Abstract
Psychotropic drugs are frequently used for functional dyspepsia (FD); however, the efficacy of these drugs for treating FD remains controversial. We aimed to comprehensively compare the relative efficacies of different psychotropic drugs for FD in adults.
To conduct this study, we searched the PubMed, Embase, and Cochrane Library databases on March 10, 2019, and conducted a frequentist network meta-analysis on the search results. The primary outcome was treatment efficacy estimated by the proportion of patients who achieved a certain percentage decrease in symptoms or who dropped below the threshold of the global FD symptom scores. The secondary outcome was acceptability, defined as all-cause discontinuation. Odds ratios (ORs) were reported with 95% confidence intervals (CIs).
We deemed 10 trials to be eligible for analysis, and these trials included 970 participants and 10 psychotropic drugs. Flupentixol + melitracen (F + M) (OR, 10.00; 95% CI, 1.59 to 62.73), tandospirone (3.24, 1.38 to 7.60), imipramine (2.21, 1.02 to 4.79), and amitriptyline (1.71, 1.06 to 3.09) were significantly superior to placebo. According to the surface under the cumulative ranking curve, the most effective treatment was F + M (89.0%), whereas the least effective was R137696 (13.6%). In terms of acceptability, escitalopram (0.32, 0.11 to 0.92) was ranked as the worst drug (12.6%), followed by imipramine and sertraline.
The present network meta-analysis suggests that F + M, tandospirone, imipramine, and amitriptyline are more effective than placebo as treatment for FD. Our results indicate that among the ten psychotropic drugs included, F + M is likely to be the most effective drug for alleviating dyspepsia symptoms.
Keywords: functional dyspepsia, functional gastrointestinal disorders, network meta-analysis, psychotropic drugs
1. Introduction
Functional dyspepsia (FD) affects 10-30% of the global population and is one of the most common functional gastrointestinal disorders (FGIDs).[1] It is defined by four main symptoms, based on the Rome criteria (presence of bothersome postprandial fullness, early satiation, epigastric pain, and epigastric burning), which occur in the absence of any structural disease that would otherwise explain the symptoms. The disease can also be categorized into three subtypes based on the symptom profile: postprandial distress syndrome (PDS), characterized by postprandial fullness or early satiation; epigastric pain syndrome (EPS), characterized by epigastric pain or burning; and PDS-EPS overlapping syndrome.[2,3]
The FD mechanism involves complex pathophysiological processes, including delayed gastric emptying,[4] decreased fundic accommodation,[5] gastroduodenal inflammation,[6] visceral hypersensitivity,[7] disorder of the brain-gut axis,[8] and psychiatric comorbidity.[9] Over the last few decades, several pharmacological therapies such as proton pump inhibitor therapy and prokinetic drugs have been introduced.[10] Although they are widely used, a substantial number of patients do not sufficiently respond to these treatments. Hence, many alternative therapies such as psychotropic drugs have been proposed in recent years.[2,11,12]
Psychotropic drugs, such as tricyclic antidepressants (TCADs), selective serotonin reuptake inhibitors (SSRIs), and 5-hydroxytryptamine (5-HT)-1A receptor agonists (tandospirone, imipramine), are used to treat various FGIDs, including FD and irritable bowel syndrome.[12] These drugs are believed to alleviate FD symptoms via their curative effects on comorbid psychological diseases, enhancement of gastric accommodation, and amelioration of pain perception. However, the efficacy of these drugs for treating FD remains controversial, as physicians may be reluctant to consider using them because of their side effect profiles.
To gain a better understanding about these drugs, numerous randomized controlled trials (RCTs)[13–26] and traditional pairwise meta-analyses[27,28] addressing these issues have been conducted. The results of these studies indicate that psychotropic drugs are more effective than placebo for treating FD. Nonetheless, the current evidence from traditional pairwise meta-analyses is based solely on direct comparisons, which limits its interpretation. Here, we employed a network meta-analysis, which is more useful for comparing all relevant treatments based on both direct and indirect comparisons. This new methodological approach allows for the integration of direct and indirect evidence from multiple treatment comparisons to estimate the interrelations across all treatments.
2. Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network Meta-Analyses.[29] The protocol was approved by the Ethics Committee of University-Town Hospital of Chongqing Medical University and registered on PROSPERO (CRD42019141195).
2.1. Search strategy and selection criteria
This network meta-analysis was performed by searching for all publications indexed in the PubMed, EMBASE, and Cochrane Library databases from study inception until March 10, 2019. There were no search restrictions on language, publication year, or publication type. The references of previous meta-analyses, reviews, and all included studies were also screened. Searches were performed using Medical Subject Headings (MeSH) terms and free keywords. The specific search strategies are described in detail online (Table S1, Supplemental Digital Content , which illustrates the search strategy used during this study). The inclusion criteria were as follows:
-
1.
