Abstract
Objectives:
The standard initial approach in patients with hematuria or other symptoms suggestive of bladder cancer (BC) is a combination of cystoscopy and urine cytology (UC); however, UC has low sensitivity particularly in low-grade tumors. The aim of the present review was to critically analyze and compare results in the literature of promising molecular urinary tests for the initial diagnosis of BC.
Methods:
We searched in the Medline and Cochrane Library databases for literature from January 2009 to January 2019, following the PRISMAguidelines.
Results:
In terms of sensitivity, ImmunoCyt showed the highest mean and median value, higher than UC. All tests analyses showed higher mean and median sensitivity when compared with UC. In terms of specificity, only UroVysion and Microsatellite analyses showed mean and median values similar to those of UC, whereas for all other tests, the specificity was lower than UC. It is evident that the sensitivity of UC is particularly low in low grade BC. Urinary tests mainly had improved sensitivity when compared to UC, and ImmunoCyt and UroVysion had the highest improvement in low grade tumors.
Conclusions:
Most of the proposed molecular markers were able to improve the sensitivity with similar or lower specificity when compared to UC. However, variability of results among the different studies was strong. Thus, as of now, none of these markers presented evidences so as to be accepted by international guidelines for diagnosis of BC.
Keywords: Bladder cancer, Cytology, Hematuria, MCM5, Urinary markers
1. Introduction
Bladder cancer (BC) represents the 4th most common neoplasia in men with a significant morbidity and mortality. [1] The standard initial approach in patients with hematuria or other symptoms suggestive of BC is a combination of cystoscopy and urine cytology (UC). [2] Cystoscopy is an invasive method whereas UC exhibits low sensitivity in detecting BC.3,4 UC has high sensitivity in high-grade tumors (84%), but low sensitivity in low-grade tumors (16%). [5] A positive UC can indicate a urothelial tumor in the urinary tract; however, a negative cytology does not exclude the presence of a tumor. Cytological interpretation is user-dependent, and evaluation can be hampered by low cellular yield, urinary tract infections, and stones. However, in experienced hands the specificity exceeds 90%. [6]
Several non-invasive molecular tumor tests have been developed to improve the sensitivity of UC. None of these markers have been accepted for diagnosis or follow-up in routine practice or clinical guidelines. [2]
The following conclusions have been drawn by EAU guidelines regarding the existing tests:
-
-
Sensitivity is usually higher at the cost of lower specificity, compared to UC.
-
-
Benign conditions may influence the results of many urinary marker tests.
-
-
The wide range in performance of the markers and low reproducibility may be explained by patient selection and the complicated laboratory methods required.
-
-
UroVysion fluorescent in situ hybridization (FISH), ImmunoCyt/uCyt, NMP-22, BTA, and microsatellite analysis are interesting tests. However, a variable range of sensitivity and specificity in different clinical trials is reported in the literature.
Different mechanisms of action are used by these tests. NMP-22 is a nuclear matrix protein involved in the proper distribution of chromatin during replication. It is a quantitative ELISA test using 2 antibodies.7,8 Microsatellite analysis is carried out by polymerase chain reaction using DNA primers on polymorphic short tandem DNA repeats in the genome. [9]
ImmunoCyt is an immunocytological test using fluorescence that combines 3 monoclonal antibodies to detect tumor-associated cellular antigens. [10] The UroVysion test is a multitarget multicolour FISH assay, taking advantage of the high occurrence of chromosomal abnormalities in BC.11,12 The BTA stat quantitative assay detects the human complement factor H-related protein and the complement factor H by immunochromatography.13,14 MCM5 is a minichromosome maintenance protein examined by immunofluorometric assay using monoclonal antibodies. [15]
There is still a need for defining a useful urine biomarker that may help in the initial diagnosis and follow-up of BC, thus replacing UC.
2. Materials and methods
2.1. Objective
The aim of the present review was to critically analyze and compare results in the literature with promising molecular urinary tests for the initial diagnosis of BC. We limited our analysis to urine tests such as UroVysion, NMP-22, ImmunoCyt/uCyt, the BTA stat test, microsatellite analysis, and the MCM5 test. We compared sensitivity, specificity, and performance of these tests and that of UC in detecting BC.
2.2. Search strategy
We searched in the Medline and Cochrane Library databases (primary fields: bladder neoplasm AND initial diagnosis AND urinary test OR UroVysion OR NMP-22 OR BTA OR microsatellite analysis OR ImmunoCyt/uCyt OR the MCM5 test).
Our search was performed without language restriction in the literature from January 2000 to August 2019 following PRISMA guidelines (Fig. 1). Original and review articles were included and critically evaluated. Additional references were identified from reference lists of these articles. We did not include abstracts and reports from meetings.
Figure 1.
PRISMA flow diagram.
2.3. Selection of the studies and inclusion criteria
Entry into the analysis was restricted to data collected from original studies on clinical trials including subjects with hematuria or other symptoms suggestive of an initial diagnosis of BC and verified by cystoscopy, transurethral biopsy, or resection of the bladder.
There were 2 authors (G.D.L. and F.D.G.) who independently screened the titles and abstracts of all articles using predefined inclusion criteria. The full-text articles were independently examined by 3 authors (G.D.L., M.M., and F.D.G.) to determine whether or not they met the inclusion criteria. Then, 3 authors (G.D.L., M.M., and F.D.G.) extracted data from the selected articles. Final inclusion was determined by all investigators’ evaluation discussion.
The studies selected for inclusion met the following criteria: (1) analysis for initial diagnosis of BC; (2) UroVysion, NMP-22, BTA, microsatellite analysis, ImmunoCyt/uCyt, MCM5 tests compared with UC; (3) cystoscopy or transurethral biopsy or resection of the bladder methods used to confirm results and diagnosis of BC; and (4) results expressed as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Articles were excluded if: (1) multiple reports were published on the same population; (2) data provided were insufficient for the outcomes; or (3) confirmation from at least cystoscopy was not reported. Risk of bias for all included reports was evaluated using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool for diagnostic accuracy studies. [16]
3. Results
3.1. Search results
The database searches initially yielded 91 journal article references. Of these 20 were subsequently removed due to either duplication or a failure to meet the inclusion criteria. Full-text articles were then re-evaluated and critically analyzed for the remaining 71 journal references. Of these, 36 did not meet the inclusion criteria. The remaining 35 studies were considered for our critical review (Fig. 1) (Table 1).
