Abstract
Neuroblastoma is an embryonal tumor of the autonomic nervous system with poor prognosis in children. In present study, we demonstrated the relationship of miRNA-34a-5p in the regulating of the Wnt/β-catenin signaling pathway by targeting SRY-related HMG-box (SOX4)
Reverse transcription-quantitative PCR was used to detect the expression levels of miRNA-34a-5p and SoX4. Western blotting was performed to assess the protein expression levels of SoX4, Wnt, MMP9, Bax, and Bcl-2. The proliferation, apoptosis, migration and invasion of neuroblastoma cells were determined using MTT, flow cytometry and Transwell assays.
In this study, we sought to investigate the role of miRNA-34a-5p on neuroblastoma and the possible molecular mechanism. We had performed in-vitro and in-vivo experiments to evaluate the effects of miRNA-34a-5p on neuroblastoma cell proliferation and invasion by altering its expression level via cell transfection. On the basis of our study, miRNA-34a-5p showed decreased expression levels in neuroblastoma. Subsequently, we manipulated miRNA-34a-5p expression through cell transfection and observed abnormal expression of β-catenin as well as the downstream targets of the Wnt/β-catenin pathway in neuroblastoma cells. With all these evidences, we determined that miRNA-34a-5p regulated Wnt/β-catenin pathway by targeting SOX4.
In conclusion, our study demonstrates that miRNA-34a-5p can inhibit the over-activation of the Wnt/β-catenin signaling pathway via targeting SOX4 and further regulate proliferation, invasion of neuroblastoma cells.
Keywords: miRNA-34a-5p, SRY-related HMG-box, neuroblastoma
1. Introduction
Neuroblastoma is an embryonal tumor of the autonomic nervous system, meaning that the cell of origin is thought to be a developing and incompletely committed precursor cell derived from neural-crest tissues. As may be expected with a disease of developing tissues, neuroblastomas generally occur in very young children; the median age at diagnosis is 17 months.[1,2] The incidence of neuroblastoma is 10.2 cases per million children under 15 years of age; it is the most common cancer diagnosed during the first year of life.[3,4] With respect to the prognosis, patients with neuroblastoma have improved, with 5-year survival rates increasing from 52% during the period from 1975 through 1977 to 74% during the period from 1999 through 2005.[5,6]
MicroRNAs (miRNAs), existing naturally as the most biologically stable nucleic acid molecule with only about 19 to 23 nucleotides, act as fine-tuning regulators of gene expression at post-transcriptional level through a complicated miRNA-mRNA interaction.[7] MiRNAs are emerging as a new class of regulatory molecules involved in numerous biologic processes.[8] Accumulating evidence has indicated that circulating miRNAs were involved in several pathophysiological processes and related to cancer.[9,10] Numerous reports had illuminated that the usefulness of circulating miRNAs as novel noninvasive biomarkers for cancers, such as colorectal cancer,[11] breast cancer[12,13] and renal cell carcinoma.[14] Studies have demonstrated the direct correlation of miRNA-34s family deregulation with epigenetic and genetic mechanisms in cancers of lung, skin,[15] pancreas, ovary,[16] breast, urinary bladder, kidney,[17] colon,[18] prostate,[19] liver, brain,[20] cervix,[21] esophagus,[21] and the lymphoid system.[22] With respect to neuroblastoma, Welch et al. reported that miRNA-34a functions as a potential tumor suppressor in human neuroblastoma cells.[23]
SOX4 is a member of the SOX (SRY-related HMG-box) family of transcription factors which involves in various physiological processes. Recently, SOX4 has attracted more attention since it is related with various malignant cancers.[24–27] On the other hand, genome-wide promoter analysis indicated that SOX4 was associated with a number of transcription factors, including E2F1, E2F4 and c-MYC. Moreover, it was demonstrated that SOX4 over-expression was a malignant prognostic factor for cancer patients, and that SOX4 functioned as an oncogene. The Wnt/β-catenin pathway requires Wnt ligand binding to Frizzled receptors as well as LRP5/6 co-receptors to initiate intracellular signaling via β-catenin nuclear translocation.[28–30] Deregulation of the canonical Wnt/β-catenin pathway is commonly observed in human cancers, and lots of evidences implied that the aberrant activation of the Wnt/β-catenin signaling pathway may be associated with cancer development
In the current study, we have systematically validated the relationship of miRNA-34a-5p in the regulating of the Wnt/β-catenin signaling pathway by targeting SOX4 in neuroblastoma and verified the molecular mechanism of miRNA-34a-5p on suppressing proliferation, migration and promoting apoptosis of neuroblastoma cells.
