Abstract
The purpose of this study was to explore the association between myasthenia gravis (MG) and the risk of atrial fibrillation (AF) in an Asian population. The risk was analyzed in a cohort of 5528 patients with history of MG and 5528 individuals without MG using a hospitalization claim dataset. Both groups were matched by age, sex, index year and baseline comorbidities as an original analysis. A Cox proportional hazard model was used to estimate the hazard ratio and 95% confidence interval of AF after adjusting for demographic and relevant clinical covariates. The adjusted hazard ratio of the MG group compared with that of the non-MG group was 1.03 (95% confidence interval, 0.76–1.38) for AF. A stratified analysis showed that compared with the propensity score matched non-MG group, there was no increased risk of developing AF based on age categories, gender, or comorbidities. Different time follow-up periods results showed no increased risk of AF compared with the non-MG group. Overall, in the Taiwanese cohort, MG is not associated with an increased risk of AF.
Keywords: atrial fibrillation, cohort, myasthenia gravis
1. Introduction
Myasthenia gravis (MG) is an autoimmune disease that mainly affects the neuromuscular junction. Patients are grouped according to the serological conditions, symptoms, age at onset, and thymic involvement.[1] Coexisting conditions such as secondary autoimmune disease,[2] thymoma, cardiac disease, and therapy-induced disorders represent a major challenge in the treatment of MG patients. In treating MG, medications such as pyridostigmine, corticosteroids, and other immunosuppressants are given. In addition, a thymectomy is conducted if concurrent thymoma, early onset MG without thymoma, or generalized MG with acetylcholine receptor antibodies in late onset group is present.[3] When myasthenic crisis occurs, immune globulin or plasma exchange plays a pivotal role in intensive care. Atrial fibrillation (AF) is one of the most clinically significant cardiac arrhythmias that poses a threat to the cardiovascular system. A 5-fold increased risk for stroke, a 2-fold increased risk for all-cause mortality, and subsequent cardiomyopathy have been reported.[4] The interplay between MG and the cardiac arrhythmia is postulated by different mechanisms. Autonomic dysfunction may play a role in inducing arrythmia. In MG patient, parasympathetic cardiac impairment was found, and the associated baroreflex sensitivity was decreased. Dysfunction in the baroreflex mechanism may leads to dizziness, syncope, atrioventricular block, and even lethal atrial fibrillation.[5] Critical arrhythmias such as ventricular and supraventricular arrhythmias as well as QTc prolongation, have also been reported.[6] In another recent study, myocardial inflammation was suggested to facilitate AF. Cardiomyositis is a rare but fatal complication reported in thymoma MG and late-onset MG in patients who present with anti kv-1.4 muscle antibodies,[7] while one older population study stated that MG is associated with a lower incidence of cardiac-related deaths.[8] A dog model aimed at studying AF found that cholinergic stimulation is the main factor in the development of spontaneous AF. The acetylcholinesterase inhibitor, which is commonly used to treat MG inhibits the breakdown of acetylcholine, thereby increasing both the level and duration of the acetylcholine effect in the autonomic ganglia and the neuromuscular junction.[9] However, augmentation of vagal tone causes a nodal conduction block and atrial arrhythmias.[10,11] One case series found that new-onset AF can occur during myasthenic crisis. However, to the best of our knowledge, there is no epidemiologic evidence to support this result. Thus, we performed a nationwide population-based cohort study to analyze the subsequent risk of AF in people with a history of MG.
2. Methods
2.1. Data source
This study used the data from the inpatient datasets of the National Health Insurance Research Database (NHIRD). The NHIRD was established by the National Health Insurance (NHI) program of Taiwan, which was launched in 1995. Encryption and anonymity were performed to protect the privacy of beneficiaries. The claims data contained the information regarding basic demographics, coding of disease diagnoses based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), details of inpatient orders, and inpatient admission and discharge dates. The study was approved by the Research Ethics Committee at China Medical University and Hospital (CMUH104-REC2-115(AR-4)). Because the study applied de-identified the secondary dataset, released for research purposes, the need for informed consent was waived.
2.2. Study population
The date of initial diagnosis of myasthenia gravis (MG) (ICD-9-CM: 385.0) was defined as the index date, and the end of follow-up was the date of onset of atrial fibrillation (ICD-9-CM: 427.31), the date of withdrawal or death, or December 31, 2013. Exclusion criteria were as follows: the index date could not be between the enrollment and the withdrawal date; the index year could not be between 2000 and 2012; atrial fibrillation could not be diagnosed before the index date; and patients could not be younger than 18 years of age. Subjects with MG were included in the MG cohort. Subjects without MG were included in the non-MG cohort and were randomly matched with the MG cohort in a 1:1 ratio by propensity scores based on age, gender, index year, and baseline comorbidities. Figure 1 displays the flowchart of the method used for population selection.
Figure 1.
