Abstract
Background:
Corticosteroid treatment is an effective and common therapeutic strategy for various inflammatory lung pathologies and may be an effective treatment for coronavirus disease 2019 (COVID-19). The purpose of this systematic review and meta-analysis of current literature was to investigate the clinical outcomes associated with corticosteroid treatment of COVID-19.
Methods:
We systematically searched PubMed, medRxiv, Web of Science, and Scopus databases through March 10, 2021 to identify randomized controlled trials (RCTs) that evaluated the effects of corticosteroid therapies for COVID-19 treatment. Outcomes of interest were mortality, need for mechanical ventilation, serious adverse events (SAEs), and superinfection.
Results:
A total of 7737 patients from 8 RCTs were included in the quantitative meta-analysis, of which 2795 (36.1%) patients received corticosteroids plus standard of care (SOC) while 4942 (63.9%) patients received placebo and/or SOC alone. The odds of mortality were significantly lower in patients that received corticosteroids as compared to SOC (odds ratio [OR] = 0.85 [95% CI: 0.76; 0.95], P = .003). Corticosteroid treatment reduced the odds of a need for mechanical ventilation as compared to SOC (OR = 0.76 [95% CI: 0.59; 0.97], P = .030). There was no significant difference between the corticosteroid and SOC groups with regards to SAEs and superinfections.
Conclusion:
Corticosteroid treatment can reduce the odds for mortality and the need for mechanical ventilation in severe COVID-19 patients.
Keywords: adrenal cortex hormones, coronavirus, respiratory distress syndrome, severe acute respiratory syndrome coronavirus-2
1. Introduction
There have been approximately 126.4 million cases of coronavirus disease 2019 (COVID-19), which have resulted in approximately 2.8 million deaths worldwide as of March 28, 2021.[1] COVID-19 is characterized by a hyperinflammatory response consisting of pro-inflammatory cytokines and chemokines, such as interleukin (IL)-1, IL-6, interferon-γ (IFN-γ), vascular endothelial growth factor (VEGF), and tumor necrosis factor-α (TNF-α).[2] The elevated inflammatory response as a result of COVID-19 can lead to acute respiratory distress syndrome (ARDS) and poor prognosis.[3–5]
Corticosteroids exert anti-inflammatory effects by suppressing the production of many initial phase cytokines (IL-1β, IL-6, TNF-α, etc).[6] Indeed, corticosteroid treatment is an effective and common therapeutic strategy for several inflammatory lung pathologies (e.g., asthma). However, results from studies of other respiratory viruses (e.g., influenza, Middle Eastern respiratory syndrome, severe acute respiratory syndrome coronavirus-1 [SARS-CoV-1]) failed to show a conclusive benefit with corticosteroids,[7] although these studies were largely observational and suffered from low statistical power.[8] Moreover, corticosteroids were commonly administered to patients with the greatest disease severity, which makes it difficult to compare outcomes between groups.[8] Here, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs) to investigate the clinical outcomes associated with corticosteroid treatment of COVID-19.
2. Methods
2.1. Search protocol
We systematically searched PubMed, medRxiv, Web of Science, and Scopus through March 10, 2021. We used the following search strings:
-
1.
(COVID-19 OR SARS-CoV-2 OR “novel coronavirus”) AND (steroids OR corticosteroids OR glucocorticoids);
-
2.
(COVID-19 OR SARS-CoV-2 OR “novel coronavirus”) AND (steroids OR corticosteroids OR glucocorticoids) AND (RCT OR “randomized controlled trial” OR randomized controlled trial); and
-
3.
(COVID-19 OR SARS-CoV-2 OR “novel coronavirus”) AND (steroids OR corticosteroids OR glucocorticoids) AND (RCT OR “randomized controlled trials”).
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This study did not require ethics approval or informed consent as no patient information was collected.