2. Materials and methods
2.1. Patients and samples
A total of 20 paired neuroblastoma tissues and adjacent non-cancerous tissues were collected from patients with neuroblastoma who underwent surgery resection at hospital. Participants in the present study had not received chemotherapy, radiation or other anticancer drugs before surgery. The patients included 9 males and 11 females. The histology and pathology of the fresh tissue samples were examined by at least two pathologists. adjacent non-cancerous tissues were sampled ≥3 cm away from tumor margin. The samples were collected from august 2017 to august 2019. all samples were instantly frozen in liquid nitrogen and stored at −80 °C until further analysis. The present study was approved by the ethics committee of Qingdao Municipal Hospital Affiliated to Qingdao University. Written informed consent was obtained from all participants.
2.2. Cell culture and transfection
SH-SY5Y neuroblastoma cells were purchased from the American Type Culture Collection (ATCC; Rockville, Md). They were maintained as monolayer cultures in 75 cm2 plastic culture flasks in Dulbecco's modified Eagle's medium (DMEM)/Ham's Nutrient Mixture F-12 (Sigma-Aldrich), supplemented with 10% FBS, penicillin (20 U/ml) and streptomycin (20 μg/ml) at 37 °C in a humid atmosphere containing 5% CO2. miRNA-34a-5p mimic, NC mimic miRNA-34a-5p inhibitor and NC inhibitor were purchased from RiboBio (Guangzhou, China) and used in strict accordance with the reagent instructions supplied by the company. Transfection was carried out with Lipofectamine 2000 following the manufacturer's instructions. Briefly, the materials used to transfect the cells (scramble miR mimic, miRNA-34a-5p mimic, miRNA-34a-5p inhibitor) were diluted with serum-free DMEM, respectively. Lipofectamine 2000 was also diluted with serum-free DMEM. The diluted Lipofectamine 2000 was added into the diluted plasmid, or miRNA mimic, or inhibitor, and incubated for 20 minute at room temperature, and subsequently added to the H1229 cells at ∼70% confluence in a 6-well plate. Subsequently, the cells were incubated at 37 °C in an atmosphere of 5% CO2 for 6 hour. Following incubation, the medium in each well was replaced by the DMEM supplemented with 10% FBS, and cultured for 24 hour at 37 °C prior to performance of the following assays.
2.3. Extract the total RNA
We use Recover All Total Nucleic Acid Isolation Kit to extract the Total RNA from tissue samples. We used the kit according to the manufacturer's instructions. The maximum absorption wavelength of nucleic acid is 260 nm, which can be used to calculate the concentration of nucleic acid sample. The ratio of OD value at 260 nm and 280 nm can be determined to estimate the purity of nucleic acid. The result of our experiment is that the extracted RNA was higher in content, and 260/280 analysis indicated un-degraded RNA, with higher purity, without pollution of DNA.
2.4. Cell Proliferation Assays
MTT assays were performed to measure the proliferation ability of SH-SY5Y neuroblastoma cells with different treatment. After transfected with miRNA-34a-5p mimics or inhibitor, SH-SY5Y neuroblastoma cells were harvested and plated into 96-well plate. After incubated for 0 hour, 24 hour, 48 hour or 72 hour at 37 °C, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-di- phenyltetrazoliumbromide)(10 μL,5 mg/mL)(Sig- ma-Aldrich, St. Louis, MO, USA) was added into each well, followed by an incubation for another 4 h. Subsequently, 100 μL dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) was added to solubilize the crystals. The optical density (OD) 490 was measured with a microplate reader (Bio-Rad, Hercules, CA).
