Abstract
Background:
This meta-analysis evaluates the difference of sparing organs at risk (OAR) in different position (Prone position and Supine position) with different breathing patterns (Free breathing, FB/Deep inspiration breath hold, DIBH) for breast cancer patients receiving postoperative radiotherapy and provides a useful reference for clinical practice.
Method:
The relevant controlled trials of prone position versus supine position in postoperative radiotherapy for breast cancer were retrieved from the sources of PubMed, Cochrane Library, Embase, Web of Science and ClinicalTrails.gov. The principal outcome of interest was OAR doses (heart dose, left anterior descending coronary artery dose and ipsilateral lung dose) and target coverage. We mainly compared the effects of P-FB (Prone position FB) and S-FB (Supine position FB) and discussed the effects of DIBH combined with different positions on OAR dose in postoperative radiotherapy. We calculated summary standardized mean difference (SMD) and 95% confidence intervals (CI). The meta-analysis was performed using RevMan 5.4 software.
Results:
The analysis included 751 patients from 19 observational studies. Compared with the S-FB, the P-FB can have lower heart dose, left anterior descending coronary artery (LADCA) dose, and ipsilateral lung dose (ILL) more effectively, and the difference was statistically significant (heart dose, SMD = − 0.51, 95% CI − 0.66 ∼ − 0.36, P < .00001. LADCA dose, SMD = − 0.58, 95% CI – 0.85 ∼ − 0.31, P < .0001. ILL dose, SMD = − 2.84, 95% CI − 3.2 ∼ − 2.48, P < .00001). And there was no significant difference in target coverage between the S-FB and P-FB groups (SMD = − 0.1, 95% CI − 0.57 ∼ 0.36, P = .66). Moreover, through descriptive analysis, we found that P-DIBH (Prone position DIBH) has better sparing OAR than P-FB and S-DIBH (Supine position DIBH).
Conclusion:
By this meta-analysis, compared with the S-FB we found that implementation of P-FB in postoperative radiotherapy for breast cancer can reduce irradiation of heart dose, LADCA dose and ILL dose, without compromising mean dose of target coverage. Moreover, P-DIBH might become the most promising way for breast cancer patients to undergo radiotherapy.
Keywords: breast cancer, deep inspiration breath hold, meta-analysis, prone position, radiotherapy, supine position
1. Introduction
Breast cancer is the most common malignant tumor in women.[1] Postoperative radiotherapy can effectively reduce the local recurrence rate and improve long-term survival rate for early stage breast cancer.[2] EBCTCG showed that postoperative radiotherapy reduced the risk of local recurrence by 19% in 5 years compared with those who did not receive postoperative radiotherapy and reduced the risk of breast cancer deaths by 5% in 15 years.[3] Hence postoperative radiotherapy has become the standard treatment for early stage breast cancer. However, radiotherapy will increase the risk of non-breast cancer related deaths, thereby offsetting the survival advantage of patients.[4,5]
The irradiation of breast tissue will bring a non-negligible dose to the heart and the ILL, which may lead to an increase in the mortality of heart and lung-related diseases.[3,6–11] Heart disease is one of the important reasons for the high mortality in breast cancer patients who accepted postoperative radiotherapy after surviving more than 15 years,[3] and the stenosis of the LADCA is one of the important causes of ischemic heart disease.[8] The incidence of major coronary events increased by 7.4% after every 1 Gy increase in radiation dose and there was no obvious threshold.[9] In addition to cardiac complications, increased lung doses and exposure volume of lung can cause radiation pneumonitis,[10] and the diagnosis rate of lung cancer as a second tumor increases linearly with the increase of radiation dose.[11]
In order to reduce the dose of OAR, some new radiotherapy techniques have been continuously explored. Intensity modulated radiation therapy (IMRT), volume of rotating intensity modulated radiotherapy (VMRT) and proton radiation therapy can effectively reduce the radiation dose of the OAR.[12–16] In addition to the improvement of radiotherapy equipment, DIBH and prone position as two other radiotherapy techniques show great advantages in the protection of OAR as well. DIBH required the patients inhale deeply and hold breath, while in the meantime performs radiotherapy.[17] Our previous research has showed that DIBH after postoperative radiotherapy for left-side breast cancer can reduce the heart dose, LADCA dose and left lung dose without compromising the target coverage.[18] Treatment position is also crucial in radiotherapy. For example, the prone position during radiotherapy for rectal cancer can significantly reduce the small bowel radiation dose compared to the supine position.[19,20] In recent years, clinical studies on different positions with FB or DIBH for postoperative radiotherapy of breast cancer have been reported. However, most studies are limited by small sample size and lack systematic evaluation. We therefore conducted a meta-analysis to provide evidence-based medical basis for its future clinical application.
2. Materials and methods
2.1. Search strategy
A search of the English literature up till July 15, 2020 was conducted using the following electronic databases: PubMed, Cochrane Library, Embase and Web of Science. Search terms included “Breast cancer OR “Breast tumor”; “Breast Neoplasm∗” OR “Breast Cancer∗” OR “Breast Carcinoma∗” OR “Breast Malignanc∗” OR “Breast Tumor∗” OR “Breast tumour∗” OR “Mammary Cancer∗” OR “Mammary Neoplasm ∗”; “radiotherapy”; “radiotherap∗” OR “radiat∗” OR “irradiat∗”; “prone”; “supine”. If possible, subject heading terms such as Medical Subject Headings terms were added in all searches. A search of the ClinicalTrials.gov website was also done to identify randomized controlled trials (RCTs) that had been completed but not yet published. All searches were conducted independently by two reviewers (JL and FZ); differences were checked by the two and resolved by discussion.
2.2. Inclusion and exclusion critera
The inclusion criteria were as follows:
-
1.