2.2. Study selection and risk of bias
We included all RCTs that evaluated the therapeutic effect of corticosteroids in comparison to standard of care (SOC) for treatment of moderate to severe COVID-19, provided that at least one of the prespecified outcomes of interest was reported. Search results were excluded if they were: study type – meta-analysis or review, editorial, opinion article, correspondence, letter to the editor, technical note, in vitro or in vivo study, methods article, protocol, case report, recommendations, or guidelines. Studies were also excluded if they did not report treatment for COVID-19 with corticosteroids, if they did not report patient outcomes, or if they were single-armed (had no comparison group). The literature was independently screened by at least 2 authors per article.
The risk of bias and levels of evidence of each study was scored using the Scottish.
Intercollegiate Guidelines Network (SIGN) checklists for controlled clinical trials.[9] As such, individual items on checklists were categorized as follows:
“Well covered” or “Yes”
“Adequately addressed”
“Poorly addressed”
“Not addressed” or “No”
“Not applicable (N/A)”
When individual items were determined to be only “adequately addressed” or “poorly addressed,” detailed explanations were provided in the risk of bias assessment form. The risk of bias among individual studies was coded as follows:
High quality (++)
Acceptable quality (+)
Low quality (−)
Unacceptable (0)
2.3. Data extraction
Data were extracted by YSP, SK, and MS. Extracted data were checked for accuracy independently by JMP, who also completed statistical analyses. Outcomes collected were mortality, length of hospital stay, intensive care unit (ICU) admission, mechanical ventilation, days on mechanical ventilation support, serious adverse events (SAEs), and superinfection.
2.4. Data analysis
All data were entered into a Microsoft Excel sheet and imported to R for analysis using the metafor package[10] We used Higgin I2 statistics to estimate the percentage of variability in effect estimates that is due to heterogeneity rather than sampling error.[11] Effect sizes were computed as log transformed odds ratios (ORs). To aid in interpretation, log transformed effect sizes were converted back to their original scale after performing each meta-analysis. A separate random effects model was fit for each outcome measure. Accordingly, the between-study variance component was estimated using a restricted effects maximum likelihood (REML) estimator with 95% CIs computed using the Q-profile method.[12] REML has been shown in various simulated meta-analyses to be robust to small study effects and low-frequency binary outcomes (e.g., mortality), while minimizing bias for the estimation of variance components. The Q-profile method is the default method for computing confidence intervals for REML in the metafor package and has been shown to perform better than standard Wald-type methods in simulated meta-analyses.[13] 95% prediction intervals (PIs) were also calculated for each outcome measure.[13] In brief, a 95% PI estimates where the true effects are to be expected for 95% of similar (exchangeable) studies that might be conducted in the future.[14] All statistical analyses were performed in RStudio (Version 1.3.959, RStudio, PBC).
3. Results
A total of 7737 patients from 8 RCTs were included in the quantitative meta-analysis (Fig. 1).[15–22] Six studies were open-label trials,[16,18–22] and 2 studies were double-blinded trials.[15,17] The following formulations of corticosteroids were evaluated against placebo and/or SOC: hydrocortisone (CAPE COVID; REMAP-CAP),[15,19] dexamethasone (CoDEX; RECOVERY),[16,18] and methylprednisolone (GLUCOCOVID; MetCOVID, Edalatifard et al, and Tang et al).[17,20–22] Among the study population, 2795 (36.1%) patients received corticosteroids in addition to SOC (steroids), and 4,942 (63.9%) received placebo and/or alone SOC. Study and patient characteristics among the included studies are provided in Table S1, Supplemental Digital Content .
Figure 1.
