Abstract
In this observational study, by the use of a multiplex proteomic platform, we aimed to explore associations between 92 targeted proteins involved in cardiovascular disease and/or inflammation, and phenotypes of deteriorating vascular health, with regards to ethnicity.
Proteomic profiling (92 proteins) was carried out in 362 participants from the Sympathetic activity and Ambulatory Blood Pressure in Africans (SABPA) study of black and white African school teachers (mean age 44.7 ± 9.9 years, 51.9% women, 44.5% Black Africans, 9.9% with known cardiovascular disease). Three proteins with <15% of samples below detectable limits were excluded from analyses. Associations between multiple proteins and prevalence of hypertension as well as vascular health [Carotid intima-media thickness (cIMT) and pulse wave velocity (PWV)] measures were explored using Bonferroni-corrected regression models.
Bonferroni-corrected significant associations between 89 proteins and vascular health markers were further adjusted for clinically relevant co-variates. Hypertension was associated with growth differentiation factor 15 (GDF-15) and C-X-C motif chemokine 16 (CXCL16). cIMT was associated with carboxypeptidase A1 (CPA1), C-C motif chemokine 15 (CCL15), chitinase-3-like protein 1 (CHI3L1), scavenger receptor cysteine-rich type 1 protein M130 (CD163) and osteoprotegerin, whereas PWV was associated with GDF15, E-selectin, CPA1, fatty acid-binding protein 4 (FABP4), CXCL16, carboxypeptidase B (CPB1), and tissue-type plasminogen activator. Upon entering ethnicity into the models, the associations between PWV and CPA1, CPB1, GDF-15, FABP4, CXCL16, and between cIMT and CCL-15, remained significant.
Using a multiplex proteomic approach, we linked phenotypes of vascular health with several proteins. Novel associations were found between hypertension, PWV or cIMT and proteins linked to inflammatory response, chemotaxis, coagulation or proteolysis. Further, we could reveal whether the associations were ethnicity-dependent or not.
Keywords: carotid intima media thickness, carotid intima media thickness, ethnicity, hypertension, multiplex proteomics, pulse wave velocity, vascular health
1. Introduction
Cardiovascular diseases (CVD) are the leading global cause of death, causing patients great personal suffering and is a major socioeconomic burden for society. Subclinical detection to improve risk stratification and preventive treatment selection along with the further understanding of the biological processes preceding CVD are urgently called upon. A leading CVD risk factor globally is hypertension, it is prevalence being the highest in Africa and also rising in low- and middle-income countries such as South Africa.[1,2] Atherosclerosis is the main pathophysiological process leading to CVD and hypertension is a major contributing factor to this process. Further, arteriosclerosis that is, arterial stiffness, precedes and predicts incident hypertension.[3] Carotid intima media thickness (cIMT) and pulse wave velocity (PWV), markers of early alterations of arterial morphology, structure and function, capture the early stages of the atherosclerotic and arteriosclerotic processes respectively, and are demonstrated to be strong predictors of future cardiovascular events.[4,5] Studying biomarkers’ involvement in relation to different aspects of CVD by proteins’ associations with hypertension, cIMT and PWV may further increase understanding of the complex biological processes leading to CVD. Multiplex proteomics platforms provide an excellent tool for investigating associations between multiple, targeted proteins and disease (e.g., CVD).[6,7]
The effects of ethnicity on several biomarkers’ reference intervals is known[8,9] yet often overlooked. Further, racial disparities in the prevalence of hypertension is known and cannot be fully explained by traditional risk factors.[10,11] Evaluating targeted biomarkers’ associations with cardiovascular traits, adjusted for ethnicity can help increase our understanding of possible differing pathophysiological pathways preceding CVD in different ethnic groups.
Here, we aimed to explore associations between 92 proteins implicated in inflammation/CVD and phenotypes of vascular health such as prevalent hypertension, subclinical atherosclerosis (as measured by cIMT) and arteriosclerosis, (as measured by PWV), with regards to ethnicity.
2. Methods
2.1. Study design and population
This study is part of the Sympathetic activity and Ambulatory Blood Pressure in Africans (SABPA) study of 409 black and white African school teachers (hereafter referred to as Blacks and Whites) (aged 20–62), working in the North-West Province, South-Africa. The inclusion of teachers ensured a cohort with similar socio-economic status. The examination was conducted in 2008–2009 and a detailed description of the study population is available elsewhere.[12] Out of 409 subjects, 31 did not have proteomics data, including the 19 HIV-infected subjects in the population. Of the remaining 378, 16 participants were excluded from all analyses because of missing data on any of self-reported smoking status, cIMT, PWV, estimated glomerular filtration rate (eGFR), mean arterial pressure (MAP) and heart rate (HR), resulting in 362 participants having data on all co-variates and thus included in analyses (Fig. 1).
Figure 1.