All studies that compared supine position radiotherapy versus prone position radiotherapy in patients with breast cancer after breast-conserving surgery without metastasis were eligible.
-
2.
Studies with total number of cases greater than or equal to 10.
-
3.
Studies that report on at least 1 of the outcome indicators mentioned in the succeeding portion.
-
4.
Studies in which patients had comorbidities or additional treatments and with non-human trials were excluded.
In addition, abstracts without full text, letters, expert opinions, reviews, conference abstracts without original data, and single case reports were excluded. This analysis was restricted to articles published in English.
2.3. Evaluation index
To investigate the dose homogeneity of target coverage, the mean dose (Dmean) and V95% of planning target volume (PTV) were calculated. Furthermore, we compared the dose distributions for the heart, LADCA, and ILL using standard defined parameters: the mean dose (Dmean), the maximum dose (Dmax), and the percentage of the organ volume receiving at least 5 Gy (V5), 20 Gy (V20), 30 Gy (V30) and 40Gy (V40). Data extraction was independently assessed by two reviewers (JL and FZ). Disagreements were resolved by consulting with a third reviewer (JD).
2.4. Quality evaluation
The quality of the cohort studies was assessed by the Newcastle–Ottawa Scale (NOS), judged on three broad perspectives: the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest for case–control or cohort studies, respectively. For randomized controlled trials, we used the domains suggested by the Cochrane Handbook for Systematic Reviews of Interventions, including the following aspects: adequacy of the generation of allocation sequence, allocation concealment, blinding, and the presence of incomplete outcome data, selective outcome, or other sources of bias.
2.5. Risk of bias in individual and across studies
Meta-analyses may suffer from several sources of bias. First of all, not all trials lead to a publication, which induces publication bias, and the language of the original publication might give rise to a selection bias. Due to the complexity of the implementation of our research problem (different positions, different positions with different breathing patterns) in distinct clinical treatment centers, most of the enrolled studies were cohort studies and few were randomized controlled studies. However, reporting bias, confounding and baseline differences might be more pronounced cohort studies, as compared to randomized controlled trials.
2.6. Statistical analysis
Because of the diversity in type of studies, patients, different modern radiotherapy techniques and dose prescription, a random effects model was used. Standardized mean difference (SMD) and 95% CI were used to analyze the effects for measurement data. P value < .05 was considered statistically significant. In addition, the funnel plot was used to understand the bias of literature publication. If the points in the funnel plot are symmetrically distributed on both sides around the middlly dashed line and concentrate in the middle, the possibility of publication bias is low. Otherwise, the possibility of publication bias may be high. All statistical analyses were conducted using the Cochrane RevMan 5.4 software.
3. Results
3.1. Included studies
The literature search with our search criteria found 114 articles in PubMed, 5 articles in Cochrane library, 228 articles in Embase, 90 articles in Web of Science and 0 articles in ClinicalTrials.gov. A total of 257 articles remained to be examined after the exclusion of the duplicates. After reading the title and abstract of the article, 46 articles were screened out preliminarily. According to the inclusion criteria and exclusion criteria, 27 articles were screened out again, and 19 articles were finally identified included the final meta-analysis (Fig. 1). Table 1 shows the baseline characteristics of the included studies. The eligible studies include 751 patients (CT scan data of patients in P-FB, S-FB, P-DIBH and S-DIBH group are 669, 566, 75 and 70, respectively). 17 cohort studies were scored using the Newcastle-Ottawa Scale and 2 randomized controlled studies were evaluated using the Cochrane Collaboration's tool for assessing risk of bias.
Figure 1.
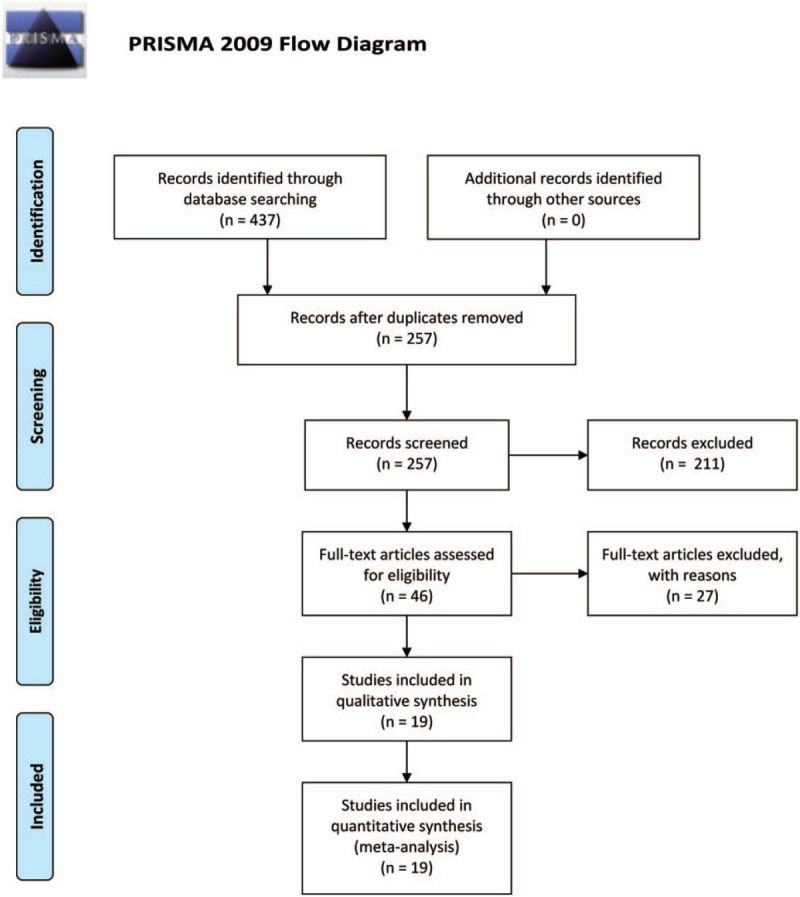
Prisma flow diagram and the process of data selection.
