Abstract
Background
Despite early antiretroviral therapy (ART), ART-suppressed people with human immunodeficiency virus (HIV) (PWH) remain at higher risk for infections and infection-related cancers than the general population. The immunologic pathways that remain abnormal in this setting, potentially contributing to these complications, are unclear.
Methods
ART-suppressed PWH and HIV-negative controls, all cytomegalovirus seropositive and enriched for HIV risk factors, were sampled from an influenza vaccine responsiveness study. PWH were stratified by timing of ART initiation (within 6 months of infection [early ART] vs later) and nadir CD4+ T-cell count among later initiators. Between-group differences in kynurenine-tryptophan (KT) ratio, interferon-inducible protein 10, soluble CD14 and CD163, soluble tumor necrosis factor receptor 2, interleukin 6, and soluble urokinase plasminogen activator receptor were assessed after confounder adjustment.
Results
Most participants (92%) were male, reflecting the demographics of early-ART initiators in San Francisco. Most biomarkers were higher among later-ART initiators. Participants in the early-ART group achieved near-normal soluble tumor necrosis factor receptor 2, interleukin 6, and soluble urokinase plasminogen activator receptor levels, but substantially higher KT ratio than those without HIV after confounder adjustment (P = .008). Soluble CD14, soluble CD163, and interferon-inducible protein 10 trended similarly.
Conclusions
While early-ART initiators restore near-normal levels of many inflammatory markers, the kynurenine pathway of tryptophan catabolism remains abnormally high. Because this pathway confers adaptive immune defects and predicts tuberculosis and cancer progression, this it may contribute to persistent risks of these complications in this setting.
Keywords: immune activation, inflammation, kynurenine, tryptophan, indoleamine 2, 3-dioxygenase-1, HIV, antiretroviral therapy, sCD14, IP-10, sCD163
People with human immunodeficiency virus starting antiretroviral therapy early remain at risk for infections, but the putative immunologic mediators remain unclear. We found that the kynurenine pathway of tryptophan catabolism remains abnormally high in this setting, potentially contributing to risks.
Antiretroviral therapy (ART) strongly reduces morbidity and mortality rates in people with human immunodeficiency virus (HIV) (PWH) regardless of CD4+ T-cell count [1, 2]. Nevertheless, ART-suppressed PWH—even those with high nadir CD4+ T-cell counts (ie, >500/μL)—remain at higher risk for several morbid conditions compared with the general population [1–4]. While health-related behaviors no doubt contribute to some of this increased morbidity risk, systemic inflammation persists despite ART and strongly predicts subsequent morbidity and mortality risks. Levels of multiple biomarkers of innate and adaptive immune activation remain abnormally elevated despite suppressive ART and also strongly predict morbidity and mortality risks [5–13].
While this elevated inflammatory state predicts a host of morbid outcomes, not all of these pathways and morbidity risks remain abnormally elevated among PWH who start ART at very early disease stages [14]. Indeed, while individuals starting ART at higher CD4+ T-cell counts remain at higher risk than the general population for tuberculosis and infection-related cancers, there is very little evidence for an increased risk of cardiovascular disease, pulmonary disease, and neurocognitive dysfunction in this setting [1–4, 15–18]. Similarly, levels of some inflammatory biomarkers, but not all of them, remain abnormal in individuals who start ART early [19, 20]. For example, among Thai PWH who initiated extremely early ART within the first 2 weeks of their infection, soluble CD14 (sCD14) levels remained abnormal compared with a control group without HIV, while other markers like interleukin 6 (IL-6) appeared to normalize [19].
It remained unclear whether the apparently persistent sCD14 elevations may have been due to confounding by health-related behaviors or efavirenz use (which has been associated with higher sCD14 levels in several clinical trials [21–24]), but these data suggested that some immune defects may remain abnormal despite ART, while others may normalize. Collectively, these observations raise the possibility that some of the root drivers of the inflammatory state (eg, HIV reservoirs, microbial translocation, and coinfections such as cytomegalovirus [CMV]) may be less active among PWH who initiated ART early, driving a more limited number of inflammatory pathways and disease manifestations [14].
To address these issues, we leveraged a study of influenza vaccine responsiveness to compare several soluble markers of immune activation between ART-suppressed PWH stratified by timing of ART initiation and CD4+ T-cell nadir and people without HIV matched for age, sex, and lifestyle. Sampling was enriched for PWH who initiated ART within the first 6 months of their infection, and all participants were required to be CMV seropositive, given the influence of this copathogen on several immune activation pathways. We also made sure to assess the kynurenine pathway of tryptophan catabolism, because it confers adaptive immune defects, predicts mortality risk, and has yet to be assessed in a cohort of durably suppressed PWH who started ART at early disease stages.
METHODS
Study Design and Participants
This prospective cohort study included all patients in an ongoing study assessing influenza vaccine responsiveness from 1 June 2014 through 31 December 2018. Enrollment of PWH was facilitated by the University of California, San Francisco, SCOPE cohort and Options project, described elsewhere [25]. Age- and sex-matched HIV-uninfected participants enriched for HIV risk factors were also enrolled.
Inclusion criteria for those with or without HIV included age between 40–65 years and CMV seropositivity. PWH were also required to have plasma HIV RNA levels <40 copies/mL for the past 12 months. Detectable “blips” <500 copies were allowed if flanked by undetectable values. Exclusion criteria included active viral hepatitis, injection drug use within the past 12 months, treatment with potent anti-inflammatory drugs within the past 4 months, acute or chronic infection requiring antibiotics or any hospitalization within the past 4 months.
PWH were stratified by timing of ART initiation. Early ART was considered within 6 months of HIV infection (using a published algorithm for estimating the date of initial HIV infection [26]) and later-ART initiators were further stratified by nadir CD4+ T-cell count (>350/μL, 200–350/μL, or <200/μL).