2.5. Cell invasion assay
Briefly, 5 × 104 cells were added into the upper chamber of a Transwell, and then, 0.7 ml DMEM was added to the lower chamber. Cells were cultured for 24 hour at 37 °C in a humidified incubator with 5% CO2. After treatment, the cells were fixed with methanol for 30 minute and stained with 1.0% crystal violet for 20 minute. The number of invasive cells penetrating the Matrigel was recorded.
2.6. Flow cytometry for apoptosis detection
The treated cells were digested and collected, and 50,000–100,000 cells were centrifuged (1000 r/min, 5 minute). The supernatant was discarded, then 195 μL of Annexin V-FITC binding solution was added and the cells were gently resuspended. Subsequently, 5 μL of Annexin V-FITC reagent was added and mixed gently, and placed at room temperature for 10 min avoiding light. Another 10 μL of propidium iodide (PI) staining solution was added, gently mixed, and placed at room temperature for 10 min avoiding light. Then, the cells were resuspended by gently adding 200 μL of Annexin V-FITC binding solution. The cell suspension was subsequently detected by flow cytometry: green fluorescence was shown after Annexin V-FITC binding and red fluorescence was shown after PI binding.
2.7. Reverse transcription-quantitative PCR (RT-qPCR).
Total RNA was extracted from Gc tissues and cells using Trlzol reagent (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Total RNA was reverse-transcribed into cDNA using the RT reagent kit (Takara Biotechnology co., ltd.) or the miRNA reverse Transcription kit (Thermo Fisher Scientific, Inc.). Subsequently, qPCR was performed using SYBr Premix ex Taq (Takara Biotechnology co., ltd.). The following primer pairs were used for qPCR: miRNA-34a-5p forward, 5′-UGGCAGUGUCUUAGCUGGUUGU-3′ and reverse, 5′-AACCAGCUAAGACACUGCAAUU-3′; SOX4 forward, 5′-GTGAGCGAGATCTCGGG-3′ and reverse, 5′-CAGGTTGGAGATGCTGGACTC-3′; GAPDH forward, 5′-CACATCGCT CAGACACCATG-3’ and reverse, 5′-TGACGGTGCCAT TGGAATTT-3′; The amplification parameters were as follows: Denaturation at 95 °C for 10 minute, followed by 40 cycles of denaturation at 95 °C for 30 second, annealing at 60 °C for 30 second and extension at 72 °C for 1 minute. mRNA and miRNA expression levels were quantified using the 2−ΔΔcq method[27] and normalized to the internal reference genes GAPDH and u6, respectively.
2.8. Western Blotting Analysis
The whole protein from PDAC cells was harvested in radioimmunoprecipitation assay (RIPA) buffer (50 mM HEPES, 150 mM NaCl, 1%(v/v) Igepal, 0.5%(w/v) Na-DOC, 0.1% SDS and 1 tablet Complete Mini protease inhibitor cocktail (Roche, Germany) and subjected to bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) for protein concentration. Then, proteins were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Roche, Basel, Switzerland), which were blocked with 5% non-fat milk in tris-buffered saline-tween (TBST) for 2 hour at room temperature and incubated with specific primary antibodies at 4 °C overnight. The following primary antibodies were used: N-Cadherin (CST, #43248, 1:200, Opal 620), E-Cadherin (CST, #3169, 1:500, Opal 650), Anti-Vimentin (Abcam, ab92574, 1:400, Opal 570). All the antibodies were diluted by PBS. Subsequently, the membranes were washed by TBST for 3 times, followed by being incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:3000, ab205718, Abcam, Cambridge, MA) for 2 hour at room temperature. All the antibodies were diluted by PBS. The results were determined by enhanced chemiluminescence kit (Pierce, Rockford, IL).