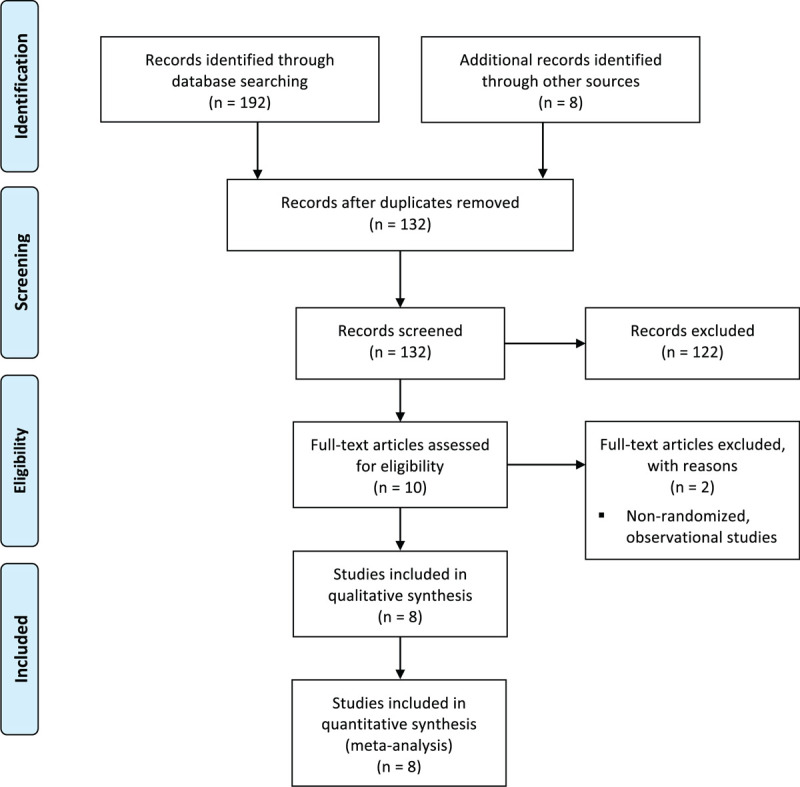
PRISMA diagram of search records and included studies.
3.1. Mortality
All included studies had data regarding mortality. Of note, 1 study evaluated mortality at 30 days,[21] 3 studies evaluated mortality at 28 days,[16–18] 1 study evaluated mortality at 21 days,[15] 1 study evaluated mortality at 14 days,[19] and 2 studies did not specify a date for mortality (time of death).[20,22] Across all available studies, the overall mortality rate in the steroids group was 22.9% (95% CI: 13.0%; 37.1%), while the overall mortality rate for the SOC group was 31.0% (95% CI: 20.3%; 44.3%). The mortality rate in the steroids group was significantly lower compared to the SOC group (OR = 0.85 [95% CI: 0.76; 0.95], P = .003; Fig. 2). The estimated between-study variability unrelated to sampling error ranged from low to high (I2 = 39.6% [95% CI: 0.0%; 73.3%]).
Figure 2.
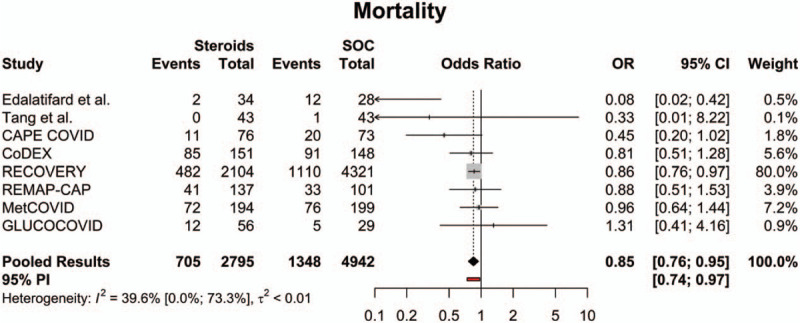
Forest plot of subgroup comparisons of mortality rates. Pooled results were computed using restricted effects maximum likelihood with 95% confidence intervals (CIs) computed using the Q-profile method. A 95% prediction interval (PI) was also computed (see red bar). OR = odds ratio, SOC = standard of care.
3.2. Need for mechanical ventilation
Of the studies included in the quantitative meta-analysis, 5 had data regarding mechanical ventilation rates.[15,17,18,20,21] Across all available studies, the overall mechanical ventilation rate in the steroids group was 14.0% (95% CI: 5.6%; 30.4%), while the overall mechanical ventilation rate for the SOC group was 16.3% (95% CI: 4.6%; 44.3%). The need for mechanical ventilation in the steroids group was significantly lower compared to the SOC group (OR = 0.76 [95% CI: 0.59; 0.97], P = .030; Fig. 3). The estimated between-study variability unrelated to sampling error ranged from low to high (I2 = 0.0% [95% CI: 0.0%; 75.1%]).