Biomarker Measurements
We selected biomarkers for analysis that have been shown to be persistently elevated in PWH receiving suppressive ART that predict morbidity and mortality risks and span overlapping but discrete inflammatory pathways. The kynurenine-tryptophan (KT) ratio reflects systemic activity of the kynurenine pathway of tryptophan catabolism, which confers adaptive immune defects and predicts incident tuberculosis and mortality risk in treated HIV infection [8–10, 27–29]. Interferon-inducible protein 10 (IP-10 or CXCL10) is an interferon response marker that predicts morbidity and mortality risks in treated HIV infection [10, 20]. Soluble tumor necrosis factor receptor 2 (sTNFR2) is one of the strongest predictors of cardiovascular events in treated HIV and predicts morbidity and mortality risk [10]. IL-6 is an inflammatory cytokine that strongly predicts subsequent morbidity and mortality risks [5, 8, 10]. sCD14 is associated with both microbial translocation and monocyte/macrophage activation and also predicts increased morbidity and mortality risks in treated HIV [8–10, 12]. Soluble urokinase plasminogen activator receptor (suPAR) and soluble CD163 (sCD163) are also associated with monocyte/macrophage activation and predict mortality risk and cardiovascular events [13, 30–32].
Plasma was cryopreserved at −80ºC. Previously unthawed aliquots were thawed for subaliquoting and refrozen just once before thawing for each assay. KT ratios were assessed on cryopreserved plasma obtained in the fasting state via a high-performance liquid chromatography tandem mass spectrometry assay [33]. The remaining biomarkers were measured with enzyme-linked immunosorbent assay (ELISA) using commercial immunoassay kits in duplicate: Quantikine ELISA sTNFR2, sCD14, sCD163, IP-10, suPAR and Quantikine HS ELISA IL-6, all from R&D Systems. The median coefficients of variation for each biomarker assessed in duplicate (ie, from the same plasma aliquot) were <5%. For all analytes except KT ratio, 2 plasma specimens from each participant were assessed approximately 1 month apart and before (or ≥1 month after) vaccination to establish a more stable within-participant average. Median within-participant standard deviations for each biomarker were as follows: sTNFR2, 0.03 log10 pg/mL; sCD14, 0.02 log10 ng/mL; sCD163, 0.03 log10 ng/mL; IP-10, 0.05 log10 ng/mL; IL-6, 0.1 log10 pg/mL; and suPAR, 0.02 log10 pg/mL.
Statistical Analysis
For participants in the early-ART group, the estimated date of detectable HIV infection was derived by a published algorithm that integrates clinical testing history and quantitative plasma HIV RNA level data, using data available from the time of Options cohort enrollment [26]. Nonparametric tests for trend were used to assess trends across ordered groups (ie, from HIV negative through to the group with nadir CD4+ T-cell counts <200/μL). Differences in biomarkers between early-ART and HIV-uninfected groups were assessed with Wilcoxon rank sum tests. Multivariate linear regression analysis was used to assess adjusted differences in immunologic biomarkers among the 5 groups. Biomarkers were log10-transformed and standard errors were calculated with heteroscedasticity-consistent covariance matrix estimators when necessary to satisfy model assumptions.
Adjusted tests for trend were performed by modeling the ordinal groups from HIV-uninfected participants to those with a CD4+ T-cell nadir <200/μL as a continuous variable (ie, linear increase across ordered groups). Age, natal sex, and men who have sex with men (MSM) status were included as potential confounders in all models. Additional covariates associated with the outcome at P < .20 in univariate analyses were considered potential confounders; these covariates included race, ethnicity, history of injection drug use, current smoker, and efavirenz-containing regimen. A covariate was maintained in the multivariate model if it affected the β coefficient of the primary predictor by≥10%. Differences between groups compared with HIV-uninfected participants were also normalized to the interquartile range (IQR) in the HIV-uninfected group (or those with HIV for analyses restricted to ART-suppressed participants) to facilitate comparisons between biomarkers with different dynamic ranges.
All statistical tests were 2-tailed, with an α level of 0.05 considered statistically significant. All statistical analyses were performed with SAS software (version 9.4).
Ethical Considerations
This study received approval of the University of California, San Francisco, Committee on Human Research. All participants provided written informed consent.
RESULTS
Patient Demographics and Characteristics
Of the 205 participants, 41 were HIV negative, and of the 164 PWH, 34 had initiated early ART within the first 6 months of their infection (median, 3 months; IQR, 1–4 months). The remaining PWH who initiated ART during chronic HIV infection were stratified by nadir CD4+ T-cell count: >350/μL (n = 32), 200–350/μL (n = 43), or <200/μL (n = 55). Most participants (92%) were male, with a median age of 54 years (IQR, 49–60 years), 40% were people of color, and 81% were MSM (Table 1). The HIV-uninfected participants were notably enriched for HIV risk factors and health-related behaviors associated with inflammation: 56% were MSM, 41% had >100 lifetime male sexual partners, 15% had ever injected drugs, and 34% were current smokers. Among PWH, the median duration of virologic suppression was 8 years (IQR, 5–11 years), and those with lower nadir CD4+ T-cell counts tended to be ART suppressed for longer periods.
Table 1.