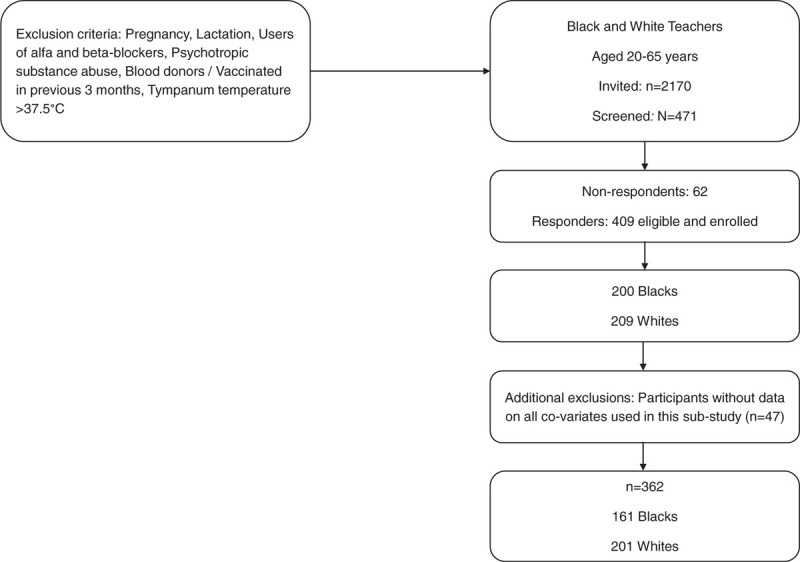
Flowchart outlining inclusion in the study.
The SABPA study conforms to the principles outlined in the Declaration of Helsinki (World Medical Association General Assembly 2004) and abided by the institutional guidelines and was approved by the Health Research Ethics Committee of the North-West University, South Africa (NWU-0003607S6). All participants provided written informed consent.
2.2. Clinical measurements
With an appropriate cuff placed on the upper left arm, after a 5 to 10-minutes rest and with the patients in the semi-Fowler's position, resting blood pressure was measured twice by a registered nurse or doctor, on the non-dominant arm by applying a suitable cuff using a stethoscope (Littman II S.E. Stethoscope 2205) and a calibrated mercury sphygmomanometer (Riester CE 0124 & 1.3 M TM). Two duplicate measures were taken, with a 3- to 5-minute resting period between each; the second of which was used for statistical analyses. Hypertension was defined as resting systolic blood pressure of ≥140 and/or diastolic blood pressure ≥90 mm Hg, or use of hypertensive medication.[13]
2.3. Carotid ultrasound
Carotid intima media thickness was obtained using a SonoSite Micromaxx ultrasound system (SonoSite, Bothell, WA) and a 6 to 13 MHz linear array transducer. Images from at least 2 optimal angles of the left and right common carotid artery were obtained,[14] and then digitalized and imported into the Artery Measurement Systems automated software[15,16] for analyses of cIMT. When image quality was satisfactory, a maximal 10 mm segment was chosen for analysis (Supplementary Figure S1). The software automatically calculates cIMT, with the possibility of manual correction if found not appropriate upon visual inspection. The mean of the far wall and near wall measurements were used. Intra-observer variability for the far wall was 0.04 mm between 2 measurements made 4 weeks apart on 10 participants.
2.4. Pulse wave velocity
The carotid-dorsalis pedis PWV was carried out across the carotid-dorsalis pedis region as a measure of arterial stiffness with the participant in a supine position (Complior SP device, Artech-Medical, Pantin, France). The distance was determined by subtracting the carotid artery to suprasternal notch distance from the distal measurement (subtraction method). All measurements were taken by the same 2 observers for all participants.[17]
2.5. Questionnaire data and anthropometry
Participants completed a lifestyle questionnaire that included information regarding their habits (ex. smoking), if on a diabetes diet, medical history (ex. CVD or diabetes), medication use. Waist circumference was measured in triplicate to the nearest 0.1 cm with an unstreachable type measure according to the standard method proposed by the International society for the advancement of kinanthropometry. CVD burden was defined as any of the following: history of physician diagnosed heart disease, use of anti-hypertensive medication, anti-diabetic medication, or statins.[18] Diabetes was defined as use of anti-diabetes drug, on a diabetes diet or HbA1c ≥6.5%.
2.6. Biochemical analyses
Sodium fluoride, plasma and serum samples from fasting blood were stored at −80°C. Serum samples for total cholesterol and gamma glutamyl transferase (GGT) were analyzed using 2 sequential multiple analyzers (Konelab 20i; Thermo Scientific, Vantaa, Finland; Unicel DXC 800 - Beckman and Coulter, Germany). The intra- and inter-coefficients of variation for all assays were below 10%.[19] HIV status was measured by a rapid anti-body test in plasma (First response kit. PMC Medical, Daman, India) and confirmed with the Pareekshak test (BHAT Bio-Tech, Bangalore, India). Estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease (MDRD) formula: 186∗[serum creatinine (μmol/l)88.4]-1.154∗[age]-0.203. Consider:∗1.210 (if Black) and/or ∗ 0.742 (if female).[20]
2.7. Proteomic profiling
Plasma levels of proteins were analyzed by the Proximity Extension Assay (PEA) technique, PCR-based affinity proteomics technology, using the Proseek Multiplex CVD III 96 × 96 reagents kit (Olink Bioscience, Uppsala, Sweden). The new PEA technique has opened up for large-scale analyzing of multiple proteins and encompasses high specificity and sensitivity using only small samples of blood.[21] The CVD III panel[22–24] comprises 92 proteins, with either established or suggested associations with CVD and inflammation. Three proteins were below detectable limits in >15% samples (NT-proBNP, pulmonary surfactant-associated protein and spondin-1). Hence, 89 proteins were taken forward to analyses. Across all 92 assays, the mean intra-assay and inter-assay variations were observed to be 7% and 14%, respectively. Validation data and coefficients of variance for all proteins can be found in the online supplemental material (Validation data CVD III) and further technical information about the assays is available on the Olink homepage (http://www.olink.com). A quality control of the technical performance of the assays, as well as the samples, is carried out during the analyses. The quality control is carried out using 4 internal controls that are spiked into all samples and external controls in every analysis:
-
1.