2.9. Statistical methods
Continuous variables were expressed as mean ± SD (standard deviation) and compared using a two-tailed unpaired Student t test; categorical variables were compared using χ2 or Fisher analysis. All statistical evaluations were carried out using SPSS software (Statistical Package for the Social Science, version 15.0, SPSS Inc, Chicago, IL). A value of P < .05 was considered to be statistically significant in all the analyses.
3. Results
3.1. Negative correlation between miRNA-34a-5p and SOX4 expression in Neuroblastoma
The quantitative polymerase chain reaction (qPCR) assay results showed that the expression of miRNA-34a-5p was significantly decreased and that of SOX4 was significantly increased in Neuroblastoma tissues compared with adjacent tissues (Fig. 1A–D). This finding suggested that miRNA-34a-5p and SOX4 were involved in the development of Neuroblastoma. To further verify this discovery, correlation analysis was performed and the results revealed that the expression levels of miRNA-34a-5p and SOX4 were significantly negatively correlated (Fig. 1E). To further investigate the tumor suppressive roles of miRNA-34a-5p in neuroblastoma cell line SH-SY5Y, we perform cell transfection to confirm the expression of miRNA-34a-5p and SOX4 in various groups using qPCR. Briefly, the overexpression and inhibition of miRNA-34a-5p were obtained by transiently transfecting miRNA-34a-5p mimics and inhibitor into SH-SY5Y cells. We found that the expression of miRNA-34a-5p in different groups had significant differences in both miRNA-34a-5p expression (Fig. 1F) and SOX4 expression (Fig. 1G). In particular, the miRNA-34a-5p expression was negatively associated with SOX4 expression as we previously showed.
Figure 1.
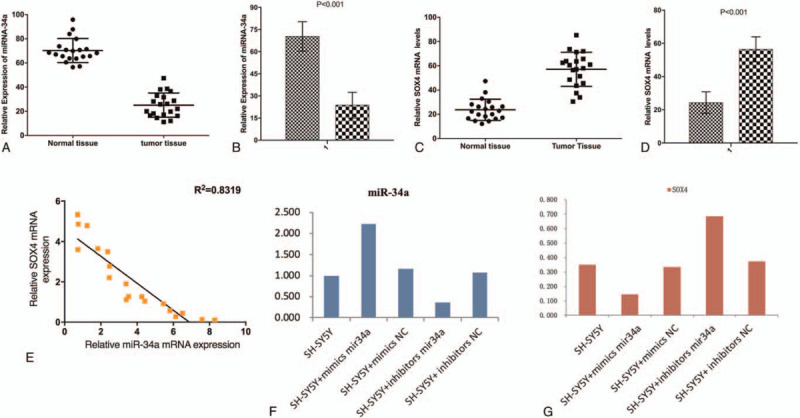
Negative correlation between miRNA-34a-5p and SOX4 expression in Neuroblastoma.
3.2. miRNA-34a-5p regulates SOX4 expression to inhibit the proliferation and invasion and to promote apoptosis of SH-SY5Y cells
To investigate the biological function of miRNA-34a-5p in Neuroblastoma cells, gain- and loss-of-function experiments were performed. SH-SY5Y cells were transfected with miRNA-34a-5p mimic, NC mimic, miRNA-34a-5p inhibitor and NC inhibitor. After successful transfection, proliferation, invasion, and apoptosis of SH-SY5Y cells were detected by MTT assay, Transwell assay, and flow cytometry, respectively. The results showed that compared with NC group, the cell proliferation rate and cell invasion ability were significantly decreased in the miRNA-34a-5p inhibitor group, while apoptosis was significantly increased (P < .05). Further, compared with the NC group, the cell proliferation rate and cell invasion ability were significantly decreased in the miRNA-34a-5p mimic group, while apoptosis was significantly increased (P < .05). Compared with SH-SY5Y cells group, the cell proliferation rate and cell invasion ability were significantly increased in the miRNA-34a-5p mimic group, while apoptosis was significantly decreased (P < .05). No significant difference was observed between the NC mimic group and the NC inhibitor group (P > .05) (Fig. 2).