Table 1.
Main data from the 35 studies considered in the review.
| Author, year, location | Study design | Sample size, n | Median participant age, y | Sampling method | Biomarker | Confirmatory test | Statistical results, % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stober et al., [17] 2002, UK | Monocenter prospective | 353 | 70 (62–78) | Voided urine | – MCM5 – Cytology | – Cystoscopy – Biopsy | MCM5 – Sens: 87 – Spec: 87 – NPV: 96 – PPV: 64 | Cytology – Sens: 48 – Spec: 97 – NPV: 88 – PPV: 82 | |||
| Kelly et al., [18] 2012, UK | Monocenter prospective blinded randomized | 1677 | 63 (49–73) | Voided urine | – MCM5 – NMP-22 – Cytology | – Cystoscopy – Bladder resection | MCM5 – Sens: 69 – Spec: 69 – NPV: 93 – PPV: 26 | NMP-22 – Sens: 53 – Spec: 84 – NPV: 92 – PPV: 36 | Cytology – Sens: 9 – Spec: 88 – NPV: 87 – PPV: 10 | ||
| Brems-Eskildsen et al., [19] 2010, Denmark | Monocenter prospective | 117 | 71 | Voided urine | – MCM5 – Cytology | – Cstoscopy – Biopsy | MCM5 – Sens: 62.5 – Spec: 65.9 – NPV: 60 – PPV: 68.2 | Cytology – Sens: 41.7 – Spec: 87.9 – NPV: 59.3 – PPV: 78.1 | |||
| Virk et al., [32] 2017, USA | Monocenter retrospective | 377 | 67 | Voided, catheterized, bladder borbotage washing | – UroVysion | – Cystoscopy – Bladder resection/biopsy | UroVysion – Sens: 44.6 – Spec: 81.8 – NPV: 80.3 – PPV: 47.2 | ||||
| Schomler et al., [33] 2010, USA | Monocenter prospective | 108 | 65.7 (30–99)∗ | Voided/borbotage | – UroVysion | – Cystoscopy – Bladder resection | UroVysion – Sens: 57.1 – Spec: 100 – NPV: 25 – PPV: 100 | ||||
| Lotan et al., [34] 2008, USA | Monocenter prospective | 50 | 65 (14.4)+ | Voided/ borbotage | – UroVysion | – Cystoscopy | UroVysion – Sens: 77.8 – Spec: 100 – NPV: 60 – PPV: 100 | ||||
| Kehinde et al., [38] 2011, Kuwait | Monocenter Prospective blinded randomized | 43 | 53 (16–77)∗ | Voided urine | – UroVysion – NMP-22 – Cytology | – Cystoscopy – Biopsy | UroVysion – Sens: 80 – Spec: 48 – NPV: 71.2 – PPV: 61 | NMP-22 – Sens: 82 – Spec: 66 – NPV: 78.8 – PPV: 71.3 | Cytology – Sens: 28 – Spec: 95 – NPV: 84.1 – PPV: 84.9 | ||
| Gopalakrishna et al., [35] 2017, USA | Monocenter retrospective | 1022 | 66 (56–75) | Voided urine | – UroVysion – Cytology | – Cystoscopy | UroVysion – Sens: 37 – Spec: 84 – NPV: NS – PPV: NS | Cytology – Sens: 63 – Spec: 41 – NPV: NS – PPV: NS | |||
| Dimashkien et al., [36] 2013, USA | Monocenter retrospective | 652 | NS | Voided, catheterized and bladder washing | – UroVysion – Cytology | – Cystoscopy | UroVysion – Sens: 60 – Spec: 93.4 – NPV: 97.5 – PPV: 35.5 | Cytology – Sens: 57.8 – Spec: 88.6 – NPV: 97.2 – PPV: 23.4 | |||
| Daniely et al., [39] 2005, Israel | Monocenter prospective | 41 | 72.4 (12.2) | Voided urine | – UroVysion – Cytology | – Cystoscopy – Bladder resection/biopsy | UroVysion – Sens: 100 – Spec: 100 – NPV: NS – PPV: NS | Cytology – Sens: 61.9 – Spec: 100 – NPV: NS – PPV: NS | |||
| Yafi et al., [5] 2015, Canada | Monocenter prospective | 109 | 69 (33–96)§ | Voided, washing, catheterized | – NMP-22 – BTA stat – ImmunoCyt – Cytology | – Cystoscopy – Biopsy | NMP-22 – Sens: 58 – Spec: 85 – NPV: 39 – PPV: 92 | BTA stat – Sens: 61 – Spec: 78 – NPV: 38 – PPV: 89 | ImmunoCyt – Sens: 62 – Spec: 79 – NPV: 37 – PPV: 91 | Cytology – Sens: 48 – Spec: 86 – NPV: 34 – PPV: 90 | |
| Toma et al., [20] 2004, Germany | Monocenter prospective | 126 | NS | Voided and bladder washing | – ImmunoCyt – BTA stat – NMP-22 – UroVysion – Cytology | – Cystoscopy | ImmunoCyt – Sens: 78.3 – Spec: 73.8 – NPV: 85.5 – PPV: 63.2 | BTA stat – Sens: 66.6 –Spec: 78.2 – NPV: 75.4 – PPV: 71.4 | NMP-22 – Sens: 68.5 – Spec: 65.2 – NPV: 77.9 – PPV: 53.6 | UroVysion – Sens: 68.8 – Spec: 89.1 – NPV: 77.8 – PPV: 83.8 | Cytology – Sens 84.6 – Spec: 80 – NPV: 88 – PPV: 75 |
| Soyuer et al., [40] 2009, Turkey | Monocenter prospective | 90 | 66 (46–80)∗ | Voided | – ImmunoCyt – Cytology | – Cystoscopy – Bladder resection/biopsy | ImmunoCyt – Sens: 83.3 – Spec: 86.1 – NPV: 79.5 – PPV: 90 | Cytology – Sens: 75.9 – Spec: 66.7 – NPV: 88.9 – PPV: 77.3 | |||
| Deininger et al., [21] 2017, Germany | Monocenter retrospective | 444 | 67 (18–93)§ | Midstream voided, catheter | – ImmunoCyt | – Cystoscopy – Bladder resection | ImmunoCyt – Sens: 86.8 – Spec: 78.7 – NPV: 97 – PPV: 42 | ||||
| Comploj et al., [22] 2013, Italy | Monocenter prospective | 2217 | 69.5 (15–99)∗ | Voided urine | – ImmunoCyt – Cytology | – Cystoscopy – Bladder resection/biopsy | ImmunoCyt – Sens: 68.1 – Spec: 72.3 – NPV: 95.2 – PPV: 22 | Cytology – Sens: 34.5 – Spec: 97.9 – NPV: 92.9 – PPV: 65.2 | |||
