T, Lesch KP, Levinson DF, Lewis CM, Li J, Lichtenstein P, Lieberman JA, Lin DY, Linszen DH, Liu C, Lohoff FW, Loo SK, Lord C, Lowe JK, Lucae S, MacIntyre DJ, Madden PA, Maestrini E, Magnusson PK, Mahon PB, Maier W, Malhotra AK, Mane SM, Martin CL, Martin NG, Mattheisen M, Matthews K, Mattingsdal M, McCarroll SA, McGhee KA, McGough JJ, McGrath PJ, McGuffin P, McInnis MG, McIntosh A, McKinney R, McLean AW, McMahon FJ, McMahon WM, McQuillin A, Medeiros H, Medland SE, Meier S, Melle I, Meng F, Meyer J, Middeldorp CM, Middleton L, Milanova V, Miranda A, Monaco AP, Montgomery GW, Moran JL, Moreno-De-Luca D, Morken G, Morris DW, Morrow EM, Moskvina V, Muglia P, Muhleisen TW, Muir WJ, Muller-Myhsok B, Murtha M, Myers RM, Myin-Germeys I, Neale MC, Nelson SF, Nievergelt CM, Nikolov I, Nimgaonkar V, Nolen WA, Nothen MM, Nurnberger JI, Nwulia EA, Nyholt DR, O'Dushlaine C, Oades RD, Olincy A, Oliveira G, Olsen L, Ophoff RA, Osby U, Owen MJ, Palotie A, Parr JR, Paterson AD, Pato CN, Pato MT, Penninx BW, Pergadia ML, Pericak-Vance MA, Pickard BS, Pimm J, Piven J, Posthuma D, Potash JB, Poustka F, Propping P, Puri V, Quested DJ, Quinn EM, Ramos-Quiroga JA, Rasmussen HB, Raychaudhuri S, Rehnstrom K, Reif A, Ribases M, Rice JP, Rietschel M, Roeder K, Roeyers H, Rossin L, Rothenberger A, Rouleau G, Ruderfer D, Rujescu D, Sanders AR, Sanders SJ, Santangelo SL, Sergeant JA, Schachar R, Schalling M, Schatzberg AF, Scheftner WA, Schellenberg GD, Scherer SW, Schork NJ, Schulze TG, Schumacher J, Schwarz M, Scolnick E, Scott LJ, Shi J, Shilling PD, Shyn SI, Silverman JM, Slager SL, Smalley SL, Smit JH, Smith EN, Sonuga-Barke EJ, St Clair D, State M, Steffens M, Steinhausen HC, Strauss JS, Strohmaier J, Stroup TS, Sutcliffe JS, Szatmari P, Szelinger S, Thirumalai S, Thompson RC, Todorov AA, Tozzi F, Treutlein J, Uhr M, van den Oord EJ, Van Grootheest G, Van Os J, Vicente AM, Vieland VJ, Vincent JB, Visscher PM, Walsh CA, Wassink TH, Watson SJ, Weissman MM, Werge T, Wienker TF, Wijsman EM, Willemsen G, Williams N, Willsey AJ, Witt SH, Xu W, Young AH, Yu TW, Zammit S, Zandi PP, Zhang P, Zitman FG, Zollner S, Devlin B, Kelsoe JR, Sklar P, Daly MJ, O'Donovan MC, Craddock N, Sullivan PF, Smoller JW, Kendler KS & Wray NR (2013). Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature Genetics 45, 984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Magi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Hua Zhao J, Zhao W, Chen J, Fehrmann R, Hedman AK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Mateo Leach I, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stancakova A, Strawbridge RJ, Ju Sung Y, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Arnlov J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Bluher M, Bohringer S, Bonnycastle LL, Bottcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Ida Chen YD, Clarke R, Daw EW, de Craen AJ, Delgado G, Dimitriou M, Doney AS, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Grassler J, Gronberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson A, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindstrom J, Sin Lo K, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PK, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Muller G, Muller-Nurasyid M, Musk AW, Nagaraja R, Nothen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Vernon Smith A, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundstrom J, Swertz MA, Swift AJ, Syvanen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gadin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JR, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Van't Hooft FM, Vinkhuyzen AA, Westra HJ, Zheng W, Zondervan KT, Heath AC, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrieres J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hypponen E, Illig T, Jacobs KB, Jarvelin MR, Jockel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJ, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimaki T, Lyssenko V, Mannisto S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PA, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PE, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tonjes A, Tregouet DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Volker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PI, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, Marz W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njolstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Perusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O'Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJ & Speliotes EK (2015). Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaelsson K, Neovius M, Stephansson O & Ye W (2016). Registers of the Swedish total population and their use in medical research. European Journal of Epidemiology 31, 125–36. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M & Olausson PO (2011). External review and validation of the Swedish national inpatient register. BMC Public Health 11, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Walters RK, Demontis D, Mattheisen M, Lee SH, Robinson E, Brikell I, Ghirardi L, Larsson H, Lichtenstein P, Eriksson N, Werge T, Bo Mortensen P, Giørtz Pedersen M, Mors O, Nordentoft M, Hougaard DM, Bybjerg-Grauholm J, Wray N, Franke B, Faraone SV, O'Donovan MC, Thapar A, Børglum AD & Neale BM (2017). A genetic investigation of sex bias in the prevalence of attention deficit hyperactivity disorder. bioRxiv. [Google Scholar]
- McGough JJ, Smalley SL, McCracken JT, Yang M, Del'Homme M, Lynn DE & Loo S (2005). Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. American Journal of Psychiatry 162, 1621–7. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR & North Atlantic Treaty Organization. Scientific Affairs Division. (1992). Methodology for genetic studies of twins and families. Kluwer Academic Publishers: Dordrecht ; Boston. [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, Estabrook R, Bates TC, Maes HH & Boker SM (2016). OpenMx 2.0: Extended Structural Equation and Statistical Modeling. Psychometrika 81, 535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NT & Varela JE (2017). Bariatric surgery for obesity and metabolic disorders: state of the art. Nature Reviews: Gastroenterology & Hepatology 14, 160–169. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Johnstone JM, Musser ED, Long HG, Willoughby M & Shannon J (2016). Attention-deficit/hyperactivity disorder (ADHD) and being overweight/obesity: New data and meta-analysis. Clinical psychology review 43, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J & Rohde LA (2007). The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry 164, 942–8. [DOI] [PubMed] [Google Scholar]
- Sjolander A (2016). Regression standardization with the R package stdReg. European Journal of Epidemiology 31, 563–74. [DOI] [PubMed] [Google Scholar]
- Skoglund C, Chen Q, D'Onofrio BM, Lichtenstein P & Larsson H (2014). Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. Journal of Child Psychology and Psychiatry and Allied Disciplines 55, 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Sweden (2011). Longitudinell integrationsdatabas foör Sjukfoörsaäkrings- och Arbetsmarknadsstudier (LISA) 1990-2009. Statistics Sweden: Sweden. [Google Scholar]
- Statistics Sweden (2013). Multi-generation register 2012. A description of contents and quality. Stockholm. [Google Scholar]
- Swedish Medical Products Agency (2016). Läkemedel vid adhd - behandlingsrekommendation. Information från Läkemedelsverket 27, 13–23. [Google Scholar]
- Tao W, Holmberg D, Naslund E, Naslund I, Mattsson F, Lagergren J & Ljung R (2016). Validation of Obesity Surgery Data in the Swedish National Patient Registry and Scandinavian Obesity Registry (SOReg). Obesity Surgery 26, 1750–6. [DOI] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E & Glasziou P (2015). Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 135, e994–1001. [DOI] [PubMed] [Google Scholar]
- Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP & Aubrey-Bassler K (2016). The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Family Practice 17, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hulzen KJ, Scholz CJ, Franke B, Ripke S, Klein M, McQuillin A, Sonuga-Barke EJ, Kelsoe JR, Landen M, Andreassen OA, Lesch KP, Weber H, Faraone SV, Arias-Vasquez A & Reif A (2017). Genetic Overlap Between Attention-Deficit/Hyperactivity Disorder and Bipolar Disorder: Evidence From Genome-wide Association Study Meta-analysis. Biological Psychiatry 82, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundstrom A, Westerholm B & Rosen M (2007). The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiology and Drug Safety 16, 726–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