Figure 3.
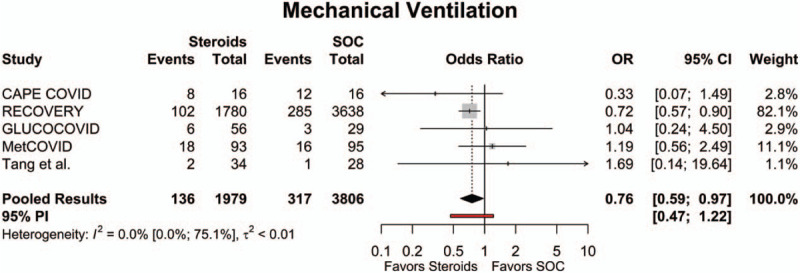
Forest plot of subgroup comparisons of need for mechanical ventilation. Pooled results were computed using restricted effects maximum likelihood with 95% confidence intervals (CIs) computed using the Q-profile method. A 95% prediction interval (PI) was also computed (see red bar). OR = odds ratio, SOC = standard of care.
3.3. Serious adverse events (SAEs)
Of the studies included in the quantitative meta-analysis, 4 had data regarding SAE rates.[15,16,19,22] As determined by the trial investigators, some SAEs included in the analysis were deemed to be unattributable to the interventions studied (discussed below). Across all available studies, the overall SAE rate in the steroids group was 3.6% (95% CI: 2.1%; 6.0%), while the overall SAE rate for the SOC group was 3.7% (95% CI: 1.4%; 9.5%). There was no significant difference in SAE rates between the steroids group and the SOC group (OR = 1.09 [95% CI: 0.37; 3.33], P = .871; Fig. 4). The estimated between-study variability unrelated to sampling error ranged from low to high (I2 = 22.2% [95% CI: 0.0%; 88.1%]).
Figure 4.
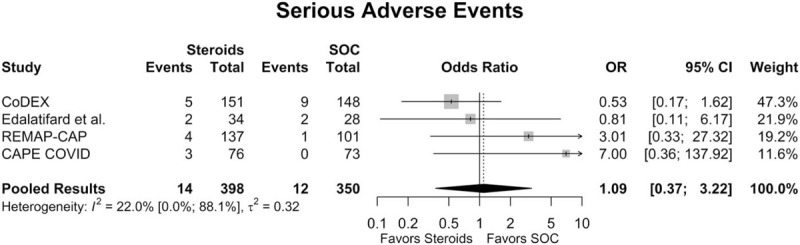
Forest plot of subgroup comparisons of serious adverse event rates. Pooled results were computed using restricted effects maximum likelihood with 95% confidence intervals (CIs) computed using the Q-profile method. OR = odds ratio, SOC = standard of care.
3.4. Superinfection
Of the studies included in the quantitative meta-analysis, 3 had data regarding superinfection rates.[15,16,22] Across all available studies, the overall superinfection rate in the steroids group was 18.3% (95% CI: 5.1%; 48.5%), while the overall superinfection rate for the SOC group was 22.4% (95% CI: 5.7%; 58.1%). There was no significant difference in superinfection rates between the steroids group and the SOC group (OR = 0.75 [95% CI: 0.50; 1.13], P = .172; Fig. 5). The estimated between-study variability unrelated to sampling error ranged from low to high (I2 = 0.0% [95% CI: 0.0%; 73.2%]).
Figure 5.