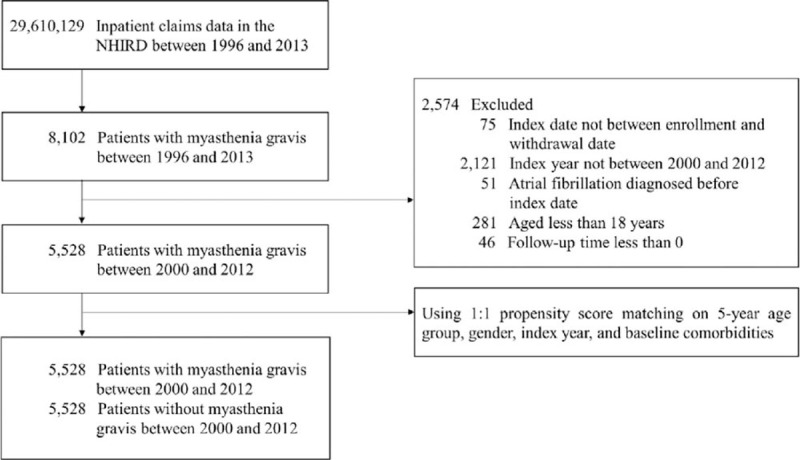
Flowchart of the study population selection.
2.3. Comorbidities and procedures
The baseline comorbidities included hypertension (ICD-9-CM: 401-405), diabetes (ICD-9-CM: 250), hyperlipidemia (ICD-9-CM: 272), coronary heart disease (ICD-9-CM: 410-414), heart failure (ICD-9-CM: 428), chronic kidney disease (ICD-9-CM: 585), chronic obstructive pulmonary disease (ICD-9-CM: 490- 496), sleep disorders (ICD-9-CM: 307.4 and 780.5), hyperthyroidism (ICD-9-CM: 242), gout (ICD-9-CM: 274), and peripheral arterial occlusive disease (PAOD) (ICD-9-CM: 440.0, 440.2, 440.3, 440.8, 440.9, 443, 444.0, 444.22, 444.8, 447.8, 447.9). Procedures included thymectomy (ICD-9-CM Procedure Code: 07.8) and plasmapheresis (ICD-9-CM Procedure Code: 99.71).
2.4. Statistical analysis
Descriptive statistics included numbers and percentages for categorical variables and means and standard deviations (SDs) for continuous variables. Standardized mean differences (SMDs) were performed to test whether there were differences in characteristics between cohorts. The incidence rate was calculated by dividing the number of events by the number of person-years in the follow-up period. The cumulative incidence was estimated using the Kaplan–Meier approach. The log-rank test was conducted to assess the differences in cumulative incidence curves between cohorts. To assess the risk of developing subsequent AF, we performed Cox regression analysis to obtain crude and adjusted hazards ratios between the two cohorts by adjusting age, gender, baseline comorbidities, and relevant procedures. Confidence intervals of 95% (95% CIs) and adjusted hazard ratios were derived by univariate and multivariate Cox proportional hazards models respectively. The criterion for determining the significance was a p-value of <0.05. Data analyses and plotting were completed using SAS 9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Characteristics of the study population
Figure 1 shows the cohort assembling process. There were 5528 subjects with MG and 5528 subjects without MG (propensity score-matched cohort) in this study, with similar distributions of age, sex, and clinical covariates. In the MG group, the mean age at diagnosis was 50.1 (16.7) years. Most were diagnosed in the younger age group (<50years, 47.02%) and there was a higher proportion of females (56.82%). The average follow-up time for the non-MG cohort (6.45 years) was negligibly different from that of the MG cohort (6.09 years) (SMD = 0.0951). The baseline characteristics and comorbidities were well balanced between the groups after matching. (Table 1)
Table 1.
Characteristics of subjects with and without MG.
| Total | Non-MG | MG | ||
| N = 11,056 | N = 5,528 | N = 5,528 | ||
| Variable | n | n (%)/Mean ± SD | n (%)/Mean ± SD | SMD§ |
| Age (yr) | ||||
| <50 | 5,198 | 2,599 (47.02) | 2,599 (47.02) | 0.0000 |
| 50-59 | 2,148 | 1,080 (19.54) | 1,068 (19.32) | 0.0055 |
| 60-69 | 1,684 | 835 (15.10) | 849 (15.36) | 0.0070 |
| 70-79 | 1,510 | 750 (13.57) | 760 (13.75) | 0.0053 |
| > = 80 | 516 | 264 (4.78) | 252 (4.56) | 0.0103 |
| Mean ± SD | 51.81 ± 17.35 | 51.83 ± 17.24 | 0.0011 | |
| Gender | ||||
| Female | 6,215 | 3,074 (55.61) | 3,141 (56.82) | 0.0244 |
| Male | 4,841 | 2,454 (44.39) | 2,387 (43.18) | 0.0244 |
| Comorbidities | ||||
| Hypertension | 1,625 | 810 (14.65) | 815 (14.74) | 0.0026 |
| Diabetes | 974 | 504 (9.12) | 470 (8.50) | 0.0217 |
| Hyperlipidemia | 504 | 272 (4.92) | 232 (4.20) | 0.0347 |
| Coronary heart disease | 715 | 365 (6.60) | 350 (6.33) | 0.0110 |
| Heart failure | 310 | 184 (3.33) | 126 (2.28) | 0.0636 |
| Chronic kidney disease | 144 | 101 (1.83) | 43 (0.78) | 0.0926 |
| COPD | 603 | 316 (5.72) | 287 (5.19) | 0.0231 |
| Sleep disorders | 190 | 108 (1.95) | 82 (1.48) | 0.0362 |