Table 1.
Baseline characteristics of the included studies.
| Studies | Patients numbersL R | Median age, yearP/S | CT scan data(P-FB/S-FB/P-DIBH/S-DIBH) | Stage of cancer | Dose prescription | |
| Buijsen J 2007[21] | 7 | 3 | NA | 10/10/0/0 | ES or CIS | 50Gy/25F |
| Basanta M A 2009[22] | 12 | 8 | NA | 20/20/0/0 | Stage 0-II | 50Gy/25F |
| Varga Z 2009[23] | 34 | 27 | 56 | 61/61/0/0 | ES | 50Gy/25F |
| Veldeman L 2010[24] | 14 | 4 | NA | 18/18/0/0 | ES or CIS | 50Gy/25F |
| Hannan R 2012[25] | 60 | 69 | 61.65/66.03 | 97/32/0/0 | Stage 0-II | 42.4Gy/16F+9.6Gy/4F |
| Chen J L-Y 2013[26] | 21 | 0 | 50.6 | 21/21/0/0 | Stage 0-I | 50Gy/25F |
| Montero F-L A 2013[27] | 6 | 4 | 50.5 | 10/10/0/0 | Stage 0-II | 50Gy/25F |
| Krengli M 2013[28] | 17 | 24 | 54.9 | 41/41/0/0 | Tis-2N0-1 | 50Gy/25F+10Gy/5F |
| Mulliez T(1) 2013[29] | 12 | 6 | NA | 18/18/0/0 | NA | 50Gy/25F |
| Mulliez T(2) 2013[30] | 50 | 50 | 58.1/59.6 | 50/50/0/0 | Tis-2N0 | 40.05Gy/15F |
| Cammarota F 2014[31] | 6 | 6 | 53 | 12/12/0/0 | ES | 42.56/16F |
| Fan L-L 2014[32] | 10 | 0 | 38 | 10/10/0/0 | NA | 50Gy/25F |
| Mulliez T 2015[33] | 50 | 0 | 55 | 50/12/50/12 | ES | 40.05Gy/15F |
| Kim H 2016[34] | 21 | 0 | 54 | 21/21/0/0 | Stage0-IA | 50.4Gy/28F |
| Takahashi K 2016[35] | 9 | 13 | 50 | 22/22/0/0 | Stage 0-II | 50Gy/25F |
| Kahán Z 2018[36] | 100 | 0 | NA | 100/100/0/0 | ES | 50Gy/25F |
| Saini A S 2018[37] | 33 | 0 | NA | 33/33/0/33 | T1-2N0 | 42.56/16F |
| Chung Y 2019[38] | 50 | 0 | 48 | 50/50/0/0 | Tis-2N0-x | 50Gy/25F |
| Saini A S 2019[39] | 25 | 0 | NA | 25/25/25/25 | T1-2N0 | 42.56/16F |
3.2. Target coverage
We investigated the difference of target coverage between the P-FB group and S-FB group. Our result showed that there was no significant difference in Dmean of planning target volume (SMD = − 0.1, 95% CI − 0.57 ∼ 0.36, P = .66) (Fig. 2).
Figure 2.
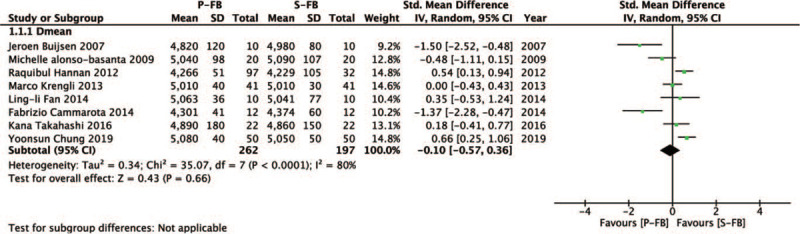
Forest plot of target coverage between the P-FB group and S-FB group.
3.3. Heart dose
We also investigated the difference in heart dose (Dmean, Dmax, V5, and V30) between the P-FB group and S-FB group. Compared with the S-FB group, the P-FB group can lower heart dose more effectively and the difference was statistically significant (SMD = − 0.51, 95% CI − 0.66 ∼ − 0.36, P < .00001). Dmean (SMD = − 0.47, 95% CI − 0.75 ∼ − 0.19, P = 0.0009), Dmax (SMD = − 0.57, 95% CI − 0.82 ∼ − 0.32, P < .00001), V5 (SMD = − 0.40, 95% CI − 0.63 ∼ − 0.16, P = .001), V20 (SMD = − 0.66, 95% CI − 1.05∼ − 0.26, P = .001) (Fig. 3).
Figure 3.