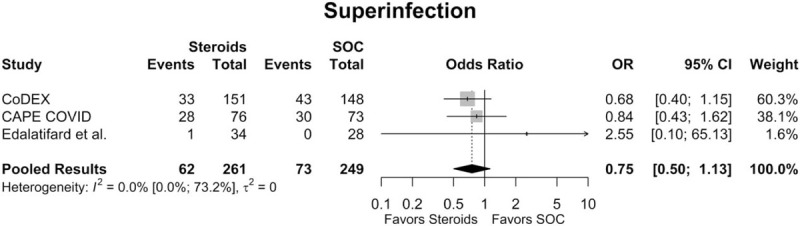
Forest plot of subgroup comparisons of superinfection rates. Pooled results were computed using restricted effects maximum likelihood with 95% confidence intervals (CIs) computed using the Q-profile method. OR = odds ratio, SOC = standard of care.
3.5. Risk of bias and qualitative synthesis
Based on the SIGN method for controlled trials, 2 studies were considered high quality, 4 studies were considered acceptable quality, and 2 studies were considered low quality. The results of our quality appraisal are summarized in File S1, Supplemental Digital Content . Outcome reporting was highly heterogenous among included RCTs, with only mortality, mechanical ventilation, SAEs, and superinfection being reported in at least 3 studies. Accordingly, further quantitative synthesis was only performed on these outcomes. Study-specific outcomes and conclusions for all included studies are provided in Table S2, Supplemental Digital Content . Five trials (CoDEX, GLUCOCOVID, RECOVERY, REMAP-CAP, and Edalatifard et al) recommended corticosteroid therapy for moderate or severe COVID-19 cases, each demonstrating superiority in primary clinical outcomes compared to standard therapy. However, the results from GLUCOCOVID were considered a low-quality early report, which only showed benefit with the composite primary outcome (ICU admission, NIV, or death) and had unclear evidence regarding individual clinical outcomes. In addition, Edalatifard et al had a large proportion of patients with protocol deviations in the SOC group (6/34, 17.6%), potentially impacting the results of the trial. The CAPE COVID, MetCOVID, and Tang et al trials each detected no differences in primary or secondary clinical outcomes with corticosteroid therapy. However, these trials were terminated early following release of the RECOVERY trial results; thus, these 3 trials were underpowered to detect statistically significant results.
4. Discussion
Here, we performed a systematic review and meta-analysis of randomized controlled clinical trials that investigated the efficacy of corticosteroid treatment for COVID-19. Corticosteroid therapy reduced the odds of mortality and the need for mechanical ventilation in COVID-19 patients, although the therapeutic benefit occurred in patients that required oxygen support (e.g., severe to critical status). Corticosteroid therapy did not increase the odds for SAEs or superinfection. These data suggest that corticosteroid treatment can improve clinical outcomes in moderate and severe-critical COVID-19 patients, and it is not associated with greater odds of adverse outcomes above and beyond standard therapies.
Corticosteroids reduce inflammation at the transcriptional level by enhancing anti-inflammatory mechanisms while reducing pro-inflammatory mechanisms.[23] Well-described mechanisms of action along with the widespread availability of corticosteroids make them a desirable therapeutic option for a wide variety of pathologies. However, the efficacy of corticosteroid treatment for respiratory viruses has been questionable, especially given the greater risk of poor outcomes and adverse events observed with treatment of other respiratory syndromes (e.g., influenza, SARS-CoV-1, Middle Eastern respiratory syndrome).[7]
We detected an overall lower odds of mortality with corticosteroid treatment as compared to standard therapies. Our results were largely influenced by the RECOVERY trial,[18] which consisted of approximately 83% of the total number of patients in the analysis. In the RECOVERY trial, corticosteroid (dexamethasone) therapy provided greater mortality benefits in patients that required invasive mechanical ventilation at randomization (29.3% vs 41.4%) and in patients that required oxygen support without invasive mechanical ventilation at randomization (23.3% vs 26.2%). In contrast, there was no mortality benefit with corticosteroids in patients that required no respiratory support at randomization (17.8% vs 14.0%). Of the remaining 7 studies, 1 study reported lower mortality with corticosteroid treatment,[22] and 6 failed to detect significant differences in odds of mortality.