2 incubation controls (non-human antigens with matching antibodies);
-
2.
extension control (IgG antibodies conjugated with matching oligo-pair) and
-
3.
detection control (synthetic double-stranded DNA).
For the entire sample plate, standard deviations are calculated for both the detection control and the incubation controls; in order to pass quality control, the standard deviations should be below a predetermined threshold.
2.8. Statistical methods
Proteins are expressed on a log2 scale and presented as normalized protein expression (NPX; arbitrary units), which corresponds to a 2-fold change in protein concentration. Variables that were skewed (serum gamma glutamyl transferase and mean arterial blood pressure) were ln-transformed. Blacks and Whites were compared using one-way ANOVA tests for continuous variables, or χ2 tests for binary variables. Complete data was available in 362 subjects, and univariate regressions were considered significant if they met the Bonferroni-corrected (P < .05/89 = 5.6 × 10−4) significance threshold for each set of analyses with hypertension, cIMT and PWV as the dependent variables and the 89 proteins as the main independents. Logistic regression analyses were carried out to examine possible associations between hypertension and each one of the 89 proteins in unadjusted models. Bonferroni-corrected significant associations were further adjusted for age and sex (Model 1), and if significant (P < .05), additional adjustment was carried out for clinically relevant co-variates (waist circumference, smoking, estimated glomerular filtration rate (eGFR), alcohol use as measured by GGT, prevalent CVD, diabetes and heart rate (Model 2a)). Ethnicity was further entered on top of Model 2a. In order to examine possible associations between each one of the 89 proteins and cIMT, cIMT was log2-transformed and unadjusted linear regression analyses were carried out. Associations that met the Bonferroni-correction were further adjusted for age and sex (Model 1), and if significant (P < .05), model 2b was further adjusted for known cardiovascular risk factors as well as factors that may be in the pathway of CVD/inflammation and atherosclerosis (waist circumference, systolic blood pressure, smoking, cholesterol, statin use, diabetes, prevalent CVD and anti-hypertensive treatment).[25] Further, ethnicity was entered on top of Model 2b. Associations between each of the 89 proteins and log2-transformed PWV were carried out unadjusted in linear regression analyses. Associations that met Bonferroni-corrected significance where then adjusted for age and sex (Model 1) and, if significant, further adjusted for waist circumference, smoking, diabetes, use of statins, total cholesterol, anti-hypertensive treatment and mean arterial blood pressure[26] (Model 2c). Ethnicity was entered on top of Model 2c (A flowchart illustrating the statistical analysis is given in Fig. 2).
Figure 2.
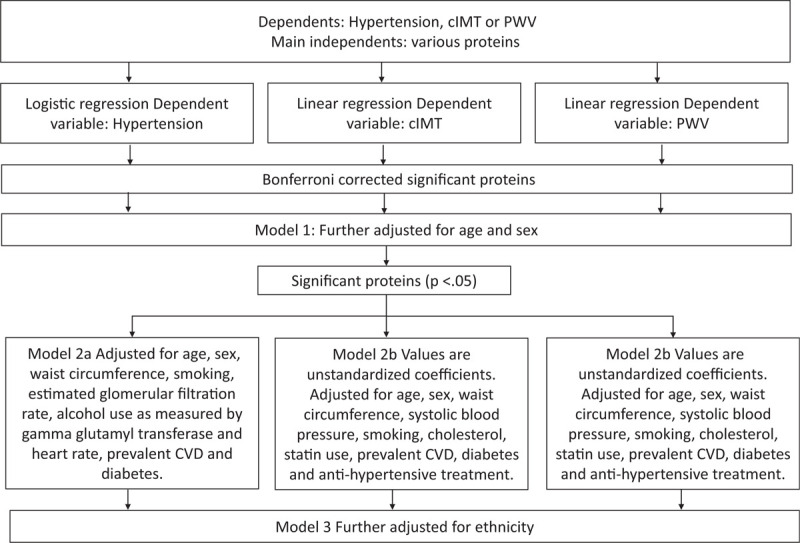
Flowchart illustrating the statistical analysis.
All analyses were performed using SPSS Windows version 26.0.
3. Results
The baseline characteristics of the study population are presented in Table 1. Blacks had overall significantly worse metabolic status overall compared to whites with higher systolic BP (P < .001), diastolic BP (P = < .001), GGT (P < .001) and prevalence diabetes (16.8% vs 8.5%, P = .02), with the exception that whites had higher total cholesterol levels (P < .001) and poorer kidney function (eGFR) (P < .001) than blacks. Regarding the markers of vascular health, PWV (P < .001), cIMT (P = .001) and prevalence of hypertension (75.2% vs 48.8% P = < .001) were all significantly higher in the Black population.
Table 1.