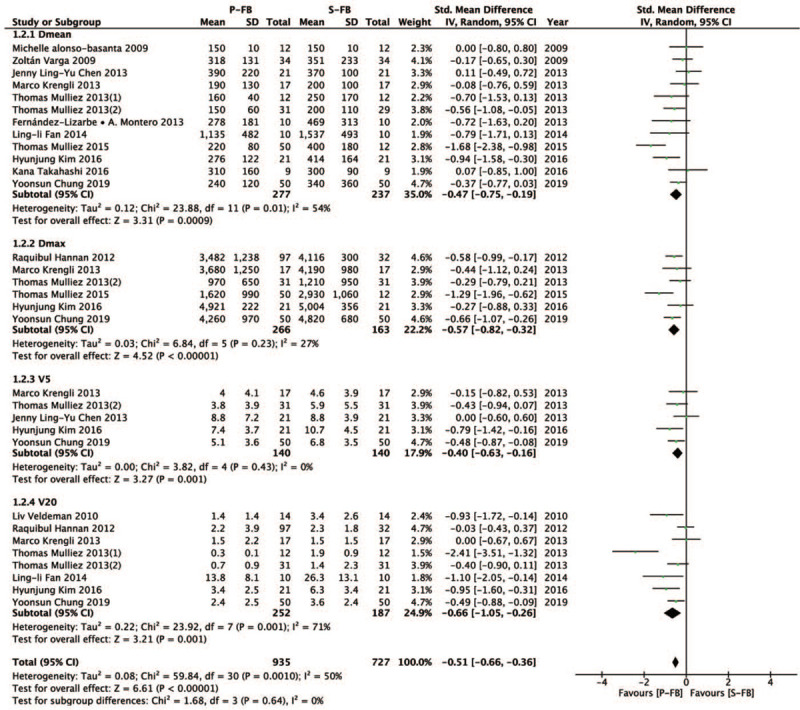
Forest plot of heart dose between the P-FB group and S-FB group.
3.4. LADCA dose
Then, we investigated the difference in LADCA dose (Dmean, Dmax, V40) between the P-FB group and S-FB group. Compared with the S-FB group, the P-FB group can reduce LADCA dose more effectively and the difference was statistically significant (SMD = − 0.58, 95% CI – 0.85 ∼ − 0.31, P < .0001). Dmean (SMD = − 0.53, 95% CI − 0.89 ∼ − 0.16, P = .005), Dmax (SMD = − 0.80, 95% CI − 1.52 ∼ − 0.09, P = .03), V40 (SMD = − 0.47, 95% CI – 0.85 ∼ − 0.09, P = .01) (Fig. 4).
Figure 4.
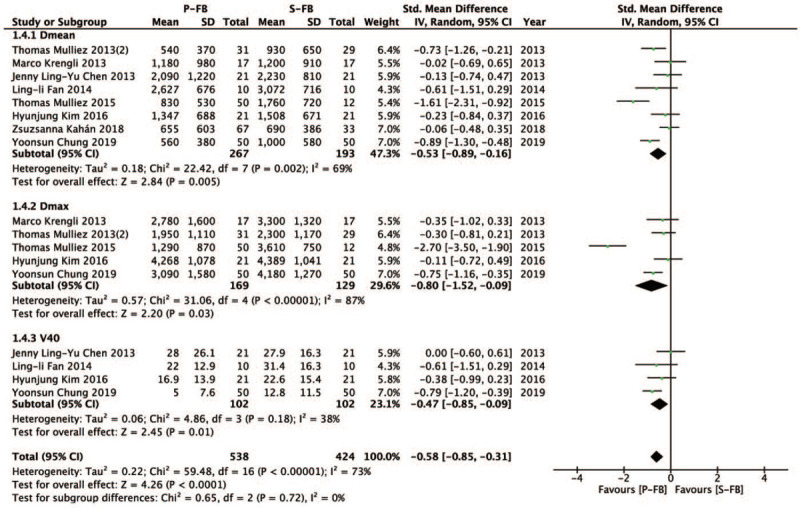
Forest plot of LADCA dose between the P-FB group and S-FB group.
3.5. ILL dose
Further, we investigated the difference in ILL dose (Dmean, Dmax, V5, and V20) between the P-FB group and S-FB group. Compared with the S-FB group, the P-FB group can also have lower ipsilateral lung dose, and the difference was statistically significant (SMD = − 2.84, 95% CI − 3.2 ∼ − 2.48, P < .00001). Dmean (SMD = − 3.36, 95% CI − 3.95 ∼ − 2.77, P < .00001), Dmax (SMD = − 1.89, 95% CI − 2.57 ∼ − 1.2, P < .00001), V5 (SMD = − 3.21, 95% CI – 4.09 ∼ − 2.34, P < .00001), V20 (SMD = − 2.54, 95% CI – 3.06 ∼ − 2.02, P < .00001) (Fig. 5).
Figure 5.
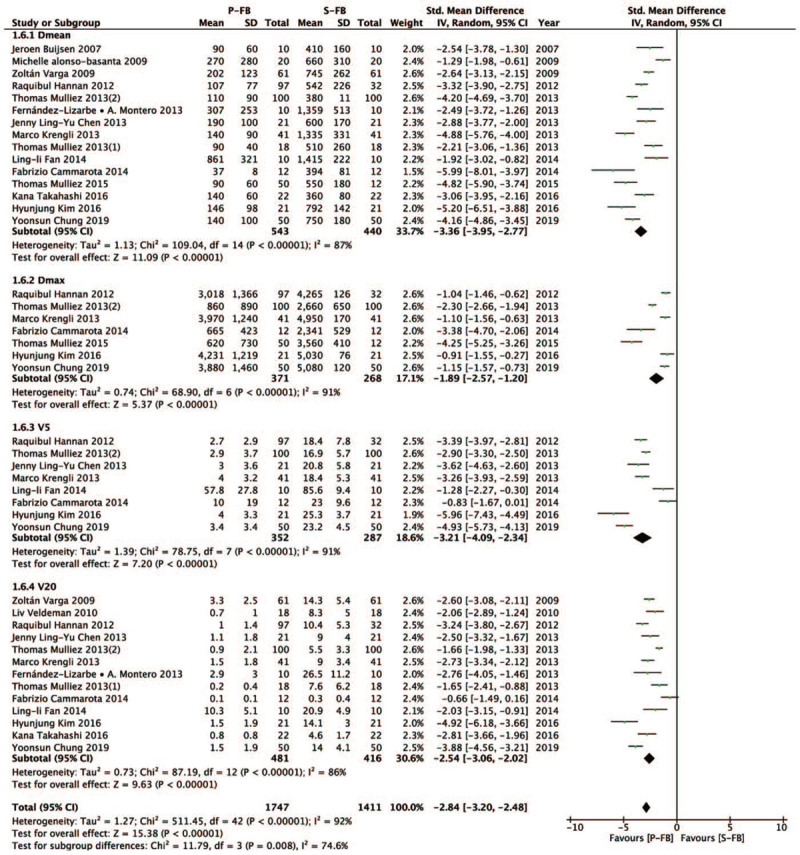
Forest plot of ILL dose between the P-FB group and S-FB group.