| Todenhofer et al., [23] 2013, Germany | Monocenter prospective | 808 | 67 (20–92)§ | Voided urine | – NMP-22 – UroVysion – ImmunoCyt – Cytology | – Cystoscopy – Bladder resection/biopsy | NMP-22 – Sens: 84.4 – Spec: 41.3 – NPV: 94.1 – PPV: 19.2 | UroVysion – Sens: 71.3 – Spec: 86.3 – NPV: 94.8 – PPV: 46.3 | ImmunoCyt – Sens: 73.9 – Spec: 76.6 – NPV: 94.7 – PPV: 34.4 | Cytology – Sens: 67.8 – Spec: 87.5 – NPV: 94.3 – PPV: 47.3 | |
| Sankhwar et al., [41] 2013, India | Monocenter retrospective | 1331 | 58.7 (14.3)# (18–96)¶ | Voided urine | – NMP-22 – Cytology | – Cystoscopy – Bladder resection | NMP-22 – Sens: 55.7 – Spec: 85.7 – NPV: 96.8 – PPV: 19.7 | Cytology – Sens: 15.8 – Spec: 99.2 – NPV: 94.9 – PPV: 94.9 | |||
| Ritter et al., [24] 2014, Germany | Monocenter prospective | 198 | 70 (20–90)§ | Midstream voided urine | – NMP-22 – Cytology | – Cystoscopy – Bladder resection | NMP-22 – Sens: 16.4 – Spec: 95.3 – NPV: 70.5 – PPV: 62.5 | Cytology – Sens: 51.7 – Spec: 78.1 – NPV: 78.1 – PPV: 51.7 | |||
| Jeong et al., [42] 2012, Korea | Monocenter prospective | 250 | 57 (50–65) | Midstream voided urine | – NMP-22 | – Cystoscopy – Bladder resection/biopsy | NMP-22 – Sens: 84.9 – Spec: 82.8 – NPV: NS – PPV: NS | ||||
| Dogan et al., [43] 2013, Turkey | Monocenter prospective | 87 | 60 (21–98)∗ | Voided urine | – NMP-22 – Cytology | – Cystoscopy | NMP-22 – Sens: 70 – Spec: 80 – NPV: 81 – PPV: 68 | Cytology – Sens: 27 – Spec: 96 – NPV: 68 – PPV: 82 | |||
| Grossman et al., [8] 2005, USA | Monocenter retrospective | 1331 | 58.7 (14.3)# (18–96)¶ | Voided urine | – NMP-22 – Cytology | – Cystoscopy – Bladder resection/biopsy | NMP-22 – Sens: 55.7 – Spec: 85.7 – NPV: 96.8 – PPV: 19.7 | Cytology – Sens: 15.8 – Spec: 99.2 – NPV: 94.9 – PPV: 54.6 | |||
| Breen et al., [48] 2015, New Zealand | Multicenter retrospective | 939 | NS | Voided urine | – NMP-22 – UroVysion – Cytology | – Cystoscopy | NMP-22 – Sens: 44.9 – Spec: 89 – NPV: NS – PPV: NS | UroVysion – Sens: 40 – Spec: 87.3 – NPV: NS – PPV: NS | Cytology – Sens: 45.5 – Spec: 96.3 – NPV: NS – PPV: NS | ||
| Bangma et al., [25] 2013, The Netherlands | Prospective multicenter | 409 | 50–75 ¶ | Voided urine | – NMP-22 – Microsatellite analysis | – Cystoscopy | NMP-22 – Sens: 25 – Spec: 96.6 – NPV: 99.2 – PPV: 7.1 | Microsatellite analysis – Sens: 50 – Spec: 91.9 – NPV: 99.4 – PPV: 6.1 | |||
| Liang et al., [44] 2010, China | Monocenter prospective | 64 | NS | Voided urine | – Microsatellite analysis | – Bladder resection | Microsatellite analysis – Sens: 62.5 – Spec: n.s. – NPV: 100 – PPV: 62.5 | ||||
| Wild et al., [26] 2009, Switzerland | Monocenter prospective | 119 | NS | Voided urine | – Microsatellite analysis – Cytology | – Cystoscopy – Biopsy | Microsatellite analysis – Sens: 72.1 – Spec: 88.2 – NPV: 61.2 – PPV: 92.5 | Cytology – Sens: 88.2 – Spec: 79.4 – NPV: 77.1 – PPV: 89.6 | |||
| Cha et al., [27] 2012, Germany | Multicenter prospective | 1182 | 65 (18–93)§ | Midstream voided urine | – ImmunoCyt – Cytology | – Cystoscopy – Biopsy | ImmunoCyt – Sens: 82.4 – Spec: 86.6 – NPV: 95 – PPV: 61.6 | Cytology – Sens: 46.5 – Spec: 94.9 – NPV: 87.2 – PPV: 70.4 | |||
| Friedrich et al., [28] 2002, Germany | Monocenter prospective | 115 | NS | Voided, bladder washing | – BAT stat – NMP-22 | – Cystoscopy – Bladder resection/biopsy | BAT stat – Sens: 70.3 – Spec: 70.6 – NPV: 80.2 – PPV: 58.4 | NMP-22 – Sens: 68.5 – Spec: 65.2 – NPV: 77.9 – PPV: 53.6 | |||
| Giannopoulos et al., [29] 2001, Greece | Monocenter prospective | 234 | 66 (25–93)∗ | Voided urine | – BAT stat – NMP-22 | – Cystoscopy – Biopsy | BAT stat – Sens: 72.9 – Spec: 73.1 – NPV: 70.1 – PPV: 67.7 | NMP-22 – Sens: 63.5 – Spec: 75 – NPV: 66.9 – PPV: 72.1 | |||
| Gutierrez Banos et al., [30] 2001, Spain | Monocenter prospective | 150 | 67.8 (11.3)# (20–91)¶ | Voided urine | – BAT stat – NMP-22 – Cytology | – Cystoscopy – Bladder resection | BAT stat – Sens: 72.4 – Spec: 89.1 – NPV: 75.9 – PPV: 87.3 | NMP-22 – Sens: 76.3 – Spec: 90.5 – NPV: 78.8 – PPV: 89.2 | Cytology – Sens: 69.7 – Spec: 93.2 – NPV: 75 – PPV: 91.4 | ||
| Halling et al., [37] 2002, USA | Monocenter prospective | 265 | 71 | Voided urine | – BAT stat – UroVysion | – Cystoscopy – Bladder resection/biopsy | BAT stat – Sens: 78 – Spec: 74 – NPV: NS – PPV: NS | UroVysion – Sens: 81 – Spec: 96 – NPV: NS – PPV: NS | |||