RCTs, including crossover trials;
-
2.
studies including adult patients (age >18 years);
-
3.
studies including patients with a diagnosis of FD based on international diagnostic criteria, such as Rome I, Rome II, Rome III, and Rome IV;
-
4.
studies comparing psychotropic drugs with either other psychotropic drugs or placebo;
-
5.
studies that continued for a minimum duration of 14 days;
-
6.
studies with outcomes showing an improvement in global FD symptoms; and
-
7.
studies reporting dropout data due to any reason.
2.2. Data extraction and quality assessment
Two researchers (ZW and NG) independently extracted data from the included studies according to a predefined data abstraction form. The following clinical data were extracted for each trial: participant characteristics (e.g., criteria used to define FD, age, percentage of females), intervention characteristics (e.g., dose ranges or mean doses of study drugs, trial duration), study characteristics (e.g., publication year, country), and outcome measures. To avoid carry-over effects, only phase one data from crossover trials were included. The primary outcome was efficacy, estimated by the proportion of patients who achieved a symptom decrease of a certain percentage or moved below the threshold of global FD symptom scores. Intention-to-treat data were used where available; otherwise, we used data from completers. The secondary outcome was acceptability, defined as all-cause discontinuation. We assessed the risk of bias following the instructions in the Cochrane Collaboration Handbook.[30]
2.3. Data analysis
We performed a network meta-analysis within a frequentist framework using STATA software version 14.0. The odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for categorical outcomes. We examined the geometry of the evidence by producing a network plot with the node and connection size corresponding to the sample size and number of studies, respectively. A comparison-adjusted funnel plot was constructed to detect publication bias.[31] Inconsistencies were statistically assessed globally (by comparison of consistency and inconsistency models) and locally (by calculation of the difference between direct and indirect estimates within the network).[32] We estimated the ranking probabilities for all treatments analyzed. The treatment hierarchy was then compiled based on the surface under the cumulative ranking curve (SUCRA).[31]
3. Results
3.1. Characteristics of the included trials
A total of 1569 records were identified. Initially, 317 duplicate records were excluded, and 1190 more records were excluded after reading the title and abstract. This left a total of 62 studies that were categorized as potentially eligible studies. Of these, 52 studies were excluded for various reasons. Finally, we included 10 articles in the quantitative analyses. These studies included a total of 970 FD patients, which we randomized for further analysis (Fig. 1).[13–16,18,20,21,24–26] The 10 trials, which included 9 trials with a parallel design and one with a crossover design, were published between 1986 and 2019. In total, 10 different psychotropic drugs, including two SSRIs (sertraline and escitalopram), three TCADs (amitriptyline, imipramine, and nortriptyline), one antipsychotic + TCAD (F + M), and one tetracyclic antidepressant (mirtazapine), were investigated in the included RCTs. The duration of the different treatments ranged from 2 to 12 weeks. Detailed characteristics of the individual RCTs are provided in Table 1. We rated one of the trials as having a high risk of bias, 6 trials were rated as having a moderate risk, whereas 3 trials were rated as having a low risk (see Figure S1, Supplemental Digital Content , which shows the risk-of-bias assessment for this study). Figure 2 shows a network plot of all eligible comparisons that could be made from the 10 included articles. All psychotropic drugs were assessed using placebo-controlled comparisons, except for one drug, which was assessed using a head-to-head comparison.
Figure 1.