3.6. Influence of DIBH with different positions
We further explored the dosimetric effects of DIBH technique with different positions on OAR. Due to the lack of articles containing DIBH with different positions, we conducted only a descriptive analysis for this part data of the included articles rather than quantitative synthesis. Firstly, we analyzed the OAR dose of P-FB vs. S-DIBH (Table 2). Compared to the P-FB, Mulliez et al found that S-DIBH can reduce the dose of heart (P < .001), and P-FB reduce the dose of LADCA and ILL (P < .001). Saini et al also confirmed that P-FB reduce the dose of ILL (P < .001), and there was no significant difference in heart and LADCA between the two groups. Then, we analyzed the OAR dose of P-DIBH vs. S-DIBH (Table 3). Compared to S-DIBH, P-DIBH show dosimetric advantage in OAR (heart, LADCA and ILL) in both studies. Although LADCA dose had no significant statistical difference in Saini's study, the dose of LADCA in P-DIBH is less than S-DIBH.
Table 2.
The OAR dose of P-FB vs. S-DIBH.
| P-FB | S-DIBH | |||||||
| OAR | Studies | Dmean | SD/Min-Max | N | Dmean | SD/Min-Max | N | P value |
| Heart | Mulliez T 2015[33] | 250 | 110 | 50 | 220 | 120 | 12 | <.001 |
| Saini A S 2018[37] | 98 | 83–115 | 33 | 108 | 120–220 | 33 | .114 | |
| LADCA | Mulliez T 2015[33] | 830 | 530 | 50 | 1090 | 780 | 12 | <.001 |
| Saini A S 2018[37] | 657 | 399–949 | 33 | 630 | 259–798 | 33 | .122 | |
| ILL | Mulliez T 2015[33] | 90 | 69 | 50 | 500 | 180 | 12 | <.001 |
| Saini A S 2018[37] | 61 | 47-80 | 33 | 554 | 429–642 | 33 | <.001 | |
Table 3.
The OAR dose of P-DIBH vs. S-DIBH.
| P-DIBH | S-DIBH | |||||||
| OAR | Studies | Dmean | SD/Min-Max | N | Dmean | SD/Min-Max | N | P value |
| Heart | Mulliez T 2015[33] | 130 | 30 | 50 | 220 | 120 | 12 | <.001 |
| Saini A S 2019[39] | 77 | 55–92 | 25 | 97 | 68–123 | 25 | ≦.001 | |
| LADCA | Mulliez T 2015[33] | 330 | 180 | 50 | 1090 | 780 | 12 | <.001 |
| Saini A S 2019[39] | 349 | 345–656 | 25 | 388 | 259–798 | 25 | ≦.194 | |
| ILL | Mulliez T 2015[33] | 90 | 40 | 50 | 500 | 180 | 12 | <.001 |
| Saini A S 2019[39] | 88 | 62–131 | 25 | 541 | 480–675 | 25 | ≦.001 | |
3.7. Publication bias
From the funnel plot (Fig. 6), it can be seen that the most point estimates are symmetrically distributed on both sides and centralized in the middle, showing no evidence of publication bias.
Figure 6.
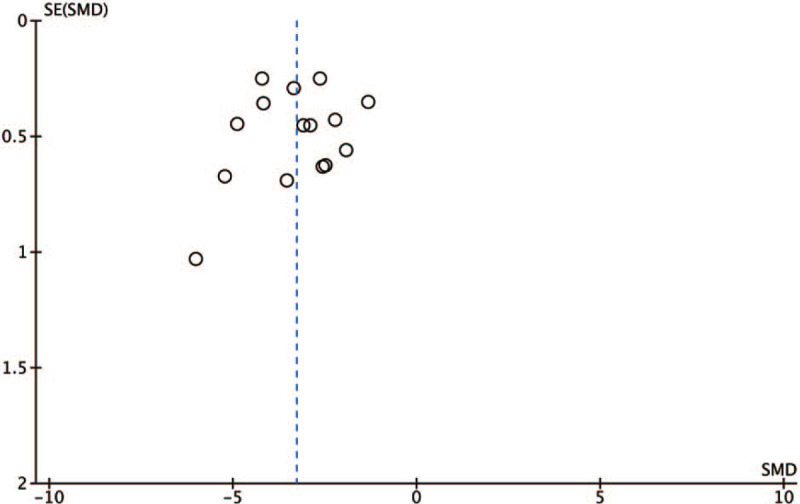
Funnel plot to explore the presence of publication bias.
4. Discussion
How to reduce the dose of OAR in postoperative radiotherapy for breast cancer has been a question that researchers continue to explore. Even if the radiotherapy equipment is updated, radiotherapy is not absolutely safe. Hence, researchers hope to further reduce the dose of OAR by discovering new techniques. Prone positions and DIBH that are two important techniques may have better sparing OAR. In order to explore the sparing OAR of different positions with different breathing patterns in breast cancer patients undergoing postoperative radiotherapy, our study conducted a meta-analysis of OAR doses for P-FB vs. S-FB and do a descriptive analysis that compared OAR doses by using DIBH in different positions.
