Abstract
Objectives:
This study aimed to compare the efficacy and toxicity of intravesical Bacillus Calmette-Guérin (BCG) therapy between elderly and younger patients with non-muscle-invasive bladder cancer (NMIBC).
Material and methods:
This retrospective study included 87 NMIBC patients who received intravesical BCG between January 2011 and December 2018. We compared the treatment outcomes of patients ≥75 and <75 years old. Our primary endpoint was intravesical recurrence-free survival after treatment. The secondary endpoint was the toxicity caused by intravesical BCG.
Results:
The elderly and younger patients included 38 and 49 patients with mean ages of 80.6 and 66.3 years (p < 0.01), respectively. Their baseline parameters were similar, aside from age. The elderly and younger patients’ 5-year recurrence-free survival rates were 59.4% and 51.6%, respectively, and were not significantly different (log-rank test, p = 0.72). Moderate to severe pain on micturition requiring prescription medications was observed more frequently in the elderly patients than in the younger patients (p < 0.01). No elderly patients required hospitalization for any adverse events. However, 3 (6.1%) younger patients were treated for urinary tract infection in the hospital.
Conclusions:
The efficacy and toxicity of intravesical BCG therapy for NMIBC patients are not associated with age. Therefore, elderly patients with high-risk NMIBC should be treated in the same manner as younger patients in clinical practice.
Keywords: Bacillus Calmette-Guérin, Bladder cancer, Elderly patients
1. Introduction
Bladder cancer (BC) is the 9th most common cancer in the world, with 390,000 new cases diagnosed each year. [1] Of these, nearly 75% are non-muscle-invasive BC (NMIBC) cases. During the treatment of NMIBC, as many as 50% of cases can recur, and 9% of cases involve invasion of the muscularis propria. [2] Radical cystectomy is the mainstay of therapy for muscle-invasive BC (MIBC). However, despite well-performed surgeries, cure rates for surgery alone remain between 66% and 80%.3,4 Therefore, preventing the progression of MIBC is critical in managing NMIBC.
In clinical practice, NMIBC is typically treated via transurethral resection of bladder tumors (TURBT) followed by intravesical therapy, depending on the risks of recurrence and progression. [5] Intravesical therapy is usually performed using chemotherapeutic agents or Bacillus Calmette-Guérin (BCG). In a recent randomized controlled trial, BCG was compared to a combination of epirubicin and interferon-alpha2b, [6] mitomycin C, [7] or epirubicin alone, [8] and it was confirmed that BCG was superior in preventing tumor recurrence in intermediate-risk and high-risk NMIBC patients. Additionally, meta-analyses demonstrated that intravesical BCG reduces the risk of tumor progression.9,10,11 However, intravesical BCG is associated with more adverse events than intravesical chemotherapy, [8] and its intolerance rate was reported to be as high as 70%. [12] Furthermore, it was reported that intravesical BCG may carry a non-negligible risk of adverse events in elderly patients.13,14 Therefore, whether intravesical BCG therapy for elderly patients can be performed in the same manner as in younger patients has often been debated.
Currently, the proportion of elderly people has been increasing dramatically worldwide. Old age is now widely accepted as the most important independent risk factor for developing BC. In the future, BC will become an even bigger public health challenge. Although the current standard of care for high-risk NMIBC patients is intravesical BCG therapy following TURBT, [15] the relationship between age and clinical outcomes of this therapy remains controversial. Therefore, we aimed to assess the effectiveness and toxicity of intravesical BCG therapy in elderly patients, compared to younger patients.
2. Materials and methods
2.1. Patients and study design
This retrospective study included high-risk NMIBC patients who received intravesical BCG after TURBT at Kagawa University Hospital in Kagawa, Japan, between January 2011 and December 2018. Tumor grade (high or low), number of tumors (solitary or multiple), T-stage (Ta, T1, or Tis), and concomitant carcinoma in situ (CIS) in TaT1 BC (yes or no) were recorded for every surgery. Additionally, prior recurrence status, a history of upper tract urothelial cancer (UTUC) and BCG therapy, urinary cytology before transurethral resection (TUR), and whether immediate single instillation with pirarubicin (THP) was administered were retrospectively investigated.