Characteristics of Antiretroviral Therapy–Suppressed Participants and Those Without Human Immunodeficiency Virus
| Characteristic | HIV-Negative Participants (n = 41), No. (%)a | HIV-Positive Participants, No. (%)a | |||
|---|---|---|---|---|---|
| Later ART by CD4+ T-Cell Nadir | |||||
| Early ART (n = 34) | >350/μL (n = 32) | 200–350/μL (n = 43) | <200/μL (n = 55) | ||
| Age, median (IQR), y | 57 (51–60) | 51 (47–53) | 51 (46–56) | 55 (49–61) | 57 (53–62) |
| Male sex | 36 (88) | 34 (100) | 32 (100) | 37 (86) | 50 (91) |
| Race/ethnicity | |||||
| White | 20 (49) | 28 (82) | 18 (56) | 27 (63) | 32 (58) |
| Black | 11 (27) | 1 (3) | 5 (16) | 10 (23) | 9 (16) |
| Latino | 1 (2) | 1 (3) | 4 (13) | 4 (9) | 6 (11) |
| Other | 9 (22) | 4 (12) | 5 (16) | 2 (5) | 8 (15) |
| MSM | 23 (56) | 33 (97) | 29 (91) | 34 (79) | 47 (85) |
| Lifetime male sex partners | |||||
| 0–10 | 18 (44) | 1 (3) | 4 (13) | 4 (11) | 6 (12) |
| 11–100 | 6 (15) | 6 (18) | 6 (19) | 9 (24) | 17 (33) |
| >100 | 17 (41) | 26 (79) | 22 (68) | 25 (66) | 28 (55) |
| IDU ever | 6 (15) | 3 (9) | 2 (6) | 7 (17) | 19 (35) |
| Current smoker | 13 (34) | 2 (6) | 2 (6) | 6 (14) | 12 (22) |
| EFV regimen | … | 4 (12) | 3 (9) | 9 (27) | 17 (52) |
| Duration of viral suppression, median (IQR), yb | … | 7 (4–9) | 6 (4–7) | 8 (5–11) | 9 (7–13) |
| Nadir CD4+ T-cell count, median (IQR), cells/μL | … | 361 (277–501) | 433 (373–499) | 264 (221–309) | 79 (28–138) |
| Current CD4+ T-cell count, median (IQR), cells/μL | 760 (644–976) | 741 (610–846) | 763 (659–956) | 632 (553–841) | 523 (328–686) |
Abbreviations: ART, antiretroviral therapy; EFV, efavirenz; HIV, human immunodeficiency virus; IDU, injection drug use; IQR, interquartile range; MSM, men who have sex with men.
aData represent no. (%) of participants unless otherwise specified.
bViral suppression defined as viral load (plasma HIV RNA level) <40 copies/mL.
Higher Levels of Most Biomarkers Among Later-ART Initiators
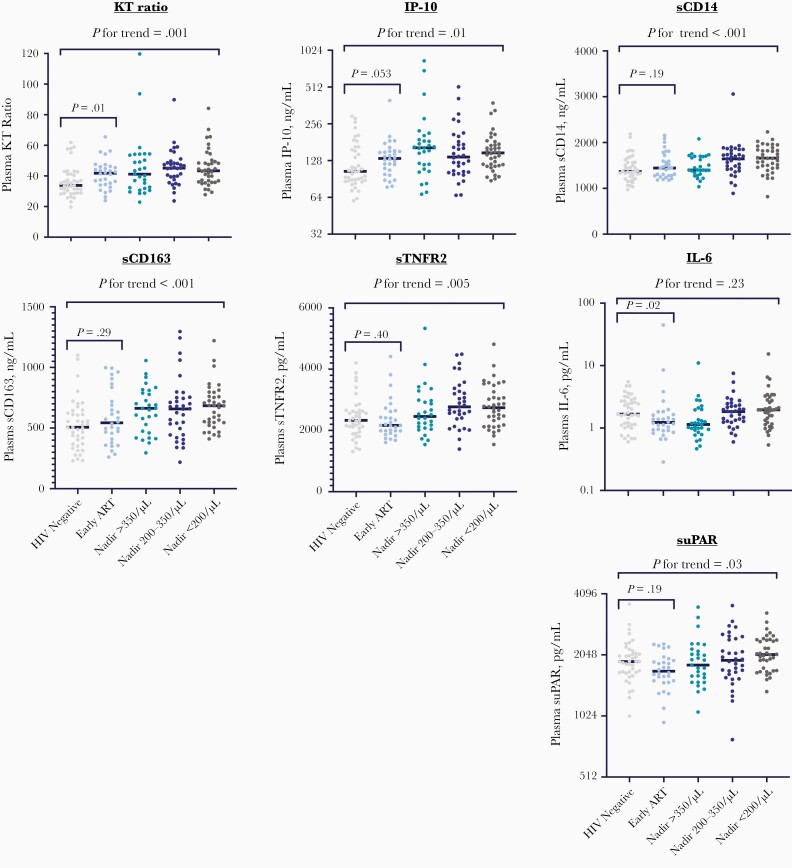
As we stratified our sampling of ART-suppressed individuals across a broad range of ART initiation timing (ie, within the first 6 months of infection or later across a range of nadir CD4+ T-cell counts), we were able to assess the degree to which timing of ART initiation and CD4+ T-cell nadir were associated with each biomarker of interest. Most biomarkers tended to increase with later timing of ART initiation in comparison with HIV-uninfected individuals (Figure 1 and Supplementary Table 1). Notably, there is substantial overlap in most biomarkers across the range of ART initiation timing, suggesting that other factors besides the timing of ART contribute to the “inflammatory set point.” For each biomarker, we next assessed additional potential confounders in adjusted analyses (Table 2). After adjustment for confounders, the trends for increasing biomarkers with delayed ART initiation remained significant for KT ratio (P = .03), sCD163 (P < .001), IP-10 (P = .04), sTNFR2 (P = .047), and suPAR (P = .03). While sCD14 and IL-6 demonstrated no evidence for an association with delayed ART initiation when modeling across ordered groups, when restricting to later-ART participants (n = 130) and modeling nadir CD4+ T-cell count as a continuous variable, lower CD4+ T-cell nadir was associated with both higher IL-6 (ρ = −0.22; P = .01) and sCD14 (ρ = −0.22; P = .01) levels.
Figure 1.