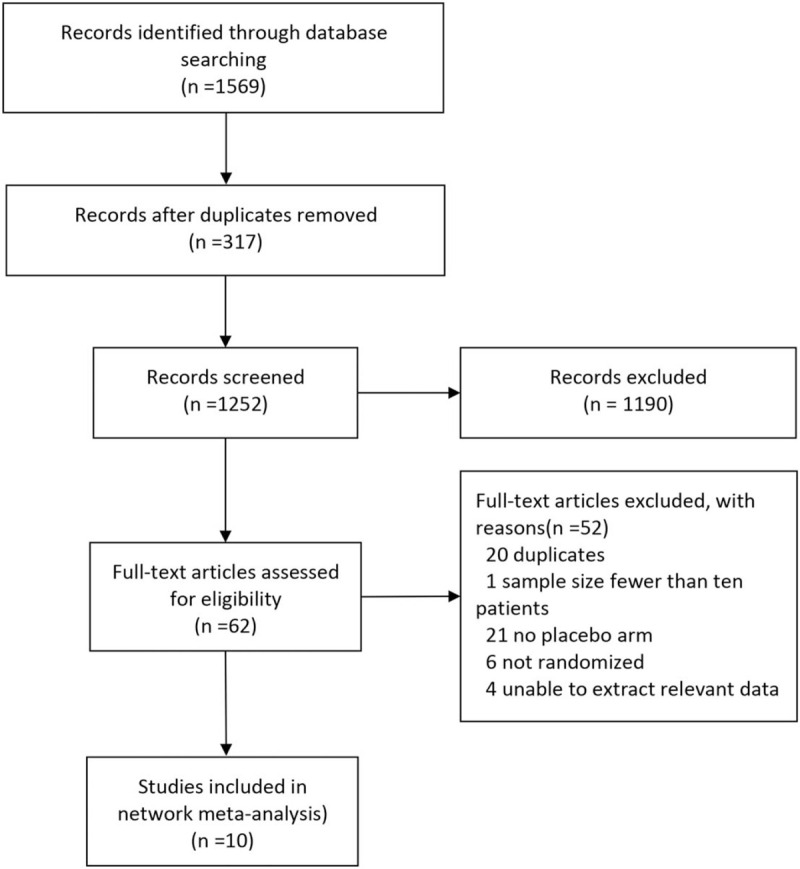
Flowchart of the study selection.
Table 1.
Characteristics of included trials.
| Study | country | diagnostic criteria | sample size (% female) | treatment duration (weeks) | treatment, n (dose range) | Drug class used | Criteria used to define symptom improvement following therapy |
| Hashash et al, 2008 | Lebanon | Rome III | 25 (56.0) | 2 | F + M, 13(1 mg/day + 20 mg/day) Placebo, 12 | antipsychotics and TCADs | Patient-reported subjective feeling of global symptom relief |
| Tack et al, 2009 | Belgium, Germany and The Netherlands | Rome II | 53 (66.0) | 4 | R137696, 29(6 mg/day) Placebo, 24 | 5-HT1A receptor agonists | 30% improvement in patient assessment of upper gastrointestinal symptom severity |
| Miwa et al, 2009 | Japan | Rome II | 150 (73.3) | 4 | Tandospirone, 75(30 mg/day) Placebo, 75 | 5-HT1A receptor agonists | Patient-reported total abdominal symptom score of 0 or 1 on a modified gastrointestinal symptom rating scale |
| Braak et al, 2011 | The Netherlands | Rome II | 38 (60.5) | 8 | Amitriptyline, 18(25 mg/day) Placebo, 20 | TCADs | 30% improvement in patient assessment of upper gastrointestinal symptom severity |
| Wu et al, 2011 | Hong Kong, China | Rome II | 107 (80.4) | 12 | Imipramine, 55(50 mg/day) Placebo, 52 | TCADs | Patient-reported relief of global symptoms |
| Tack et al, 2012 | Belgium | Rome II | 17 (76.5) | 4 | Buspirone, 7(30 mg/day) Placebo, 10 | 5-HT1A receptor agonists | 30% improvement in patient-reported dyspepsia symptom severity |
| Tan et al, 2012 | Hong Kong, China | Rome II | 193 (72.0) | 8 | Sertraline, 98(50 mg/day) Placebo, 95 | SSRIs, | Patient-reported relief of global symptoms |
| Tack et al, 2015 | Belgium | Rome III | 34 (85.3) | 8 | Mirtazepine, 17(15 mg/day) Placebo, 17 | tetracyclic antidepressants | 30% improvement in patient-reported dyspepsia symptom severity |
| Talley et al, 2015 | USA and Canada | Rome II | 292 (75.0) | 12 | Amitriptyline, 97(25-50 mg/day) escitalopram, 98(10 mg/day) Placebo, 97 | TCADs SSRIs | Patient-reported adequate relief of global symptoms for 50% of weeks during weeks 3–12 |
| Kaosombatwattana U et al, 2018 | Bangkok, Thailand | Rome III | 61(70.0) | 8 | Nortriptyline, 28(10 mg/day) Placebo, 33 | TCADs | 50% improvement in patient-reported dyspepsia symptom score |
Figure 2.