| Hyperthyroidism | 162 | 73 (1.32) | 89 (1.61) | 0.0241 |
| Gout | 233 | 141 (2.55) | 92 (1.66) | 0.0617 |
| PAOD | 77 | 53 (0.96) | 24 (0.43) | 0.0631 |
| Follow-up duration (year) | 6.45 ± 3.75 | 6.09 ± 3.82 | 0.0951 | |
3.2. Risk factors of atrial fibrillation and subgroup analysis
Table 2 gives the results of the Cox regression analysis of each variable associated with atrial fibrillation. Among the mentioned variables, being older than 50 years and having some comorbidities were associated with a higher risk of developing AF. Patients who had a history of MG had no significant risk of developing AF (adjusted hazard ratio [aHR] = 1.03, 95% CI 0.76–1.38), after adjusting for full covariates listed in Table 2. Compared with the reference patient group (aged <50), those aged older than 50 years had a higher risk of developing AF (50-59 age group: aHR = 7.71, 95% CI 3.46–17.22; 60-69 age group: aHR = 13.20, 95% CI 6.05–28.81; 70-79 age group: aHR = 33.71, 95% CI 16.03–70.89; > = 80 age group: aHR = 57.54, 95% CI 25.96–127.54). Males had no higher risk of developing AF compared to females (aHR = 1.25, 95% CI 0.93-1.70). A higher risk of developing AF was shown in patients with hypertension (aHR = 1.48, 95%CI 1.02–2.13), hyperlipidemia (aHR = 1.66, 95% CI 1.05–2.64), heart failure (aHR = 1.85, 95% CI 1.11–3.10), chronic kidney disease (aHR = 2.08, 95% CI 1.07–4.03), and chronic obstructive pulmonary disease (aHR = 1.56, 95% CI 1.03–2.38).
Table 2.
Cox regression analyses of each risk factor associated with atrial fibrillation among subjects.
| Event | IR | Crude | Adjusted∗ | ||||
| Variable | n = 177 | Person-Year | 1,000 person-years | HR (95% CI) | P-value | HR (95% CI) | P-value |
| MG | |||||||
| No | 95 | 35,645 | 2.67 | 1 (Reference) | 1 (Reference) | ||
| Yes | 82 | 33,654 | 2.44 | 0.91 (0.68, 1.22) | .5339 | 1.03 (0.76, 1.38) | .8608 |
| Age (year) | |||||||
| <50 | 8 | 37,298 | 0.21 | 1 (Reference) | 1 (Reference) | ||
| 50-59 | 24 | 13,186 | 1.82 | 8.57 (3.85, 19.10) | <.0001 | 7.71 (3.46, 17.22) | <.0001 |
| 60-69 | 34 | 9,363 | 3.63 | 17.13 (7.92, 37.02) | <.0001 | 13.20 (6.05, 28.81) | <.0001 |
| 70-79 | 75 | 7,644 | 9.81 | 46.44 (22.36, 96.44) | <.0001 | 33.71 (16.03, 70.89) | <.0001 |
| > = 80 | 36 | 1,809 | 19.90 | 94.95 (43.88, >100) | <.0001 | 57.54 (25.96, 127.54) | <.0001 |
| Gender | |||||||
| Female | 78 | 40,273 | 1.94 | 1 (Reference) | 1 (Reference) | ||
| Male | 99 | 29,026 | 3.41 | 1.75 (1.30, 2.35) | .0002 | 1.25 (0.93, 1.70) | .1398 |
| Comorbidities | |||||||
| Hypertension | 73 | 7,407 | 9.86 | 5.75 (4.25, 7.78) | <.0001 | 1.48 (1.02, 2.13) | .0368 |
| Diabetes | 38 | 4,328 | 8.78 | 3.95 (2.75, 5.68) | <.0001 | 1.10 (0.74, 1.64) | .6402 |
| Hyperlipidemia | 28 | 2,416 | 11.59 | 5.02 (3.35, 7.53) | <.0001 | 1.66 (1.05, 2.64) | .0318 |
| Coronary heart disease | 40 | 3,452 | 11.59 | 5.41 (3.80, 7.71) | <.0001 | 1.26 (0.81, 1.94) | .3038 |
| Heart failure | 21 | 1,277 | 16.45 | 6.87 (4.34, 10.86) | <.0001 | 1.85 (1.11, 3.10) | .0192 |
| Chronic kidney disease | 11 | 477 | 23.05 | 8.86 (4.79, 16.37) | <.0001 | 2.08 (1.07, 4.03) | .0309 |
| COPD | 31 | 2,799 | 11.08 | 4.89 (3.31, 7.21) | <.0001 | 1.56 (1.03, 2.38) | .0379 |
| Sleep disorders | 3 | 1,044 | 2.87 | 1.10 (0.35, 3.45) | .8689 | 0.52 (0.16, 1.67) | .2718 |
| Hyperthyroidism | 4 | 847 | 4.72 | 1.83 (0.68, 4.93) | .2324 | 2.38 (0.87, 6.53) | .0924 |
| Gout | 14 | 1,062 | 13.18 | 5.31 (3.07, 9.17) | <.0001 | 1.52 (0.85, 2.70) | .1583 |
| PAOD | 2 | 380 | 5.26 | 2.01 (0.50, 8.09) | .3271 | 0.53 (0.13, 2.21) | .3860 |
Figure 2 displays the cumulative incidence of AF in subjects with and without MG using the Kaplan–Meier method. The result of the log rank test was P = .5330. There was no difference in cumulative incidence curves between the non-MG and the MG cohorts.