All patients received initial TURBT followed by intravesical BCG. Moreover, repeat TUR was performed in all high-grade pTa and pT1 BC patients before adjuvant BCG instillation. Intravesical BCG was administered once a week for 6 or 8 weeks, and 80 mg of Tokyo 172 strain in 40 mL of saline or 81 mg of Connaught strain in 40 mL of saline were instilled per treatment with 2 h of retention time. Maintenance BCG (3 weekly instillations at 3, 6, and 12 months post-treatment initiation) was considered for high-risk BC. This has been offered to our institution's patients since 2018.
This study's primary endpoint was intravesical recurrence-free survival (RFS) after initial TUR implementation. We defined “younger” as <75 years old and “elderly” as ≥75 years old. Cystoscopy and urine cytology were repeated every 3 months during the follow-up period. The European Association of Urology risk group stratification [15] was used to stratify the type of NMIBC risk as high and low. Recurrence was defined as histology-proven tumor recurrence (any grade) or the appearance of CIS. Progression was defined as the development of a new tumor with pathologically proven muscle invasion. Our secondary analysis included evaluating toxicity according to the National Cancer Institute Common Terminology Criteria version 5.0. The cutoff date for survival and treatment-related events was December 2019.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Kagawa University, Faculty of Medicine (Permission number, 2020–046). A waiver of informed consent was obtained, given the retrospective nature of the study.
2.2. Statistical analysis
Comparisons of clinical characteristics and oncological outcomes between younger and elderly patients were performed using Fisher's exact test and the Mann–Whitney U test. Survival curves were generated using the Kaplan–Meier method and compared using the log-rank test. p-Values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 12 for Windows (SPSS Inc., Chicago, IL).
3. Results
3.1. Clinical and demographic characteristics
Table 1 lists patients’ tumor characteristics stratified by age group. In total, 87 patients’ intravesical BCG courses were evaluated: 38 elderly and 49 younger patients. The mean ages of elderly and younger patients were 80.6 and 66.3 years, respectively (p < 0.01). There were no significant differences between the 2 groups in terms of mean follow-up period, prior recurrence status, a history of UTUC and BCG therapy, T-stage, concomitant CIS status, tumor grade, tumor characteristics of TUR specimens, and whether immediate single instillation with THP was performed.
Table 1.
Tumor characteristics according to age.
| Characteristics | Total | Elderly patients | Younger patients | p |
|---|---|---|---|---|
| Evaluable treatment course, n | 87 | 38 | 49 | |
| Mean age, y | 72.6 (50–92) | 80.6 (75–92) | 66.3 (50–74) | <0.01 |
| Mean follow-up period, mo | 29.7 (2–89) | 31.9 (3–89) | 29.0 (2–80) | 0.18 |
| Sex, n (%) | 1.00 | |||
| Male | 85 (97.7) | 37 (97.4) | 48 (98.0) | |
| Female | 2 (2.3) | 1 (2.6) | 1 (2.0) | |
| Prior recurrence status, n (%) | 0.66 | |||
| Primary | 52 (59.8) | 24 (63.2) | 28 (47.1) | |
| Recurrent | 35 (40.2) | 14 (36.8) | 21 (42.9) | |
| History of UTUC, n (%) | 0.69 | |||
| Yes | 7 (8.0) | 4 (10.5) | 3 (6.1) | |
| No | 80 (92.0) | 34 (89.5) | 46 (93.9) | |
| Tumor focality, n (%) | 0.45 | |||
| Solitary | 21 (24.1) | 11 (28.9) | 10 (20.4) | |
| Multiple | 66 (75.9) | 27 (71.1) | 39 (79.6) | |
| History of BCG therapy, n (%) | 0.78 | |||
| Yes | 15 (17.2) | 6 (15.8) | 9 (18.4) | |
| No | 72 (82.8) | 32 (84.2) | 40 (81.6) | |
| T-stage, n (%) | 0.75 | |||
| Ta | 24 (27.6) | 9 (23.7) | 15 (30.6) | |
| T1 | 38 (43.7) | 17 (44.7) | 21 (42.9) | |
| CIS | 25 (28.7) | 12 (31.6) | 13 (26.5) | |
| Concomitant CIS, n (%) | 0.64 | |||
| Yes | 60 (69.0) | 25 (65.8) | 35 (71.4) | |
| No | 27 (31.0) | 13 (34.2) | 14 (28.6) | |
| Tumor grade, n (%) | 0.44 | |||
| Low | 1 (1.1) | 1 (2.6) | 0 (0.0) | |
| High | 86 (98.9) | 37 (97.4) | 49 (100.0) | |
| Immediate single instillation with THP, n (%) | 0.35 | |||
| Yes | 60 (69.0) | 24 (63.2) | 36 (73.5) | |
| No | 27 (31.0) | 14 (36.8) | 13 (26.5) |
Table 2 shows the intravesical BCG treatment characteristics for both age groups. The average period between initial TURBT and intravesical BCG therapy initiation was 6 weeks for both groups (p = 0.35). In total, 33 (86.8%) elderly patients received the standard intravesical BCG dose, while all younger patients received the standard dose (p < 0.05). There were no significant differences in the number of instillations and the duration of the induction course between the 2 groups, regardless of whether maintenance intravesical BCG was performed and whether antibiotic prophylaxis was administered during BCG instillation.