From our meta-analysis, P-FB group significantly reduced the dose of heart (SMD = − 0.51, 95% CI − 0.66 ∼ − 0.36, P < .00001), LADCA (MD = − 0.58, 95% CI − 0.85 ∼ − 0.31, P < .0001) and ILL (SMD = − 2.84, 95% CI − 3.2 ∼ − 2.48, P < .00001). This result shows that radiotherapy in the prone position can reduce the lower doses than supine position, without compromising Dmean of target coverage (SMD = − 0.1, 95% CI − 0.57 ∼ 0.36, P = .66). Actually, in previous studies, researchers concluded that patients with larger breasts size had better sparing OAR when treated in the prone position.[21,27,28,30,35] Montero et al reported that P-FB radiotherapy for patients with larger breasts can effectively reduce Dmean of heart by 190cGy (P = 0.005) and Dmean of ILL by 1051cGy(P = .047), compared with S-FB.[27] In another study, P-FB can significantly reduce the Dmean of the ILL by 270cGy (P < .001), the LADCA by 390cGy (P = .007) and reduce the Dmean of heart by 50cGy(P = .08) moderately.[30] This is because large size breasts will fold in the supine position, particularly at the inframammary area, which will cause dose inhomogeneity in the target and increase acute and late skin toxicities.[40] It is generally believed that due to the effect of gravity during prone radiotherapy, the dropping breast tissue has a relatively good shape, which can improve the homogeneity of dose in the target area. Moreover, the chest wall obstructed by the treatment bed so that it cannot go down. Hence stretched breast tissue increases the distance between it and the OAR. A concern raised regarding prone breast irradiation is the displacement of the heart anteriorly when prone. Compared to the supine position, researcher found that the mean displacement of the heart was 19 mm anteriorly in prone position (P < .001).[41] In patients with small breast size, breast stretching is not obvious when treated in prone position. Therefore, patients with small size breast needed to cautiously decide whether use prone radiotherapy, which may could increase the dose of heart. Interestingly, in recent years, several studies found that prone radiotherapy in patients with small breast size not only can reduce the ILL dose but also reduce the dose of heart and LADCA.[34,38] But in other studies, regardless of breast size, the results have shown that prone position can significantly reduce the dose to ILL but not the heart.[23,25] The first part of our study also showed that the prone radiotherapy has the advantage of sparing OAR.
In the second part of our research, we found that P-DIBH has better sparing OAR than P-FB and S-DIBH. But due to the lack of evidence, we cannot consider that the above conclusion is necessarily correct. DIBH is an advanced technique through the expanded lung tissue can push the heart away from the chest wall, thereby reducing the dose of the heart. And due to the expansion of lung tissue, the number of alveoli of the same volume irradiated under the same radiotherapy technology is reduced, so that the radiation dose received by the lung tissue is also relatively reduced. Our preliminary a meta-analysis of DIBH versus FB in postoperative radiotherapy for left-side breast cancer[18] had showed that compared with FB group, DIBH group can lower heart dose, LADCA dose and left lung dose more effectively, and the difference was statistically significant (Heart dose, SMD = − 1.36, 95%CI: − 1.64 ∼ − 1.09, P < .01. LADCA dose, SMD = − 1.45, 95%CI: − 1.62 ∼ − 1.27, P < .01. Left lung dose, SMD = − 0.52, 95%CI: − 0.81∼ − 0.23, P < .01). And there was no significant difference in target coverage between the two groups (SMD = 0.03, 95%CI: -0.11∼0.18, P = .64). Hjelstuen et al reported a decrease in Dmean of heart for patients with left side breast cancers from 6.2 Gy with FB to 3.1 Gy with DIBH. The V20 of heart decreased from 7.8% to 2.3% (P < .001), and V40 of heart decreased from 3.4% to 0.3% (P < .001).[42] DIBH has shown great advantages in reducing the dose of OAR, whether the combination of DIBH in different positions can bring further dosimetry benefits to patients. In Mulliez's study,[33] they found that reductions in heart Dmean with P-DIBH compared to P/S-FB according to breast volume < 750 cc, 750–1500 cc and > 1500 cc were 1.3 (± 0.9 Gy), 0.7 (± 0.7 Gy) and 0.4 (± 0.4 Gy), respectively. The results showed that P-DIBH nearly consistently reduced Dmean of heart to less than 2 Gy, regardless of breast volume. Moreover, patients with smaller breast volume seem to benefit the most from P-DIBH. In addition, P-DIBH also can reduce the ILL (P < .001) and LADCA dose (P < .001). Similarly, Saini et al found that P-DIBH could not only significantly reduce the Dmean of ILL (P ≦ .001) but also heart (P ≦ .001) compared other position combined with different breathing patterns.[39] Hence, patients with large breasts or with small breasts both can benefit from P-DIBH radiotherapy. Combined with the above analysis, P-DIBH might be a good choice for breast cancer patients, especially for patients with small size breasts.
Several limitations of our study should be acknowledged when interpreting the results. First, some heterogeneity was observed in this study due to uncontrolled confounding factors and selection bias. We solved this problem by adopting a random-effects model. Second, only articles published and written in English were included this meta-analysis, which might have resulted in some degree of publication bias. However, no significant publication bias was detected, indicating that no noticeable harm was done by potential publication bias.
Through this meta-analysis of P-FB versus S-FB in postoperative radiotherapy for breast cancer, we found that using P-FB for breast cancer patients allows for a significant reduction in heart dose, LADCA dose, and ILL dose while maintaining Dmean of target coverage. Moreover, P-DIBH might become the most promising way for breast cancer patients to undergo radiotherapy. It is worth further exploration by more researchers.