Inflammatory biomarkers by human immunodeficiency virus (HIV) status and timing of prior antiretroviral therapy (ART) initiation. Plasma biomarker levels are compared between HIV-uninfected controls (n = 41) and ART-suppressed people with HIV, stratified by timing of prior ART initiation: early ART (within 6 months of infection; n = 34) or later ART stratified by nadir CD4+ T-cell count (>350/μL [n = 32], 200–350/μL [n = 43], or <200/μL [n = 55]). The kynurenine-tryptophan (KT) ratio is the ratio of the kynurenine level (in nanomoles per liter) to the tryptophan level (in micromoles per liter). P for trend reflects an unadjusted nonparametric test of trend across all ordered groups from HIV negative to CD4+ T-cell nadir <200/μL, and the P value between early-ART participants and HIV-uninfected controls reflects a Wilcoxon rank sum test. Abbreviations: IL-6, interleukin 6; IP-10, interferon-inducible protein 10; sCD14, soluble CD14; sCD163, soluble CD163; sTNFR2, soluble tumor necrosis factor receptor 2; suPAR, soluble urokinase plasminogen activation receptor.
Table 2.
Independent Associations Between Timing of Antiretroviral Therapy (ART) Initiation and Inflammatory Biomarkers During ART-Mediated Viral Suppression
| Biomarkers and Covariates | Mean Fold Change per IQR in HIV-Negative Participants (95% CI) | P Value |
|---|---|---|
| KT ratio | ||
| HIV negative | Reference | … |
| Early ART | 2.9 (1.3–6.2) | .008 |
| Later ART: CD4+ T-cell nadir | ||
| >350/μL | 3.6 (1.7–7.9) | .001 |
| 200–350/μL | 3.0 (1.5–6.0) | .003 |
| <200/μL | 2.4 (1.2–4.7) | .01 |
| Age, per year | 1.1 (1.03–1.1) | .002 |
| Female sex | 1.6 (.6–4.5) | .38 |
| Male sex, not MSM | Reference | … |
| Male sex, MSM | 1.1 (.5–2.3) | .76 |
| sCD163 | ||
| HIV negative | Reference | … |
| Early ART | 2.6 (1.0–6.9) | .046 |
| Later ART: CD4+ T-cell nadir | ||
| >350/μL | 5.0 (1.9–12.9) | .001 |
| 200–350/μL | 3.8 (1.6–8.8) | .003 |
| <200/μL | 4.6 (2.0–10.4) | <.001 |
| Age, per year | 1.03 (.99–1.1) | .13 |
| Female sex | 1.1 (.3–3.9) | .84 |
| Male sex, not MSM | Reference | … |
| Male sex, MSM | 0.5 (.2–1.2) | .14 |
| Current smoking | 0.6 (.3–1.3) | .20 |
| IP-10 | ||
| HIV negative | Reference | … |
| Early ART | 2.2 (.8–5.7) | .11 |
| Later ART: CD4+ T-cell nadir | ||
| >350/μL | 5.1 (2.0–13.2) | <.001 |
| 200–350/μL | 2.6 (1.1–6.0) | .03 |
| <200/μL | 2.6 (1.2–6.0) | .02 |
| Age, per year | 1.04 (1.0–1.1) | .05 |
| Female sex | 0.6 (.2–2.0) | .39 |
| Male sex, not MSM | Reference | … |
| Male sex, MSM | 0.7 (.3–1.8) | .52 |
| Current smoking | 0.5 (.3–1.1) | .09 |
| sCD14 | ||
| HIV negative | Reference | … |
| Early ART | 2.1 (1.1–4.06) | .04 |
| Later ART: CD4+ T-cell nadir | ||
| >350/μL | 1.7 (.9–3.4) | .11 |
| 200–350/μL | 2.0 (1.1–3.7) | .03 |
| <200/μL | 1.8 (1.0–3.3) | .07 |
| Age, per year | 1.05 (1.02–1.1) | <.001 |
| Female sex | 10.3 (4.2–25.5) | <.001 |
| Male sex, not MSM | Reference | … |
| Male sex, MSM | 1.8 (.97–3.5) | .06 |
| EFV | 3.8 (2.2–6.5) | <.001 |
| sTNFR2 | ||
| HIV negative | Reference | … |
| Early ART | 1.2 (.5–2.9) | .66 |
| Later ART: CD4+ T-cell nadir | ||
| >350/μL | 2.1 (.9–5.0) | .08 |
| 200–350/μL | 2.6 (1.2–5.6) | .02 |
| <200/μL | 1.7 (.8–3.7) | .14 |
| Age, per year | 1.1 (1.03–1.1) | <.001 |
| Female sex | 2.8 (.9–9.0) | .08 |
| Male sex, not MSM | Reference | … |
| Male sex, MSM | 1.1 (.5–2.5) | .79 |
| suPAR | ||
| HIV negative | Reference | … |
| Early ART | 1.0 (.4–2.4) | .98 |
| Later ART: CD4+ T-cell nadir | ||
| >350/μL | 2.2 (.9–5.2) | .07 |
| 200–350/μL | 2.0 (.9–4.3) | .08 |
| <200/μL | 1.8 (.9–3.8) | .11 |
| Age, per year | 1.05 (1.02–1.1) | .004 |
| Female sex | 2.4 (.8–7.4 | .12 |
| Male sex, not MSM | Reference | … |
| Male sex, MSM | 0.4 (.2–0.8) | .02 |
| Current smoking | 2.8 (1.5–5.3) | .002 |
| IL-6 | ||
| HIV negative | Reference | … |
| Early ART | 1.0 (.4–2.2) | .97 |
| Later ART: CD4+ T-cell nadir | ||
| >350/μL | 0.7 (.3–1.4) | .28 |
| 200–350/μL | 1.1 (.5–2.2) | .80 |
| <200/μL | 1.2 (.6–2.4) | .58 |
| Age, per year | 1.04 (1.01–1.08) | .006 |
| Female sex | 2.7 (.9–7.9) | .06 |
| Male sex, not MSM | Reference | … |
| Male sex, MSM | 0.6 (.3–1.2) | .12 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; IL-6, interleukin 6; IP-10, interferon-inducible protein 10; IQR, interquartile range; KT, kynurenine-tryptophan; MSM, men who have sex with men; sCD14, soluble CD14; sCD163, soluble CD163; sTNFR2, soluble tumor necrosis factor receptor 2; suPAR, soluble urokinase plasminogen activation receptor.