| O'Sullivan et al., [49] 2012, New Zealand | Multicenter prospective | 485 | 69 (59–77) | Midstream voided urine | – NMP-22 – Cytology | – Cystoscopy – Biopsy | NMP-22 – Sens: 50 – Spec: 88 – NPV: NS – PPV: NS | Cytology – Sens: 56.1 – Spec: 94.5 – NPV: NS – PPV: NS | |||
| Song et al., [45] 2010, South Korea | Monocenter prospective | 602 | 62∞ | Voided urine | – UroVysion – Cytology | – Cystoscopy – Bladder resection | UroVysion – Sens: 53.5 – Spec: 94.9 – NPV: NS – PPV: NS | Cytology – Sens: 23.9 – Spec: 99 – NPV: NS – PPV: NS | |||
| Smrkolj et al., [31] 2011, Slovenia | Monocenter prospective | 108 | 68.3 (9.9) | Voided urine | – NMP-22 – Cytology | – Cystoscopy – Bladder resection | NMP-22 – Sens: 45.2 – Spec: 75 – NPV: NS – PPV: NS | Cytology – Sens: 37 – Spec: 100 – NPV: NS – PPV: NS | |||
| Hwang et al., [46] 2011, Korea | Monocenter prospective | 424 | 65∞ | Voided urine | – NMP-22 – Cytology | – Cystoscopy – Bladder resection | NMP-22 – Sens: 40.6 – Spec: 96.3 – NPV: NS – PPV: NS | Cytology – Sens: 40.6 – Spec: 99.7 – NPV: NS – PPV: NS | |||
| Sagnak et al., [47] 2011, Turkey | Monocenter prospective | 164 | 30.8 (6.4) | Voided urine | – NMP-22 – Cytology | – Cystoscopy – Biopsy | NMP-22 – Sens: 100 – Spec: 85.2 – NPV: 100 – PPV: 7.7 | Cytology – Sens: 0 – Spec: 96.9 – NPV: 98.7 – PPV: 0 | |||
IQR = interquartile range; NS = not specified; Sens = sensitivity; Spec = specificity.
+ = Median (SD); § = median (range); ∗ = mean (range); # = mean (SD); ¶ = range; ∞ = mean.
Quality of studies: of the 35 studies entered into the review, 15 were conducted in Europe,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31 8 in America,5,8,32,33,34,35,36,37 10 in Asia,38,39,40,41,42,43,44,45,46,47 and 2 in Oceania.48,49 Of the 35 studies, 2 were randomized studies,18,38 7 were retrospective mono- or multi-center,8,21,32,35,36,41,48 and 28 were prospective mono- or multi-center.5,17,18,19,20,22,23,24,25,26,27,28,29,30,31,33,34,37,38,39,40,42,43,44,45,46,47,49 Each study comparatively considered one or more recognized biomarkers for BC diagnosis, and compared them with one or more confirmatory tests such as cystoscopy, transurethral bladder biopsy, or resection (Table 1). Risk of bias for all included reports was evaluated using the QUADAS-2 tool for diagnostic accuracy studies (fig. S1, supplementary material). The major bias within the studies focused upon “patient selection” and “index test.” In general, 21 out of the 35 studies were of upper middle quality.
3.2. Study sample size, mean age, and inclusion/exclusion criteria
In the 35 studies, the sample size of cases strongly varied from 41 to 2217 cases analyzed (total sample 16,691 cases). The range of mean age across the studies varied from 30.8 to 72.4 years. No study had as exclusion criteria data such as age, race, or other registries. All studies had as inclusion criteria hematuria or other symptoms requiring initial investigation for BC.
3.3. Sampling methods
All 35 studies used voided urine as the main method for sample collection. Some studies used in association others collecting methods. In particular, 3 trials used bladder barbotage,32,33,34 4 catheter,5,21,32,36 and 5 bladder washing.5,20,28,32,36
3.4. Biomarker used
Most of the analyzed studies considered one or more recognized biomarker for the initial diagnosis of BC.
MCM5 was used in 3 studies on 2147 cases17,18,19; UroVysion (uFISH) in 12 studies on 5033 cases20,23,32,33,34,35,36,37,38,39,45,48; NMP-22 in 19 studies on 8988 cases 5,8,18,20,23,24,25,28,29,30,31,38,41,42,43,46,47,48,49; microsatellite analysis in 3 studies on 592 cases25,26,44; BTA stat in 6 studies on 999 cases5,20,28,29,30,37; and ImmunoCyt/uCyt in 7 studies on 4976 cases.5,20,21,22,23,27,40 Twenty-five studies also included UC as a diagnostic tool in comparison with the new tests (14,375 cases) (Table 1).5,8,17,18,19,20,22,23,24,26,27,30,31,35,36,38,39,40,41,43,45,46,47,48,49
3.5. Comfirmatory test
In all analyzed studies, at least cystoscopy was used as the confirmatory test to verify biomarker results. In addition to cystoscopy, 28 studies also performed transurethral bladder biopsy or resection.5,8,17,18,19,21,22,23,24,26,27,28,29,30,31,32,33,37,38,39,40,41,42,44,45,46,47,49
3.6. Diagnostic accuracy analysis of the different biomarkers
Table 2 summarizes sensitivity, specificity, PPV, and NPV of each test analyzed in the studies in terms of prediction for initial diagnosis of BC. Results were analyzed across all grades and risk categories of BC.
