Abstract
Background:
Modified Runchang-Tang (MRCT), a Chinese herbal medicine, is widely used to treat functional constipation (FC), which is a common digestive system disease. However, its efficacy has not been evaluated systematically and objectively. Thus, a meta-analysis was conducted to assess the efficacy and safety of MRCT for treating functional constipation.
Methods:
We searched for relevant publications from Embase, Medline, The Cochrane Library, Chinese Biomedical Literature Database, China National Knowledge Infrastructure, Chinese Scientific Journals Database, and Wanfang Data for relevant literature. The timeframe of retrieval was set from the founding date of each database to July 15, 2020.
Result:
A total of 26 randomized controlled trials with 2103 individuals were included in this meta-analysis. All trials were conducted in mainland China and were written in Chinese. The results showed that MRCT monotherapy provided better symptom relief in FC patients compared to prokinetic agent monotherapy (odds ratio, [OR] = 4.06), osmotic laxatives (OR = 4.39) and stimulant laxatives (OR = 2.99). Additionally, there were no obvious adverse effects in MRCT group compared with control group.
Conclusion:
MRCT treatment is an efficient and safe treatment for FC. However, considering the limitations of this study, further well-designed randomized controlled trials are required to validate this conclusion.
Keywords: functional constipation, herbal formula, laxatives, modified Runchang-Tang, traditional Chinese medicine
1. Introduction
Functional constipation (FC) is a benign disease diagnosed through reduced frequency of bowel movements, dry stools and/or difficulty in bowel movements.[1] The prevalence of constipation is about 16% globally,[2] 6% in China,[3] and 33.5% in the population aged over 60.[4] Although constipation is not a life-threatening condition, it may directly or indirectly increase the probability of developing other mental or physical illnesses.[5,6] Its impact on the life quality may be as great as other chronic diseases. Due to the severity and high prevalence, constipation put a considerable burden on health care resources.[7,8]
Modified Runchang-Tang (MRCT), a well-known traditional Chinese medicine prescription, is developed from the herbal adjustment of Runchang-Tang according to syndrome differentiation (Principles of Chinese medicine treatment). It predominantly contains the following traditional Chinese herbs (Fig. 1, Table 1): Danggui (dried roots of Angelica sinensis Diels, Fig. 1A), Shengdi (dried fresh roots of Rehmannia glutinosa Libosch, Fig. 1B), Maren (kernel of Cannabis sativa L mature seed, Fig. 1C), Taoren (kernel of Prunus persica Batsch or P davidiana Franch mature seed, Fig. 1D), Zhike (dried immature fruit of Citrus aurantium L. and its cultivars, Fig. 1E). In the original literature, Runchang-Tang is mainly used to treat constipation characterized by “intestinal dryness and fluid depletion” (from traditional Chinese medicine theory). For people with the stagnation of qi, add Chenpi (dried mature fruit peel of Citrus reticulata Blanco) and Houpo (dried bark of Magnilia officinalis Rehd. et Wils or M officinalis Rehd. et Wils. var. biloba Rehd. et Wils.), with deficiency of qi, add Renshen (dried roots of Panax ginseng C. A. Mey.), with deficiency of yang, add Fuzi (dried roots of Aconitum carmichaeli Debx) and Rougui (dried bark of Cinnamomum cassia Presl). As long as permitted by local laws, the above herbs can be easily obtained outside of China. The procedure of making MRCT is as follows: Mix all the herbs, add an appropriate amount of water, boil and simmer for a certain period, and filter out the herb residue. The resulting herb solution should be taken orally 2 or 3 times a day. Because of the merits of safety, effectiveness, low cost and low toxic-side effects, MRCT has been commonly used to treat FC for centuries.
Figure 1.

The prescription of Modified Runchang-Tang (MRCT) containing 5 herbs.
Table 1.
Each herb concentration in MRCT.
| No | Name | Dose (gram) | King, Ministers, Adjutants, and Messengers |
| a | Danggui (the dried roots of Angelica sinensis Diels). | 10g | King |
| b | Shengdi (dried fresh roots of Rehmannia glutinosa Libosch). | 30g | |
| c | Maren (the kernel of Cannabis sativa L mature seed). | 15g | Ministers |
| d | Taoren (the kernel of Prunus persica Batsch or P. davidiana Franch mature seed). | 10g | |
| e | Zhike (dried immature fruit of Citrus aurantium L. and its cultivars). | 10g | Adjutants |
Increasing number of clinical trials have reported that MRCT successfully alleviates clinical symptoms of FC. However, the efficacy and safety of MRCT has not been systematically and objectively evaluated. Therefore, we conducted a meta-analysis to provide reliable assessment for clinical treatment of FC.
2. Methods
2.1. Ethics statement
As all analyses were based on previously published studies, no ethical approval or patient consent was required.
2.2. Literature search
Publications dated prior to July 15, 2020 and indexed in Medline, Embase, The Cochrane Library, Chinese Biomedical Literature Database, China National Knowledge Infrastructure, Chinese Scientific Journals Database or Wanfang Data were searched using keywords “Runchang” or “Run chang”. Regardless of the language and publication area, the studies returned from the search were included if they met the conditions defined in 2.2 and 2.3.
2.3. Inclusion criteria
The included studies should meet the following PICOS approach.
-
(1)
Patients were diagnosed using recognized criteria of FC.
-
(2)
Intervention group received oral administration of MRCT component for a complete treatment process.
-
(3)
Control group received monotherapy of other recognized drugs for a complete treatment process.