Association of Efavirenz Use with Selective Increase in sCD14 Levels
Given prior randomized controlled trials suggesting that efavirenz-based regimens result in less robust declines in sCD14 or intestinal fatty acid binding protein than comparator regimens [21–24], we assessed whether efavirenz-containing regimens might be associated with the biomarkers in this study. Among all ART-suppressed participants, and after multivariate adjustment, those taking efavirenz-containing regimens (n = 33) had a mean 3.9-fold IQR greater sCD14 level than those taking efavirenz-sparing regimens (95% confidence interval [CI], 2.2–6.8; P < .001) (Table 3 and Supplementary Figure 1). There was little evidence for an association between efavirenz use and any of the other measured biomarkers.
Table 3.
Association Between Efavirenz Use and Inflammatory Biomarkers in Antiretroviral Therapy–Suppressed Participants
| Biomarker | Adjusted Relative Difference in EFV- vs Non-EFV Regimen Normalized to IQR of ART-Suppressed Participants (95% CI)a | P Value |
|---|---|---|
| KT ratio | 0.53 (.27–1.1) | .07 |
| sCD163 | 0.56 (.29–1.1) | .09 |
| IP-10 | 0.89 (.42–1.9) | .75 |
| sCD14 | 3.9 (2.2–6.8) | <.001b |
| sTNFR2 | 0.67 (.36–1.2) | .20 |
| suPAR | 0.88 (.46–1.7) | .69 |
| IL-6 | 0.75 (.43–1.32) | .32 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; EFV, efavirenz; IL-6, interleukin 6; IP-10, interferon-inducible protein 10; IQR, interquartile range; KT, kynurenine-tryptophan; sCD14, soluble CD14; sCD163, soluble CD163; sTNFR2, soluble tumor necrosis factor receptor 2; suPAR; soluble urokinase plasminogen activation receptor.
aRelative differences were adjusted with covariates listed in Table 2.
bSignificant at P < .05.
Abnormal Levels of Certain Biomarkers in Early-ART Participants Compared with HIV-Uninfected Controls
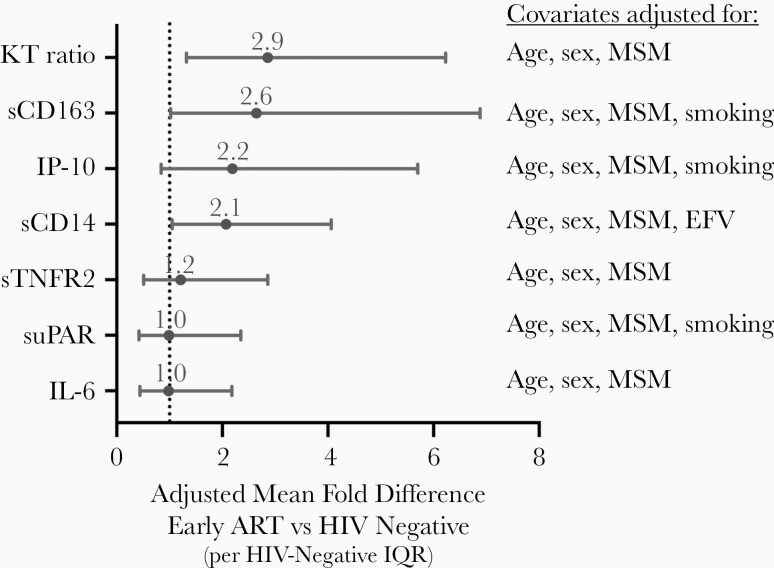
We next assessed the degree to which biomarker levels remained abnormal among PWH who initiated ART in the first 6 months of their infection compared with participants without HIV. After adjustment for age, sex, and other health-related behaviors, KT ratio and sCD14 and sCD163 levels remained significantly higher in early-ART participants than those without HIV (Table 2 and Figure 2). After normalizing to the IQR of each biomarker in those without HIV to facilitate comparisons between biomarkers, early-ART participants had a mean 2.9-fold IQR higher plasma KT ratio (95% CI, 1.3–6.2) than those without HIV after adjustment for age, sex at birth, and MSM status (P = .008). Similar trends were observed when excluding early-ART participants who had CD4+ T-cell counts below the group median (ie, <361/μL) during acute/early HIV infection. After adjustment for confounders, sCD163 and sCD14 levels were a mean 2.6-fold and 2.1-fold IQRs higher than HIV-negative controls, but both CIs bordered closely on 1 (1.02–6.9 and 1.05–4.1, respectively). IP-10 levels tended to be higher in early-ART participants than in those without HIV, with a mean 2.2-fold IQRs higher (95% CI, .8–5.7-fold), but this difference did not reach statistical significance (P = .11). There was no evidence for a difference in sTNFR2, IL-6, or suPAR levels between early-ART participants and HIV-uninfected controls after adjustment for confounders (P > .65 for all).
Figure 2.
Adjusted difference between early antiretroviral therapy (ART) and human immunodeficiency virus (HIV)–uninfected participants. The mean fold difference in plasma biomarkers between early-ART and HIV-uninfected participants is plotted after normalization to the interquartile range (IQR; in the HIV-uninfected group) and after adjustment for the indicated confounders. Mean fold differences are denoted by circles, with bars spanning 95% confidence intervals. The vertical dotted line reflects a relative fold change of 1.0 (ie, no difference between early-ART and HIV-uninfected groups). Abbreviations: EFV, efavirenz; IL-6, interleukin 6; IP-10, interferon-inducible protein 10; KT, kynurenine-tryptophan; MSM, men who have sex with men; sCD14, soluble CD14; sCD163, soluble CD163; sTNFR2, soluble tumor necrosis factor receptor 2; suPAR, soluble urokinase plasminogen activation receptor.