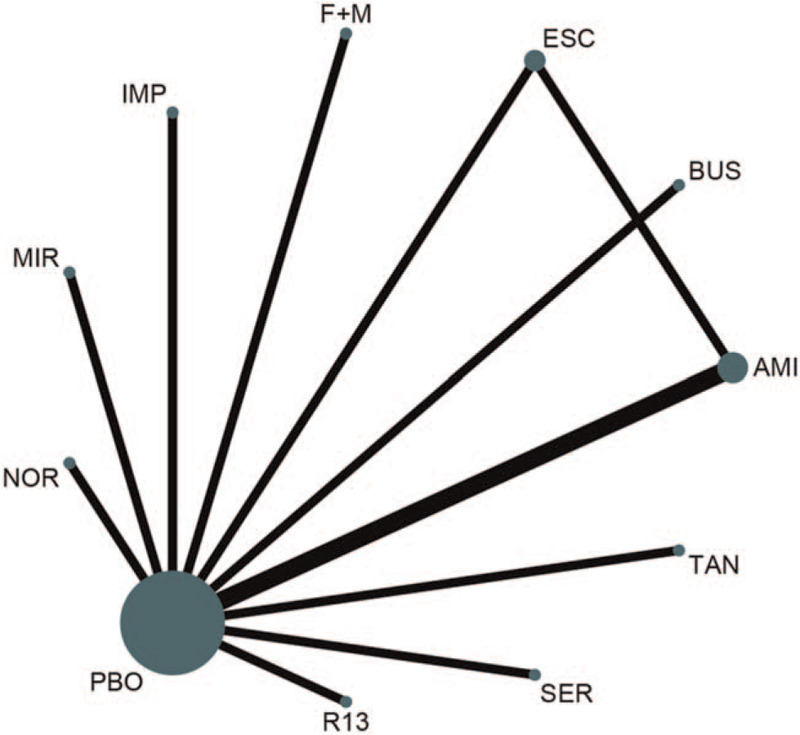
Network of eligible comparisons for all relevant articles. The thickness of the lines between the nodes represents the number of trials comparing every pair of treatments, and the size of the nodes is proportional to the sample number. F + M = flupentixol + melitracen; BUS = buspirone; MIR = mirtazapine; TAN = tandospirone; IMI = imipramine; AMI = amitriptyline; PBO = placebo; ESC = escitalopram; SER = sertraline; NOR = nortriptyline; R13 = R137696.
3.2. Assessment of inconsistency
Fitting the inconsistency model provided no evidence of statistically significant inconsistencies for efficacy and acceptability (global Wald test: P = .35 and P = .13, respectively). One closed loop was produced, and there was no evidence of local inconsistency (P = .38 and P = .14, respectively) (see Table S2, Supplemental Digital Content , which shows the assessment of the inconsistency analysis for this study).
3.3. Results of the network meta-analysis
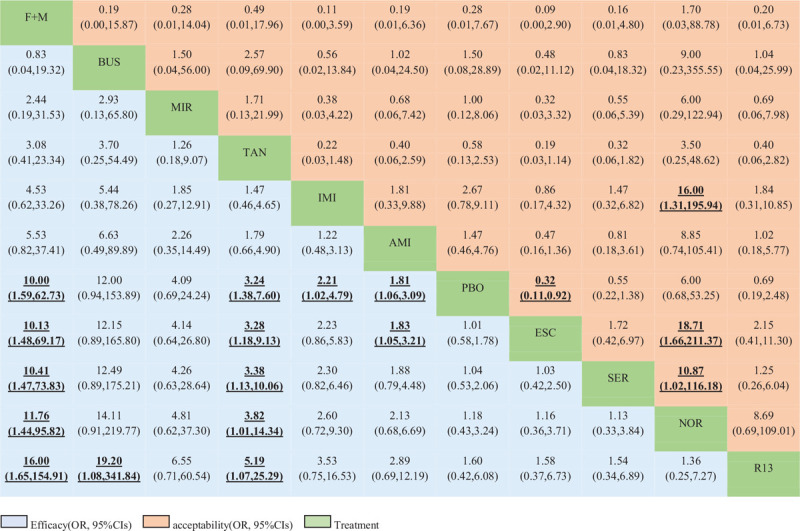
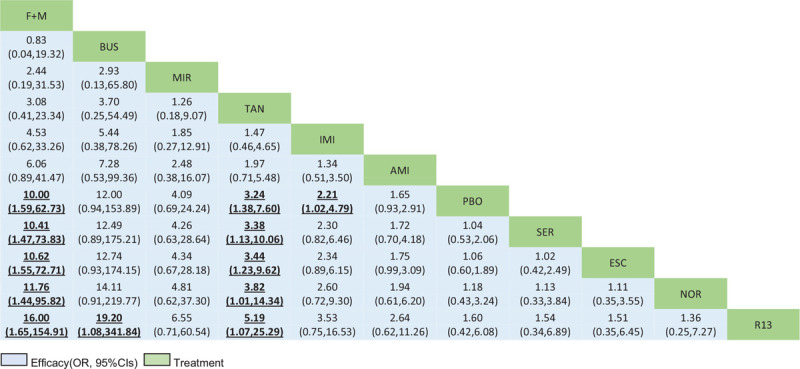
Table 2 shows the results of the network meta-analysis for the primary and secondary outcomes. In terms of efficacy, F + M (OR, 10.00; 95% CI, 1.59 to 62.73), tandospirone (3.24, 1.38 to 7.60), imipramine (2.21, 1.02 to 4.79), and amitriptyline (1.81, 1.06 to 3.09) were more effective than placebo. No significant differences between these drugs were observed (see Table S3, Supplemental Digital Content , which shows the treatment ranking and SUCRA plot for each outcome). The most effective treatment was F + M (89.0%), followed by buspirone (88.1%) and mirtazapine (73.1%). In terms of acceptability, only escitalopram (0.32, 0.11 to 0.92) was associated with higher dropout rates than placebo and was ranked as the worst drug (12.6%) for treating FD, followed by imipramine and sertraline. Nortriptyline (90.0%) had the highest probability of being most accepted.
