Abstract
Background:
The aim of this study is to evaluate the alterations in bone mineral density and other surrogate markers for osteoporosis in obese patients with type 2 diabetes mellitus (T2DM) who received Roux-en-Y gastric bypass (RYGB) versus medical treatment as control.
Methods:
We searched 4 electronic databases and reference lists of relevant studies for eligible research published before December, 2019. After quality assessment, eligible studies were synthesized for relevant outcomes, including lumbar spine bone mineral density (L-spine BMD) change, total hip BMD change, osteocalcin level, C-terminal telopeptide level, and parathyroid hormone level.
Results:
Three randomized clinical trials and 2 observational studies concerning 307 total obese T2DM patients were included. Follow-up ranged from 12 to 60 months. Patients underwent RYGB surgery were associated with both higher L-spine BMD loss (mean difference: −2.90, 95% CI: −2.99∼−2.81, P < .00001) and total hip BMD loss (mean difference: −5.81, 95% CI: −9.22∼−2.40, P = .0008). As to biochemical markers of bone metabolism, we found significantly higher osteocalcin level in medical treatment (control) group compared with RYGB group (mean difference: 11.16, 95% CI: 8.57–13.75, P < .00001). However, higher C-terminal telopeptide level and parathyroid hormone level were noted in medical treatment group (control) compared with RYGB group (mean difference: 0.29, 95% CI: 0.11–0.48, P = .002; mean difference: 1.56, 95% CI: 0.84–2.27, P < .0001).
Conclusions:
RYGB surgery is associated with negative impact on bone metabolism and increase the risk of osteoporosis in obese patients with T2DM. We suggest that clinicians acknowledge the adverse effects of surgery and keep monitoring bone mineral components in post-RYGB populations. Further studies regarding the optimal amount of perioperative and postsurgical supplementation should be evaluated.
Keywords: bariatric surgery, bone markers, bone metabolism, medical therapy, obesity, roux-en-Y gastric bypass, type 2 diabetes mellitus
1. Introduction
Obesity is an emerging epidemiological health crisis. In 2015, approximately 108 million children and 604 million adults were classified obese globally, with the associated comorbidities such as cardiovascular disease and diabetes mellitus also increasing.[1] Improving metabolic syndromes can efficiently decrease the incidence of major adverse cardiovascular events in the future.[2] The use of bariatric surgical procedure has dramatically increased worldwide in the last decade and has helped obese patients achieve weight reduction.[3] Obese patients who undergo bariatric surgery have lower long-term mortality rates[4] and improved renal functions.[5]
Among all types of bariatric surgery, Roux-en-Y gastric bypass (RYGB) is one of the most commonly performed procedures.[6] In RYGB, a small gastric pouch limits oral intake, and the small bowel is reconfigured. This reconfiguration then provides additional mechanisms, such as dumping physiology, positive hormonal changes, and mild malabsorption, which are all efficacious for weight reduction. Literature reviews showed that compared to medical treatment, RYGB resulted in higher type 2 diabetes mellitus (T2DM) remission rates and improved serum levels of high-density lipoprotein cholesterol.[7] However, evidence revealed unexpected adverse effects of RYGB such as loss in bone density and high bone turnover rates after surgery.[8] Based on the physiological effects of the food bypassing a great part of the stomach, duodenum, and proximal jejunum following RYGB, which leads to decreased absorption of minerals and fat-soluble vitamins,[9,10] patients who underwent RYGB were advised to take supplements of vitamin D, vitamin B12, calcium, iron, and multivitamins for the rest of their lives.[11–13] The relationship between dysregulation of bone metabolism and RYGB has been reported in several studies.[14,15] However, a consensus has not been reached regarding the deleterious impact of bariatric surgery on bone metabolism.[11] Therefore, we aimed to meta-analyze randomized controlled trial and cohort studies to evaluate the alterations in bone mineral density and other surrogate markers for osteoporosis in obese patients with T2DM who received RYGB versus medical treatment as a control.
2. Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic reviews and Meta-analyses guidelines.[16] Because this meta-analysis synthesized data from published articles, this study was exempt from institutional review board approval. The systematic review was accepted by the online PROSPERO international prospective register of systematic reviews of the National Institute for Health Research.
2.1. Search strategy
We conducted a systematic review of the English-language literature published until December 2019 by searching relevant keywords in PubMed, EMBASE, Cochrane Library, Cochrane Clinical Trials Registry, and Web of Science electronic databases. The following terms and Boolean operator were used in MeSH and free-text searches: (bariatric surgery OR obesity surgery OR metabolic surgery OR Roux-en-Y gastric bypass), (medical therapy OR nonsurgical treatment), (type 2 diabetes), AND (bone OR bone markers OR bone turnover). The “related articles” facility in PubMed was used to broaden the search. We attempted to identify additional studies by searching the reference lists of relevant papers and contacting known experts in the field.
2.2. Selection criteria
We reviewed randomized control trials, retrospective and prospective cohort studies, case reports, letters, and reviews in the literature that evaluated the outcomes of RYGB, performed laparoscopically or open, versus medical treatment in obese patient with T2DM. The inclusion criteria were as follows: investigated participants with obesity and T2DM, interventions that compared groups between RYGB and medical treatment, and outcomes regarding bone metabolism. The details of study characteristics are shown in Table 1. The exclusion criteria were as follows: studies did not meet the inclusion criteria, only compared groups between RYGB and sleeve gastrectomy, did not directly evaluate the parameters of bone turnover, did not clearly state the outcomes of alterations in bone metabolism, involved the duplicate reporting of patient cohorts, and animal studies.
Table 1.
Characteristics of studies that fulfilling the meta-analysis inclusion criteria.
| Author [year] | Study design [country] | Population | Number of patients | Age, year | Duration fromRYGB, year | Postsurgical supplement |
| Crawford [2018] | RCT [USA] | Obesity + T2DM | RYGB: 37IMT: 25 | RYGB: 47.4 ± 8.8∗IMT: 51.0 ± 7.6∗ | 5 | Vitamin D and calcium; (compliance unknown) |
| Tangalakis [2019] | Prospective cohort [USA] | Obesity + T2DM | RYGB: 24DSE: 27 | RYGB: 49.6 ± 1.4∗DSE: 47.4 ± 1.5∗ | 1 | Vitamin D 800 IU/d Calcium carbonate 1200 mg/d |
| Madsen [2019] | Retrospective cohort [Denmark] | Obesity + T2DM | RYGB: 96control: 49 | RYGB: 55.3 (48.1; 61.8) †Control: 56.2 (51.1; 60.3) † | 6.1 (5.4; 7.0)‡ | Vitamin D and calcium (86% patients followed the instruction) |
| Crawford [2017] | RCT [USA] | Obesity + T2DM | RYGB: 10IMT: 4 | 50 ± 7∗ | 6.7 ± 1.3∗ | Not mentioned |
| Maghrabi [2015] | Prospective cohort [USA] | Obesity + T2DM | RYGB: 18IMT: 17 | 48 ± 4∗ | 2 | Ergocalciferol 50000 IU/wk (40% patients followed) Vitamin D3 2000 IU/d (30% patients followed) Vitamin D3 1000 IU/d (30% patients followed) |
After the final comprehensive search, 2 researchers independently searched through literature, selected studies, assessed quality, and extracted and verified data from articles. Any disagreement was resolved by consulting a third reviewer.
2.3. Methodological quality appraisal
Two reviewers independently appraised the methodological quality of each study using the Cochrane Risk of Bias tool (Bristol, UK) [17] for randomized control trials and Modified Newcastle-Ottawa Quality Assessment Scale [18] for cohort studies. Using the Cochrane Risk of Bias tool, we evaluated sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases in randomized clinical trials. For cohort studies, representativeness of the cohort, comparability based on the design or analysis of the cohort, assessment of outcome, as well as overall power were assessed. In cases of any conflict, the third reviewer's comments should be obtained, and further discussion was performed until a final consensus.