-
(4)
Outcome was measured by treatment efficacy, such as total effective rate or symptom scores.
-
(5)
Studies included in the meta-analysis were randomized controlled trials (RCTs).
2.4. Exclusion criteria
A study will be excluded if it meets any of the following PICOS criteria:
-
(1)
patients with unclear diagnosis criteria for FC, individuals <18 years old, pregnant or puerperia,
-
(2)
intervention group including intervention methods other than MRCT receiving MRCT with composition not derived from the decoction of “Shen's Zunsheng Book”, or with non-oral administration of MRCT,
-
(3)
control group involving monotherapy of unrecognized drugs or combination therapy,
-
(4)
outcome measured using non-dichotomy statistical results or having no statistical results, and
-
(5)
study type being report and review, animal experiments, non-clinical findings, duplicate publications, unverified RCT studies, or with no or inappropriate control group.
2.5. Study selection
Articles were extracted according to the following steps. Firstly, we retrieved the publications from the database and browsed titles, abstracts and keywords to determine whether they were appropriate. Relevant publications were summarized by authors’ names, publication year, diagnostic criteria, type of control group, treatment of duration, age and the number of individuals, intervention drugs, symptom improvement and adverse events. The summary was independently checked and reconfirmed to ensure the accuracy of extracted data. Finally, the extracted data were used for meta-analysis.
2.6. Outcome categorization
The following results were considered as successful relief of constipation symptoms: the term “patient with significantly improved symptoms” was defined as bowel movements 2 to 3 times a week, with soft stools but poor bowel movements. The term “asymptomatic patient” was defined as bowel movements more than 3 times a week and no other symptoms.
2.7. Quality critical appraisal
The quality of literature was carefully evaluated to ensure the accuracy of the final analysis outcome. Quality evaluations were independently conducted via Cochrane Bias Risk Tool, which includes the following 6 aspects: selection bias, performance bias, detection bias, attritions bias, reporting bias, and other biases.
2.8. Statistical analysis
Review Manager 5.3 (Cochrane Collaboration) software was used for statistical analysis. P and I2 were used to assess the statistical heterogeneity between studies. When P > .1 and I2 < 50%, a fixed-effects model was used. Otherwise, a random-effects model was applied. For the dichotomous data, the odds ratio (OR) and corresponding 95% confidence interval (CI) were calculated. P < .05 is considered statistically significant.
3. Results
3.1. Literature search
A total of 1669 studies were retrieved according to the above strategies and methods. The unrelated studies were excluded based on titles, abstracts, and keywords. Then, the remaining 150 articles were previewed in full-text. Finally, 26 RCTs[9–34] that included 2,103 individuals were analyzed. All studies in the final sample were conducted in mainland China and written in Chinese (Fig. 2).
Figure 2.
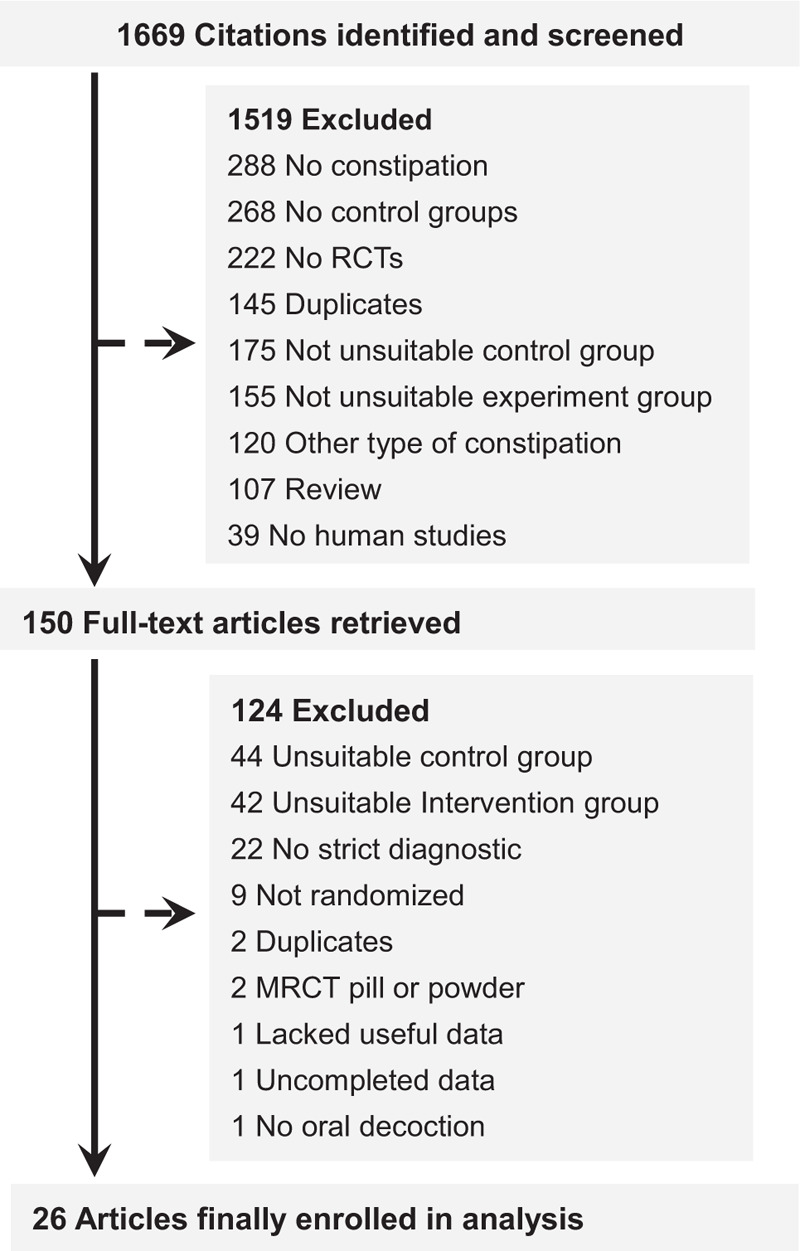
Study selection process for the meta-analysis with exclusion criteria noted.