Table 2.
Network meta-analysis of efficacy and acceptability.
3.4. Publication bias and sensitivity analysis
A comparison-adjusted funnel plot was used to evaluate publication bias. Figure 3 shows that the comparison-adjusted funnel plot for efficacy is slightly asymmetric. We believe that this asymmetry derives from the study by Braak et al.[16] By excluding this study from the sensitivity analysis, we found that there was no statistically significant difference between amitriptyline (1.47, 0.46 to 4.76) and placebo (Table 3). No obvious publication bias was found for the acceptability parameter (see Figure S2, Supplemental Digital Content , which shows a comparison-adjusted funnel plot for each outcome).
Figure 3.
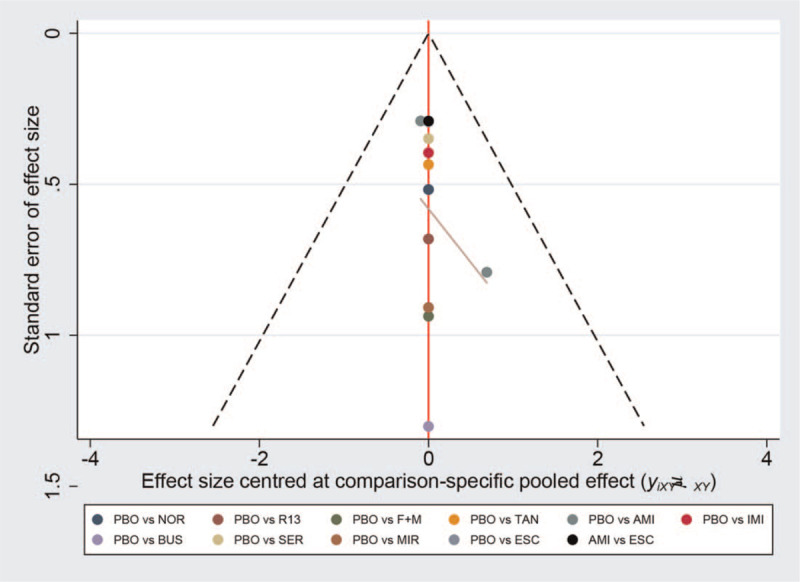
The comparison-adjusted funnel plot of the efficacy of the analyzed drugs.
Table 3.
Sensitivity analysis after omitting Braak et al.
4. Discussion
In this network meta-analysis, we performed a comprehensive synthesis of data for ten psychotropic drugs (nortriptyline, F + M, tandospirone, mirtazapine, amitriptyline, R137696, buspirone, sertraline, imipramine, and escitalopram) used for the treatment of adults with FD. The primary aim of this study was to compare the relative efficacies of these psychotropic drugs. In line with previous traditional meta-analyses, we demonstrated the overall efficacy of F + M, tandospirone, imipramine, and amitriptyline for treating FD. However, when the study by Braak et al[16] was excluded from the sensitivity analysis, amitriptyline appeared to be ineffective. According to the SUCRA analysis, F + M was ranked as the most effective drug. However, the large CI raises the question as to whether this evidence is sufficiently robust to inform clinical practice. In terms of acceptability, only escitalopram was associated with higher dropout rates than placebo.