DISCUSSION
While it is now widely acknowledged that immune activation persists in many PWH despite ART-mediated viral suppression, few studies to date have carefully discriminated biomarkers that remain abnormally elevated between those who started ART at early versus late disease stages. Using a robust control group of people without HIV who were enriched for HIV risk factors and health-related behaviors associated with the inflammatory state, we were able to address this gap in the literature.
We made several important observations. First, delayed ART initiation (and lower CD4+ T-cell nadir) was associated with greater persistent immune activation for most analytes assessed. Second, we identified a striking association between efavirenz use and sCD14 (but not other biomarkers), which may have confounded earlier studies addressing the degree to which immune activation normalizes sCD14 during very early ART [19]. Finally, while many biomarkers seemed to normalize in PWH who started ART in the first 6 months of their infection, the KT ratio, a biomarker of the immunoregulatory kynurenine pathway of tryptophan catabolism, remained strikingly high. Other associated pathways (sCD163, sCD14, and perhaps IP-10) remained elevated but to a lesser degree. While the clinical consequences of these differences remain unclear, these data may provide clues as to the discrete immunologic pathways that mediate the persistently increased risk of infections and infection-related cancers even among PWH who initiate ART at very early disease stages.
As in many prior studies, we confirmed that later ART initiation is associated with a greater immune activation set point in PWH maintaining ART-mediated viral suppression. Indeed, very early observational studies identified a link between lower nadir CD4+ T-cell counts and T-cell activation during suppressive ART [34]. Many subsequent observational studies confirmed that markers of innate immune activation and inflammation tended to remain more abnormal in those initiating ART at lower nadir CD4+ T-cell counts, and particularly those with poor CD4+ T cell recovery [35, 36]. Most recently, the START trial provided more definitive evidence that PWH with relatively early-stage infection (ie, CD4+ T-cell counts >500/μL) randomized to immediate ART initiation achieved a lower inflammatory set point (defined by IL-6 and D-dimer levels) during viral suppression than those randomized to delayed ART initiation [37].
Our findings are broadly consistent with this prior literature and highlight how biomarkers spanning several immunologic pathways that predict morbidity and mortality risks remain abnormal in PWH who initiate ART at advanced disease stages. This is particularly true for biomarkers that strongly predict cardiovascular events (sTNFR2, sCD163, and suPAR [10, 32, 38, 39]), neurocognitive decline (sCD14 and sCD163 [40–42]), and pulmonary disease (sCD14, sCD163, and IL-6 [43–46]). These relationships may also help explain why lower CD4+ T-cell nadirs have been consistently associated with a greater risk of these particular morbid conditions.
Our finding that efavirenz use is associated with substantially higher sCD14 levels (but not other biomarkers) is also important. Indeed, several prior trials established that efavirenz-based regimens result in less robust declines in sCD14 or greater increases in intestinal fatty acid binding protein than comparator regimens [21–24]. These observations may suggest a direct effect of efavirenz on microbial translocation, oxidized low-density lipoproteins (another Toll-like receptor 4 ligand) [47, 48], or some other mechanism that specifically increases sCD14 levels. Arguing against a direct impact of efavirenz on microbial translocation is the trend toward lower KT ratio (another biomarker associated with microbial translocation [29]) in efavirenz-treated participants, though efavirenz may suppress kynurenine levels directly by inhibiting hepatic tryptophan 2,3-dioxygenase [49], an observation supported by another clinical study [50].
Regardless of the mechanism, these findings are important to consider in assessing the degree to which biomarkers normalize during ART, because HIV-uninfected comparator groups are always efavirenz naive. For example, the RV254 study from Thailand previously reported that sCD14 levels fail to normalize in PWH starting ART in the first 2 weeks of their infection, but because all of these individuals were receiving an efavirenz-based regimen, it is impossible to know how much of this persistent elevation is explained by efavirenz versus an irreversible effect of HIV [19]. Our study demonstrates that PWH who started ART within the first 6 months of their infection do in fact have higher sCD14 levels than HIV-uninfected individuals but that this difference is significantly attenuated after accounting for efavirenz use.
Finally, we found that only some biomarkers (KT ratio and to a lesser degree sCD163 and sCD14) remained abnormal in PWH who initiated ART in the first 6 months of their infection. The higher levels of sCD14 (and trend toward higher IP-10) in the early-ART compared with HIV-uninfected participants observed in our study is consistent with the findings from the RV254 study of Thai HIV-infected individuals who initiated ART in the first 2 weeks of their infection [19, 20]. Two smaller studies also suggested that KT ratio might not normalize in PWH initiating ART early, but our study is the first to report that durably suppressed early-ART initiators continue to have abnormally high plasma KT ratios when adjusted for confounders, compared with HIV-negative controls [51, 52]. Plasma KT ratio reflects the immunoregulatory kynurenine pathway of tryptophan catabolism, which has been implicated in the pathogenesis of HIV, tuberculosis, and cancer [27, 29, 53].
Tryptophan catabolism to kynurenine normally occurs at low levels by the constitutively active tryptophan deoxygenase enzyme in the liver, but it can also be induced at high levels by myeloid cell expression of indoleamine 2,3-dioxygenase (IDO) 1 in response to inflammatory stimuli, including type I and II interferons (or by similar enzymes in gut-resident bacteria) [29, 54]. IDO-1 activity is strongly induced by HIV, tuberculosis, and several cancers, and downstream catabolites of the kynurenine pathway confer adaptive immune defects by suppressing proliferation of T, B, and natural killer cells, suppressing T-helper 17 cells, and expanding regulatory T cells [29, 55, 56].