Characteristics of the study population.
| Total n = 362 | Blacks n = 161 | Whites n = 201 | P | |
| Age (yrs) | 44.8 (±9.9) | 44.5 (±8.5) | 45.0 (±10.9) | .61 |
| Sex, n female (%) | 188 (51.9) | 86 (53.4) | 102 (50.7) | .61 |
| eGFR | 102.8 (±24.0) | 113.2 (±27.1) | 94.6 (±17.2) | <.001 |
| Total cholesterol (mmol/L) | 5.1 (±1.3) | 4.6 (±1.1) | 5.5 (±1.3) | <.001 |
| Waist circumference (cm)Lifestyle and medication | 93.5 (±15.9) | 94.0 (±15.5) | 93.1 (±16.2) | .63 |
| Smoker, n (%) | 53 (14.6) | 25 (15.5) | 28 (13.9) | .67 |
| GGT | 27 (16.0–46.8) | 40.0 (27.4–68.4) | 18.0 (12.0–28.5) | <.001 |
| Use of statins, n (%) | 11 (3) | 2 (1.2) | 9 (4.5) | .08 |
| Use of antihypertensives, n (%) | 86 (23.8) | 60 (37.3) | 26 (12.9) | <.001 |
| Hypertension, n (%) | 219 (60.5) | 121 (75.2) | 98 (48.8) | <.001 |
| Prevalent CVD, n (%) | 36 (9.9) | 13 (8.1) | 23 (11.4) | .29 |
| Diabetes, n (%)Cardiovascular measurements | 44 (12.2) | 27 (16.8) | 17 (8.5) | .02 |
| Off SBP (mm Hg) | 135.7 (±20.2) | 141.0 (±20.6) | 131.5 (±15.2) | <.001 |
| Off DBP (mm Hg) | 88.7 (±13.5) | 93.4 (±13.6) | 85.0 (12.1) | <.001 |
| Pulse wave velocity (m/s) | 8.3 (7.2 – 9.3) | 8.6 (7.4 – 9.6) | 8.2 (7.1 – 9.0) | <.001 |
| cIMT (mm) | 0.652 (0.57–0.74) | 0.665 (0.60–0.75) | 0.64 (0.55–0.73) | .001 |
Names and acronyms of all of the 92 proteins included in the CVD III panel are presented in Supplementary Table S1. Bonferroni-corrected univariate logistic regression analyses of associations between 89 proteins and prevalence of hypertension resulted in 11 significant findings (Supplementary Table S2). Ten proteins remained significant when further adjusted for age and sex (Supplementary Table S3). After further adjustment for waist circumference, smoking, eGFR, GGT, prevalent CVD, diabetes and heart rate, growth differentiation factor 15 (GDF15; odds ratio (OR) 2.06, 95% confidence interval (CI95%) 1.04–4.07, P = .04) and C-X-C motif chemokine 16 (CXCL16; OR 2.33, CI95% 1.06–5.11, P = .03) remained significantly associated with hypertension (Table 2, Supplementary Table S4).
Table 2.
Associations between proteins and HT, cIMT or PWV.
| HTOR (CI95%) P value | cIMTβ (P value) | PWVβ (P value) | ||||
| Proteins | Model 2a | Model 3 | Model 2b | Model 3 | Model 2c | Model 3 |
| GDF15 | 2.06 (1.04–4.07).04 | 1.96 (0.97–3.96).06 | 0.032 (.20) | –∗ | 0.086 (.004) | 0.080 (.006) |
| CPA1 | –∗ | –∗ | 0.031 (.048) | 0.020 (0.20) | 0.050 (.01) | 0.041 (.03) |
| CCL15 | –∗ | –∗ | 0.065 (<.001) | 0.045 (.02) | –∗ | –∗ |
| CHI3L1 | 1.33 (0.98–1.80).07 | –∗ | 0.028 (.02) | 0.020 (.10) | 0.021 (.14) | –∗ |
| OPG | –∗ | –∗ | 0.074 (.01) | 0.041 (.17) | –∗ | –∗ |
| SELE | –∗ | –∗ | 0.033 (.08) | –∗ | 0.049 (.03) | 0.038 (.09) |
| FABP4 | 0.89 (0.63–1.28).53 | –∗ | 0.036 (.05) | –∗ | 0.055 (.01) | 0.045 (.04) |
| CXCL16 | 2.33 (1.06–5.11).03 | 1.47 (0.62–3.50).38 | –∗ | –∗ | 0.119 (.001) | 0.089 (.03) |
| tPA | 1.12 (0.97–1.28).12 | –∗ | –∗ | –∗ | 0.014 (.04) | 0.010 (.16) |
| CD163 | 1.26 (0.76–2.09).37 | –∗ | 0.044 (.04) | 0.031 (.15) | 0.016 (.52) | –∗ |
| CPB1 | –∗ | –∗ | –∗ | –∗ | 0.043 (.04) | 0.045 (.03) |
When ethnicity was entered upon model 2 none of the proteins remained significantly associated with prevalence of hypertension (Table 2).
As for associations between 89 proteins and cIMT, Bonferroni-corrected univariate linear regression analyses resulted in 14 significant findings (Supplementary Table S5). Eleven proteins remained significant when further adjusted for age and sex Supplementary Table S6). After further adjustment for waist circumference, systolic blood pressure, smoking, total cholesterol, statin use, prevalent CVD, diabetes and anti-hypertensive treatment, 5 proteins remained significantly associated with increasing cIMT: carboxypeptidase A (CPA1; β 0.031; P = .048), C-C motif chemokine 15 (CCL15; β 0.065, P < .001), CHI3L1 (β 0.028, P = .02), scavenger receptor cysteine-rich type 1 protein M130 (CD163; β 0.044; = .04) and osteoprotegerin (OPG; β 0.074, P = .01) (Table 2, Supplementary table S7). When ethnicity was entered upon the adjusted model, only CCL15 remained significantly associated with increasing cIMT (β 0.031, P = .02) (Table 2).