Table 2.
Intravesical BCG for NMIBC patients.
| Characteristics | Total | Elderly patients | Younger patients | p |
|---|---|---|---|---|
| Mean time interval between TUR and BCG therapy, wk | 5.3 (2–15) | 5.6 (2–15) | 4.9 (2–11) | 0.35 |
| BCG dose, n (%) | 0.014 | |||
| Standard | 82 (94.3) | 33 (97.4) | 49 (100.0) | |
| Low | 5 (5.7) | 5 (2.6) | 0 (0.0) | |
| Number of instillations for induction course, n | 6.9 (1–-8) | 6.9 (1–8) | 6.8 (1–8) | 0.865 |
| Maintenance BCG therapy, n (%) | 0.288 | |||
| Yes | 9 (10.3) | 2 (5.3) | 7 (14.3) | |
| No | 78 (89.7) | 36 (94.7) | 42 (85.7) | |
| Antibiotic prophylaxis, n (%) | 0.348 | |||
| Yes | 25 (28.7) | 13 (34.2) | 12 (24.5) | |
| No | 62 (71.3) | 25 (65.8) | 37 (75.5) |
3.2. Effectiveness
Table 3 shows the intravesical BCG therapy results for both age groups. Tumor recurrence was reported in 39.5% of elderly patients and in 49.0% of younger patients; the difference was not significant (p = 0.395). The Kaplan–Meier curves for RFS showed no significant difference between the 2 groups (Fig. 1). Tumors progressed in 3.4% of elderly patients, whereas tumor progression occurred in 5.3% of younger patients; the difference was not significant (p = 0.414). Additionally, no significant difference was observed in histological findings of recurrent tumors between the 2 groups (p = 0.485).
Table 3.
BCG outcomes in patients with NMIBC.
| Clinical outcomes | Total | Elderly patients | Younger patients | p |
|---|---|---|---|---|
| Recurrence, n (%) | 0.395 | |||
| Yes | 39 (44.8) | 15 (39.5) | 24 (49.0) | |
| No | 48 (55.2) | 23 (60.5) | 25 (51.0) | |
| Progression, n (%) | 0.414 | |||
| Yes | 3 (3.4) | 2 (5.3) | 1 (2.0) | |
| No | 84 (96.6) | 36 (94.7) | 48 (98.0) | |
| Histological findings of recurrent tumors, n (%) | 0.485 | |||
| Low-grade Ta | 2 (6.3) | 0 (0) | 2 (11.1) | |
| High-grade Ta | 4 (12.5) | 1 (7.1) | 3 (16.7) | |
| High-grade T1 | 8 (25.0) | 3 (21.4) | 5 (27.8) | |
| High-grade Tis | 15 (46.9) | 8 (57.1) | 7 (38.9) | |
| High-grade T2 | 3 (9.4) | 2 (14.3) | 1 (5.6) |
Figure 1.