3.2. Methodological quality of studies included
Using a Cochrane Bias Risk Tool, we conducted a risk of bias analysis for these included studies (Fig. 3). All studies mentioned the approach of random assignment of participants. However, only 8 studies[9,10,13,14,16,22,26,27] mentioned specific randomization methods: six[9,14,16,22,26,27] used a random number table, 1[10] used lottery, and 1[13] performed randomization by dice-throwing. None of the studies mentioned allocation concealment, blinding of outcome assessment, or blinding of participants and personnel. All studies have completed outcome data, low reporting and other biases. Hence, all included studies were of medium quality.
Figure 3.
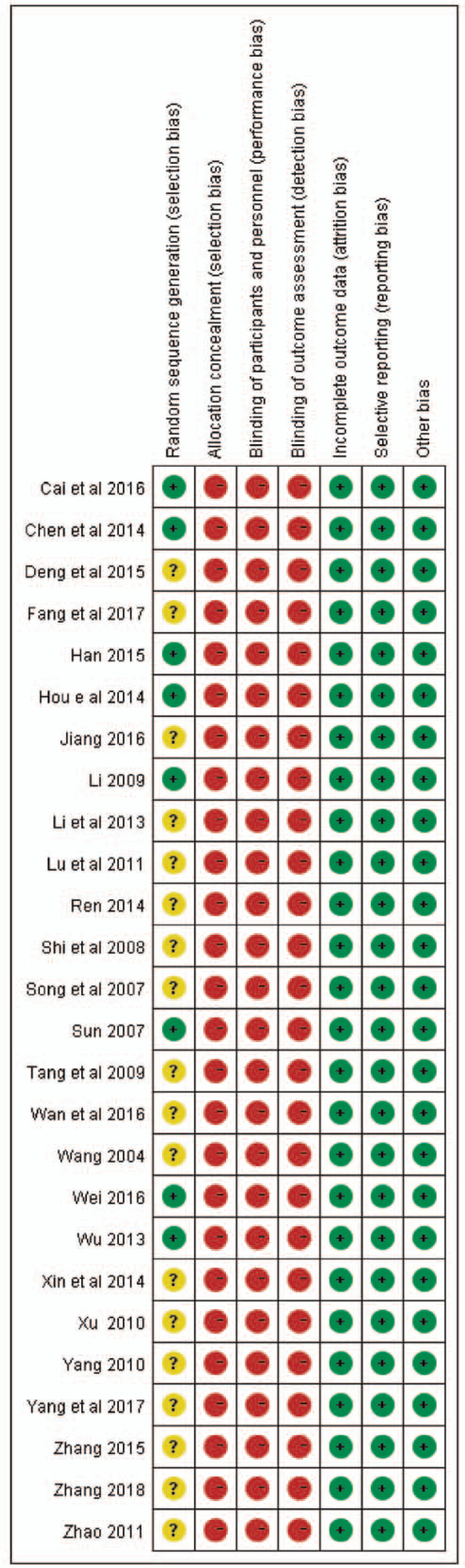
Methodological quality judgments about each risk of bias item for each study.
3.3. Study characteristics
The study characteristics were summarized in Table 2. The age of the participants ranged from 16 to 84 years. The studies were published between 2004 and 2018. The intervention duration ranged from 10 days to 8 weeks. Eighteen studies employed the Roman criteria for diagnosis of FC. In 4 studies[10,11,17,25] the authors applied Diagnostic efficacy of standard TCM syndrome (DESS). The remaining 4 trials[15,18,24,29] used the guidelines for diagnosis and treatment of chronic constipation in China (GDTC). Nine studies[9,10,12,13,16,19,26,27,34] reported adverse events. Eleven studies[9,10,14,17,21,22,25,29,30,33,34] compared MRCT treatment with prokinetic agents (N = 1010), ten studies[11–13,19,20,23,24,26,27,31] compared MRCT treatment with osmotic laxatives (N = 762), and 5 studies[15,16,18,28,32] compared MRCT treatment with stimulant laxatives (N = 331) (Fig. 4).
Table 2.
Characteristics of the included studies in meta-analysis.