Table 2.
Performance of different urinary biomarkers to predict BC.
| Biomarker n° of studies (n° of pts) | Cytology n = 25 (14,375) | MCM-5 n = 3 (2147) | Microsatellite n = 3 (592) | UroVysion n = 12 (5033) | BTA stat n = 6 (999) | ImmunoCyt n = 7 (4976) | NMP-22 n = 19 (8988) |
|---|---|---|---|---|---|---|---|
| Sensitivity, % | |||||||
| Mean ± SD | 45.5 ± 23.1 | 72.8 ± 12.7 | 61.5 ± 11.1 | 64.3 ± 19.0 | 70.2 ± 5.8 | 76.4 ± 8.9 | 60.1 ± 21.0 |
| Median | 46.5 | 69.0 | 62.5 | 64.4 | 71.4 | 78.3 | 58.0 |
| Range | 9.0–88.2 | 62.5–87.0 | 50.0–72.1 | 37.0–100.0 | 61.0–78.0 | 62.0–86.8 | 16.4–100.0 |
| Specificity, % | |||||||
| Mean ± SD | 89.7 ± 13.2 | 74.0 ± 11.4 | 90.1 ± 2.6 | 88.4 ± 14.2 | 77.2 ± 6.5 | 79.0 ± 5.6 | 80.6 ± 13.6 |
| Median | 94.9 | 69.0 | 90.1 | 91.3 | 76.0 | 78.7 | 85.0 |
| Range | 41.0–100 | 65.9–87.0 | 88.2–91.9 | 48.0–100.0 | 70.6–89.1 | 72.3–86.6 | 41.3–96.6 |
| NPV, % | |||||||
| Mean ± SD | 82.6 ± 16.1 | 83.0 ± 20.0 | 86.9 ± 22.2 | 72.4 ± 24.6 | 67.9 ± 17.1 | 83.4 ± 21.4 | 82.1 ± 16.6 |
| Median | 87.6 | 93.0 | 99.4 | 77.8 | 75.4 | 94.7 | 79.9 |
| Range | 34.0–98.7 | 60–96.0 | 61.2–100.0 | 25.0–97.5 | 38.0–80.2 | 37.0–97.0 | 39.0–100.0 |
| PPV, % | |||||||
| Mean ± SD | 64.9 ± 28.5 | 52.7 ± 23.2 | 53.7 ± 43.9 | 67.7 ± 26.8 | 74.8 ± 13.1 | 57.7 ± 26.7 | 48.0 ± 29.5 |
| Median | 76.2 | 64.0 | 62.5 | 61.0 | 71.4 | 61.6 | 53.6 |
| Range | 10.0–94.9 | 26–68.2 | 6.1–92.5 | 35.5–100.0 | 58.4–89.0 | 22.0–91.0 | 7.1–92.0 |
pts = patients; SD = standard deviation.
In the 25 studies that analyzed UC in comparison with the new tests, sensitivity, specificity, PPV, and NPV ranges were 9.0%–88.2%, 41.0%–100%, 10.0%–94.9%, and 34.0%–98.7%, respectively.
Values for MCM5 and microsatellites analysis were only reported in 3 studies.
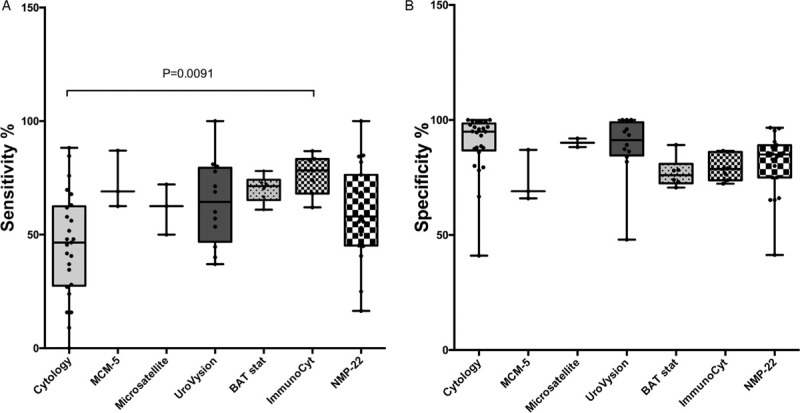
All 6 urinary tests analyzed, showed a strong variability of results in terms of diagnostic accuracy among studies (Table 2) (Fig. 2).
Figure 2.
Sensitivity (A) and specificity (B) of different urinary biomarkers in predicting BC.
Microsatellite analysis, UroVysion, and NMP-22 tests showed a higher mean and median specificity than sensitivity whereas for all the other tests sensitivity and specificity showed similar mean and median values.
In terms of sensitivity, ImmunoCyt showed the highest mean and median values, higher than UC. All 6 tests showed higher mean and median sensitivity when compared with UC.
In terms of specificity, only UroVysion and microsatellite analysis showed mean and median values similar to those of UC, whereas for all other tests, specificity was lower than UC. Mean and median values of false negatives where particularly low using microsatellite, ImmunoCyt, and MCM5 tests (NPV).