Associations between 89 proteins and PWV were carried out in Bonferroni-corrected univariate linear regressions, and resulted in 14 significant findings (Supplementary Table S8). All 14 proteins remained significant when further adjusted for age and sex (Supplementary Table S9). After further adjustment for waist circumference, smoking, diabetes, mean arterial blood pressure, total cholesterol, use of statins and anti-hypertensive treatment, 7 proteins presented significant associations with PWV: GDF15 (β 0.086, P = .004), SELE (β 0.049, P = .03), CPA1 (β 0.050, P = .01), FABP4 (β 0.055, P = .01), CXCL16 (β 0.119, P = .001), CPB1 (0.043; P = 0.04), and tPA (β 0.020, P = .03) (Table 2, Supplementary table S10).
Additional adjustment for ethnicity resulted in 5 significant associations between GDF15 (β 0.080, P = .006), CPA1 (β 0.050, P = .04), FABP4 (β 0.045, P = .04), CXCL16 (β 0.119, P = .03) and CPB1 (0.045, P = .03), and PWV (Table 2).
4. Discussion
Here, we used a multiplex proteomic panel in a bi-ethnic cohort to explore associations between vascular traits such as hypertension, cIMT and PWV and multiple proteins involved in CVD and inflammation. We could confirm previously demonstrated associations (between PWV and GDF15, FABP4 and SELE[27–29]; between hypertension and GDF-15[30]; and between cIMT and Osteoprotegerin (OPG), CD163 and Chitinase-3-like protein 1 (CHI3L1)[31–33]). CXCL16, involved in inflammatory response, revealed novel associations with hypertension. Four proteins (CXCL16, CPA1, CPB1, and tPA), playing a role in inflammatory response, chemotaxis, coagulation or proteolysis, represent novel associations with increased PWV and 1 protein (CCL15), also with an important role in inflammation through chemotaxis, represents novel associations with cIMT. We could reveal whether the associations were dependent of ethnicity or not which can be relevant to take into consideration, if, for example, developing reference intervals for the various proteins or, if investigating possible ethnic variations in the biological processes preceding CVD.
Although being different phenotypes of vascular health with differing mechanisms, hypertension, PWV and cIMT are all well documented predictors of the development of CVD.[4,5,34] Placing multiplex proteomics in a pre-clinical atherosclerotic, arteriosclerotic and hypertensive context by studying their relation to hypertension, PWV and cIMT, may further increase understanding of the complex biological processes leading to CVD.
4.1. Proteins with novel and ethnicity-independent associations
4.1.1. Carboxypeptidase A1 and B1
CPA1 and CPB1 are biomarkers related to proteolysis and inflammation. Studies on CPA1's association with vascular health/CVD are scarce. Kulasingam et al[35] compared the plasma levels of biomarkers related to cardiovascular disease in the acute phase of ST-elevation myocardial infarction (STEMI), with the protein levels in the stable phase 3 months after STEMI. They found that concentrations of CPA1 were increased at the three-month follow-up, compared to the acute phase. To our knowledge, the positive associations between PWV and CPA1/CPB1 found in this study are novel. Further, when adjusted for ethnicity, the associations remained significant.
4.1.2. Chemokine (C-C motif) ligand 15
CCL15, also named Leukotactin-1 (Lkn-1), is a chemotactic factor involved in leukocyte trafficking and activation, and thus, plays an important role in inflammation.[36] Chemokines recruit leukocytes into the arterial wall and CCL15 has been shown to contribute to plaque destabilization in the progression of atherosclerosis.[37,38] In this light, the positive associations between cIMT and CCL15 demonstrated here are sound. To our knowledge, this is the first time CCL15 has been implicated in subclinical atherosclerosis as measured by cIMT. Furthermore, another finding of this study is that cIMT remained significantly associated with CCL15 when adjusted for ethnicity. Additionally, repeated alcohol exposure induced changes in the CC chemokine family should also be considered as it revealed a link between CCL cytokines and stress.[39] As ethnicity influenced associations between cIMT and CCL15; the higher alcohol consumption in the Black cohort may contribute to their higher mean inflammatory status. However, upon entering cGGT (as a marker of alcohol use) in the model, the association between cIMT and CCL15 remained significant; suggesting the association observed is independent of cGGT. On the other hand, previously black Africans with low grade inflammation demonstrated higher alcohol consumption in relation to coronary artery disease risk and stress.[40]
4.1.3. CXC Ligand 16
CXCL16 is a chemokine expressed at sites of inflammation and has been suggested to play a functional role in atherosclerosis.[41] It has been proposed to have adverse effects on cardiomyocytes in patients with CVD[42] and associated to adverse clinical outcomes in patients with acute coronary syndrome.[43] Here, we found a significant association with CXCL16 and PWV, which remained after adjusting for ethnicity. This is, to the best of our knowledge, a novel finding. Membrane-bound CXCL16 has been suggested to transform vascular smooth muscular cells from contractile to a proliferative form, thereby promoting vascular remodeling,[44] suggesting this could be a reason for CXCL16's correlation to arterial stiffness found in this study. In contrast to Wang et al,[45] we found no association between CXCL16 and cIMT; however, the study population involved atherosclerotic stroke patients and was older than the population of this study.