[15–17,19–21] However, 4 of the 6 studies exhibited trends for lower odds of mortality with corticosteroid treatment.[15,16,19,21] Moreover, 4 studies (CoDEX, CAPE COVID, REMAP-CAP, Tang et al) were terminated early due to the results from the RECOVERY trial or a lack of patients and were underpowered to detect statistically significant differences in outcomes. The odds for mortality in the CoDEX trial (OR: 0.81),[16] which examined the effects of dexamethasone treatment in COVID-19 patients with moderate to severe ARDS, was of the same magnitude to that observed in the RECOVERY trial (OR: 0.86). Moreover, the odds of mortality were lower with corticosteroid treatment in CAPE COVID (hydrocortisone) and REMAP-CAP trials (hydrocortisone), which both consisted of COVID-19 patients admitted to the ICU (i.e., severe-critical disease).[15,19] The odds of mortality could have been lower with corticosteroid therapy in the REMAP-CAP trial as 15% of patients in the standard care group received corticosteroid treatment as needed (e.g., postextubation stridor). The OR for mortality with corticosteroid treatment (methylprednisolone) was similar to standard treatment in the MetCOVID trial.[17] However, there was a distinct difference in mortality as a product of age and treatment. Patients aged 60 years and older that received corticosteroids exhibited lower rates of mortality at 28 days, while patients under the age of 60 exhibited greater rates of mortality with corticosteroids. Older patients expressed a greater systemic inflammatory response as evidenced by elevated C-reactive protein levels as compared to younger patients. While not statistically significant, patients who received corticosteroid treatment were ventilated for a longer duration before receiving treatment, which could have influenced the treatment effect. The preliminary results of the GLUCOCOVID trial (methylprednisolone) were obtained from patients not in the ICU (less severe disease); however, the initial mortality data from this ongoing study were highly variable.[20] Taken together, these data indicate that corticosteroid treatment does provide a mortality benefit in COVID-19 patients that require oxygen support. Mortality benefits with corticosteroid treatment may be greater in patients that require mechanical ventilation.
Five studies reported a need for mechanical ventilation following treatment, and the results largely reflected the quality and magnitude of results observed with mortality. The odds for a need to mechanically ventilate a patient were lower with corticosteroid treatment in CAPE COVID and RECOVERY trials.[15,18] In the RECOVERY trial, the need for mechanical ventilation was further reduced in a subanalysis that examined only those patients that required oxygen support at randomization. In contrast, the odds of a need for mechanical ventilation in the GLUCOCOVID trial,[20] which consisted of patients with relatively lesser forms of disease, did not suggest a benefit with corticosteroid treatment. Aside from mortality, there were no further analyses performed in the MetCOVID trial to examine the effects of age (=60 years of age) on clinical outcomes.[17] Thus, it was unclear if the need for mechanical ventilation would have been different in the subpopulation of patients 60 years of age or older in this trial. The CoDEX trial consisted of patients already receiving mechanical ventilation at enrolment.[16] Patients that received corticosteroids had a significantly greater number of ventilator-free days as compared to standard therapy (6.6 vs 4.0). Collectively, these data indicate that corticosteroid treatment lowers the odds of a need for mechanical ventilation in severe-critical COVID-19 patients.