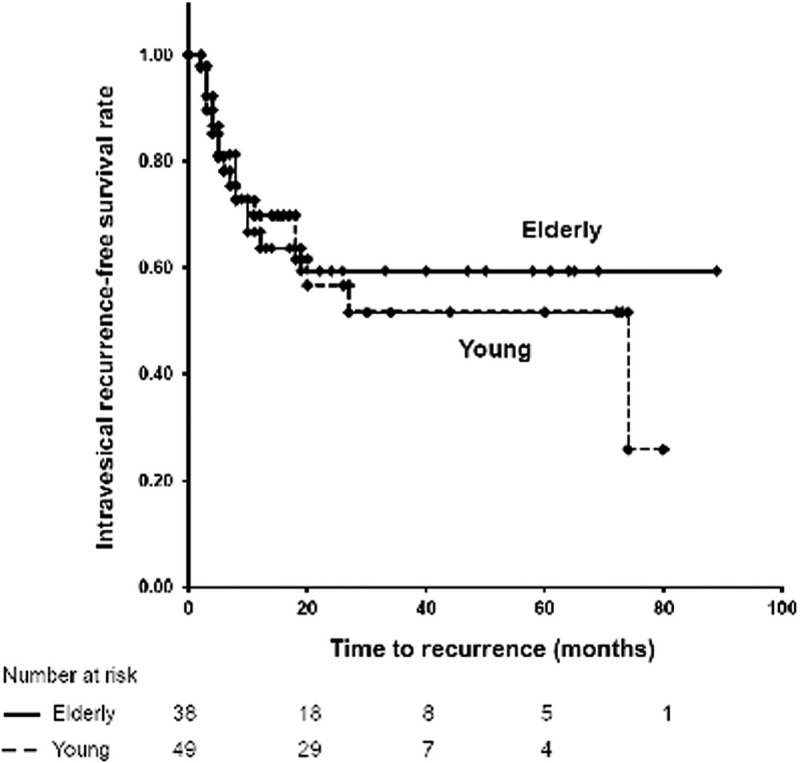
Kaplan–Meier curves of the RFS rates for the elderly group and young groups (log-rank test, p = 0.72).
3.3. Toxicity evaluation
Overall, 76.3% and 63.2% of elderly and younger patients, respectively, experienced some form of adverse events; however, the difference was not significant (p = 0.80) (Table 4). The common adverse events observed in both age groups were hematuria, pollakiuria, micturition pain, urinary retention, fever, fatigue, edema, and urinary tract infection. In terms of adverse events of all grades, there was no significant difference in the number of patients who experienced each adverse event between the 2 groups. As regards grade 2 and higher adverse events, micturition pain occurred significantly more frequently in elderly patients than in younger patients (p < 0.01). However, no elderly patients required hospitalization, whereas three (6.1%) younger patients were treated for urinary tract infection in the hospital.
Table 4.
Adverse events in NMIBC patients.
| All adverse event grades | Adverse event grade ≥2 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Adverse events | Elderly patients | Younger patients | p | Elderly patients | Younger patients | p |
| Total, n (%) | 29 (76.3) | 39 (63.2) | 0.80 | 14 (36.8) | 11 (22.4) | 0.16 |
| Hematuria, n (%) | 3 (7.8) | 4 (8.1) | 1.00 | 0 (0.0) | 1 (2.0) | 1.00 |
| Pollakiuria, n (%) | 7 (18.4) | 7 (14.2) | 0.77 | 3 (7.8) | 3 (6.1) | 1.00 |
| Micturiton pain, n (%) | 14 (36.8) | 16 (32.7) | 0.82 | 10 (26.3) | 2 (4.1) | <0.01 |
| Urinary retention, n (%) | 0 (0.0) | 1 (2.0) | 1.00 | 0 (0.0) | 1 (2.0) | 1.00 |
| Fever, n (%) | 8 (21.1) | 7 (14.2) | 0.57 | 1 (2.6) | 1 (2.0) | 1.00 |
| Fatigue, n (%) | 1 (2.6) | 4 (8.1) | 0.38 | 0 (0.0) | 0 (0.0) | |
| Edema, n (%) | 1 (2.6) | 0 (0.0) | 0.44 | 0 (0.0) | 0 (0.0) | |
| Urinary tract infection, n (%) | 0 (0.0) | 3 (6.1) | 0.25 | 0 (0.0) | 3 (6.1) | 0.25 |
4. Discussion
In clinical practice, clinicians are concerned about the safety of intravesical BCG for elderly patients. Therefore, we analyzed retrospective data to compare the efficacy of and tolerance to intravesical BCG in elderly and younger high-risk NMIBC patients. Our study demonstrated that response to BCG was not influenced by age and that advanced age was not a risk factor for complications in patients receiving intravesical BCG. These results suggest that intravesical BCG therapy for elderly patients (≥75 years) should not be avoided.