2.4. Outcome assessment
The outcome data included lumbar spine bone mineral density change, total hip bone mineral density (BMD) change, osteocalcin level, C-terminal telopeptide (CTX) level, and parathyroid hormone (PTH) level. Means and standard deviations were extracted for continuous data. When the original study reported a 95% confidence interval (CI), we calculated the standard deviation (SD) according to the formula: 95% CI = x̄ ± 1.96 (SD/√n).
2.5. Statistical analyses
Statistical analysis was conducted using the Review Manager software version 5.3 (Cochrane Collaboration, Oxford, England, UK). The precision of an effective size was reported as 95% CI. The data were pooled only for studies exhibiting adequate clinical and methodological similarities. Statistical heterogeneity was assessed using the I2 test that quantified the proportion of the total outcome variability that was attributable to variability among the studies. When I2 was higher than 50%, the pooled result was judged as having high heterogeneity.
3. Results
3.1. Characteristics of the studies
This systematic review identified 394 references from 4 electronic databases. The references were from PubMed (n = 94), EMBASE (n = 272), Cochrane Library (n = 20), and Web of Science (n = 8). A total of 229 duplications were eliminated using the Endnote system and manual screening. After the screening of titles and abstracts, 150 studies were deemed ineligible and were excluded because they were not relevant (Fig. 1). Next, we retrieved the full text of the 15 remaining studies for further review. Ten articles were excluded from our final analysis for the following reasons: 8 used different comparisons and 2 revealed inappropriate measurements. The remaining 5 eligible studies were included in our analysis,[19–23] and their characteristics are shown in Table 1. The 5 studies were published between 2015 and 2018, with sample sizes ranging from 14 to 145 patients. Three of them are randomized control trials,[19–21] whereas the other 2 are observational studies.[22,23] Four of the studies were conducted in the US,[19–21,23] and one was conducted in Denmark.[22] The mean age in each study ranged from 47 to 56, and the evaluation of final outcome was conducted between 1 to 5 years after RYGB surgery. The quality of the included studies is shown in Table 2.
Figure 1.
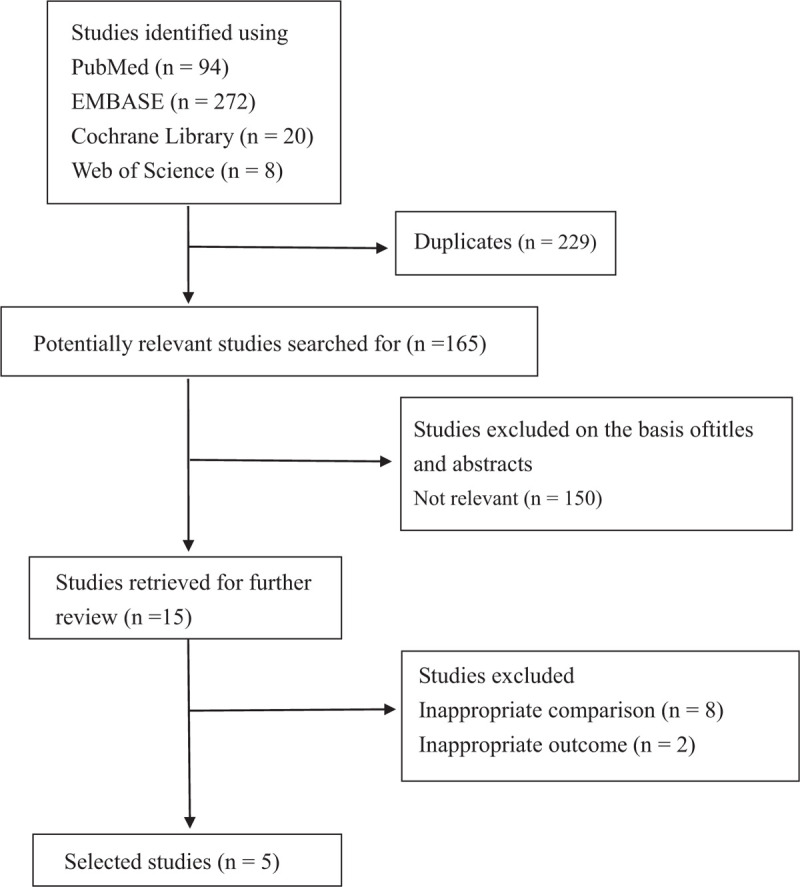
Flowchart of the study inclusion criteria.