| Age (years) | Number of patients | Intervention drugs | Percentage of symptom improvement | |||||||||
| Study | Diagnostic criteria | Control group | Treatment duration | Case | Control | Case | Control | Case | Control | Case | Control | Adverse event |
| Cai et al (2016)[9] | RIIIC/CDTC | Prokinetic agents | 1 mo | 49.11 ± 6.48 | 48.76 ± 6.24 | 34 | 34 | MRCT(TID) | Mosapride (5 mg, TID) | 94.12% (32/34) | 79.41% (27/34) | Included |
| Chen et al (2014)[10] | DESS | Prokinetic agents | 10 d | 72.3 ± 2.45 | 74.2 ± 2.13 | 50 | 50 | MRCT(BID) | Cisapride (5 mg, BID) | 92% (46/50) | 82% (41/50) | Included |
| Deng et al (2015)[11] | DESS | Osmotic laxatives | 15 d | 54-70 | 54-70 | 50 | 50 | MRCT(BID) | Lactulose Oral Solution (10 g, SO, bid) | 96.00% (48/50) | 92.00% (46/50) | NR |
| Fang et al (2017)[12] | RIIIC/ CGND | Osmotic laxatives | 28 d | 45.30 ± 6.71 | 43.56 ± 6.81 | 31 | 29 | MRCT(BID) | Macrogol 4000 Powder (10 g, BID) | 97% (30/31) | 79% (23/29) | Included |
| Han (2015)[13] | RIIIC/ CGND | Osmotic laxatives | 3 wk | 57.6 ± 11.6 | 60.8 ± 10.2 | 56 | 56 | MRCT(BID) | Macrogol 4000 Powder (10 g, BID) | 96.43% (54/56) | 82.14% (46/56) | Included |
| Hou et al (2014)[14] | RIIIC/ DESS | Prokinetic agents | 4 wk | 43.28 ± 8.51 | 42.36 ± 8.13 | 30 | 30 | MRCT(BID) | Mosapride (5 mg, TID) | 90% (27/30) | 76.67% (23/30) | NR |
| Jiang (2016)[15] | GDTC | Stimulant laxatives | 8 wk | 65-78 | 65-78 | 32 | 32 | MRCT(BID) | Phenolphthalein (100 mg, BID) | 90.63% (29/32) | 75% (24/32) | NR |
| Li (2009)[16] | RIIIC/ CGND | Stimulant laxatives | 4 wk | 56.26 ± 8.81 | 57.45 ± 8.41 | 34 | 31 | MRCT(BID) | Bisacodyl Enteric-coated Tablets (10 mg, QN) | 79.41% (27/34) | 74.19% (23/31) | Included |
| Li et al (2013)[17] | DESS | Prokinetic agents | 2 wk | 50-72 | 50-72 | 30 | 30 | MRCT(BID) | Mosapride (5 mg, TID) | 93.33% (28/30) | 66.67% (20/30) | NR |
| Lu et al (2011)[18] | GDTC | Stimulant laxatives | 2 wk | 62-78 | 60-75 | 25 | 25 | MRCT(BID) | Phenolphthalein (100 mg, QN) | 92.00% (23/25) | 68.00% (17/25) | NR |
| Ren (2014)[19] | RIIIC/ CDTC | Osmotic laxatives | 8 wk | 43.6 ± 11.63 | 42.7 ± 11.05 | 30 | 30 | MRCT(BID) | Macrogol 4000 Powder (20 g, QD) | 86.7% (26/30) | 90% (27/30) | Included |
| Shi et al (2008)[20] | RIIIC | Osmotic laxatives | 4 wk | 32-68 | 30-66 | 30 | 30 | MRCT(BID) | Macrogol 4000 Powder (10 g, BID) | 96.7% (29/30) | 80% (24/30) | NR |
| Song et al (2007)[21] | RIIC | Prokinetic agents | 4 wk | 26-62 | 24-60 | 36 | 36 | MRCT(BID) | Cisapride (3 mg,TID) | 97.2% (35/36) | 88.89% (32/36) | NR |
| Sun (2007)[22] | RIIIC/DTSS | Prokinetic agents | 1 mo | 16∼68 | 16∼68 | 100 | 100 | MRCT(BID) | Mosapride (10 mg,TID) | 88% (88/100) | 58% (58/100) | NR |
| Tang et al (2009)[23] | RIIIC/ CGND | Osmotic laxatives | 4 wk | 42.75 ± 13.98 | 44.59 ± 15.80 | 51 | 49 | MRCT(BID) | Macrogol 4000 Powder (10 g, BID) | 96.08% (49/51) | 79.59% (39/49) | NR |
| Wan et al (2016)[24] | GDTC | Osmotic laxatives | 3 wk | 62.00 ± 3.50 | 64.00 ± 4.10 | 45 | 45 | MRCT(BID) | Lactulose Oral Solution (10 g, SO, bid) | 97.78% (44/45) | 80.00% (36/45) | NR |
| Wang (2004)[25] | DESS | Prokinetic agents | 3 wk | 20-52 | 18-50 | 36 | 30 | MRCT(BID) | Cisapride (10 mg, TID) | 91.7% (33/36) | 73.3% (22/30) | NR |
| Wei (2016)[26] | RIIIC/ CGND | Osmotic laxatives | 1 mo | 69.67 ± 5.63 | 70.00 ± 5.62 | 30 | 30 | MRCT(BID) | Macrogol 4000 Powder (10 g, BID) | 93.33% (28/30) | 73.33% (22/30) | Included |
| Wu (2013)[27] | RIIIC/ CGND | Osmotic laxatives | 4 wk | 59.97 ± 8.84 | 60.90 ± 9.58 | 30 | 30 | MRCT(BID) | Macrogol 4000 Powder (10 g, BID) | 93.33% (28/30) | 73.33% (22/30) | Included |
| Xin et al (2014)[28] | RIIIC/DESS | Stimulant laxatives | 4 wk | 68.2 ± 7.5 | 70.7 ± 8.7 | 35 | 35 | MRCT(BID) | Phenolphthalein (100 mg, QN) | 94.3% (33/35) | 80.0% (28/35) | NR |
| Xu (2010)[29] | GDTC | Prokinetic agents | 30 d | >60 | >60 | 80 | 50 | MRCT(QD) | Mosapride (10 mg, TID) | 93.75% (75/80) | 70% (35/50) | NR |
| Yang (2010)[30] | RIIIC | Prokinetic agents | 3 wk | 46.62 ± 8.93 | 45.95 ± 9.12 | 47 | 47 | MRCT(BID) | Mosapride (5 mg, TID) | 89.4% (42/47) | 76.6% (36/47) | NR |
| Yang et al (2017)[31] | RIIIC/ CDTC | Osmotic laxatives | 4 wk | 25-76 | 25-76 | 30 | 30 | MRCT(BID) | Lactulose Oral Solution (6.67 g, SO, TID) | 93.3% (28/30) | 76.7% (23/30) | NR |
| Zhang (2015)[32] | RIIC | Stimulant laxatives | 4 wk | 43.06 ± 9.67 | 42.38 ± 10.41 | 41 | 41 | MRCT(BID) | Phenolphthalein (200 mg, QN) | 92.70% (38/41) | 75.60% (31/41) | NR |
| Zhang (2018)[33] | RIIIC/CDTC | Prokinetic agents | 4 wk | 45.76 ± 5.07 | 46.01 ± 5.12 | 38 | 38 | MRCT(TID) | Mosapride (5 mg, TID) | 94.74% (36/38) | 78.95% (30/38) | NR |
| Zhao (2011)[34] | RIIC | Prokinetic agents | 3 wk | 62-81 | 60-84 | 42 | 42 | MRCT(BID) | Mosapride (10 mg, BID) | 88% (37/42) | 76% (32/42) | Included |
Figure 4.