The KT ratio is also one of the strongest immunologic predictors of mortality risk in ART-suppressed Africans with HIV [9, 28]. Thus, the abnormal increase in the kynurenine pathway among early-ART participants might provide a plausible mechanistic explanation for the persistently increased risk of tuberculosis and infection-related cancers in early-ART initiators. Indeed, the kynurenine pathway is induced even in latent tuberculosis infection, appears to play a critical role in containing tuberculosis in granulomas in nonhuman primate models of tuberculosis, and predicts incident tuberculosis in a South African cohort [27, 57, 58]. The kynurenine pathway is also mechanistically linked to the other biomarkers that tended to be abnormal in early-ART initiators (sCD14, sCD163, and IP-10). IP-10, like IDO, is expressed in response to type I and II interferons, and the kynurenine pathway–mediated suppression of T-helper 17 cells may also contribute to microbial translocation, increasing sCD14 directly and macrophage activation more generally (sCD163).
Our study did have some important limitations. The relatively small sample size may have limited our ability to detect clinically significant differences in some biomarkers between early-ART initiators and HIV-uninfected participants. Indeed, the nonsignificant trend for IP-10 was nevertheless substantial in effect size relative to the dynamic range observed in HIV-uninfected individuals. The small sample size may have also limited our ability to adjust for multiple confounders simultaneously (particularly in the early-ART group). Nonetheless, we adjusted for a substantial number of confounders that typically have been neglected in the prior literature, including CMV serostatus (by excluding seronegative participants), sexual risk behavior, smoking, and injection drug use (largely by excluding active users), all of which are known to affect immune activation. In addition, our study was too small to evaluate the association between biomarkers and clinical outcomes, particularly in the early-ART group, so we cannot establish the clinical relevance of these findings in the current study. Finally, because we had a small number of female participants in this cohort, constrained by the low prevalence of female early-ART initiators in our setting [59], we may not have been able to adequately assess the influence of sex and sex-related hormones.
In summary, we found that the inflammatory set point for most biomarkers of immune activation during ART-mediated viral suppression tends to be higher in individuals who started ART at more advanced disease stages. These data further support current guidelines suggesting ART initiation as soon as possible after HIV diagnosis. Nevertheless, only a subset of biomarkers of immune activation—particularly those reflecting the kynurenine pathway of tryptophan catabolism—remain abnormal in individuals who started ART within the first 6 months of their infection. This finding may plausibly explain why the risk of tuberculosis and infection-related cancers remains elevated even among individuals who start ART early and may highlight the kynurenine pathway of tryptophan catabolism as an important interventional target in future studies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Virtual 2020 Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, March 8–11, 2020; abstract 206.
Financial support. This study was supported by the National Institutes of Health (grants R01AI110271, R38HL143581 and K24AI145806) and the University of California San Francisco–Gladstone Institute of Virology & Immunology, Center for National Institute of Allergy and Infectious Diseases (grant P30 AI027763).
Potential conflicts of interest. S. G. D. reports grants from Gilead and Merck, personal fees from AbbVie and Enochian Biosciences, and other support from ByroLogyx. P. W. H. has received research funding from Gilead Sciences; honoraria from Gilead, ViiV, and Janssen; and consulting fees from Biotron and ViiV. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Danel C, Moh R, Gabillard D, et al. . A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 2. Lundgren JD, Babiker AG, Gordin F, et al. . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia Garrido HM, Mak AMR, Wit FWNM, et al. . Incidence and risk factors for invasive pneumococcal disease and community-acquired pneumonia in human immunodeficiency virus-infected individuals in a high-income setting. Clin Infect Dis 2020; 71:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One 2012; 7:e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wada NI, Jacobson LP, Margolick JB, et al. . The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sunil M, Nigalye M, Somasunderam A, et al. . Unchanged levels of soluble CD14 and IL-6 over time predict serious non-AIDS events in HIV-1-infected people. AIDS Res Hum Retroviruses 2016; 32:1205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hunt PW, Sinclair E, Rodriguez B, et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee S, Byakwaga H, Boum Y, et al. . Immunologic pathways that predict mortality in HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis 2017; 215:1270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tenorio AR, Zheng Y, Bosch RJ, et al. . Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boulware DR, Hullsiek KH, Puronen CE, et al. ; INSIGHT Study Group . Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandler NG, Wand H, Roque A, et al. ; INSIGHT SMART Study Group . Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knudsen TB, Ertner G, Petersen J, et al. . Plasma soluble CD163 level independently predicts all-cause mortality in HIV-1-infected individuals. J Infect Dis 2016; 214:1198–204. [DOI] [PubMed] [Google Scholar]
- 14. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016; 214:S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rasmussen LD, May MT, Kronborg G, et al. . Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV 2015; 2:e288–98. [DOI] [PubMed] [Google Scholar]
- 16. Wright EJ, Grund B, Robertson KR, et al. ; INSIGHT START Neurology Substudy Group . No neurocognitive advantage for immediate antiretroviral treatment in adults with greater than 500 CD4+ T-cell counts. AIDS 2018; 32:985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker JV, Sharma S, Achhra AC, et al.Changes in cardiovascular disease risk factors with immediate versus deferred antiretroviral therapy initiation among HIV-positive participants in the START (Strategic Timing of Antiretroviral Treatment) Trial. J Am Heart Assoc 2017; 6:e004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kunisaki KM, Niewoehner DE, Collins G, et al. ; INSIGHT START Pulmonary Substudy Group . Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. Lancet Respir Med 2016; 4:980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sereti I, Krebs SJ, Phanuphak N, et al. ; RV254/SEARCH 010, RV304/SEARCH 013 and SEARCH 011 protocol teams . Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis 2017; 64:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hellmuth J, Slike BM, Sacdalan C, et al. . Very early initiation of antiretroviral therapy during acute HIV infection is associated with normalized levels of immune activation markers in cerebrospinal fluid but not in plasma. J Infect Dis 2019; 220:1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hileman CO, Kinley B, Scharen-Guivel V, et al. . Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis 2015; 212:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vesterbacka J, Nowak P, Barqasho B, et al. . Kinetics of microbial translocation markers in patients on efavirenz or lopinavir/r based antiretroviral therapy. PLoS One 2013; 8:e55038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thompson M, Saag M, DeJesus E, et al. . A 48-week randomized phase 2b study evaluating cenicriviroc versus efavirenz in treatment-naive HIV-infected adults with C-C chemokine receptor type 5-tropic virus. AIDS 2016; 30:869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asundi A, Robles Y, Starr T, et al. . Immunological and neurometabolite changes associated with switch from efavirenz to an integrase inhibitor. J Acquir Immune Defic Syndr 2019; 81:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serrano-Villar S, Sainz T, Lee SA, et al. . HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pilcher CD, Porco TC, Facente SN, et al. ; Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA) . A generalizable method for estimating duration of HIV infections using clinical testing history and HIV test results. AIDS 2019; 33:1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adu-Gyamfi CG, Snyman T, Hoffmann CJ, et al. . Plasma indoleamine 2, 3-dioxygenase, a biomarker for tuberculosis in human immunodeficiency virus-infected patients. Clin Infect Dis 2017; 65:1356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Byakwaga H, Boum Y 2nd, Huang Y, et al. . The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis 2014; 210:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Favre D, Mold J, Hunt PW, et al. . Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2010; 2:32ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirkegaard-Klitbo DM, Mejer N, Knudsen TB, et al. . Soluble CD163 predicts incident chronic lung, kidney and liver disease in HIV infection. AIDS 2017; 31:981–8. [DOI] [PubMed] [Google Scholar]
- 31. Burdo TH, Lentz MR, Autissier P, et al. . Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoenigl M, Moser CB, Funderburg N, et al. ; Adult Clinical Trials Group NWCS 411 study team . Soluble urokinase plasminogen activator receptor is predictive of non-AIDS events during antiretroviral therapy-mediated viral suppression. Clin Infect Dis 2019; 69:676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O’Brien MP, Hunt PW, Kitch DW, et al. . A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus-infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect Dis 2017; 4:ofw278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hunt PW, Martin JN, Sinclair E, et al. . T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43. [DOI] [PubMed] [Google Scholar]
- 35. Ghislain M, Bastard JP, Meyer L, et al. ; ANRS-COPANA Cohort Study Group . Late Antiretroviral Therapy (ART) initiation is associated with long-term persistence of systemic inflammation and metabolic abnormalities. PLoS One 2015; 10:e0144317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lederman MM, Calabrese L, Funderburg NT, et al. . Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011; 204:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baker JV, Grund B, Sharma S, et al. . Long-term elevated IL-6 and D-dimer after delayed art initiation in the start trial. Presented at: 2020 Conference on Retroviruses and Opportunistic Infections; 8–11 March 2020; Boston, MA. Abstract 240. [Google Scholar]
- 38. Burdo TH, Lo J, Abbara S, et al. . Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Subramanian S, Tawakol A, Burdo TH, et al. . Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lyons JL, Uno H, Ancuta P, et al. . Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr 2011; 57:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Imp BM, Rubin LH, Tien PC, et al. . Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. J Infect Dis 2017; 215:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Attia EF, Akgün KM, Wongtrakool C, et al. . Increased risk of radiographic emphysema in HIV is associated with elevated soluble CD14 and nadir CD4. Chest 2014; 146:1543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crothers K, Petrache I, Wongtrakool C, Lee PJ, Schnapp LM, Gharib SA. Widespread activation of immunity and pro-inflammatory programs in peripheral blood leukocytes of HIV-infected patients with impaired lung gas exchange. Physiol Rep 2016; 4:e12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. North CM, Muyanja D, Kakuhikire B, et al. . Brief report: systemic inflammation, immune activation, and impaired lung function among people living with HIV in Rural Uganda. J Acquir Immune Defic Syndr 2018; 78:543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fitzpatrick ME, Nouraie M, Gingo MR, et al. . Novel relationships of markers of monocyte activation and endothelial dysfunction with pulmonary dysfunction in HIV-infected persons. AIDS 2016; 30:1327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Estrada V, Monge S, Gómez-Garre MD, et al. ; CoRIS and the HIV Biobank integrated in the Spanish AIDS Research Network . Relationship between plasma bilirubin level and oxidative stress markers in HIV-infected patients on atazanavir- vs. efavirenz-based antiretroviral therapy. HIV Med 2016; 17:653–61. [DOI] [PubMed] [Google Scholar]
- 48. Zidar DA, Juchnowski S, Ferrari B, et al. . Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J Acquir Immune Defic Syndr 2015; 69:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zheve GT Neuroprotective mechanisms of nevirapine and efavirenz in model of neurodegeneration. Rhodes, Greece: Rhodes University, 2007. [Google Scholar]
- 50. Keegan MR, Winston A, Higgs C, Fuchs D, Boasso A, Nelson M. Tryptophan metabolism and its relationship with central nervous system toxicity in people living with HIV switching from efavirenz to dolutegravir. J Neurovirol 2019; 25:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gelpi M, Hartling HJ, Ueland PM, Ullum H, Trøseid M, Nielsen SD. Tryptophan catabolism and immune activation in primary and chronic HIV infection. BMC Infect Dis 2017; 17:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sortino O, Phanuphak N, Schuetz A, et al. . Impact of acute HIV infection and early antiretroviral therapy on the human gut microbiome. Open Forum Infect Dis 2019. doi: 10.1093/ofid/ofaa381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu X, Newton RC, Friedman SM, Scherle PA. Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer therapy. Curr Cancer Drug Targets 2009; 9:938–52. [DOI] [PubMed] [Google Scholar]
- 54. Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. . Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 2009; 31:974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 2002; 196:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gautam US, Foreman TW, Bucsan AN, et al. . In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2018; 115:E62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Collins JM, Siddiqa A, Jones DP, et al. . Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight 2020; 5:e137131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jain V, Liegler T, Vittinghoff E, et al. . Transmitted drug resistance in persons with acute/early HIV-1 in San Francisco, 2002-2009. PLoS One 2010; 5:e15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.