Figure 2.
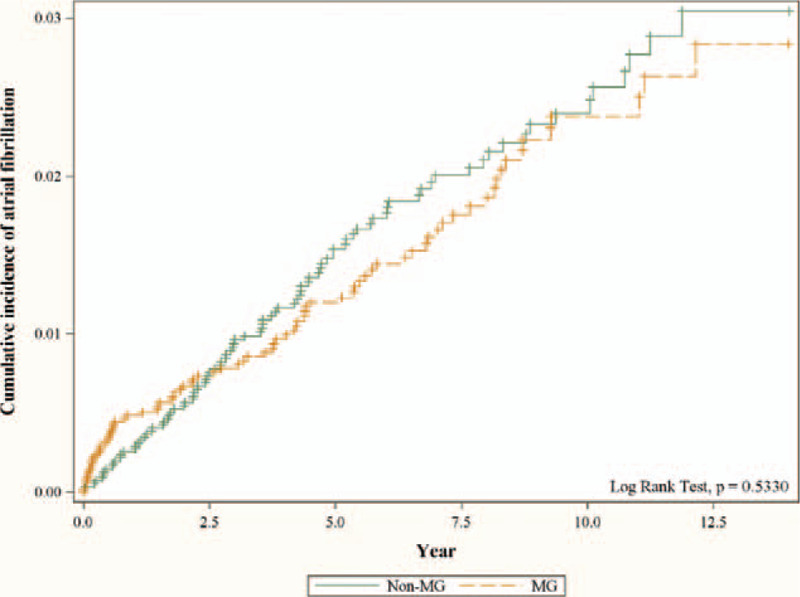
Cumulative incidence of atrial fibrillation in subjects with and without myasthenia gravis using the Kaplan-Meier method. The result showed no difference in cumulative incidence of atrial fibrillation between the non-MG and the MG cohorts.
Table 3 represents the association between MG and the risk of developing AF in terms of age subgroups, gender subgroups, and the comorbidity subgroups. In the age subgroups analysis, no statistically significant adjusted HR was found. In the gender subgroup analysis, although males had a higher incidence rate of developing AF compared with females in both the MG and non-MG group (3.38 vs. 1.77; 3.43 vs 2.1, respectively), there was no gender difference in risk of AF after adjusting for age, and comorbidities (male aHR = 1.19, 95% CI 0.80–1.79 vs. female aHR = 0.88, 95% CI 0.56–1.38). In the comorbidity subgroup analysis, gout subjects with MG were less likely to develop AF compared to those without MG (aHR = 0.19, 95% CI 0.04–0.90).
Table 3.
Comparisons of incidence of atrial fibrillation between subjects with and without MG in the different stratifications.
| Non-MG | MG | MG: Non-MG | ||||||||
| Event | IR | Event | IR | Crude | Adjusted∗ | |||||
| Variable | n = 95 | Person-Year | 1,000 person-years | n = 82 | Person-Year | 1,000 person-years | HR (95% CI) | P-value | HR (95% CI) | P-value |
| All | 95 | 35,645 | 2.67 | 82 | 33,654 | 2.44 | 0.91 (0.68, 1.22) | .5339 | 1.03 (0.76, 1.38) | .8608 |
| Age (yr) | ||||||||||
| <50 | 4 | 18,850 | 0.21 | 4 | 18,447 | 0.22 | 1.01 (0.25, 4.06) | .9838 | 1.01 (0.24, 4.21) | .9843 |
| 50-59 | 15 | 6,818 | 2.20 | 9 | 6,368 | 1.41 | 0.65 (0.28, 1.48) | .3007 | 0.66 (0.28, 1.54) | .3334 |
| 60-69 | 16 | 4,893 | 3.27 | 18 | 4,470 | 4.03 | 1.25 (0.64, 2.45) | .5201 | 1.26 (0.64, 2.48) | .5098 |
| 70-79 | 39 | 4,096 | 9.52 | 36 | 3,548 | 10.15 | 1.06 (0.68, 1.67) | .7908 | 1.08 (0.68, 1.71) | .7459 |
| > = 80 | 21 | 988 | 21.26 | 15 | 821 | 18.27 | 0.85 (0.44, 1.65) | .6296 | 1.03 (0.52, 2.06) | .9237 |
| Gender | ||||||||||
| Female | 43 | 20,504 | 2.10 | 35 | 19,769 | 1.77 | 0.84 (0.54, 1.31) | .4419 | 0.88 (0.56, 1.38) | .5844 |
| Male | 52 | 15,141 | 3.43 | 47 | 13,885 | 3.38 | 0.98 (0.66, 1.46) | .9292 | 1.19 (0.80, 1.79) | .3918 |
| Comorbidities | ||||||||||
| Hypertension | 42 | 3,768 | 11.15 | 31 | 3,638 | 8.52 | 0.76 (0.48, 1.21) | .2544 | 0.85 (0.53, 1.36) | .4848 |
| Diabetes | 20 | 2,301 | 8.69 | 18 | 2,028 | 8.88 | 1.03 (0.54, 1.94) | .9357 | 1.06 (0.55, 2.05) | .8627 |
| Hyperlipidemia | 18 | 1,315 | 13.69 | 10 | 1,101 | 9.08 | 0.66 (0.31, 1.44) | .3008 | 0.76 (0.33, 1.73) | .5152 |
| Coronary heart disease | 24 | 1,804 | 13.30 | 16 | 1,648 | 9.71 | 0.73 (0.39, 1.38) | .3362 | 0.95 (0.49, 1.84) | .8780 |
| Heart failure | 14 | 784 | 17.85 | 7 | 492 | 14.22 | 0.76 (0.31, 1.89) | .5610 | 0.85 (0.33, 2.21) | .7391 |
| Chronic kidney disease | 10 | 342 | 29.23 | 1 | 135 | 7.40 | 0.27 (0.03, 2.08) | .2067 | 0.22 (0.02, 2.05) | .1850 |