Author contributions
Conceptualization: Junming Lai, Fangyan Zhong.
Data curation: Jianxiong Deng.
Formal analysis: Fangyan Zhong.
Funding acquisition: Junming Lai.
Investigation: Shuang Hu, Ruoyan Shen.
Methodology: Yongbiao Luo, Hui Luo.
Project administration: Junming Lai, Fangyan Zhong.
Software: Fangyan Zhong.
Supervision: Junming Lai.
Validation: Jianxiong Deng.
Writing – original draft: Fangyan Zhong.
Writing – review & editing: Junming Lai.
Footnotes
Abbreviations: CI = confidence intervals, DIBH = Deep inspiration breath hold, Dmax = max dose, Dmean = mean dose, FB = Free breathing, ILL = ipsilateral lung, LADCA = left anterior descending coronary artery, OAR = Organs at risk, P = Prone position, RCTs = randomized controlled trials, S = Supine position, SMD = standardized mean difference, V20 = the percentage of the organ volume receiving at least 20 Gy, V30 = the percentage of the organ volume receiving at least 30 Gy, V40 = the percentage of the organ volume receiving at least 40 Gy, V5 = the percentage of the organ volume receiving at least 5 Gy.
How to cite this article: Lai J, Zhong F, Deng J, Hu S, Shen R, Luo H, Luo Y. Prone position versus supine position in postoperative radiotherapy for breast cancer: A meta-analysis. Medicine. 2021;100:20(e26000).
This study was supported by grants from The First batch of Yiwu Science and Technology Project in 2019 [Grant Number:19-3-03].
Junming Lai and Fangyan Zhong Junming Lai and Fangyan Zhong contributed equally to this work.
Ethics approval: All the data will be extracted from the published studies through database without directly relate to patients’ data, thus not ethical approval is required.
Consent for publication: Not applicable
Availability of data and materials: Not applicable
The authors declare that they have no competing interests.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
CIS = Carcinoma in situ, DIBH = Deep inspiration breath hold, ES = Early stage, FB = Free breathing, L = left-side breast cancer patients, NA = Not available, P = Prone, R = right-side breast cancer patients, S = Supine.
DIBH = Deep inspiration breath hold, FB = Free breathing, ILL = Ipsilateral lung, Max = The maximum value, Min = The minimum value, N = Total number of patients, OAR = Organs at risk, P = Prone, S = Supine. The unit of all Dmean data is ‘cGy’.
DIBH = Deep inspiration breath hold, FB = Free breathing, ILL = Ipsilateral lung, Max = The maximum value, Min = The minimum value, N = Total number of patients, OAR = Organs at risk, P = Prone, S = Supine. The unit of all Dmean data is ‘cGy’.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 1994;12:447–53. [DOI] [PubMed] [Google Scholar]
- [3].(EBCTCG) EBCTCG. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–106. [DOI] [PubMed] [Google Scholar]
- [4].EBCTCG) EBCTCG. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet 2000;355:1757–70. [PubMed] [Google Scholar]
- [5].Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–41. [DOI] [PubMed] [Google Scholar]
- [6].Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer 2013;108:179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Taylor CW, Povall JM, McGale P, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys 2008;72:501–7. [DOI] [PubMed] [Google Scholar]
- [8].Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol 2012;30:380–6. [DOI] [PubMed] [Google Scholar]
- [9].Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- [10].Gagliardi G, Bjöhle J, Lax I, et al. Radiation pneumonitis after breast cancer irradiation: analysis of the complication probability using the relative seriality model. Int J Radiation Oncology Biol Phys 2000;46:373–81. [DOI] [PubMed] [Google Scholar]
- [11].Grantzau T, Thomsen MS, Vaeth M, et al. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol 2014;111:366–73. [DOI] [PubMed] [Google Scholar]
- [12].Schubert LK, Gondi V, Sengbusch E, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol 2011;100:241–6. [DOI] [PubMed] [Google Scholar]
- [13].Yin Y, Chen J, Sun T, et al. Dosimetric research on intensity-modulated arc radiotherapy planning for left breast cancer after breast-preservation surgery. Med Dosim 2012;37:287–92. [DOI] [PubMed] [Google Scholar]
- [14].Muren LP, Maurstad G, Hafslund R, et al. Cardiac and pulmonary doses and complication probabilities in standard and conformal tangential irradiation in conservative management of breast cancer. Radiother Oncol 2002;62:173–83. [DOI] [PubMed] [Google Scholar]
- [15].Iorio GC, Franco P, Gallio E, et al. Volumetric modulated arc therapy (VMAT) to deliver nodal irradiation in breast cancer patients. Med Oncol 2017;35:01–8. [DOI] [PubMed] [Google Scholar]
- [16].Ares C, Khan S, Macartain AM, et al. Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys 2010;76:685–97. [DOI] [PubMed] [Google Scholar]
- [17].Bergom C, Currey A, Desai N, Tai A, Strauss JB. Deep inspiration breath hold: techniques and advantages for cardiac sparing during breast cancer irradiation. Front Oncol 2018;8:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lai J, Hu S, Luo Y, et al. Meta-analysis of deep inspiration breath hold (DIBH) versus free breathing (FB) in postoperative radiotherapy for left-side breast cancer. Breast Cancer 2020;27:299–307. [DOI] [PubMed] [Google Scholar]