Figure 2.
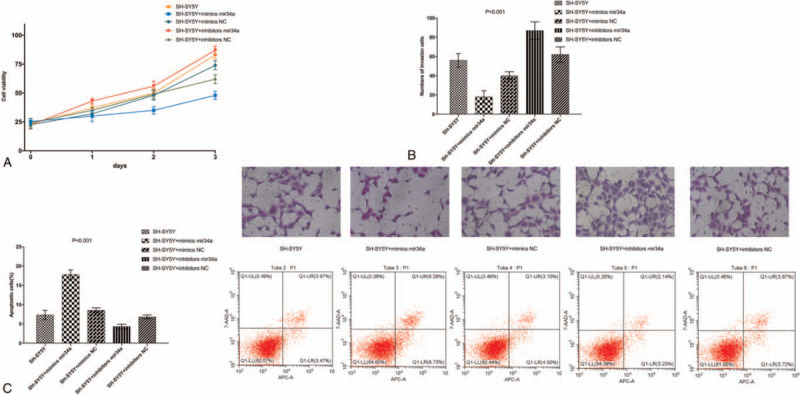
miRNA-34a-5p regulates SOX4 expression to inhibit the proliferation and invasion and to promote apoptosis of SH-SY5Y cells.
3.3. Effect of miRNA-34a-5p targeting SOX4 on the Wnt/β-catenin signaling pathway in SH-SY5Y cells
In order to verify miRNA-34a-5p inhibits the Wnt/β-catenin signaling pathway to affect proliferation, invasion, apoptosis, and other biological functions in Neuroblastoma, cells were transfected with miRNA-34a-5p mimic, NC mimic, miRNA-34a-5p inhibitor and NC inhibitor. After transfection, the expression levels of the corresponding proteins of the Wnt/β-catenin signaling pathway (Wnt, MMP9, Bax, and Bcl-2) were detected. The results of western blot showed that the protein levels of Wnt, MMP9 and Bcl-2 in the miRNA-34a-5p mimic group were significantly decreased compared with those in the NC group (P < .05). Further, the protein levels of Wnt, MMP9 and Bcl-2 in the miRNA-34a-5p inhibitor group were significantly increased compared with those in the NC group (P < .05). Meanwhile, the protein levels of Bax in the miRNA-34a-5p mimic group were significantly increased compared with those in the NC group (P < .05). The protein levels of Bax in the miRNA-34a-5p inhibitor group were significantly decreased compared with those in the NC group (P < .05). No significant difference was observed between the NC groups (P > .05) (Fig. 3). These results suggested that miRNA-34a-5p mediated the proliferation, invasion, apoptosis, and other biological functions of Neuroblastoma through inhibiting the Wnt/β-catenin signaling pathway by targeting SOX4.
Figure 3.
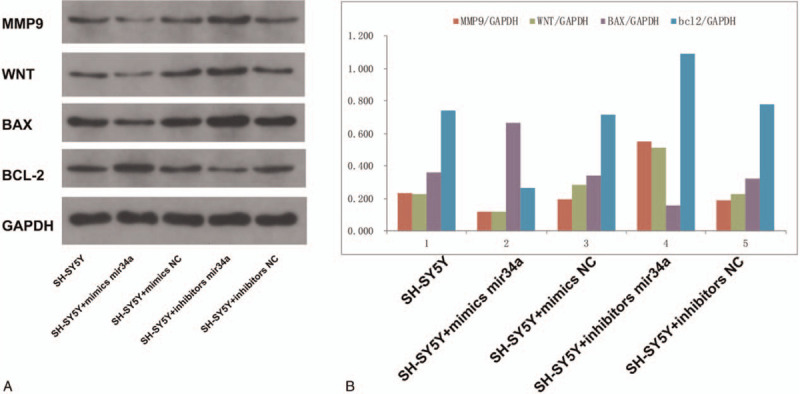
Effect of miRNA-34a-5p targeting SOX4 on the Wnt/β-catenin signaling pathway in SH-SY5Y cells.