| COPD | 19 | 1,562 | 12.16 | 12 | 1,237 | 9.70 | 0.79 (0.38, 1.63) | .5277 | 0.81 (0.38, 1.70) | .5702 |
| Sleep disorders | 3 | 573 | 5.24 | 0 | 471 | 0.00 | NA | NA | ||
| Hyperthyroidism | 2 | 352 | 5.68 | 2 | 495 | 4.04 | 0.77 (0.11, 5.45) | .7894 | NA | |
| Gout | 12 | 652 | 18.40 | 2 | 410 | 4.88 | 0.26 (0.06, 1.18) | .0813 | 0.19 (0.04, 0.90) | .0362 |
| PAOD | 2 | 248 | 8.06 | 0 | 132 | 0.00 | NA | NA | ||
Table 4 shows comparisons of incidence of AF in subjects with or without MG and with or without accordant procedures. Subjects with MG and receiving thymectomy or plasmapheresis had a decreased risk of getting AF, but not statistically significant no matter when compared to subjects without MG (aHR = 0.55, 95% CI 0.25–1.19) or subjects with MG but not receiving any procedures (aHR = 0.49, 95% CI 0.22–1.08).
Table 4.
Comparisons of incidence of atrial fibrillation in subjects with or without MG and with or without procedures.
| Event | IR | Crude | Adjusted∗ | ||||||||
| Variable | n = 177 | Person-Year | 1,000 person-years | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Subgroup | |||||||||||
| Non-MG | 95 | 35,645 | 2.67 | 1 (Reference) | 1 (Reference) | ||||||
| MG without PP and OP | 75 | 24,537 | 3.06 | 1.14 (0.84, 1.54) | .4118 | 1 (Reference) | 1.12 (0.82, 1.52) | .4806 | 1 (Reference) | ||
| MG with PP or OP | 7 | 9,118 | 0.77 | 0.29 (0.14, 0.63) | .0017 | 0.26 (0.12, 0.56) | .0006 | 0.55 (0.25, 1.19) | .1287 | 0.49 (0.22, 1.08) | .0759 |
Table 5 shows comparisons of the incidence of AF between subjects with and without MG in the different follow-up periods. For subjects with less than 5 years of follow up, subjects with MG did not have a higher risk of developing AF compared to subjects without MG (aHR = 0.98, 95% CI 0.68–1.41). Similarly, for subjects with more than 5 years of follow up, subjects with MG did not have higher risk of developing AF compared to subjects without MG (aHR = 1.21, 95% CI 0.70–2.07).
Table 5.
Comparisons of incidence of atrial fibrillation between subjects with and without MG in different follow-up periods.
| Non-MG | MG | MG: Non-MG | ||||||||
| Variable | Event | IR | Event | IR | Crude | Adjusted∗ | ||||
| n = 95 | Person-Year | 1,000 person-years | n = 82 | Person-Year | 1,000 person-years | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Follow-up duration (yr) | ||||||||||
| <5 | 69 | 22,577 | 3.06 | 55 | 21,629 | 2.54 | 0.83 (0.58, 1.18) | .3025 | 0.98 (0.68, 1.41) | .9142 |
| > = 5 | 26 | 13,068 | 1.99 | 27 | 12,025 | 2.25 | 1.13 (0.66, 1.93) | .6592 | 1.21 (0.70, 2.07) | .4926 |
4. Discussion
This is the first nationwide study aimed at investigating the risk of developing atrial fibrillation (AF) among Asian patients with a history of MG. Comorbidities that frequently accompany AF were identified in a previous study.[12] We compared the MG cohort with a matched non-MG cohort using propensity score matching to analyze the risk of developing AF. The results showed MG patients had no higher risk of developing AF than the non-MG cohort.