3.7. Sensitivity in relation to BC grade
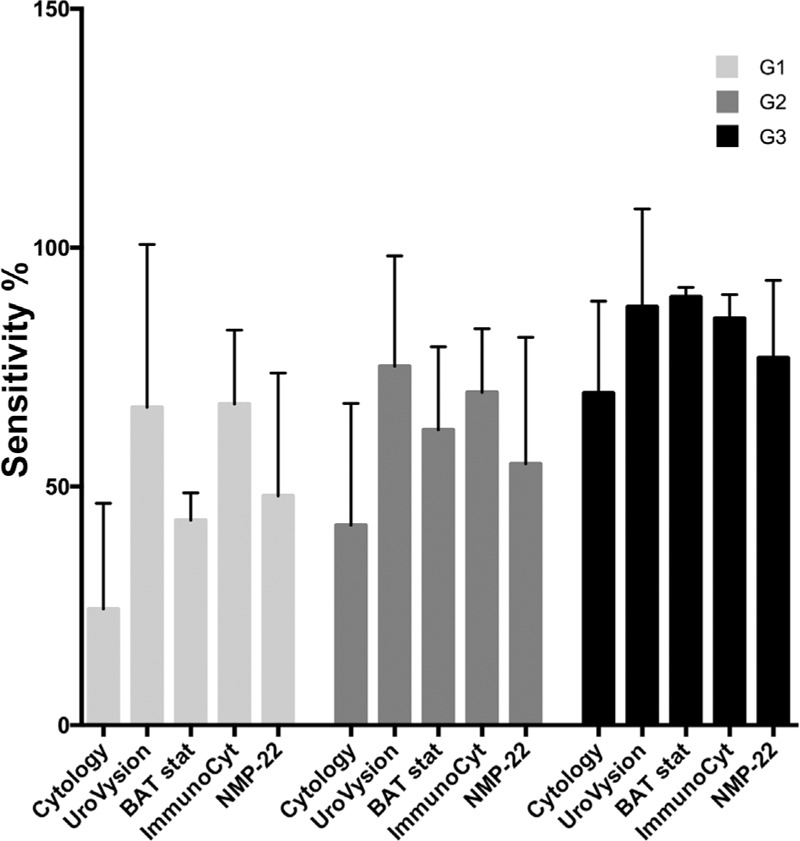
Table 3 shows sensitivity, specificity, NPV, and PPV for each urinary test in relation to the BC histological grade (Table 3) (Fig. 3). No data were found for microsatellite and MCM5 tests.
Table 3.
Sensitivity of different urinary biomarkers to predict BC by histological grade.
| Biomarker n° of studies (n° of pts) | Cytology n = 14 (7585) | UroVysion n = 6 (1997) | BTA stat n = 4 (584) | ImmunoCyt n = 5 (3350) | NMP-22 n = 11 (4867) |
|---|---|---|---|---|---|
| G1 | |||||
| Mean ± SD | 24.4 ± 22.2 | 66.5 ± 34.2 | 42.9 ± 5.7 | 67.3 ± 15.5 | 48.0 ± 25.7 |
| Median | 16.0 | 61.5 | 42.9 | 69.2 | 48.2 |
| Range | 0–67.7 | 20.0–100.0 | 36–50.0 | 47.0–85.7 | 5.1–100.0 |
| G2 | |||||
| Mean ± SD | 41.9 ± 25.5 | 75.2 ± 23.1 | 61.9 ± 17.4 | 69.7 ± 13.3 | 54.8 ± 26.5 |
| Median | 36.1 | 63.0 | 69.1 | 75.0 | 53.3 |
| Range | 16.0–87.0 | 51.4–100.0 | 36.0–73.3 | 47–79.9 | 5.1–100.0 |
| G3 | |||||
| Mean ± SD | 69.6 ± 19.2 | 95.5 ± 7.3 | 89.7 ± 2.0 | 85.2 ± 5.0 | 76.9 ± 16.2 |
| Median | 74.0 | 100.0 | 89.7 | 83.3 | 76.9 |
| Range | 37.5–100.0 | 83.3–100.0 | 87.5–91.7 | 79.0–91.3 | 36.4–100.0 |
pts = patients; SD = standard deviation.
Figure 3.
Sensitivity of different urinary biomarkers in predicting BC according to grade.
It is evident that sensitivity of UC is particularly low in low grade BC. The urinary tests mainly improved sensitivity compared to UC, and ImmunoCyt and UroVysion had the highest improvement in low grade tumors (ImmunoCyt G1 vs. UC G1: mean difference 42.9, 95%CI 3.67–82.13, p = 0.0186; UroVysion G1 vs. UC G1: mean difference 42.2, 95%CI 2.83–81.39, p = 0.0229).
4. Critical analysis and conclusion
A number of noninvasive tests to detect BC have been developed. It is interesting to note that none of these urinary markers have a better sensitivity than cytology but have similar or mainly lower specificity (Table 2) (Fig. 2). These markers can be divided into 2 categories based on whether urine (soluble urine markers: BTA stat, NMP-22) or exfoliated cells (cell-associated markers: microsatellite, ImmunoCyt, UroVysion, MCM5) are used for the assay. [4]
Considering the high rates of variability of results in terms of sensitivity, specificity, NPV, and PPV for each test among the different studies, it is not possible to define a real advantage of one test over the others. A direct comparison among at least 3 different tests in the same study and on the same population was performed in only 3 studies and different tests were compared each time.5,20,23
The sensitivity and specificity of these urinary tests as predictors for an initial diagnosis of BC varied from 16.4–100% to 41.0–100%, respectively. As reported by guidelines, [2] this variability of results was in part related to patient selection and complicated laboratory methods.
The performance of these tests on the basis of BC grades was not clearly reported by most of the studies. Tetu et al. [4] underlined that whereas for BTA stat, NMP-22, and UroVysion tests, the improvement in sensitivity when compared to UC was lower for low grade tumors, ImmunoCyt was able to improve sensitivity for low or high grade BC. In our analysis, Table 3 and Figure 3 show that either ImmunoCyt (p = 0.0186) or UroVysion (p = 0.0229) strongly improved sensitivity in low grade tumors when compared with UC.
In conclusion, the low sensitivity of UC drives interest in new urinary markers for the initial diagnosis of BC that can substitute or be used in combination with UC. Most of the proposed molecular markers are able to improve sensitivity with similar or lower specificity when compared to UC. As of now, none of these markers showed evidences so as to be accepted by international guidelines for the diagnosis of BC. [2]
Acknowledgments
None.
Statement of ethics
This review has been carried out according to the internationally-accepted standards for research practice and reporting.
Conflict of interest statement
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors listed gave a substantive contribution to this study and to this original article. A. Sciarra: conceptualization, writing - original draft preparation; G. Di Lascio: conceptualization, formal analysis and investigation; F. Del Giudice: formal analysis and investigation; P.P. Leoncini: statistical analysis; S. Salciccia: methodology;A. Gentilucci: methodology;A. Porreca: formal analysis and investigation; B.I. Chung: conceptualization; G. Di Pierro: writing - review and editing, G.M. Busetto: formal analysis and investigation; E. De Berardinis: writing - review and editing, M. Maggi: writing - original draft preparation, formal analysis and investigation.