Table 2.
Assessment of methodological quality of included studies.
| RCTs evaluated by RoB 2.0 | |||
| RCT [year] | Crawford [2018] | Crawford [2017] | Maghrabi [2015] |
| Selection bias | Low risk | Moderate risk∗ | Low risk |
| Allocation bias | Low risk | Low risk | Low risk |
| Performance bias | Moderate risk† | Moderate risk† | Moderate risk† |
| Detection bias | Moderate risk† | Moderate risk† | Moderate risk† |
| Attrition bias | Moderate risk‡ | Moderate risk‡ | Moderate risk‡ |
| Reporting bias | Low risk | Low risk | Low risk |
| Overall risk of bias | Moderate | Moderate | Moderate |
| Observational studies evaluated by Modified Newcastle-Ottawa Scale | |||
| Author [year] | Tangalakis [2019] | Madsen [2019] | |
| Selection | Representativeness of exposed cohort (Maximum:★) | ★ | ★ |
| Selection of non-exposed cohort (Maximum:★) | ★ | ★ | |
| Ascertainment of exposure (Maximum:★) | ★ | ★ | |
| Comparability | On the basis of the design or analysis (Maximum:★★)§ | ★ | ★★ |
| Outcome | Assessment of outcome (Maximum:★) | ★ | ★ |
| Adequacy of follow up of cohort (Maximum:★) | – | ★ | |
| Total score (out of 7) | ★★★★★(5) | ★★★★★★(6) | |
| Power | Adequately powered | Adequately powered | |
3.2. Lumbar spine bone mineral density change
Two studies reported lumbar spine bone mineral density changes in 86 patients from baseline to 1 year after surgery. The overall pooling result demonstrated higher lumbar spine bone mineral density changes in the RYGB group than in the medical treatment (control) group after 1-year follow-up, with the pooled mean difference of −2.90 (95% CI: −2.99∼−2.81). The results indicated no significant heterogeneity across the studies (I2 = 0%, P < .00001) (Fig. 2).
Figure 2.
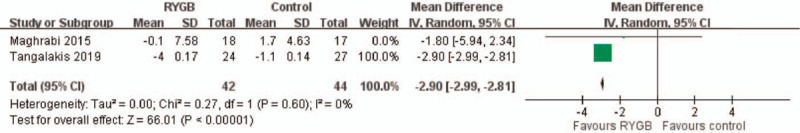
L-spine BMD change. The pooling result demonstrated significantly higher L-spine BMD change in RYGB group compared with medical treatment (control) group after 1 year follow-up. (Mean difference: −2.90; 95% CI: −2.99∼− 2.81).
3.3. Total hip bone mineral density change
Three studies reported total hip BMD changes from baseline to 1 year after surgery. The overall pooling result demonstrated higher total hip BMD changes in the RYGB group than in the medical treatment (control) group after 1 year follow-up, with the pooled mean difference of −5.81 (95% CI: −9.22∼−2.40). The results indicated no significant heterogeneity across the studies (I2 = 84%, P = .0008) (Fig. 3).
Figure 3.
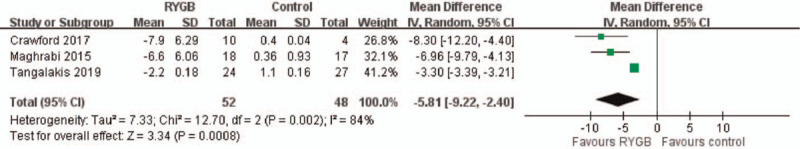
Total hip BMD change. The pooling result revealed significantly higher total hip BMD change in RYGB group compared with medical treatment (control) group after 1 year follow- up. (Mean difference: −5.81, 95% CI: −9.22∼−2.40).