A recent case series reported eight patients with myasthenic crisis and concurrent AF during hospitalization. The majority of these patients were males with an average age above 50, seropositive for the acetylcholine receptor (AChR) antibody, and diagnosed with late-onset MG. AF status subsided once myasthenic crisis or MG activity stabilized. The authors speculated that autoantibodies targeted at cardiac muscle or autonomic instability were possible mechanisms.[13] Anti-Kv1.4 antibodies were found to be associated with myocarditis in MG patients. Suzuki et al found that 10% of 650 MG patients were seropositive for anti-Kv1.4 antibodies, and eight patients who were clinically diagnosed with myocarditis were all anti-Kv1.4 antibody positive. Cardiac abnormalities including ventricular tachycardia, complete atrioventricular block, atrial fibrillation, and even sudden death were found.[14] MG patients with thymoma, those who were AChR antibody positive without thymoma, and those who were MuSK antibody positive were prone to developing autonomic dysfunction and subsequent cardiac arrhythmia.[15] AChR antibodies are classified into two groups (muscular or neuronal) based on their targets. Upto 90% of MG patients were found to be seropositive for muscle AChR antibodies that were highly targeted to the neuromuscular synaptic junction (muscle AChR α1 subunit). Ganglionic AchR antibodies were targeted mainly to the α3 subunit of neuronal AChR and were responsible for pathogenesis in autoimmune autonomic ganglionopathy, a disease that presents with dysautonomia due to impaired cholinergic transmission at the ganglionic synaptic junction.[16] Although AChR antibodies in MG were thought to be highly specific to muscular AChR, dysautonomia still occurred in MG patients. One study reported that 3% of MG patients were seropositive for both muscle and ganglionic AChR antibodies.[17] Further, 8% of cases with MG with thymoma were seropositive for both AChR antibodies.[18] Muscular AChR antibodies might be cross reactive with neuronal AChR to some extent.[19] Based on the aforementioned theories, acetylcholinesterase inhibitor used for routine MG treatment seems to be a potential therapy for autonomic disorders as well.[16] However, Sharifov et al, who conducted test using dog models, postulated that cholinergic stimulation is the pivotal factor in spontaneous AF initiation, while adrenergic stimulation has both initiation and maintenance roles.[20] One randomized study studied the effect of botulinum injection on epicardial fat, and found that it inhibited acetylcholine release and decreased the AF burden.[21] Aside from autonomic dysfunction, interleukin-6 (IL-6) may cause interplay between MG and AF. Anti-AchR antibodies were found to increase IL-6 production in a muscle biopsy study.[22] Pyridostigmine stimulated rather than suppressed pro-inflammatory cytokines including IL-6.[23] Post-thymectomy induced myasthenic crisis was observed with concurrent overproduction of IL-6.[24] Dai et al demonstrated positive correlations between serum IL-6 and TNF-α levels with AF inducibility and duration using rat models.[25] Another study have determined that the chronic inflammatory process that occurs via the upregulation of IL-6 is an important mediator in the pathophysiology of AF.[26] Whether or not the overproduction of IL-6 in MG will actually lead to the induction of AF is unknown. Further study is needed to clarify the detailed mechanism responsible for this. However, from an epidemiology aspect, in our cohort, we observed that individuals with MG did not have and increased AF risk (aHR = 1.03). There was no increased risk, even after different follow-up periods (<5 years, aHR = 0.98, 95% CI 0.68–1.41; > = 5 years, aHR = 1.21, 95% CI 0.70–2.07).
One study analyzed non-cardiac thoracic surgery and found that undergoing a lobectomy, esophagectomy, or thymectomy may increase the risk of developing AF.[27] The use of plasma exchange is indicated in diseases such as Guillain-Barré syndrome, thrombotic thrombocytopenic purpura, rheumatoid arthritis (RA), systemic lupus erythematosus, and MG.[28] In a Swedish study, 1% of patients who underwent plasma exchange developed complications, such as hypotension or arrhythmias, that needed medication or interrupted of the treatment.[29] In our cohort, however, we observed that there was no increased risk of AF in MG subgroups with or without procedures comparing to non MG subgroup.
Paroxysmal AF is often asymptomatic, but its prevalence in general population has been reported to be up to 12.4%.[30] Patients may not seek medical help due to being asymptomatic. Diagnosis of AF is time and resource consuming, as it requires a 24-hr Holter scan, serial electrocardiography, loop recorders, in-hospital monitoring, and use of an insertable cardiac monitor.[31] Although the results for our cohort did not detect a higher risk of developing AF in MG patients, it is still possible that occult AF exists among MG patients without diagnosis. Furthermore, whether paroxysmal AF occurs, especially in patients with hemodynamically unstable MG status, is unknown. Thus, further study is needed.