Supplementary Material
References
- [1]. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65 (1):5–29. [DOI] [PubMed] [Google Scholar]
- [2]. Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 2017;71 (3):447–461. [DOI] [PubMed] [Google Scholar]
- [3]. Krajewski W, Kościelska-Kasprzak K, Rymaszewska J, Zdrojowy R. How different cystoscopy methods influence patient sexual satisfaction, anxiety, and depression levels: a randomized prospective trial. Qual Life Res 2017;26 (3):625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Têtu B. Diagnosis of urothelial carcinoma from urine. Mod Pathol 2009;22 Suppl 2:S53–59. [DOI] [PubMed] [Google Scholar]
- [5]. Yafi FA, Brimo F, Steinberg J, Aprikian AG, Tanguay S, Kassouf W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol 2015;33 (2). 66.e25-31. [DOI] [PubMed] [Google Scholar]
- [6]. Raitanen MP, Aine R, Rintala E, et al. Differences between local and review urinary cytology in diagnosis of bladder cancer. An interobserver multicenter analysis. Eur Urol 2002;41 (3):284–289. [DOI] [PubMed] [Google Scholar]
- [7]. Shelfo SW, Soloway MS. The role of nuclear matrix protein 22 in the detection of persistent or recurrent transitional-cell cancer of the bladder. World J Urol 1997;15 (2):107–111. [DOI] [PubMed] [Google Scholar]
- [8]. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293 (7):810–816. [DOI] [PubMed] [Google Scholar]
- [9]. van Rhijn BW, Lurkin I, Chopin DK, et al. Combined microsatellite and FGFR3 mutation analysis enables a highly sensitive detection of urothelial cell carcinoma in voided urine. Clin Cancer Res 2003;9 (1):257–263. [PubMed] [Google Scholar]
- [10]. Fradet Y, Lockhart C. Performance characteristics of a new monoclonal antibody test for bladder cancer: ImmunoCyt trade mark. Can J Urol 1997;4 (3):400–405. [PubMed] [Google Scholar]
- [11]. Halling KC, King W, Sokolova IA, et al. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J Urol 2000;164 (5):1768–1775. [PubMed] [Google Scholar]
- [12]. Bubendorf L, Grilli B, Sauter G, Mihatsch MJ, Gasser TC, Dalquen P. Multiprobe FISH for enhanced detection of bladder cancer in voided urine specimens and bladder washings. Am J Clin Pathol 2001;116 (1):79–86. [DOI] [PubMed] [Google Scholar]
- [13]. Leyh H, Marberger M, Conort P, et al. Comparison of the BTA stat test with voided urine cytology and bladder wash cytology in the diagnosis and monitoring of bladder cancer. Eur Urol 1999;35 (1):52–56. [DOI] [PubMed] [Google Scholar]
- [14]. Pode D, Shapiro A, Wald M, Nativ O, Laufer M, Kaver I. Noninvasive detection of bladder cancer with the BTA stat test. J Urol 1999;161 (2):443–446. [PubMed] [Google Scholar]
- [15]. Stoeber K, Halsall I, Freeman A, et al. Immunoassay for urothelial cancers that detects DNA replication protein Mcm5 in urine. Lancet 1999;354 (9189):1524–1525. [DOI] [PubMed] [Google Scholar]
- [16]. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155 (8):529–536. [DOI] [PubMed] [Google Scholar]
- [17]. Stoeber K, Swinn R, Prevost AT, et al. Diagnosis of genito-urinary tract cancer by detection of minichromosome maintenance 5 protein in urine sediments. J Natl Cancer Inst 2002;94 (14):1071–1079. [DOI] [PubMed] [Google Scholar]
- [18]. Kelly JD, Dudderidge TJ, Wollenschlaeger A, et al. Bladder cancer diagnosis and identification of clinically significant disease by combined urinary detection of Mcm5 and nuclear matrix protein 22. PLoS One 2012;7 (7):e40305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Brems-Eskildsen AS, Zieger K, Toldbod H, et al. Prediction and diagnosis of bladder cancer recurrence based on urinary content of hTERT, SENP1, PPP1CA, and MCM5 transcripts. BMC Cancer 2010;10 (1):646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Toma MI, Friedrich MG, Hautmann SH, et al. Comparison of the ImmunoCyt test and urinary cytology with other urine tests in the detection and surveillance of bladder cancer. World J Urol 2004;22 (2):145–149. [DOI] [PubMed] [Google Scholar]
- [21]. Deininger S, Todenhöfer T, Hennenlotter J, et al. Impact of variant microscopic interpretation of the uCyt+ immunocytological urine test for the detection of bladder cancer. Diagn Cytopathol 2018;46 (2):111–116. [DOI] [PubMed] [Google Scholar]
- [22]. Comploj E, Mian C, Ambrosini-Spaltro A, et al. uCyt+/ImmunoCyt and cytology in the detection of urothelial carcinoma: an update on 7422 analyses. Cancer Cytopathol 2013;121 (7):392–397. [DOI] [PubMed] [Google Scholar]
- [23]. Todenhöfer T, Hennenlotter J, Esser M, et al. Combined application of cytology and molecular urine markers to improve the detection of urothelial carcinoma. Cancer Cytopathol 2013;121 (5):252–260. [DOI] [PubMed] [Google Scholar]
- [24]. Ritter R, Hennenlotter J, Kühs U, et al. Evaluation of a new quantitative point-of-care test platform for urine-based detection of bladder cancer. Urol Oncol 2014;32 (3):337–344. [DOI] [PubMed] [Google Scholar]
- [25]. Bangma CH, Loeb S, Busstra M, et al. Outcomes of a bladder cancer screening program using home hematuria testing and molecular markers. Eur Urol 2013;64 (1):41–47. [DOI] [PubMed] [Google Scholar]
- [26]. Wild PJ, Fuchs T, Stoehr R, et al. Detection of urothelial bladder cancer cells in voided urine can be improved by a combination of cytology and standardized microsatellite analysis. Cancer Epidemiol Biomarkers Prev 2009;18 (6):1798–1806. [DOI] [PubMed] [Google Scholar]