4. Discussion
Among the children, Neuroblastoma accounts up to 10% of total pediatric cancer cases, with incidence rate of 1 case per 7000 live births. A highly sensitive and specific approach to classify NB patients into high-risk and low-risk groups based on the level of expression of a small subset of miRNAs was suggested in the past.[31–33] The involvement of miRNAs in the diverse aspects of neuroblastoma pathogenesis strongly suggests their diagnostic and therapeutic potential in the clinical management of neuroblastoma.[34–36] Hence, we have every reason to believe that identification of neuroblastoma related miRNAs as well as their downstream targets, their biological functions and the molecular mechanism would contribute to neuroblastoma treatments.
Wnt/β-catenin signaling is a key developmental pathway which is dysregulated in a wide variety of cancers. Activation of this pathway can conversely lead to oncogenic or tumour suppressive effects in different cancer entities. in neuroblastoma contradictory evidence is emerging about the role played by Wnt/β-catenin signaling with both pathway activation and inhibition being mooted as potential therapeutic strategies, and β-catenin activation being linked to chemoresistance. miRNAs are reported as key regulator for Wnt/β-catenin signaling in neuroblastoma. In this study, we sought to investigate the role of miRNA-34a-5p on neuroblastoma and the possible molecular mechanism. We had performed in-vitro and in-vivo experiments to evaluate the effects of miRNA-34a-5p on neuroblastoma cell proliferation and invasion by altering its expression level via cell transfection. On the basis of our study, miRNA-34a-5p showed decreased expression levels in neuroblastoma, which was in accordance with previous studies performed in other cancers. Thus, it was surmisable that miRNA-34a-5p might serve as a tumor suppressor. Researchers reported that the invasion and migration of suppressive effects of miRNA-34a-5p on cell proliferation, migration and invasion in other cancers.[37,38] Similarly, our results also indicated that miRNA-34a-5p repressed the development of neuroblastoma. Up-regulation of miRNA-34a-5p attenuated cell proliferation and migration while stimulated apoptosis in neuroblastoma.
On the other hand, Several SOX proteins have proven to be important developmental regulators in various diseases. One such protein is the group E protein SOX4, which is prominently expressed in the early neural crest and in glial lineages of the peripheral and central nervous systems both during development and in the adult. In this study, we also investigated the biological functions of SOX4 in neuroblastoma cells. SOX4 was previously considered to be an oncogene according to many other researches and was confirmed with our findings in neuroblastoma. We determined that SOX4 was up-regulated in neuroblastoma cells. For this reason, we subsequently focused on the specific relationship between miRNA-34a-5p and SOX4 as well as the potential downstream signaling pathways. Our further studies indicated that SOX4 and Wnt/β-catenin were involved in the molecular mechanism of miRNA-34a-5p's tumor suppressive effect. There have been some studies investigating the regulatory relationship between SOX4 and Wnt/β- catenin pathway.[39–41] We verified the regulator relationship between miRNA-34a-5p and SOX4 at first and our results suggested that miRNA-34a-5p directly targeted SOX4. Subsequently, we manipulated miRNA-34a-5p expression through cell transfection and observed abnormal expression of β-catenin as well as the downstream targets of the Wnt/β-catenin pathway in neuroblastoma cells. With all these evidences, we determined that miRNA-34a-5p regulated Wnt/β-catenin pathway by targeting SOX4.
By the way, this study is in small size and retrospective design. On the other hand, the relationship between SOX4 and the potential possibilities of miRNA-34a-5p suppressing neuroblastoma was not clearly explained by changing of Wnt/β-catenin signaling. Moreover, the change of Wnt/β-catenin signaling after treatment apart from pretreatment can be investigated in future studies.