3.4. Osteocalcin level
Two studies reported osteocalcin levels 5 years after surgery. The overall pooling result demonstrated higher osteocalcin levels in the medical treatment (control) group than in the RYGB group, with the pooled mean difference of 11.16 (95% CI: 8.57–13.75). The results indicated no significant heterogeneity across the studies (I2 = 0%, P < .00001) (Fig. 4).
Figure 4.
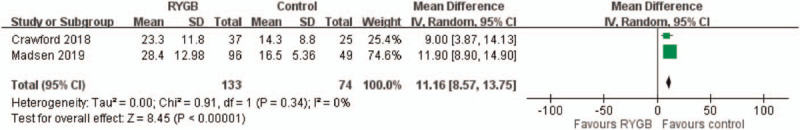
Osteocalcin level. Significantly higher osteocalcin level was found in medical treatment (control) group compared with RYGB group, with the pooled mean difference of 11.16. (95% CI: 8.57–13.75).
3.5. C-terminal telopeptide level
Three studies reported CTX levels from 1 to 5 years after surgery. The overall pooling result demonstrated significantly higher CTX levels in the medical treatment group (control) than in the RYGB group, with the pooled mean difference of 0.29 (95% CI: 0.11–0.48). The results indicated no significant heterogeneity across the studies (I2 = 90%, P = .002) (Fig. 5).
Figure 5.
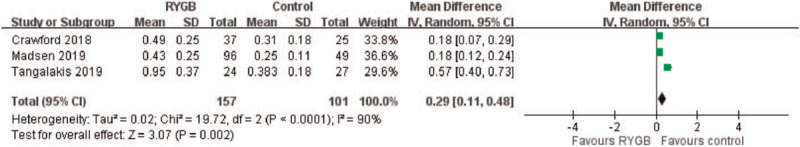
C-terminal telopeptide (CTX) level. Significantly higher CTX level was found in medical treatment group (control) compared with RYGB group, with the pooled mean difference of 0.29. (95% CI: 0.11–0.48).
3.6. Parathyroid hormone level
Two studies reported PTH levels 5 years after surgery. The overall pooling result demonstrated higher PTH levels in the medical treatment group (control) than in the RYGB group, with the pooled mean difference of 1.56 (95% CI: 0.84–2.27). The results indicated no significant heterogeneity across the studies (I2 = 0%, P < .0001) (Fig. 6).
Figure 6.
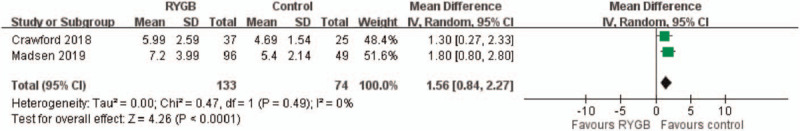
Parathyroid hormone (PTH) level. The overall pooling result showed significantly higher PTH level in medical treatment group (control) compared with RYGB group. (Mean difference: 1.56; 95% CI: 0.84–2.27).
4. Discussion
This study demonstrates that obese patients with T2DM who underwent RYGB surgery have higher bone loss when compared to those who received medical treatment alone. To our knowledge, this is the first meta-analysis study focusing on bone metabolism in patients with T2DM following RYGB versus medical treatment. Our findings are also consistent among previous randomized clinical trials and observational studies.