Corticosteroid therapy attenuates the immune response, which could render patients more susceptible to infection and/or other adverse events.[23] We did not detect a difference in the odds for SAEs or superinfection with corticosteroid treatment. It is important to note that there were substantially fewer patients included in SAE (n = 748) and superinfection (n = 510) analyses as compared to mortality (n = 7,737) and the need for mechanical ventilation (n = 5785) analyses. Aside from limited data, there was substantial variability across studies in the odds for SAEs; however, the overall rate of SAEs was relatively low (SOC: 3.4%, Steroids: 3.5%). Two of 4 studies (REMAP-CAP, CAPE COVID) exhibited higher, albeit statistically insignificant, odds for SAEs with corticosteroid treatment.[15,19] None of the 3 SAEs were attributed to corticosteroid treatment in the CAPE COVID trial, while only 2 of the 4 SAEs (severe neuromyopathy and fungemia) were attributed to corticosteroid treatment in the REMAP-CAP trial. Similarly, the odds for superinfection were the same for patients that received corticosteroid or standard therapy. Corticosteroids can dysregulate metabolic (carbohydrate, lipid), immune, and inflammatory processes, which can lead to a wide variety of adverse events.[23] Greater rates of hyperglycemia or a need for insulin were reported in patients that received corticosteroids in MetCOVID and GLUCOCOVID trials,[17,20] while similar rates of hyperglycemia or a need for insulin were reported across study groups in the CoDEX trial.[16] Aside from hyperglycemia, adverse event rates were similar between corticosteroid and standard care arms, which was consistent with observations from studies that utilized corticosteroid treatment for other severe disease states (e.g., ARDS).[24,25] Taken together, corticosteroid treatment did not increase the odds for SAEs or superinfection in COVID-19 patients, although these limited data were obtained in patients with severe to critical forms of COVID-19.
The success of corticosteroid treatment of COVID-19 may depend on the stage of disease at the time of treatment. In SARS-CoV-1, the peak of viral replication and shedding occurs during the 2nd week of symptom onset; however, the peak of these processes occurs earlier (1st week) with COVID-19 (SARS-CoV-2).[26–28] The median duration of symptoms in the above mentioned trials was in the second week of illness; as such, there were limited data available to comment on the usefulness of corticosteroid treatment in the early phase of the disease.[15–18,21] Corticosteroid therapy was associated with substantial reductions in CRP levels,[20] and superior clinical outcomes (e.g., mortality) were observed in subpopulations of patients with elevated CRP levels at baseline.[17] These data suggest that corticosteroid therapy is efficacious during the hyperinflammatory stage of COVID-19.
4.1. Limitations
Due to limited data, small-study bias assessments were not performed; as such, the pooled effect sizes for each outcome is assumed to be affected by small study bias to some degree. Furthermore, a limited number of studies and an inability to access patient-level data prevented us from exploring further sources of heterogeneity that may have impacted differences in clinical outcomes between treatment groups. In addition, the steroids group also consisted of different dosing regimens, SOC arms among studies were not consistent, and outcome reporting was heterogenous. Another limitation is that some included trials are still ongoing and have inconclusive results. Most of the data in the present analysis were obtained from the RECOVERY trial. Four of the other 7 studies were terminated early following publication of the RECOVERY results or due to a lack of patients.[15,16,19,21] The varying quality of studies included in our meta-analysis may have also impacted the conclusions of our study, with only 2 studies being considered high quality, double-blinded RCTs while the rest were either moderate or low quality open-label studies. Nevertheless, each study was deemed to be applicable to the target population and were of sufficient quality for inclusion based on the SIGN methodology for assessing the risk of bias and quality of evidence of individual RCTs.
5. Conclusions
Based on a random effects meta-analysis of 8 RCTs, corticosteroid treatment can reduce both the need for mechanical ventilation support and mortality in moderate to severe COVID-19 patients. Ongoing RCTs will provide evidence regarding the clinical benefit of steroids compared to other investigational treatments.
Author contributions
Conceptualization: Yashwitha Sai Pulakurthi, Kavitha Saravu, Nitin Gupta, Charan Thej Reddy Vegivinti.
Data curation: Yashwitha Sai Pulakurthi, John M Pederson, Shelby Kamrowski, Megan Schmidt, Natalie L. Reierson, Kirk W Evanson.
Formal analysis: Yashwitha Sai Pulakurthi, John M Pederson, Kavitha Saravu, Nitin Gupta, Prasanth Balasubramanian, Charan Thej Reddy Vegivinti, Mahmoud Dibas, Sailaja Pisipati, Betsy Ann Joseph, Pragadeesh Thamarai Selvan, Adam A Dmytriw, Praneeth Reddy Keesari, Varsha Sriram, Spandana Chittajallu, Waleed Brinjikji, Rewanth R Katamreddy, Richa Chibbar, Ameer E Hassan.