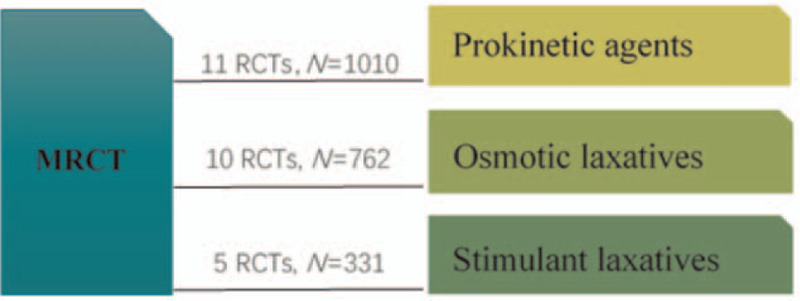
Network formed by interventions and their direct comparisons included in the analyses.
3.4. Results of functional constipation meta-analysis
All of the 11 RCTs[9,10,14,17,21,22,25,29,30,33,34] comparing MRCT treatment with prokinetic agents reported that MRCT showed better remission ability in FC (OR = 4.06, 95%CI:[2.80, 5.88]; P < .00001) (Fig. 5). The heterogeneity test (P = .95, I2 = 0%) also indicated no heterogeneity between the studies. Ten clinical trials[11–13,19,20,23,24,26,27,31] compared MRCT treatment with osmotic laxatives. Meta-analysis showed that MRCT was more effective for symptomatic relief in patients with FC than osmotic laxatives [OR = 4.39, 95%CI: (2.59,7.44); P < .00001] (Fig. 5). The heterogeneity test showed no heterogeneity (P = .61, I2 = 0%) across the studies. Five trails[15,16,18,28,32] tested the efficacy of MRCT treatment against stimulant laxatives in FC. Meta-analysis revealed that MRCT was significantly better in relieving constipation symptoms [OR = 2.99, 95% CI: (1.61, 5.54); P = .0005] (Fig. 5). Heterogeneity test showed homogeneity across the 5 trails (P = .61, I2 = 0%).
Figure 5.
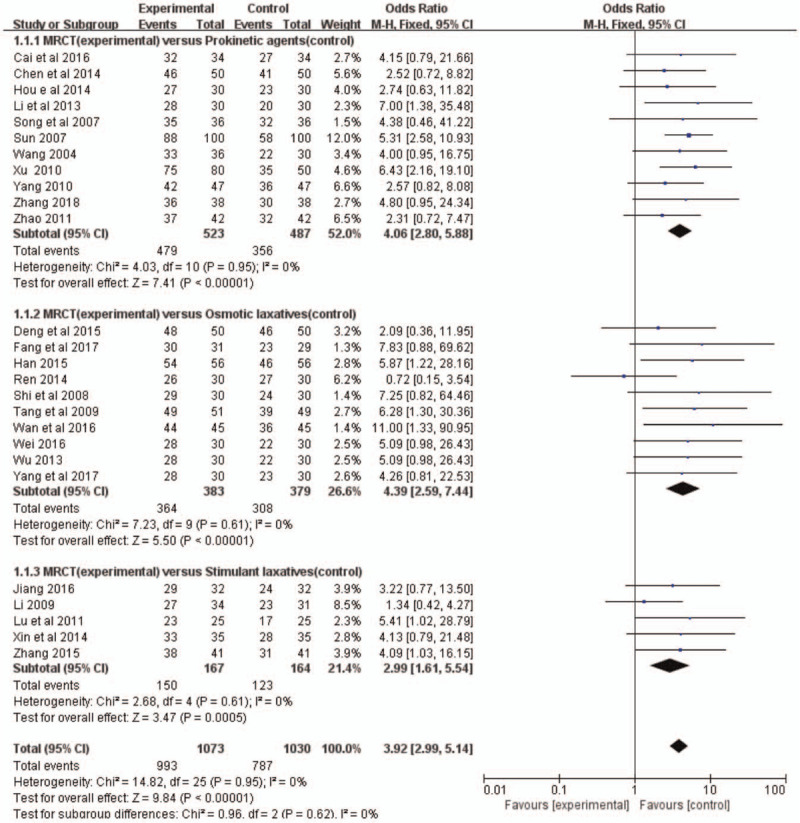
Treatment effects of MRCT on clinical response in patients with functional constipation. MRCT = Modified Run-Chang-Tang.