There were some limitations in our study. First, the diagnoses of MG and AF were dependent on ICD-9 codes. The disease status of MG patients upon diagnosis and subgroup classification were unknown. Also, the NHIRD does not provide detailed patient information, such as body mass index, tobacco consumption, dietary habits, and lifestyle factors. Secondly, the hospitalization claim dataset does not include laboratory reports, indication of treatment (thymectomy and plasma exchange), or drug information. Third, according to one cohort study, autoimmune disease is associated with 40% increase in risk of atrial fibrillation.[32] Chang et al reported that patients with MG had higher overall incidence rate of autoimmune diseases.[2] However, we did not analyze the risk of developing AF in patient subgroup of MG with concurrent second autoimmune diseases due to our study design. Further study should focus on exploring the risk of MG with second autoimmune disease and subsequent AF. Unknown confounders may also have existed due to our retrospective cohort study design, and we attempted to eliminate the potential biases by using multivariable models. Finally, the majority of patients included in NHIRD are Taiwanese, thus the result of our study may have population bias and it would not be generalizable to population of other ethnics. Further clinical studies should include patients with diverse nationalities to extrapolate the observed results.
5. Conclusion
Our population-based cohort showed that individuals with MG had no higher risk of subsequent development of AF than the general population in Taiwan. However, we still need to be aware of occult AF, especially when hemodynamical instability or cardiac involvement occurs in MG patients.
Acknowledgments
The authors expressed appreciation to the Department of Medical Education and Research and Research Center of Medical Informatics in Kaohsiung Municipal United Hospital for the comments.
Author contributions
Collection and assembly of data: Yaw-Tzeng Liou, James Cheng-Chung Wei, Kai-Chieh Hu, Yao-Min Hung, Renin Chang.
Conception/Design: Yaw-Tzeng Liou, James Cheng-Chung Wei, Kai-Chieh Hu, Yao-Min Hung, Renin Chang.
Data analysis and interpretation: Yaw-Tzeng Liou, Kai-Chieh Hu, Yao-Min Hung, Renin Chang.
Data curation: Kai-Chieh Hu.
Final approval of manuscript: Yaw-Tzeng Liou, James Cheng-Chung Wei, Kai-Chieh Hu, Yao-Min Hung, Mei-Chia Chou, Renin Chang.
Formal analysis: Kai-Chieh Hu.
Manuscript preparation and revision: Yaw-Tzeng Liou, Yao-Min Hung, Renin Chang.
Methodology: Renin Chang.
Project administration: Renin Chang.
Provision of study materials and patients: Yaw-Tzeng Liou, Yao-Min Hung, Renin Chang.
Resources: Renin Chang.
Supervision: James Cheng-Chung Wei, Renin Chang, Mei-Chia Chou.
Visualization: James Cheng-Chung Wei.
Writing – original draft: Yaw Tzeng Liou, Renin Chang.
Writing – review & editing: Yao-Min Hung, Mei-Chia Chou.
Footnotes
Abbreviations: AChR = acetylcholine receptor, AF = atrial fibrillation, aHR = adjusted hazard ratio, CI = confidence interval, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, MG = myasthenia gravis, NHIRD = National Health Insurance Research Database.
How to cite this article: Liou YT, Wei JC, Hu KC, Hung YM, Chou MC, Chang R. Risk of subsequent atrial fibrillation in patients with myasthenia gravis: a population-based cohort study. Medicine. 2021;100:20(e26008).
The authors have no conflicts of interest to disclose.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), MOST Clinical Trial Consortium for Stroke (MOST 109-2321-B-039-002), China Medical University Hospital (DMR-109-231), Tseng-Lien Lin Foundation, Taichung, Taiwan.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
COPD, chronic obstructive pulmonary disease; MG, myasthenia gravis; PAOD, peripheral arterial occlusive disease; SD, standard deviation; SMD, standardized mean difference.
A standardized mean difference of ≤0.1 indicates a negligible difference between the two cohorts.
COPD, chronic obstructive pulmonary disease; CI, confidence interval; HR, hazard ratio; IR, incidence rate; MG, myasthenia gravis; PAOD, peripheral arterial occlusive disease.
Adjusted for age, sex, and baseline comorbidities.
COPD, chronic obstructive pulmonary disease; CI, confidence interval; HR, hazard ratio; IR, incidence rate; MG, myasthenia gravis; NA, not available; PAOD, peripheral arterial occlusive disease.
Adjusted for age, sex, and baseline comorbidities.
CI, confidence interval; HR, hazard ratio; IR, incidence rate; MG, myasthenia gravis; OP, operation; PP, plasmapheresis.
Adjusted for age, sex, and comorbidities.
CI, confidence interval; HR, hazard ratio; IR, incidence rate; MG, myasthenia gravis.
Adjusted for age, sex, and baseline comorbidities.