Investigation: Yashwitha Sai Pulakurthi, John M Pederson.
Methodology: John M Pederson.
Project administration: John M Pederson, Shelby Kamrowski, Amber R Davis, Kevin M Kallmes.
Resources: John M Pederson, Kevin M. Kallmes.
Software: John M Pederson.
Supervision: John M Pederson, Shelby Kamrowski, Natalie L Reierson, Amber R Davis, Kevin M Kallmes.
Validation: John M Pederson.
Visualization: John M Pederson, Manashree Malpe, Hemant K Mishra, Kirk W Evanson.
Writing – original draft: Yashwitha Sai Pulakurthi, John M Pederson, Prasanth Balasubramanian, Kirk W Evanson.
Writing – review & editing: Yashwitha Sai Pulakurthi, John M Pederson, Kavitha Saravu, Nitin Gupta, Prasanth Balasubramanian, Kirk W Evanson.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, COVID-19 = coronavirus disease 2019, ICU = intensive care unit, OR = odds ratio, RCT = randomized controlled trial, SAE = serious adverse event, SARS-CoV = severe acute respiratory syndrome coronavirus, SOC = standard of care.
How to cite this article: Pulakurthi YS, Pederson JM, Saravu K, Gupta N, Balasubramanian P, Kamrowski S, Schmidt M, Vegivinti CT, Dibas M, Reierson NL, Pisipati S, Joseph BA, Selvan PT, Dmytriw AA, Keesari PR, Sriram V, Chittajallu S, Brinjikji W, Katamreddy RR, Chibbar R, Davis AR, Malpe M, Mishra HK, Kallmes KM, Hassan AE, Evanson KW. Corticosteroid therapy for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2021;100:20(e25719).
The authors have no funding and conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Supplemental digital content is available for this article.
References
- [1]. Weekly epidemiological update on COVID-19-30 March 2021. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---31-march-2021. Published 2021 [accessed March 31, 2021] [Google Scholar]
- [2].Nile SH, Nile A, Qiu J, et al. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 2020;53:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cross LJ, Matthay MA. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin 2011;27:355–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020;26:845–8. [DOI] [PubMed] [Google Scholar]
- [5].Li L, Huang Q, Wang DC, et al. Acute lung injury in patients with COVID-19 infection. Clin Transl Med 2020;10:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10: Suppl 2: 81–90. discussion 91-82. [DOI] [PubMed] [Google Scholar]
- [7].Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shang L, Zhao J, Hu Y, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020;395:683–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Sign 50: A guideline developer's handbook. Available at: https://www.sign.ac.uk/assets/sign50_2011.pdf. Published 2011. Accessed November 1, 2020. [Google Scholar]
- [10].Viechtbauer W. Package ’metafor’. Meta-Analysis Package for R Web site. https://cran.r-project.org/web/packages/metafor/metafor.pdf. Published 2020. Accessed November 1, 2020 [Google Scholar]
- [11].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van Aert RCM, van Assen M, Viechtbauer W. Statistical properties of methods based on the Q-statistic for constructing a confidence interval for the between-study variance in meta-analysis. Res Synth Methods 2019;10:225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Henmi M, Copas JB. Confidence intervals for random effects meta-analysis and robustness to publication bias. Stat Med 2010;29:2969–83. [DOI] [PubMed] [Google Scholar]
- [14].Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- [15].Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 2020;324:1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA 2020;324:1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 2020;72:e373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 preliminary report. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020;324:1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: An open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr 2021;133:303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tang X, Feng YM, Ni JX, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in nonintensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration 2021;100:116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J 2020;56:2002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol 2013;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006;354:1671–84. [DOI] [PubMed] [Google Scholar]
- [25].Villar J, Ferrando C, Martinez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomized controlled trial. Lancet Respir Med 2020;8:267–76. [DOI] [PubMed] [Google Scholar]
- [26].Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581:465–9. [DOI] [PubMed] [Google Scholar]
- [27].To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020;20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