In conclusion, our study demonstrates that miRNA-34a-5p can inhibit the over-activation of the Wnt/β-catenin signaling pathway via targeting SOX4 and further regulate proliferation, invasion of neuroblastoma cells.
Author contributions
Conceptualization: Yue Wang, Lirong Sun.
Data curation: Yue Wang, Dehua Li, Lirong Sun.
Formal analysis: Dehua Li.
Investigation: Enqing Guan.
Methodology: Enqing Guan.
Validation: Yue Wang.
Visualization: Yue Wang.
Writing – original draft: Yue Wang, Lirong Sun.
Writing – review & editing: Yue Wang, Lirong Sun.
Footnotes
Abbreviations: DMEM = Dulbecco's modified Eagle's medium, miRNAs = microRNAs, SOX = SRY-related HMG-box.
How to cite this article: Wang Y, Guan E, Li D, Sun L. miRNA-34a-5p regulates progression of neuroblastoma via modulating the Wnt/β-catenin signaling pathway by targeting SOX4. Medicine. 2021;100:20(e25827).
Funding was not applicable.
The authors have no conflicts of interest to disclose.
The data used to support the findings of this study are available from the corresponding author upon request.
References
- [1].Hoehner JC, Gestblom C, Hedborg F, Sandstedt B, Olsen L, Påhlman S. A developmental model of neuroblastoma: differentiating stroma-poor tumors’ progress along an extra-adrenal chromaffin lineage. Lab Invest 1996;75:659–75. [PubMed] [Google Scholar]
- [2].London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol 2005;23:6459–65. [DOI] [PubMed] [Google Scholar]
- [3].Carlsen NL. How frequent is spontaneous remission of neuroblastomas? Implications for screening. Br J Cancer 1990;61:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009;27:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Elzomor H, Ahmed G, Elmenawi S, et al. Survival outcome of intermediate risk neuroblastoma at Children Cancer Hospital Egypt. J Egypt Natl Canc Inst 2018;30:21–6. [DOI] [PubMed] [Google Scholar]
- [6].Tolbert VP, Matthay KK. Neuroblastoma: clinical and biological approach to risk stratification and treatment. Cell Tissue Res 2018;372:195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Naidu S, Magee P, Garofalo M. MiRNA-based therapeutic intervention of cancer. J Hematol Oncol 2015;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A 2009;106:4402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 2011;108:5003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nonaka R, Nishimura J, Kagawa Y, et al. Circulating miR-199a-3p as a novel serum biomarker for colorectal cancer. Oncol Rep 2014;32:2354–8. [DOI] [PubMed] [Google Scholar]
- [12].Mar-Aguilar F, Mendoza-Ramirez JA, Malagon-Santiago I, et al. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers 2013;34:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Si H, Sun X, Chen Y, et al. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol 2013;139:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Redova M, Poprach A, Nekvindova J, et al. Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J Transl Med 2012;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bommer GT, Gerin I, Feng Y, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 2007;17:1298–307. [DOI] [PubMed] [Google Scholar]
- [16].Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 2007;26:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vogt M, Munding J, Gruner M, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch 2011;458:313–22. [DOI] [PubMed] [Google Scholar]
- [18].Li N, Fu H, Tie Y, et al. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett 2009;275:44–53. [DOI] [PubMed] [Google Scholar]
- [19].Tryndyak VP, Ross SA, Beland FA, Pogribny IP. Down-regulation of the microRNAs miR-34a, miR-127, and miR-200b in rat liver during hepatocarcinogenesis induced by a methyl-deficient diet. Mol Carcinog 2009;48:479–87. [DOI] [PubMed] [Google Scholar]
- [20].Feinberg-Gorenshtein G, Avigad S, Jeison M, et al. Reduced levels of miR-34a in neuroblastoma are not caused by mutations in the TP53 binding site. Genes Chromosomes Cancer 2009;48:539–43. [DOI] [PubMed] [Google Scholar]