References
- [1].Gilhus NE, Tzartos S, Evoli A, et al. Myasthenia gravis. Nat Rev Dis Primers 2019;5:30. [DOI] [PubMed] [Google Scholar]
- [2].Chang CC, Lin TM, Chang YS, et al. Thymectomy in patients with myasthenia gravis increases the risk of autoimmune rheumatic diseases: a nationwide cohort study. Rheumatology (Oxford) 2019;58:135–43. [DOI] [PubMed] [Google Scholar]
- [3].Gilhus NE. Myasthenia Gravis. N Engl J Med 2016;375:2570–81. [DOI] [PubMed] [Google Scholar]
- [4].Zimetbaum P. Atrial Fibrillation. Ann Intern Med 2017;166:ITC33–48. [DOI] [PubMed] [Google Scholar]
- [5].Gunal DI, Afsar N, Tanridag T, et al. Autonomic dysfunction in multiple sclerosis- correlation with disease-related parameters. Eur Neurol 2002;48:01–5. [DOI] [PubMed] [Google Scholar]
- [6].Peric S, Rakocevic-Stojanovic V, Nisic T, et al. Cardiac autonomic control in patients with myasthenia gravis and thymoma. J Neurol Sci 2011;307:30–3. [DOI] [PubMed] [Google Scholar]
- [7].Gilhus NE, Nacu A, Andersen JB, et al. Myasthenia gravis and risks for comorbidity. Eur J Neurol 2015;22:17–23. [DOI] [PubMed] [Google Scholar]
- [8].Owe JF, Daltveit AK, Gilhus NE. Causes of death among patients with myasthenia gravis in Norway between 1951 and 2001. J Neurol Neurosurg Psychiatry 2006;77:203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase inhibitors- pharmacology and toxicology. Curr Neuropharmacol 2013;11:315–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Khan MS, Tiwari A, Khan Z, et al. Pyridostigmine induced prolonged asystole in a patient with myasthenia gravis successfully treated with hyoscyamine. Case Rep Cardiol 2017;2017:6956298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rowland JP, Rigby J, Harper AC, et al. Cardiovascular monitoring with acetylcholinesterase inhibitors: a clinical protocol. Adv Psychiatr Treat 2018;13:178–84. [Google Scholar]
- [12].Chen DY, Lin CH, Chen YM, et al. Risk of atrial fibrillation or flutter associated with periodontitis: a nationwide, population-based. Cohort Study PLoS One 2016;11:e0165601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jacobs DH. Myasthenia gravis crisis and atrial fibrillation. Neurologist 2020;25:01–3. [DOI] [PubMed] [Google Scholar]
- [14].Suzuki S, Baba A, Kaida K, et al. Cardiac involvements in myasthenia gravis associated with anti-Kv1.4 antibodies. Eur J Neurol 2014;21:223–30. [DOI] [PubMed] [Google Scholar]
- [15].Nikolic A, Peric S, Nisic T, et al. The presence of dysautonomia in different subgroups of myasthenia gravis patients. J Neurol 2014;261:2119–27. [DOI] [PubMed] [Google Scholar]
- [16].Vernino S, Sandroni P, Singer W, et al. Invited article: autonomic ganglia: target and novel therapeutic tool. Neurology 2008;70:1926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci 2009;146:03–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vernino S, Lennon VA. Autoantibody profiles and neurological correlations of thymoma. Clin Cancer Res 2004;10:7270–725. [DOI] [PubMed] [Google Scholar]
- [19].Vernino S, Lindstrom J, Hopkins S, et al. Characterization of ganglionic acetylcholine receptor autoantibodies. J Neuroimmunol 2008;197:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sharifov OF, Fedorov VV, Beloshapko GG, et al. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol 2004;43:483–90. [DOI] [PubMed] [Google Scholar]
- [21].Romanov A, Pokushalov E, Ponomarev D, et al. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: three-year follow-up of a randomized study. Heart Rhythm 2019;16:172–7. [DOI] [PubMed] [Google Scholar]
- [22].Maurer M, Bougoin S, Feferman T, et al. IL-6 and Akt are involved in muscular pathogenesis in myasthenia gravis. Acta Neuropathol Commun 2015;3:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dinan TG, Clarke G, Quigley EM, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol 2008;103:2570–6. [DOI] [PubMed] [Google Scholar]
- [24].Endo S, Hasegawa T, Sato Y, et al. Inhibition of IL-6 overproduction by steroid treatment before transsternal thymectomy for myasthenia gravis- does it help stabilize perioperative condition? Eur J Neurol 2005;12:768–73. [DOI] [PubMed] [Google Scholar]
- [25].Dai H, Wang X, Yin S, et al. Atrial fibrillation promotion in a rat model of rheumatoid arthritis. J Am Heart Assoc 2017;6:e007320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sharma G, Shetkar S, Bhasin A, et al. High sensitive C-reactive protein and interleukin 6 in atrial fibrillation with rheumatic mitral stenosis from Indian cohort. Indian Heart J 2017;69:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vaporciyan AA, Correa AM, Rice DC, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg 2004;127:779–86. [DOI] [PubMed] [Google Scholar]
- [28].Clark WF, Rock GA, Buskard N, et al. Therapeutic plasma exchange: an update from the Canadian Apheresis Group. Ann Intern Med 1999;131:453–62. [DOI] [PubMed] [Google Scholar]
- [29].Norda R, Stegmayr BG. Therapeutic apheresis in Sweden: update of epidemiology and adverse events. Transfus Apher Sci 2003;29:159–66. [DOI] [PubMed] [Google Scholar]
- [30].Primo J, Gonçalves H, Macedo A, et al. Prevalence of paroxysmal atrial fibrillation in a population assessed by continuous 24-hour monitoring. Rev Port Cardiol 2017;36:535–46. [DOI] [PubMed] [Google Scholar]
- [31].Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- [32].Lindhardsen J, Ahlehoff O, Gislason GH, et al. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ 2012;344:e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]