Psychological factors such as anxiety and depression are known to be associated with FD.[9] The mechanisms by which anxiety or depression may drive FD are likely to be multifactorial. The brain-gut axis, which refers to the bidirectional communication between the central nervous system and the enteric nervous system, may play a key role in patients with FD and psychiatric comorbidities.[8] Numerous studies addressing disorders of the brain-gut axis have been conducted. A prospective longitudinal population-based study published in 2014 reported that the brain-gut pathway is bidirectional and that both brain-to-gut and gut-to-brain dysfunction may occur in patients with FD.[33] Another study published in 2016 revealed that higher levels of anxiety and depression at baseline were predictors of developing FD and reported that individuals with documented FD at baseline had higher levels of anxiety and depression at 1-year follow-up.[34] Based on these studies, we believe that the relationship between FD and psychological factors is clear.
The bidirectional communication between the central nervous system and the enteric nervous system is not well understood. Nonetheless, the common embryologic basis for the brain-gut axis is clear: the enteric nervous system develops from the embryonic endoderm, which is populated by the down ganglia from the brain and spinal cord during fetal development. Thus, the central nervous system shares common neurotransmitters (e.g., serotonin, dopamine) and receptors with the enteric nervous system. These neurotransmitters have critical functions in both the brain and gut.[12] As the majority of antidepressants act on these shared neurotransmitters and receptors, they will also have effects on gastrointestinal symptoms.
Psychotropic drugs have been proposed as potential treatments for FD for many years. Many clinical guidelines recommend them as second-choice drugs, especially when PPI therapy and prokinetic drugs fail to alleviate FD symptoms.[3,11] A traditional meta-analysis published in 2017 showed that TCADs and antipsychotics were more effective than placebo for treating FD.[28] Similarly, in our network meta-analysis, F + M, a mixture of the TCADs melitracen, imipramine, and amitriptyline and the classical antipsychotic flupentixol, was more effective than placebo. Therefore, TCADs could be a good choice for treating FD.
Impaired fundic accommodation to a meal is considered an important pathophysiological mechanism of FD.[5] The 5-HT1A receptor agonist, a fundus-relaxing drug, has been implicated in the improvement in FD symptoms by inducing the relaxation of the gastric fundus.[35] Consequently, in theory, tandospirone, buspirone, and R137696 should have all performed well in the treatment of FD, especially when the patients being administered these drugs had meal-induced dyspeptic symptoms, such as early satiation and postprandial fullness. However, our network meta-analysis showed that tandospirone performed better than buspirone and R137696, neither of which showed a statistically significant difference from placebo. This may be because these RCTs did not distinguish between the FD subtypes, each of which may involve different mechanisms. For instance, impaired gastric accommodation is likely to induce symptoms of PDS rather than EPS.[36] In light of this, additional studies on the effects of 5-HT1A receptor agonists on different FD subtypes should be considered in the future.
SSRIs have been used to treat symptoms of irritable bowel syndrome, a highly prevalent FGID characterized by chronic abdominal pain and alterations in bowel habits.[37] The rationale for this is that SSRIs may improve visceral hypersensitivity and treat psychiatric comorbidities. Thus, SSRIs may be a good choice for treating FD. However, we found that the SSRIs escitalopram and sertraline[21,26] were not better than placebo for treating FD; in fact, they ranked among the worst drugs analyzed in our study. Furthermore, both had higher dropout rates than the other drugs in our analysis. These results suggest that SSRI drugs should be used with more caution when treating patients with FD.
The present study had some limitations. First, the majority of the included studies exhibited unclear risk of bias, particularly in terms of random sequence generation and allocation concealment. Notably, the overall risk of bias was deemed high for 1 trial (9%). Second, the number of included trials was relatively small, which restricts the interpretation of the findings. This limited our ability to draw definitive conclusions for individual drugs, such as F + M. Third, the Rome criteria for FD have undergone multiple revisions over the past 30 years. Thus, there are variations between individual trial populations. In addition, different definitions of improvement in global FD symptoms may have resulted in comparisons of different patient populations. Fourth, because most psychotropic drugs were compared to placebo, comparisons between different psychotropic drugs were primarily based on indirect evidence. Because of the non-negligible heterogeneity across the studies, we should treat these results with caution, and head-to-head RCTs are needed to confirm our results. Finally, only short-term effects were investigated in the included studies, meaning that the long-term efficacy of psychotropic drugs for the treatment of FD remains undetermined.
5. Conclusion
In summary, our network meta-analysis supports the use of psychotropic drugs, especially TCADs, for the treatment of adult patients with FD. This information may assist clinicians in choosing a second-line therapeutic strategy for FD. However, a definitive conclusion regarding which drug is best cannot currently be made because of the limited number of studies and patients for each drug. Additional well-designed, head-to-head studies are needed in the future.
Author contributions
Conceptualization: Wan Zhou, Xia Li, Gang Nie, Dongdong Zhou.
Investigation: Yin Huang, Yan Liu.