3.5. Adverse events
Adverse events were measured in 9 studies. Six[9,10,12,19,26,27] reported no adverse event in either the experimental group or the control group. In the other 3 RCTs,[13,16,34] all of the adverse events (abdominal pain and diarrhea) occurred in the control group. The probability of adverse events was 26.2% in prokinetic agents,[34] 10.7% in osmotic laxatives[13] and 21.2% in stimulant laxatives.[16] Therefore, the available data showed that MRCT had a lower rate of adverse events compared with prokinetic agents, osmotic and stimulant laxatives.
4. Discussion
MRCT is a traditional Chinese medicine prescription and it is a unified whole. The combinational use of herbal medicines is at the heart of traditional Chinese herb medicine. It is the use of synergistic compound prescriptions that a main therapeutic tool, rather than a single herb. In actual application, the traditional Chinese medicine doctors use a prescription to treat diseases instead of using a single herb. In the existing literature, we have not found a comparison study of single herb effect and MRCT. Thus, the effect of MRCT and single herb are not comparable.
Runchang-Tang is formed according to the traditional principles of combining medicinal substances (King, Ministers, Adjutants, and Messengers). King (Danggui, Shengdi) is the substance that provides the main therapeutic effect in the prescription. Ministers (Maren, Taoren) enhance or assist the therapeutic actions of King. Adjutants (Zhike) provide 1 or more of the following functions: treating accompanying symptoms, moderating the harshness or toxicity of the primary substances, assisting King and Ministers in accomplishing their main objectives, or providing assistance from another therapeutic direction. Messengers either guide the other herbs in the formula to a specific channel or organ or exert a harmonizing influence[35] (Table 1). It is generally believed that adding or subtracting in types or doses of herbs will not change the main status of King and Ministers herbs, nor will it change the main therapeutic effect of Runchang-Tang on FC. Therefore, the comparison of these RCTs in this study is reasonable.
The current medications for FC treatment are predominantly prokinetic agents, osmotic and stimulant laxatives. However, their efficacies are limited. Up to 47% of individuals were dissatisfied with these treatments,[36,37] and prolonged treatment duration could increase the incidence of adverse reactions. This meta-analysis found that MRCT monotherapy showed better efficacy of symptom relief in FC and lower incidence of adverse events compared with prokinetic agents, osmotic and stimulant laxatives.
Previous research[38] found that herbal medicine was better than placebo (OR = 3.83, I2 = 62%) and similar to conventional pharmacological drugs (OR = 1.19, I2 = 22%) in treating FC, thus indicating that herbal medicine is a potentially well-tolerated and effective treatment for FC. However, their outcomes were based on a few subgroups, insufficiently included studies, and larger heterogeneity than the present study. Moreover, it could not define which formula had the highest probability in improving FC symptoms. Furthermore, another research found the effects of MBYT (Modified Buzhong-Yiqi-Tang) monotherapy on FC were superior to stimulant laxatives (OR = 1.21), osmotic laxatives (OR = 1.32) and prokinetic agents (RR = 1.18).[39] However, our meta-analysis found that FC patients with MRCT treatment displayed 4.06-fold higher probability of symptom relief than prokinetic agents, 2.99-fold higher than stimulant laxatives and 4.39-fold higher than osmotic laxatives. Moreover, no heterogeneity between studies (I2 = 0%) was detected. Taken together, these findings suggest that MRCT monotherapy may be a more effective treatment than MBYT for the relief of clinical symptoms of FC.
Although adverse events were rarely mentioned in the studies included in this study, the available data showed that MRCT had less adverse events compared with prokinetic agents, stimulant and osmotic laxatives. This is consistent with the results of a previous study.[39] Hence, MRCT can be considered a relatively safe treatment.
Although the mechanisms of MRCT on FC treatment are unclear, results from mouse model of slow transit constipation indicated that MRCT treatment may act through restoration of the stem cell factor/c-kit pathway, increasing the count of interstitial cells of Cajal, and enhancing its function by increasing intracellular Ca2+ concentration.[40] In addition, a study from Korea indicated that herbal intervention could decrease phylum Firmicutes and genus Blautia in fecal microbiota, suggesting its potential to be used as a prebiotic.[41]
There are some limitations in the present study. Specifically, all of the included RCTs were of medium quality. Many of them did not describe the randomization methods, allocation concealment, blinding of participants, personnel and outcome assessment. Most of the studies did not record adverse events during the study. Moreover, A common problem[42] of traditional Chinese medicine prescriptions study at home and abroad also exists in this study, that is, the instability of the prescriptions, which will more or less affect the efficacy evaluation of MRCT. Finally, all of the studies were conducted in mainland China and written in Chinese, therefore publication bias is highly possible, the meta-analysis outcomes can only be confirmed to some extent. More clinical trials of higher methodological quality, larger sample size, and possibly multi-centered, should be incorporated to further confirm this conclusion.
5. Conclusion
This meta-analysis demonstrates that MRCT is an efficient and safe herbal medicine for FC treatment. However, considering the limitation of this study, further well-designed RCTs are required to confirm this conclusion.