In order to provide optimal treatment for elderly patients with high-risk NMIBC, it should be determined whether age can affect the efficacy and toxicity of intravesical BCG. However, evidence-based practice guidelines regarding BC management are limited for such elderly patients, because elderly patients are physiologically, psychologically, and socially different from younger patients. [16] While NMIBC treatments are generally well tolerated by elderly patients, minor complications, such as high fever, increased urinary frequency, discomfort on urination, hematuria, clot retention, and the need for repeated catheterization, can be more problematic for elderly patients than younger patients, because urinary, vascular, and cardiac functions are often decreased in elderly patients. Additionally, aging progressively weakens the immune system. [17] Therefore, some clinicians caution against using intravesical therapy for the elderly. [18]
Previous studies reported on the relationship between age and intravesical BCG. With regard to efficacy, Herr et al. [19] demonstrated that age can affect patients’ response to BCG. Although they found no difference in the initial response to BCG, after 5 years, only 27% of patients older than 70 years were cancer-free, whereas 37% of younger patients were cancer-free by that time. Additionally, Oddens et al. [20] reported that patients on BCG who were over 70 years old had worse long-term prognoses than younger patients. Alternatively, Yuge et al. [21] explored the association between age and BC recurrence. They reported that patient age was not an independent predictor of tumor recurrence.
With regard to toxicity, Oddens et al. [22] reported that the percentage of patients who discontinued BCG therapy due to toxicity was 17.9% for patients aged ≤60 years, 21.9% for patients aged 61–70 years, 22.9% for patients aged 71–75 years, and 16.4% for patients aged >75 years. In terms of systemic and local side effects, there was no significant difference between each age group. However, Heiner et al. [23] reported that the complication rate for intravesical BCG was 17.6% for patients <70 years old and 48.6% for patients ≥70 years old. They concluded that BCG should be administered with caution in patients aged >70 years and should be avoided in patients aged >80 years.
Therefore, the relationship between age and the efficacy and toxicity of BCG treatment remains controversial. Additionally, most reports set the criteria for “younger” and “elderly” at <70 years and ≥70 years, respectively. This is inadequate considering the rising age of BC patients. In our study, we analyzed retrospective data to compare the efficacy of and tolerance to BCG in elderly and younger patients, who were defined as ≥75 years old and <75 years old, respectively.
Our data showed that the efficacy of intravesical BCG was not affected by age. At 5 years post-treatment, 60.5% of elderly patients continued to be disease-free, whereas 51.0% of younger patients were disease-free; the difference between the groups was nonsignificant. Additionally, the risk of intravesical BCG-related adverse events was low in elderly patients, despite including patients over 90 years old. Furthermore, elderly patients did not require hospitalization for adverse events.
Our study had several limitations. First, the small sample size made estimating the treatment effect less robust. Second, our data came from a single institution. Therefore, selection bias may have affected our results. As such, our sample size may reflect that younger patients were offered intravesical BCG treatment more often than elderly patients. However, the number of patients in each group was similar.
5. Conclusions
The efficacy of intravesical BCG therapy in NMIBC patients was not associated with age and increasing age is not associated with toxicity leading to cessation of intravesical BCG therapy. Therefore, we believe that elderly patients with high-risk NMIBC should be treated in the same manner as younger patients in the clinical practice.
Acknowledgments
The authors gratefully acknowledge the valuable support from the members of their department.
Statement of ethics
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Kagawa University, Faculty of Medicine (Permission number, 2020–046). A waiver of informed consent was obtained, given the retrospective nature of the study.
Conflict of interest statement
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Funding source
None.
Author contributions
Y.M. and R.T. collected the data, performed the analysis, and drafted the manuscript. All authors discussed and commented on the manuscript.