In the recent meta-analysis conducted, beneficial effects of RYGB surgery on T2DM remission were superior to medical treatment alone, with regard to improvement in obesity, hyperglycemia, hyperlipidemia, arterial hypertension, and other metabolic conditions as well as cardiovascular risks.[7] However, the potential adverse effects of bariatric surgery on bone metabolism are still debated, even though the association between bariatric surgery and reduced bone density or increased fracture risk has been proven in several studies.[24–26] According to the results of our meta-analysis, BMD loss at both hip and lumbar spine are greater in the RYGB group than in the medical treatment (control) group, and the reductions in total hip and lumbar spine BMD appear to extend beyond 5 years after surgery. Furthermore, positive site-specific BMD impact was greater in total hip BMD than in lumbar spine BMD in some of our included studies.[20–22] The greater postsurgical losses of BMD in the hip than in the vertebral spine could be because of the catabolic effect on cortical bones caused by secondary hyperparathyroidism following nutritional deficiency,[27,28] and the reduced mechanical loading at the hip due to weight loss after RYGB. We recommend clinicians keep follow-up patient's BMD level at least 5 years after operation based on current evidence in our included studies. The impact on fracture risk should be considered by patients considering bariatric surgery and should also be evaluated in further long-term studies.
Besides BMD, biochemical markers of bone metabolism have proven useful for monitoring treatment efficacy in metabolic bone disease.[29] After a comprehensive review, several bone markers including osteocalcin, CTX, PTH showed the most significance. Osteocalcin, holds particular interest because low levels of it are associated with increased fracture incidence in older male populations.[30] Osteocalcin levels were higher in the medical treatment (control) group than in the RYGB group. This finding appears to be compatible with the BMD change in both groups because osteocalcin is secreted by osteoblasts to be primarily integrated into bone matrix.
The bone resorption marker, CTX, has been studied extensively in relation to surgical weight loss. Many studies have shown increases in CTX after RYGB, ranging from 174% at 3 months post-intervention to 220% at one year when compared with baseline levels.[28,31] In our study, however, CTX levels were higher in the medical treatment group than in the RYGB group. This might be because of the post-surgical implementation of daily calcium and vitamin D supplementation based on current guidelines in most RYGB groups [Table 1], whereas there was no nutritional supplementation in the medical treatment groups. As the RYGB procedure comprised gastric restriction and intestinal bypass, malabsorption of micronutrients such as vitamins, calcium, zinc, and iron might become an issue after surgery. Adequate supplementation of calcium and vitamin D could maintain the balance of bone metabolism and thus prevent bone resorption and osteoporosis. Low concentrations of vitamin D are associated with impaired calcium absorption, negative calcium balance, and a compensatory rise in PTH, which results in excessive bone resorption. Since no supplementation was given, it is reasonable to expect higher CTX levels in the medical treatment (control) group than in the RYGB group. Similarly, higher PTH levels are noted in the medical treatment (control) group, possibly because of decreased absorption of calcium and vitamin D. A prospective study found that obese patients undergoing either RYGB or sleeve gastrectomy treated with pre- and post-surgical vitamin D, protein supplementation, and regimented exercise had a smaller degree of CTX elevation than their post-surgical counterparts who did not undergo this intervention after 24 months of follow-up.[32] Therefore, supplementation with these nutrients is needed, even though there is no consensus on the optimal amount to provide after bariatric surgery. It is important to note that biochemical markers of bone metabolism should be monitored long term after bariatric surgery, since the prolonged increase in bone turnover might place the skeleton at risk for loss of bone mass and fracture. Besides adequate vitamin D and calcium supplementation, further preventative measures such as physical activity programs and professional nutrition monitoring should also be planned . Though there are currently no evidence-based physical activity guidelines specifically for bariatric surgery patients, growing evidence shows that physical activity has played a significant role in weight loss and other postoperative outcomes.[33] Recommendations for physical activity have been made by a number of organizations, including the American Society for Metabolic and Bariatric Surgery (ASMBS), the Obesity Society, and the American Heart Association. We advocate for appropriate physical activities for bariatric surgery patients based on the common guidelines, and recommend that they should adhere to a healthful lifestyle.