4.2. Proteins with novel and ethnicity-dependent associations
4.2.1. CXC Ligand 16
In this study we found the previously described CXCL16 to be significantly associated to hypertension. We believe this is a novel finding and CXCL16's role in hypertension is relatively unknown. In animal models, Xia et al showed that it is a mediator of renal inflammation and propagates hypertensive kidney damage but genetic deletion of CXCL16 did however not affect hypertension.[46] The results suggest that hypertension could cause renal inflammation, rather than the other way around. After adjusting for ethnicity, the association between CXCL16 and hypertension in this study did not remain significant.
4.2.2. Tissue plasminogen activator
tPA is a protein found on the endothelial cells, is a part of the fibrinolytic pathway and has been proven to be an independent risk factor of a first CVD event.[47] Here, we found a significant association between elevated levels of tPA and PWV. This is in contrast to a study by Tuttolomondo et al where they did not see a relationship between tPA and PWV[48] in a population consisting of stroke patients and controls. The association found in this study was no longer significant after adjusting for ethnicity.
4.2.3. Transferrin receptor protein 1
TR is a carrier protein of transferrin, needed for import of iron into cells. In this study we found a significant association between TR and hypertension which we believe is a novel finding, the significance was however lost upon entering ethnicity in the model. In Chinese subjects, Zhu et al[49] did not find an association between soluble transferrin receptor and incident hypertension, however Naito et al reported that heterozygous mice deficient in the transferrin-1 receptor (TfR1) show protection against the development of hypoxia-induced pulmonary hypertension and vascular remodeling.[50]
4.3. Study limitations
The data in the SABPA study were collected at a single regional center, which confines replication of significant findings and limits applicability to other populations. On the other hand, the subjects in our population were of similar age and socio-economic status, and balanced with regards to sex and ethnicity. The cross-sectional associations share the usual limitations of causality and control as seen for all cross-sectional studies. The gold standard for measuring PWV is carotid-femoralis PWV. Here, due to cultural and ethical reasons we used the carotid-dorsalis PWV.
5. Conclusions
In a South African bi-ethnic cohort, we confirmed several proteins’ associations with phenotypes of vascular health using a multiplex proteomic approach. Novel associations were found between hypertension, PWV or cIMT and proteins involved in inflammatory response, chemotaxis, coagulation or proteolysis. Further, we could reveal whether the associations were dependent of ethnicity or not.
Author contributions
Conceptualization: Leone Malan.
Data curation: Leone Malan.
Formal analysis: Anna Dieden, Amra Jujic, Martin Magnusson.
Funding acquisition: Leone Malan, Martin Magnusson.
Methodology: Anna Dieden.
Project administration: Leone Malan.
Resources: Martin Magnusson.
Supervision: Amra Jujic, Martin Magnusson.
Validation: Martin Magnusson.
Writing – original draft: Anna Dieden.
Writing – review & editing: Anna Dieden, Leone Malan, Catharina MC Mels, Leandi Lammertyn, Annemarie Wentzel, Peter M Nilsson, Petri Gudmundsson, Amra Jujic, Martin Magnusson.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: cIMT = carotid intima media thickness, PWV = pulse wave velocity.
How to cite this article: Dieden A, Malan L, Mels CM, Lammertyn L, Wentzel A, Nilsson PM, Gudmundsson P, Jujic A, Magnusson M. Exploring biomarkers associated with deteriorating vascular health using a targeted proteomics chip: the SABPA study. Medicine. 2021;100:20(e25936).
AJ and MM contributed equally to this work.
This work was kindly supported by North-West University; National Research Foundation (NRF); Medical Research Council (MRC-SA); Department of Education North-West Province; ROCHE diagnostics; South Africa and Metabolic Syndrome Institute, France. AD was supported by Malmö University. AJ was supported by Lund University and the Region Skane. MM was supported by grants from the Swedish Heart and Lung Foundation, the Medical Faculty of Lund University, Skane University Hospital, the Crafoord Foundation, the Region Skane, the Knut and Alice Wallenberg Foundation and ALF from Region Skåne. PMN was funded by Research Council of Sweden (K2008-65X-20752-01-3 and K2011-65X-20752-04-6), E. Lundström Foundation and ALF from Region Skåne. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This study was previously presented as an abstract on the ESC Congress in Paris, 28 may of 2019.
AD and AJ analyzed and interpreted the patient data regarding the OLINK CVD III panel and cIMT, and were lead writers of the manuscript. LM was responsible for all collection of data and was a major contributor in writing the manuscript. PMN, CM, LM, PG, LL, AW and MM all contributed in writing and a critical revision of the manuscript. MM and AJ also contributed with statistical expertise. All authors read and approved the final manuscript.
The data that support the findings of this study are available from North-West University, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of North-West University.
For the mechanism used for reviewing the ethics of the research conducted we refer to the Health Research Ethics Committee of the North-West University, South Africa (NWU-0003607S6).
The authors have no conflicts of interests to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
Supplemental digital content is available for this article.
Values are means ± standard deviation (SD) or median (25–75 interquartile range (IQR)). cIMT = carotid intima media thickness, CVD = cardiovascular disease, eGFR = estimated glomerular filtration rate, GGT = Serum Gamma glutamyl transferase, Off DBP = Office diastolic blood pressure, Off SBP = Office systolic blood pressure, PWV = pulsed wave velocity.
the associations were not significant in the previous analyses made and were therefore not analysed in this step. CCL15 = C-C motif chemokine 15, CD163 = Scavenger receptor cysteine-rich type 1 protein M130, CHI3L1 = chitinase-3-like protein 1, CPA1 = Carboxypeptidase A1, CPB1 = carboxypeptidase B1, CXCL16 = C-C motif chemokine 16, FABP4 = fatty acid-binding protein 4, GDF15 = growth-differentiation factor 15, OPG = osteoprotegerin, SELE = E-selectin, tPA = Tissue-type plasminogen activator.