References
- [1]. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017;71 (1):96–108. [DOI] [PubMed] [Google Scholar]
- [2]. Liedberg F, Hagberg O, Holmäng S, et al. Local recurrence and progression of non-muscle-invasive bladder cancer in Sweden: a population-based follow-up study. Scand J Urol 2015;49 (4):290–295. [DOI] [PubMed] [Google Scholar]
- [3]. Zehnder P, Studer UE, Skinner EC, et al. Unaltered oncological outcomes of radical cystectomy with extended lymphadenectomy over three decades. BJU Int 2013;112 (2):E51–E58. [DOI] [PubMed] [Google Scholar]
- [4]. Mitra AP, Quinn DI, Dorff TB, et al. Factors influencing post-recurrence survival in bladder cancer following radical cystectomy. BJU Int 2012;109 (6):846–854. [DOI] [PubMed] [Google Scholar]
- [5]. Burger M, Catto JWF, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013;63 (2):234–241. [DOI] [PubMed] [Google Scholar]
- [6]. Duchek M, Johansson R, Jahnson S, et al. Bacillus Calmette-Guerin is superior to a combination of epirubicin and interferon-alpha2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. Eur Urol 2010;57 (1):25–31. [DOI] [PubMed] [Google Scholar]
- [7]. Järvinen R, Kaasinen E, Sankila A, Rintala E. FinnBladder Group. Long-term efficacy of maintenance bacillus Calmette-Guerin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20-year follow-up. Eur Urol 2009;56 (2):260–265. [DOI] [PubMed] [Google Scholar]
- [8]. Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 2010;57 (5):766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Böhle A, Bock PR. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology 2004;63 (4):682–686. [DOI] [PubMed] [Google Scholar]
- [10]. Sylvester RJ, van der Meijden APM, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 2002;168 (5):1964–1970. [DOI] [PubMed] [Google Scholar]
- [11]. Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 2003;169 (1):90–95. [DOI] [PubMed] [Google Scholar]
- [12]. Brausi M, Oddens J, Sylvester R, et al. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomized phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol 2014;65 (1):69–76. [DOI] [PubMed] [Google Scholar]
- [13]. Joudi FN, Smith BJ, O’Donnell MA, Konety BR. The impact of age on the response of patients with superficial bladder cancer to intravesical immunotherapy. J Urol 2006;175 (5):1634–1640. [DOI] [PubMed] [Google Scholar]
- [14]. Gontero P, Sylvester R, Pisano F, et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guérin: results of a retrospective multicenter study of 2451 patients. Eur Urol 2015;67 (1):74–82. [DOI] [PubMed] [Google Scholar]
- [15]. Babjuk M, Burger M, Compérat EM, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur Urol 2019;76 (5):639–657. [DOI] [PubMed] [Google Scholar]
- [16]. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med 1981;135 (6):434–440. [PMC free article] [PubMed] [Google Scholar]
- [17]. DaVeale B, Brummel T, Seroude L. Immunity and aging: the enemy within? Aging Cell 2004;3 (4):195–208. [DOI] [PubMed] [Google Scholar]
- [18]. Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz P, Meryn S, Bochner BH. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int 2010;105 (3):300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Herr HW. Age and outcome of superficial bladder cancer treated with Bacillus Calmette-Guerin therapy. Urology 2007;70 (1):65–68. [DOI] [PubMed] [Google Scholar]
- [20]. Oddens JR, Sylvester RJ, Brausi MA, et al. The effect of age on the efficacy of maintenance bacillus Calmette-Guerin relative to maintenance epirubicin in patients with stage Ta T1 urothelial bladder cancer: results from EORTC genito-urinary group study 30911. Eur Urol 2014;66 (4):694–701. [DOI] [PubMed] [Google Scholar]
- [21]. Yuge K, Kikuchi E, Matsumoto K, Takeda T, Miyajima A, Oya M. Could patient age influence tumor recurrence rate in non-muscle-invasive bladder cancer patients treated with BCG immunotherapy? Jpn J Clin Oncol 2011;41 (4):565–570. [DOI] [PubMed] [Google Scholar]
- [22]. Oddens JR, Sylvester RJ, Brausi MA, et al. Increasing age is not associated with toxicity leading to discontinuation of treatment in patients with urothelial non-muscle-invasive bladder cancer randomised to receive 3 years of maintenance bacille Calmette-Guérin: results from European Organisation for Research and Treatment of Cancer Genito-Urinary Group study 30911. BJU Int 2016;118 (3):423–428. [DOI] [PubMed] [Google Scholar]
- [23]. Heiner JG, Terris MK. Effect of advanced age on the development of complications from intravesical bacillus Calmette-Guerin therapy. Urol Oncol 2008;26 (2):137–140. [DOI] [PubMed] [Google Scholar]