- [19].White R, Foroudi F, Sia J, Marr MA, Lim Joon D. Reduced dose to small bowel with the prone position and a belly board versus the supine position in neoadjuvant 3D conformal radiotherapy for rectal adenocarcinoma. J Med Radiat Sci 2017;64:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Scobioala S, Kittel C, Niermann P, et al. A treatment planning study of prone vs. supine positions for locally advanced rectal carcinoma: comparison of 3dimensional conformal radiotherapy, tomotherapy, volumetric modulated arc therapy, and intensity-modulated radiotherapy. Strahlenther Onkol 2018;194:975–84. [DOI] [PubMed] [Google Scholar]
- [21].Buijsen J, Jager JJ, Bovendeerd J, et al. Prone breast irradiation for pendulous breasts. Radiother Oncol 2007;82:337–40. [DOI] [PubMed] [Google Scholar]
- [22].Alonso-Basanta M, Ko J, Babcock M, Dewyngaert JK, Formenti SC. Coverage of axillary lymph nodes in supine vs. prone breast radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:745–51. [DOI] [PubMed] [Google Scholar]
- [23].Varga Z, Hideghety K, Mezo T, et al. Individual positioning: a comparative study of adjuvant breast radiotherapy in the prone versus supine position. Int J Radiat Oncol Biol Phys 2009;75:94–100. [DOI] [PubMed] [Google Scholar]
- [24].Veldeman L, Speleers B, Bakker M, et al. Preliminary results on setup precision of prone-lateral patient positioning for whole breast irradiation. Int J Radiat Oncol Biol Phys 2010;78:111–8. [DOI] [PubMed] [Google Scholar]
- [25].Hannan R, Thompson RF, Chen Y, et al. Hypofractionated whole-breast radiation therapy: does breast size matter? Int J Radiat Oncol Biol Phys 2012;84:894–901. [DOI] [PubMed] [Google Scholar]
- [26].Chen JL, Cheng JC, Kuo SH, Chan HM, Huang YS, Chen YH. Prone breast forward intensity-modulated radiotherapy for Asian women with early left breast cancer: factors for cardiac sparing and clinical outcomes. J Radiat Res 2013;54:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fernandez-Lizarbe E, Montero A, Polo A, et al. Pilot study of feasibility and dosimetric comparison of prone versus supine breast radiotherapy. Clin Transl Oncol 2013;15:450–9. [DOI] [PubMed] [Google Scholar]
- [28].Krengli M, Masini L, Caltavuturo T, et al. Prone versus supine position for adjuvant breast radiotherapy: a prospective study in patients with pendulous breasts. Radiation Oncol 2013;8:232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mulliez T, Speleers B, Madani I, et al. Whole breast radiotherapy in prone and supine position: is there a place for multi-beam IMRT? Radiation Oncol 2013;8:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mulliez T, Veldeman L, van Greveling A, et al. Hypofractionated whole breast irradiation for patients with large breasts: a randomized trial comparing prone and supine positions. Radiother Oncol 2013;108:203–8. [DOI] [PubMed] [Google Scholar]
- [31].Cammarota F, Giugliano FM, Iadanza L, et al. Hypofractionated breast cancer radiotherapy. Helical tomotherapy in supine position or classic 3D- conformal radiotherapy in prone position: which is better? Anticancer Res 2014;34:1233–8. [PubMed] [Google Scholar]
- [32].Fan LL, Luo YK, Xu JH, He L, Wang J, Du XB. A dosimetry study precisely outlining the heart substructure of left breast cancer patients using intensity-modulated radiation therapy. J Appl Clin Med Phys 2014;15:4624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mulliez T, Veldeman L, Speleers B, et al. Heart dose reduction by prone deep inspiration breath hold in left-sided breast irradiation. Radiother Oncol 2015;114:79–84. [DOI] [PubMed] [Google Scholar]
- [34].Kim H, Kim J. Evaluation of the anatomical parameters for normal tissue sparing in the prone position radiotherapy with small sized left breasts. Oncotarget 2016;7:72211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Takahashi K, Morota M, Kagami Y, et al. Prospective study of postoperative whole breast radiotherapy for Japanese large-breasted women: a clinical and dosimetric comparisons between supine and prone positions and a dose measurement using a breast phantom. BMC Cancer 2016;16:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kahan Z, Rarosi F, Gaal S, et al. A simple clinical method for predicting the benefit of prone vs. supine positioning in reducing heart exposure during left breast radiotherapy. Radiother Oncol 2018;126:487–92. [DOI] [PubMed] [Google Scholar]
- [37].Saini AS, Hwang CS, Biagioli MC, Das IJ. Evaluation of sparing organs at risk (OARs) in left-breast irradiation in the supine and prone positions and with deep inspiration breath-hold. J Appl Clin Med Phys 2018;19:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chung Y, Yu JI, Park W, Choi DH. Phase II study, feasibility of prone position in postoperative whole breast radiotherapy: a dosimetric comparison. Cancer Res Treat 2019;51:1370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Saini AS, Das IJ, Hwang CS, Biagioli MC, Lee WE. Biological indices evaluation of various treatment techniques for left-sided breast treatment. Pract Radiat Oncol 2019;9:e579–90. [DOI] [PubMed] [Google Scholar]
- [40].Bentel GC, Marks LB, Whiddon CS, Prosnitz LR. Acute and late morbidity of using a breast positioning ring in women with large/pendulous breasts. Radiother Oncol 1999;50:277–81. [DOI] [PubMed] [Google Scholar]
- [41].Chino JP, Marks LB. Prone positioning causes the heart to be displaced anteriorly within the thorax: implications for breast cancer treatment. Int J Radiat Oncol Biol 2008;70:916–20. [DOI] [PubMed] [Google Scholar]
- [42].Hjelstuen MHB, Mjaaland I, Vikström J, Dybvik KI. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular- and internal mammary lymph nodes without compromising target coverage or dose restrictions to organs at risk. Acta Oncol 2011;51:333–44. [DOI] [PubMed] [Google Scholar]