There are some limitations of our study. First, potential selection bias in some of our included studies regarding the different baselines of two groups such as the diabetes duration and HbA1c levels was noted. Second, some studies lack details regarding patients’ compliance to the post-surgical vitamin D and calcium supplementation, and not all patients followed the instructions of supplementation in some studies. Third, bone turnover is known to relate to age and gender. Though the mean ages of included patients were similar, gender-matched studies should be evaluated, especially in women. We had tried to perform subgroup meta-analyses based on pre, peri and post-menopausal status of female subjects, but unfortunately failed due to lack of data. Moreover, bone markers are highly dynamic when compared to BMD; thus, the value of single marker measurement taken at one time point might be questionable. Longitudinal changes of other associated parameters for bone health such as renal calcium excretion, gastrointestinal hormones, high-calcium food intake, and daily exercise should also be evaluated because they might provide a better understanding of the underlying mechanisms for bone quality. Last, our included studies did not provide fine details about postoperative adjunct measures such as physical activity programs or professional nutrition monitoring which might have positive impact on postoperative outcome.
5. Conclusion
Our study examined the negative impact of RYGB surgery on bone turnover when compared with the impact of medical treatment alone on bone turnover. We suggest that clinicians acknowledge the potential risk of adverse effects of RYGB on bone health and continue monitoring bone mineral components in post-RYGB populations. Further investigation regarding the optimal amount of post-surgical supplementation is important. Additional research of perioperative supplementation and bone health might also be essential to understand the association between bariatric surgery and bone metabolism.
Author contributions
Data curation: Tzu-Wen Huang, Chih-Chin Kao.
Formal analysis: Tzu-Wen Huang.
Investigation: Jing-Yi Chen, Shu-Ching Yeh.
Methodology: Yueh-Lin Wu.
Supervision: Chih-Chin Kao, Yueh-Lin Wu, Shu-Ching Yeh.
Writing – original draft: Tzu-Wen Huang, Yen-Chung Lin.
Writing – review & editing: Jing-Yi Chen, Yen-Chung Lin.
Footnotes
Abbreviations: L-spine BMD = bone mineral density, CTX = C-terminal telopeptide, PTH = parathyroid hormone, RYGB = Roux-en-Y gastric bypass, T2DM = Type 2 diabetes mellitus.
How to cite this article: Huang TW, Chen JY, Wu YL, Kao CC, Yeh SC, Lin YC. Alterations of bone markers in obese patients with type 2 diabetes after bariatric surgery: a meta-analysis and systemic review of randomized controlled trials and cohorts. Medicine. 2021;100:20(e26061).
T-WH, J-YC, and Y-CL contributed equally to this work.
The authors have no conflicts of interests to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
DSE = diabetes support and education program, IMT = intensive medical treatment, RCT = randomized clinical trial, RYGB = Rou-en Y gastric bypass, T2DM = type 2 diabetes mellitus.
Indicates mean ± SD.
Indicates mean (IQR).
Indicates median (IQR).
Without sufficient information.
Without double blind.
Without using intention-to-treat analysis.
One point was allocated if there was no adjustment in the study, with an additional point given if adjusted for HbA1c, calcium, and vitamin D supplementation, PTH, gender and menopausal status, and smoking status. The total score was derived using the minimum score allocated for confounders.
References
- [1].Afshin A, Forouzanfar MH, et al. GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Park S, Lee S, Kim Y, et al. Altered risk for cardiovascular events with changes in the metabolic syndrome status: a nationwide population-based study of approximately 10 million persons. Ann Intern Med 2019;171:875–84. [DOI] [PubMed] [Google Scholar]
- [3].Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg 2013;23:427–36. [DOI] [PubMed] [Google Scholar]
- [4].Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015;313:62–70. [DOI] [PubMed] [Google Scholar]