- [27]. Cha EK, Tirsar LA, Schwentner C, et al. Immunocytology is a strong predictor of bladder cancer presence in patients with painless hematuria: a multicentre study. Eur Urol 2012;61 (1):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Friedrich MG, Hellstern A, Hautmann SH, et al. Clinical use of urinary markers for the detection and prognosis of bladder carcinoma: a comparison of immunocytology with monoclonal antibodies against Lewis X and 486p3/12 with the BTA STAT and NMP22 tests. J Urol 2002;168 (2):470–474. [DOI] [PubMed] [Google Scholar]
- [29]. Giannopoulos A, Manousakas T, Gounari A, Constantinides C, Choremi-Papadopoulou H, Dimopoulos C. Comparative evaluation of the diagnostic performance of the BTA stat test, NMP22 and urinary bladder cancer antigen for primary and recurrent bladder tumors. J Urol 2001;166 (2):470–475. [PubMed] [Google Scholar]
- [30]. Gutiérrez Baños JL, Rebollo Rodrigo MH, Antolín Juárez FM, Martín García B. NMP 22, BTA stat test and cytology in the diagnosis of bladder cancer: a comparative study. Urol Int 2001;66 (4):185–190. [DOI] [PubMed] [Google Scholar]
- [31]. Smrkolj T, Mihelič M, Sedlar A, Sterle I, Osredkar J, Sedmak B. Performance of nuclear matrix protein 22 urine marker and voided urine cytology in the detection of urinary bladder tumors. Clin Chem Lab Med 2011;49 (2):311–316. [DOI] [PubMed] [Google Scholar]
- [32]. Virk RK, Abro S, de Ubago JMM, et al. The value of the UroVysion® FISH assay in the risk-stratification of patients with “atypical urothelial cells” in urinary cytology specimens. Diagn Cytopathol 2017;45 (6):481–500. [DOI] [PubMed] [Google Scholar]
- [33]. Schlomer BJ, Ho R, Sagalowsky A, Ashfaq R, Lotan Y. Prospective validation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. J Urol 2010;183 (1):62–67. [DOI] [PubMed] [Google Scholar]
- [34]. Lotan Y, Bensalah K, Ruddell T, Shariat SF, Sagalowsky AI, Ashfaq R. Prospective evaluation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. J Urol 2008;179 (6):2164–2169. [DOI] [PubMed] [Google Scholar]
- [35]. Gopalakrishna A, Fantony JJ, Longo TA, et al. Anticipatory positive urine tests for bladder cancer. Ann Surg Oncol 2017;24 (6):1747–1753. [DOI] [PubMed] [Google Scholar]
- [36]. Dimashkieh H1, Wolff DJ, Smith TM, Houser PM, Nietert PJ, Yang J. Evaluation of urovysion and cytology for bladder cancer detection: a study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol 2013;121 (10):591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Halling KC, King W, Sokolova IA, et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J Urol 2002;167 (5):2001–2006. [PubMed] [Google Scholar]
- [38]. Kehinde EO, Al-Mulla F, Kapila K, Anim JT. Comparison of the sensitivity and specificity of urine cytology, urinary nuclear matrix protein-22 and multitarget fluorescence in situ hybridization assay in the detection of bladder cancer. Scand J Urol Nephrol 2011;45 (2):113–121. [DOI] [PubMed] [Google Scholar]
- [39]. Daniely M1, Rona R, Kaplan T, et al. Combined analysis of morphology and fluorescence in situ hybridization significantly increases accuracy of bladder cancer detection in voided urine samples. Urology 2005;66 (6):1354–1359. [DOI] [PubMed] [Google Scholar]
- [40]. Soyuer I, Sofikerim M, Tokat F, Soyuer S, Ozturk. Which urine marker test provides more diagnostic value in conjunction with standard cytology–ImmunoCyt/uCyt+ or Cytokeratin 20 expression. Diagn Pathol 2009;4 (1):20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Sankhwar M, Singh R, Sankhwar SN, Goel MM, Jain A, Sankhwar PL. Nuclear matrix protein 22 in voided urine cytology efficacy in risk stratification for carcinoma of bladder. World J Oncol 2013;4 (3):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [42]. Jeong S, Park Y, Cho Y, Kim YR, Kim HS. Diagnostic values of urine CYFRA21-1, NMP22, UBC, and FDP for the detection of bladder cancer. Clin Chim Acta 2012;414:93–100. [DOI] [PubMed] [Google Scholar]
- [43]. Doğan C, Pelit ES, Yildirim A, et al. The value of the NMP22 test for superficial bladder cancer diagnosis and follow-up. Turk J Urol 2013;39 (3):137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Liang JF, Zheng HX, Li N, et al. Fluorescent microsatellite analysis of urine sediment in patients with urothelial carcinoma. Urol Int 2010;85 (3):296–303. [DOI] [PubMed] [Google Scholar]
- [45]. Song MJ, Lee HM, Kim SH. Clinical usefulness of fluorescence in situ hybridization for diagnosis and surveillance of bladder cancer. Cancer Genet Cytogenet 2010;198 (2):144–150. [DOI] [PubMed] [Google Scholar]
- [46]. Hwang EC, Choi HS, Jung SI, Kwon DD, Park K, Ryu SB. Use of the NMP22 BladderChek test in the diagnosis and follow-up of urothelial cancer: a cross-sectional study. Urology 2011;77 (1):154–159. [DOI] [PubMed] [Google Scholar]
- [47]. Sagnak L, Ersoy H, Gucuk O, Ozok U, Topaloglu H. Diagnostic value of a urine-based tumor marker for screening lower urinary tract in low-risk patients with asymptomatic microscopic hematuria. Urol Int 2011;87 (1):35–41. [DOI] [PubMed] [Google Scholar]
- [48]. Breen V, Kasabov N, Kamat AM, et al. A holistic comparative analysis of diagnostic tests for urothelial carcinoma: a study of Cxbladder Detect, UroVysion® FISH, NMP22® and cytology based on imputation of multiple datasets. BMC Med Res Methodol 2015;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. O'Sullivan P, Sharples K, Dalphin M, et al. A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J Urol 2012;188 (3):741–747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