Author contributions
Data curation: Xiankun Zhao, Feng Qin, Hanlin Gong.
Methodology: Xiankun Zhao, Jing Ye, Wenzhu Lu.
Project administration: Hanlin Gong.
Software: Jing Ye, Wenzhu Lu.
Supervision: Feng Qin, Hanlin Gong.
Validation: Feng Qin.
Writing – original draft: Xiankun Zhao.
Writing – review & editing: Xiankun Zhao, Yuxiao Fang, Jing Ye.
Footnotes
Abbreviations: FC = functional constipation, MRCT = modified Runchang-Tang, OR = odds ratio, RCTs = randomized controlled trials.
How to cite this article: Zhao X, Fang Y, Ye J, Qin F, Lu W, Gong H. A meta-analysis of randomized controlled trials of a traditional Chinese medicine prescription, modified RunChang-Tang, in treating functional constipation. Medicine. 2021;100:20(e25760).
PROSPERO registration number: CRD42020189731.
This work was supported by the Department of Science and Technology of Sichuan Province (2015SZ0134).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
MRCT = Modified Run-Chang-Tang.
BID, twice a day; CDTC = consensus opinion on diagnosis and treatment of chronic constipation for TCM; CGND = clinical guideline of new drugs for TCM; DESS = diagnostic efficacy of standard TCM syndrome; DTSS = diagnostic and treatment routine for TCM syndrome in Shanghai; GDTC = guidelines for diagnosis and treatment of chronic constipation in China; MRCT = modified Run-Chang-Tang; NR = no record; QD, once a day; RIIC = Rome II Criteria; RIIIC = Rome III Criteria; SO = solution; TID, three times a day.
References
- [1].Gallegos-Orozco JF, Foxx-Orenstein AE, Sterler SM, et al. Chronic constipation in the elderly. Am J Gastroenterol 2012;107:18–25. [DOI] [PubMed] [Google Scholar]
- [2].Schmidt FM, Santos VL. Prevalence of constipation in the general adult population: an integrative review. J Wound Ostomy Continence Nurs 2014;41:70–6. [DOI] [PubMed] [Google Scholar]
- [3].Zhao YF, Ma XQ, Wang R, et al. Epidemiology of functional constipation and comparison with constipation-predominant irritable bowel syndrome: the Systematic Investigation of Gastrointestinal Diseases in China (SILC). Aliment Pharmacol Ther 2011;34:1020–9. [DOI] [PubMed] [Google Scholar]
- [4].Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol 2011;25:03–18. [DOI] [PubMed] [Google Scholar]
- [5].Belsey J, Greenfield S, Candy D, et al. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther 2010;31:938–49. [DOI] [PubMed] [Google Scholar]
- [6].Wald A, Scarpignato C, Kamm MA, et al. The burden of constipation on quality of life: results of a multinational survey. Aliment Pharmacol Ther 2007;26:227–36. [DOI] [PubMed] [Google Scholar]
- [7].Sethi S, Mikami S, Leclair J, et al. Inpatient burden of constipation in the United States: an analysis of national trends in the United States from 1997 to 2010. Am J Gastroenterol 2014;109:250–6. [DOI] [PubMed] [Google Scholar]
- [8].Sun SX, Dibonaventura M, Purayidathil FW, et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the national health and wellness survey. Dig Dis Sci 2011;56:2688–95. [DOI] [PubMed] [Google Scholar]
- [9].Cai LW, Wang YJ, Qian WZ. Effect of Huoxue Yiqi Runchang Decoction in the treatment of chronic intractable constipation in colonic dynamics. Chin J Biochem Pharm 2016;36:137–9. [Google Scholar]
- [10].Chen WF, Yang BK. Yiyuanrunchangtang treatment of senile constipation random parallel control study. J Pract Tradit Chin Intern Med 2014;28:20–1. [Google Scholar]
- [11].Deng YG, Fu P, Deng LL, et al. Treatment of 50 cases of habitual constipation in elderly patients with deficiency of yin-blood by Yangxue Runchang Decoction. Jiangxi J Tradit Chin Med 2015;46:52–3. [Google Scholar]
- [12].Fang X, Qian HH. Treatment of 31 cases of chronic transmission constipation with Yangyin Runchang Decoction. Nei Mongol J Tradit Chin Med 2017;36:50–1. [Google Scholar]
- [13].Han YB. A randomized parallel controlled study of Jianpi Runchang Tongji Decoction in the treatment of functional constipation of spleen deficiency and intestinal dryness type. J Pract Tradit Chin Intern Med 2015;29:42–3. [Google Scholar]
- [14].Hou YJ, Huang L, Fu P. The clinical observation on the treatment of functional constipation by combination of chinese and western medicine. J Pract Tradit Chin Med 2014;30:42–3. [Google Scholar]
- [15].Jiang C. Observation and analysis of the application effect of Jianpi Runchang Decoction in senile constipation. Contemp Med 2016;22:51–152. [Google Scholar]
- [16].Li SB. Clinical research of Yiqi Runchang Tang on treating the middle-aged and the elderly patients with functional constipation [M.S. Thesis]. Guangzhou, China: Guangzhou University of Chinese Medicine; 2009. [Google Scholar]
- [17].Li JH, Jiang YP. Self-made Yiqi Yangxue Runchang Decoction in the treatment of 30 cases of habitual constipation in the elderly. Jiangxi J Tradit Chin Med 2013;44:27–8. [Google Scholar]