Methodology: Xia Li, Dongdong Zhou, Jiayan Wang.
Software: Wan Zhou.
Supervision: Gang Nie, Dongdong Zhou.
Writing – original draft: Wan Zhou, Xia Li.
Writing – review & editing: Wan Zhou.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 5-HT = 5-hydroxytryptamine, AMI = amitriptyline, BUS = buspirone, CI = confidence interval, EPS = epigastric pain syndrome, ESC = escitalopram, FD = functional dyspepsia, FGIDs = functional gastrointestinal disorders, F+M = flupentixol + melitracen, IMI = imipramine, MeSH = Medical Subject Headings, MIR = mirtazapine, NOR = nortriptyline, ORs = odds ratios, PDS = postprandial distress syndrome, PBO = placebo, R13 = R137696, RCTs = randomized controlled trials, SER = sertraline, SSRIs = selective serotonin reuptake inhibitors, SUCRA = surface under the cumulative ranking curve, TAN = tandospirone, TCADs = tricyclic antidepressants.
How to cite this article: Zhou W, Li X, Huang Y, Xu X, Liu Y, Wang J, Nie G, Zhou D. Comparative efficacy and acceptability of psychotropic drugs for functional dyspepsia in adults: a systematic review and network meta-analysis. Medicine. 2021;100:20(e26046).
WZ and XL contributed equally to this work.
Xia Li, funded by China Scholarship Council.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
F + M = flupentixol + melitracen, TCADs = tricyclic antidepressants, 5-HT = 5-hydroxytryptamine; SSRIs = selective serotonin reuptake inhibitors.
Drugs are listed in order of efficacy ranking according to the SUCRA. For efficacy (bottom-left), an OR greater than 1 favors row-defining treatment. For acceptability (top right), an OR below 1 favors the row-defining treatment. Significant ORs of comparisons are in bold and underlined. CIs = confidence intervals F + M = flupentixol + melitracen BUS = buspirone. MIR = mirtazapine. TAN = tandospirone. IMI = imipramine. AMI = amitriptyline. PBO = placebo. ESC = escitalopram. SER = sertraline. NOR = nortriptyline. R13 = R137696. OR = odds ratio. SUCRA = surface under the cumulative ranking curve.
Drugs are reported in the order of efficacy ranking according to the SUCRA. Comparisons should be read from left to right. The estimates are located at the crossing between the column-defining treatment and row-defining treatment. For efficacy, an OR greater than 1 favors row-defining treatment. Significant results are emboldened and underlined. CIs = confidence intervals F + M = flupentixol + melitracen BUS = buspirone. MIR = mirtazapine. TAN = tandospirone. IMI = imipramine. AMI = amitriptyline. PBO = placebo. ESC = escitalopram. SER = sertraline. NOR = nortriptyline. R13 = R137696. OR = odds ratio. SUCRA = surface under the cumulative ranking curve.
References
- [1].Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol 2006;12:2661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Enck P, Azpiroz F, Boeckxstaens G, et al. Functional dyspepsia. Nat Rev Dis Primers 2017;3:17081. [DOI] [PubMed] [Google Scholar]
- [3].Drossman DA. Functional gastrointestinal disorders: history, pathophysiology. clinical features and Rome IV. Gastroenterology 2016. [DOI] [PubMed] [Google Scholar]
- [4].Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol 2003;98:783–8. [DOI] [PubMed] [Google Scholar]
- [5].Tack J, Piessevaux H, Coulie B, Tana P, Corazza GR. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 1998;115:1346–52. [DOI] [PubMed] [Google Scholar]
- [6].Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014;63:262–71. [DOI] [PubMed] [Google Scholar]
- [7].Vandenberghe J, Vos R, Persoons P, Janssens J, Tack J. Dyspeptic patients with visceral hypersensitivity: sensitisation of pain specific or multimodal pathways? Gut 2005;54:914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Keightley PC, Koloski NA, Talley NJ. Pathways in gut-brain communication: evidence for distinct gut-to-brain and brain-to-gut syndromes. Aust N Z J Psychiatry 2015;49:207–14. [DOI] [PubMed] [Google Scholar]
- [9].Van Oudenhove L, Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013;10:158–67. [DOI] [PubMed] [Google Scholar]
- [10].Tack J. Prokinetics and fundic relaxants in upper functional GI disorders. Curr Opin Pharmacol 2008;8:690–6. [DOI] [PubMed] [Google Scholar]
- [11].Hojo M, Nagahara A, Asaoka D, Watanabe S. Emerging pharmacological therapy for functional dyspepsia. Clin J Gastroenterol 2013;6:352–6. [DOI] [PubMed] [Google Scholar]