All proteins are adjusted for age and sex. Model 2a Values are odds ratios (OR) and 95% confidence intervals (CI95%). Adjusted for age, sex, waist circumference, smoking, estimated glomerular filtration rate, alcohol use as measured by gamma glutamyl transferase and heart rate, prevalent CVD and diabetes. Model 2 b Values are unstandardized coefficients. Adjusted for age, sex, waist circumference, systolic blood pressure, smoking, cholesterol, statin use, prevalent CVD, diabetes and anti-hypertensive treatment. Model 2c Values are unstandardized coefficients. Adjusted for age, sex, waist circumference, mean arterial blood pressure, smoking, cholesterol, use of statins, diabetes and anti-hypertensive treatment. Model 3 is further adjusted for ethnicity.
References
- [1].Mendis S, Puska P, Norrving B. World Health Organization., World Heart Federation., World Stroke Organization. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization; 2011. [Google Scholar]
- [2].Collaboration NCDRF. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017;389(10064):37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012;308(9):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115(4):459–67. [DOI] [PubMed] [Google Scholar]
- [5].Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55(13):1318–27. [DOI] [PubMed] [Google Scholar]
- [6].Molvin J, Jujic A, Melander O, et al. Exploration of pathophysiological pathways for incident atrial fibrillation using a multiplex proteomic chip. Open Heart 2020;7:e001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Molvin J, Pareek M, Jujic A, et al. Using a targeted proteomics chip to explore pathophysiological pathways for incident diabetes- the malmo preventive project. Sci Rep 2019;9:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tahmasebi H, Trajcevski K, Higgins V, Adeli K. Influence of ethnicity on population reference values for biochemical markers. Crit Rev Clin Lab Sci 2018;55(5):359–75. [DOI] [PubMed] [Google Scholar]
- [9].Kim CX, Bailey KR, Klee GG, et al. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. PLoS One 2010;5(2):e9065–19065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schutte AE, van Rooyen JM, Huisman HW, Kruger HS, de Ridder JH. Factor analysis of possible risks for hypertension in a black South African population. J Hum Hypertens 2003;17(5):339–48. [DOI] [PubMed] [Google Scholar]
- [11].Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci 2014;348(2):135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Malan L, Hamer M, Frasure-Smith N, Steyn HS, Malan NT. Cohort Profile: Sympathetic activity and Ambulatory Blood Pressure in Africans (SABPA) prospective cohort study. Int J Epidemiol 2015;44(6):1814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34(28):2159–219. [DOI] [PubMed] [Google Scholar]
- [14].Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007;23(1):75–80. [DOI] [PubMed] [Google Scholar]
- [15].Liang Q, Wendelhag I, Wikstrand J, Gustavsson T. A multiscale dynamic programming procedure for boundary detection in ultrasonic artery images. IEEE Trans Med Imaging 2000;19(2):127–42. [DOI] [PubMed] [Google Scholar]
- [16].Wendelhag I, Liang Q, Gustavsson T, Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke 1997;28(11):2195–200. [DOI] [PubMed] [Google Scholar]
- [17].Schutte R, Schutte AE, Huisman HW, et al. Arterial stiffness, ambulatory blood pressure and low-grade albuminuria in non-diabetic African and Caucasian men: the SABPA study. Hypertens Res 2011;34(7):862–8. [DOI] [PubMed] [Google Scholar]
- [18].Hamer M, von Känel R, Reimann M, et al. Progression of cardiovascular risk factors in black Africans: 3 year follow up of the SABPA cohort study. Atherosclerosis 2015;238(1):52–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hamer M, Malan L, Schutte AE, et al. Conventional and behavioral risk factors explain differences in sub-clinical vascular disease between black and Caucasian South Africans: the SABPA study. Atherosclerosis 2011;215(1):237–42. [DOI] [PubMed] [Google Scholar]
- [20].Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145(4):247–54. [DOI] [PubMed] [Google Scholar]
- [21].Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brunner PM, Suarez-Farinas M, He H, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep 2017;7:8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ouwerkerk W, Zwinderman AH, Ng LL, et al. Biomarker-guided versus guideline-based treatment of patients with heart failure: results from BIOSTAT-CHF. J Am Coll Cardiol 2018;71:386–98. [DOI] [PubMed] [Google Scholar]