- [18].Lu K, Yuan FY, Zhao YC, et al. Treatment of 25 cases of senile habitual constipation with Yiqi Yangxue Runchang Decoction. Mod Tradit Chin Med 2011;31:18–9. [Google Scholar]
- [19].Ren AM. The clinical study on the treatment of functional constipation of jin shortage and blood deficiency type by Modifided Runchang Wan Decoction [M.S. Thesis]. Naniing, China: Nanjing University of Chinese Medicine; 2014. [Google Scholar]
- [20].Shi C, He Y, Zhou JH. Clinical observation on 30 cases of functional constipation treated by supplementing qi and nourishing yin methods. Jilin J Chin Med 2008;28:26–7. [Google Scholar]
- [21].Song QZ, Zhang L, Tang SY. Treatment of 36 cases of constipation type irritable bowel syndrome with Jianpi Runchang Decoction. Forum Tradit Chin Med 2007;22:26–7. [Google Scholar]
- [22].Sun JH. Clinical observation of Wenpi runchang decoction in treating slow transit constipation. Shanghai J Tradit Chin Med 2007;41:44–5. [Google Scholar]
- [23].Tang SJ, Chen MW, Gu CY. Summary of 51 cases of senile functional constipation treated by Ziyin Jianpi Runchang Prescription. Hunan J Tradit Chin Med 2009;25:29–30. [Google Scholar]
- [24].Wan F, Fu P, Li LF. Treatment of 45 cases of habitual constipation in the elderly with qi and blood deficiency due to Yiqi Yangxue Runchang Decoction. Pract Clin J Integr Tradit Chin West Med 2016;16:66–7. [Google Scholar]
- [25].Wang KP. Shugan Liqi Runchang Decoction in the treatment of 36 cases of constipation type irritable bowel syndrome, compared with 30 cases of cisapride treatment. Zhejiang J Tradit Chin Med 2004;39:21. [Google Scholar]
- [26].Wei LP. Clinical research of Yiqiyangyinrunchang Decoction in the treatment of senile functional constipation of qi and yin deficiency type [M.S. Thesis]. Zhengzhou, China: Henan University of Chinese Medicine; 2016. [Google Scholar]
- [27].Wu GL. Clinical effect observation of Yiqi Runchang Daozhi Decoction treating qi and yin deficiency type constipation (slow transit) [M.S. Thesis]. Jinan, China: Shandong University of Traditional Chinese Medicine; 2013. [Google Scholar]
- [28].Xin H, Wang XP, Zhang JQ, et al. Runchang Tongbian Decoction for the treatment of senile functional constipation. Shanghai J Tradi Chin Med 2014;48:43–4. [Google Scholar]
- [29].Xu RH. Treatment of 80 cases of senile constipation with Yiqi Runchang Decoction. Chin Med Mod Dist Educ CHN 2010;8:33. [Google Scholar]
- [30].Yang XJ. Clinical observation on 47 cases of slow transit constipation treated by Sanshen Runchang Decoction. Guid J Tradit Chin Med Pharm 2010;16:37–8. [Google Scholar]
- [31].Yang JY, Liu XY, Li KG, et al. Clinical observation of Runchang Wan Decoction in treating functional constipation of jin shortage and blood deficiency type. World Latest Med Inform 2017;17:125–6. [Google Scholar]
- [32].Zhang YX. Clinical efficacy of Baizi Ziyin Runchang Decoction in the treatment of chronic functional constipation. Guangming J Chin Med 2015;30:792–3. [Google Scholar]
- [33].Zhang Y. The Therapeutic effect observation of Huoxue Yiqi Runchang Decoction on chronic intractable constipation. Chin J Ethnomed Ethnopharm 2018;27:100–1. [Google Scholar]
- [34].Zhao HX. The therapeutic effect observation of self-made Yiqi Runchang Decoction on 42 cases of senile constipation. Chin Community Doct 2011;13:177–8. [Google Scholar]
- [35].Su X, Yao Z, Li S, et al. Synergism of Chinese herbal medicine: illustrated by Danshen compound. Evid Based Complement Alternat Med 2016;7279361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007;25:599–608. [DOI] [PubMed] [Google Scholar]
- [37].van Tilburg MA, Palsson OS, Levy RL, et al. Complementary and alternative medicine use and cost in functional bowel disorders: a six month prospective study in a large HMO. BMC Complement Altern Med 2008;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tan N, Gwee KA, Tack J, et al. Herbal medicine in the treatment of functional gastrointestinal disorders: a systematic review with meta-analysis. J Gastroenterol Hepatol 2020;35:544–56. [DOI] [PubMed] [Google Scholar]
- [39].Gong H, Qin F, He H. Herbal formula Modified Buzhong-Yiqi-Tang for functional constipation in adults: a Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med 2018;9602525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jiang F, Zhou JYS Wu##J, et al. Yangyin Runchang Decoction improves intestinal motility in mice with atropine/diphenoxylate-induced slow-transit constipation. Evid Based Complement Alternat Med 2017;4249016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Han K, Bose S, Kim YM, et al. Rehmannia glutinosa reduced waist circumferences of Korean obese women possibly through modulation of gut microbiota. Food Funct 2015;6:2684–92. [DOI] [PubMed] [Google Scholar]
- [42].Ma BL, Ma YM. Pharmacokinetic herb-drug interactions with traditional Chinese medicine: progress, causes of conflicting results and suggestions for future research. Drug Metab Rev 2016;48:01–26. [DOI] [PubMed] [Google Scholar]