- [5].Lin YC, Lai YJ, Lin YC, et al. Effect of weight loss on the estimated glomerular filtration rates of obese patients at risk of chronic kidney disease: the RIGOR-TMU study. J Cachexia Sarcopenia Muscle 2019;10:756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg 2017;27:2279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yan Y, Sha Y, Yao G, et al. Roux-en-Y gastric bypass versus medical treatment for Type 2 diabetes mellitus in obese patients. A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res 2014;29:1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schafer AL, Weaver CM, Black DM, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res 2015;30:1377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition 2009;25:1150–6. [DOI] [PubMed] [Google Scholar]
- [11].Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int 2014;25:423–39. [DOI] [PubMed] [Google Scholar]
- [12].Via MA, Mechanick JI. Nutritional and micronutrient care of bariatric surgery patients: current evidence update. Curr Obes Rep 2017;6:286–96. [DOI] [PubMed] [Google Scholar]
- [13].Madsen LR, Espersen R, Rejnmark L, et al. Effect of calcium citrate vs calcium carbonate on elevated parathyroid hormone after Roux-en-Y gastric bypass. A double-blinded, randomized trial. Clin Endocrinol (Oxf) 2018;89:734–41. [DOI] [PubMed] [Google Scholar]
- [14].Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 2007;18:427–44. [DOI] [PubMed] [Google Scholar]
- [15].Leslie WD, Rubin MR, Schwartz AV, et al. Type 2 diabetes and bone. J Bone Miner Res 2012;27:2231–7. [DOI] [PubMed] [Google Scholar]
- [16].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [19].Crawford MR, Pham N, Khan L, et al. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery versus medical therapy at 5 years. Endocr Pract 2018;24:256–64. [DOI] [PubMed] [Google Scholar]
- [20].Crawford MR, Bena JF, Schauer PR, et al. Long term outcomes of bariatric surgery on bone density in obese patients with type 2 diabetes. J Diabetes Complications 2017;31:780–1. [DOI] [PubMed] [Google Scholar]
- [21].Maghrabi AH, Wolski K, Abood B, et al. Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity (Silver Spring) 2015;23:2344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Madsen LR, Espersen R, Ornstrup MJ, et al. Bone health in patients with type 2 diabetes treated by Roux-En-Y gastric bypass and the role of diabetes remission. Obes Surg 2019;29:1823–31. [DOI] [PubMed] [Google Scholar]
- [23].Tangalakis LL, Tabone L, Spagnoli A, et al. Effects of Roux-en-Y gastric bypass on osteoclast activity and bone density in morbidly obese patients with type 2 diabetes. Obes Surg 2020;30:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lu CW, Chang YK, Chang HH, et al. Fracture risk after bariatric surgery: a 12-year nationwide cohort study. Medicine (Madr) 2015;94:e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harper C, Pattinson AL, Fernando HA, et al. Effects of obesity treatments on bone mineral density, bone turnover and fracture risk in adults with overweight or obesity. Horm Mol Biol Clin Investig 2016;28:133–49. [DOI] [PubMed] [Google Scholar]
- [26].Rodríguez-Carmona Y, López-Alavez FJ, González-Garay AG, et al. Bone mineral density after bariatric surgery. A systematic review. Int J Surg 2014;12:976–82. [DOI] [PubMed] [Google Scholar]
- [27].Zibellini J, Seimon RV, Lee CM, et al. Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J Bone Miner Res 2015;30:2168–78. [DOI] [PubMed] [Google Scholar]
- [28].Stein EM, Carrelli A, Young P, et al. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab 2013;98:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cavalier E, Bergmann P, Bruyère O, et al. The role of biochemical of bone turnover markers in osteoporosis and metabolic bone disease: a consensus paper of the Belgian Bone Club. Osteoporos Int 2016;27:2181–95. [DOI] [PubMed] [Google Scholar]
- [30].Chubb SA, Byrnes E, Manning L, et al. Reference intervals for bone turnover markers and their association with incident hip fractures in older men: the Health in Men study. J Clin Endocrinol Metab 2015;100:90–9. [DOI] [PubMed] [Google Scholar]
- [31].Biagioni MFG, Mendes AL, Nogueira CR, et al. Bariatric Roux-en-Y gastric bypass surgery: adipocyte proteins involved in increased bone remodeling in humans. Obes Surg 2017;27:1789–96. [DOI] [PubMed] [Google Scholar]
- [32].Muschitz C, Kocijan R, Haschka J, et al. The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: the BABS study. J Bone Miner Res 2016;31:672–82. [DOI] [PubMed] [Google Scholar]
- [33].King WC, Bond DS. The importance of preoperative and postoperative physical activity counseling in bariatric surgery. Exerc Sport Sci Rev 2013;41:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