- [21].Wang X, Wang HK, McCoy JP, et al. Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA 2009;15:637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mraz M, Malinova K, Kotaskova J, et al. miR-34a, miR-29c and miR-17-5p are downregulated in CLL patients with TP53 abnormalities. Leukemia 2009;23:1159–63. [DOI] [PubMed] [Google Scholar]
- [23].Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene 2007;26:5017–22. [DOI] [PubMed] [Google Scholar]
- [24].Ding L, Zhao Y, Dang S, et al. Circular RNA circ-DONSON facilitates gastric cancer growth and invasion via NURF complex dependent activation of transcription factor SOX4. Mol Cancer 2019;18:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moreno CS. SOX4: the unappreciated oncogene. Semin Cancer Biol 2020;67:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang J, Xiao C, Feng Z, et al. SOX4 promotes the growth and metastasis of breast cancer. Cancer Cell Int 2020;20:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bagati A, Kumar S, Jiang P, et al. Integrin αvβ6-TGFβ-SOX4 Pathway Drives Immune Evasion in Triple-Negative Breast Cancer. Cancer Cell 2021;39: 54-67.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guo Y, Xiao L, Sun L, Liu F. Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res 2012;61:337–46. [DOI] [PubMed] [Google Scholar]
- [29].Arend RC, Londoño-Joshi AI, Straughn JM, Jr, Buchsbaum DJ. The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol Oncol 2013;131:772–9. [DOI] [PubMed] [Google Scholar]
- [30].Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev 2018;62:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Buhagiar A, Ayers D. Chemoresistance, cancer stem cells, and miRNA influences: the case for neuroblastoma. Anal Cell Pathol (Amst) 2015;2015:150634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stigliani S, Morandi F, Persico L, et al. miRNA expression profile of bone marrow resident cells from children with neuroblastoma is not significantly different from that of healthy children. Oncotarget 2018;9:19014–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chava S, Reynolds CP, Pathania AS, et al. miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol Oncol 2020;14:180–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Neviani P, Wise PM, Murtadha M, et al. Natural killer-derived exosomal mir-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res 2019;79:1151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schmittgen TD. Exosomal miRNA Cargo as Mediator of Immune Escape Mechanisms in Neuroblastoma. Cancer Res 2019;79:1293–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tao T, Shi H, Mariani L, et al. LIN28B regulates transcription and potentiates MYCN-induced neuroblastoma through binding to ZNF143 at target gene promotors. Proc Natl Acad Sci U S A 2020;117:16516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li XY, Wen JY, Jia CC, et al. MicroRNA-34a-5p enhances sensitivity to chemotherapy by targeting AXL in hepatocellular carcinoma MHCC-97L cells. Oncol Lett 2015;10:2691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ma Y, Huang YX, Chen YY. miRNA34a5p downregulation of VEGFA in endometrial stem cells contributes to the pathogenesis of endometriosis. Mol Med Rep 2017;16:8259–64. [DOI] [PubMed] [Google Scholar]
- [39].Tian Z, Yang G, Jiang P, Zhang L, Wang J, Sun S. Long non-coding RNA Sox4 promotes proliferation and migration by activating Wnt/β-catenin signaling pathway in osteosarcoma. Pharmazie 2017;72:537–42. [DOI] [PubMed] [Google Scholar]
- [40].Wang C, Zi H, Wang Y, Li B, Ge Z, Ren X. LncRNA CASC15 promotes tumour progression through SOX4/Wnt/β-catenin signalling pathway in hepatocellular carcinoma. Artif Cells Nanomed Biotechnol 2020;48:763–9. [DOI] [PubMed] [Google Scholar]
- [41].Zhao G, Yin Y. Zhao B. miR-140-5p is negatively correlated with proliferation, invasion, and tumorigenesis in malignant melanoma by targeting SOX4 via the Wnt/β-catenin and NF-κB cascades. J Cell Physiol 2020;235:2161–70. [DOI] [PubMed] [Google Scholar]