- [24].Enroth S, Maturi V, Berggrund M, et al. Systemic and specific effects of antihypertensive and lipid-lowering medication on plasma protein biomarkers for cardiovascular diseases. Sci Rep 2018;8:5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ren L, Cai J, Liang J, Li W, Sun Z. Impact of cardiovascular risk factors on carotid intima-media thickness and degree of severity: a cross-sectional study. PLoS One 2015;10(12):e0144182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gottsater M, Ostling G, Persson M, et al. Non-hemodynamic predictors of arterial stiffness after 17 years of follow-up: the Malmo Diet and Cancer study. J Hypertens 2015;33:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Andersson C, Enserro D, Sullivan L, et al. Relations of circulating GDF-15, soluble ST2, and troponin-I concentrations with vascular function in the community: the Framingham Heart Study. Atherosclerosis 2016;248:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eikendal ALM, Bots ML, Gohar A, et al. Circulating levels of P-selectin and E-selectin relate to cardiovascular magnetic resonance-derived aortic characteristics in young adults from the general population, a cross-sectional study. J Cardiovasc Magn Reson 2018;20:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tsai JP, Wang JH, Lee CJ, Chen YC, Hsu BG. Positive correlation of serum adipocyte fatty acid binding protein levels with carotid-femoral pulse wave velocity in geriatric population. BMC Geriatr 2015;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ferreira JP, Pizard A, Machu JL, et al. Plasma protein biomarkers and their association with mutually exclusive cardiovascular phenotypes: the FIBRO-TARGETS case-control analyses. Clin Res Cardiol 2020;109(1):22–33. [DOI] [PubMed] [Google Scholar]
- [31].Jafary F, Khamechi SP, Talari HR, Sharif MR, Nikoueinejad H, Sehhat M. Correlation between serum YKL-40 and carotid intima media thickness in type 1 diabetics. Int J Diabetes Developing Countries 2015;35(3):411–7. [Google Scholar]
- [32].Morisawa T, Nakagomi A, Kohashi K, et al. Osteoprotegerin is associated with endothelial function and predicts early carotid atherosclerosis in patients with coronary artery disease. Int Heart J 2015;56(6):605–12. [DOI] [PubMed] [Google Scholar]
- [33].David C, Divard G, Abbas R, et al. Soluble CD163 is a biomarker for accelerated atherosclerosis in systemic lupus erythematosus patients at apparent low risk for cardiovascular disease. Scand J Rheumatol 2020;49(1):33–7. [DOI] [PubMed] [Google Scholar]
- [34].Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation 2015;131(19):e435–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kulasingam A, Hvas AM, Grove EL, Funck KL, Kristensen SD. Detection of biomarkers using a novel proximity extension assay in patients with ST-elevation myocardial infarction. Thromb Res 2018;172:21–8. [DOI] [PubMed] [Google Scholar]
- [36].Murphy PM, Baggiolini M, Charo IF, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 2000;52(1):145–76. [PubMed] [Google Scholar]
- [37].Kwon S-H, Ju S-A, Kang J-H, Kim C-S, Yoo H-M, Yu R. Chemokine Lkn-1/CCL15 enhances matrix metalloproteinase-9 release from human macrophages and macrophage-derived foam cells. Nutr Res Pract 2008;2(2):134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sheikine Y, Hansson GK. Chemokines and atherosclerosis. Ann Med 2004;36(2):98–118. [DOI] [PubMed] [Google Scholar]
- [39].Garcia-Marchena N, Araos PF, Barrios V, et al. Plasma chemokines in patients with alcohol use disorders: association of CCL11 (Eotaxin-1) with psychiatric comorbidity. Front Psychiatry 2016;7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oosthuizen W, Malan L, Scheepers JD, Cockeran M, Malan NT. The defense response and alcohol intake: a coronary artery disease risk? The SABPA Study. Clin Exp Hypertens 2016;38(6):526–32. [DOI] [PubMed] [Google Scholar]
- [41].Bakogiannis C, Sachse M, Stamatelopoulos K, Stellos K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 2019;122:154157. [DOI] [PubMed] [Google Scholar]
- [42].Orn S, Breland UM, Mollnes TE, et al. The chemokine network in relation to infarct size and left ventricular remodeling following acute myocardial infarction. Am J Cardiol 2009;104(9):1179–83. [DOI] [PubMed] [Google Scholar]
- [43].Andersen T, Ueland T, Ghukasyan Lakic T, et al. C-X-C ligand 16 is an independent predictor of cardiovascular death and morbidity in acute coronary syndromes. Arteriosclerosis, Thrombosis Vascular Biol 2019;39(11):2402–10. [DOI] [PubMed] [Google Scholar]
- [44].Smith C, Halvorsen B, Otterdal K, et al. High levels and inflammatory effects of soluble CXC ligand 16 (CXCL16) in coronary artery disease: down-regulatory effects of statins. Cardiovasc Res 2008;79(1):195–203. [DOI] [PubMed] [Google Scholar]
- [45].Wang KD, Liu ZZ, Wang RM, et al. Chemokine CXC Ligand 16 serum concentration but not A181 V genotype is associated with atherosclerotic stroke. Clin Chim Acta 2010;411(19–20):1447–51. [DOI] [PubMed] [Google Scholar]
- [46].Xia Y, Entman Mark L, Wang Y. Critical role of CXCL16 in hypertensive kidney injury and fibrosis. Hypertension 2013;62(6):1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Thogersen AM, Jansson JH, Boman K, et al. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation 1998;98(21):2241–7. [DOI] [PubMed] [Google Scholar]
- [48].Tuttolomondo A, Di Raimondo D, Pecoraro R, et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke. Atherosclerosis 2010;213(1):311–8. [DOI] [PubMed] [Google Scholar]
- [49].Zhu Y, Chen G, Bo Y, Liu Y. Markers of iron status, blood pressure and incident hypertension among Chinese adults. Nutr Metab Cardiovasc Dis 2019;29(8):830–6. [DOI] [PubMed] [Google Scholar]
- [50].Naito Y, Hosokawa M, Sawada H, et al. Transferrin receptor 1 in chronic hypoxia-induced pulmonary vascular remodeling. Am J Hypertens 2016;29(6):713–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