- [12].Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Oudenhove LV. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a Rome foundation working team report. Gastroenterology 2018;154:1140–71. e1141. [DOI] [PubMed] [Google Scholar]
- [13].Tack J, Janssen P, Masaoka T, Farré R, Oudenhove LV. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol 2012;10:1239–45. [DOI] [PubMed] [Google Scholar]
- [14].Tack J, Ly HG, Carbone F, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol 2016;14:385–92. e384. [DOI] [PubMed] [Google Scholar]
- [15].Tack J, Van Den Elzen B, Tytgat G, et al. A placebo-controlled trial of the 5-HT1A agonist R-137696 on symptoms, visceral hypersensitivity and on impaired accommodation in functional dyspepsia. Neurogastroenterol Motil 2009;21:619–26. e623-614. [DOI] [PubMed] [Google Scholar]
- [16].Braak B, Klooker TK, Wouters MM, Lei A, van den Wijngaard RM, Boeckxstaens GE. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011;34:638–48. [DOI] [PubMed] [Google Scholar]
- [17].HUI WM, LAM SK, LOK ASF, Ma-Taing M, Wong Kee-Lam, Fok K-H. Sulpiride improves functional dyspepsia: a double-blind controlled study. J Gastroenterol Hepatol 1986;1:391–9. [Google Scholar]
- [18].Miwa H, Nagahara A, Tominaga K, et al. Efficacy of the 5-HT1A agonist tandospirone citrate in improving symptoms of patients with functional dyspepsia: a randomized controlled trial. Am J Gastroenterol 2009;104:2779–87. [DOI] [PubMed] [Google Scholar]
- [19].van Kerkhoven LA, Laheij RJ, Aparicio N, et al. Effect of the antidepressant venlafaxine in functional dyspepsia: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 2008;6:746–52. quiz 718. [DOI] [PubMed] [Google Scholar]
- [20].Hashash JG, Abdul-Baki H, Azar C, et al. Clinical trial: a randomized controlled cross-over study of flupenthixol + melitracen in functional dyspepsia. Aliment Pharmacol Ther 2008;27:1148–55. [DOI] [PubMed] [Google Scholar]
- [21].Tan VP, Cheung TK, Wong WM, Pang R, Wong BCY. Treatment of functional dyspepsia with sertraline: a double-blind randomized placebo-controlled pilot study. World J Gastroenterol 2012;18:6127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Song C-W, Chun H-J, Kim C-D, Ryu H-S, Choe J-G, Hyun J-H. Effects of levosulpiride in patients with functional dyspepsia accompanied by delayed gastric emptying. Korean J Intern Med 1998;13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arienti V, Corazza G, Sorge M, et al. The eflects of levosulpiride on gastric and gall-bladder emptying in functional dyspepsia. Clinical Trial Aliment Pharmacol Ther 1994;8:631–8. [DOI] [PubMed] [Google Scholar]
- [24].Kaosombatwattana U, Pongprasobchai S, Limsrivilai J, Monthira M, Leelakusolvong S, Tanwandee T. Efficacy and safety of nortriptyline in functional dyspepsia in Asians: a randomized double-blind placebo-controlled trial. J Gastroenterol Hepatol 2018;33:411–7. [DOI] [PubMed] [Google Scholar]
- [25].Cheong PK, Ford AC, Cheung CKY, et al. Low-dose imipramine for refractory functional dyspepsia: a randomised, double-blind, placebo-controlled trial. The Lancet Gastroenterol Hepatol 2018;3:837–44. [DOI] [PubMed] [Google Scholar]
- [26].Talley NJ, Locke GR, Saito YA, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter. Randomized Controlled Study Gastroenterology 2015;149:340–9. e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lu Y, Chen M, Huang Z, Tang C. Antidepressants in the treatment of functional dyspepsia: a systematic review and meta-analysis. PLoS One 2016;11:e0157798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ford AC, Luthra P, Tack J, Boeckxstaens GE, Moayyedi P, Talley NJ. Efficacy of psychotropic drugs in functional dyspepsia: systematic review and meta-analysis. Gut 2017;66:411–20. [DOI] [PubMed] [Google Scholar]
- [29].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [30].John Wiley & Sons, Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Vol 4. 2011. [Google Scholar]
- [31].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [32].Chaimani A, Higgins JP, Mavridis D, Panagiota S, Georgia S. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain–gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut 2012;61:1284–90. [DOI] [PubMed] [Google Scholar]
- [34].Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther 2016;44:592–600. [DOI] [PubMed] [Google Scholar]
- [35].Boeckxstaens GE, Tytgat GN, Wajs E, van Nueten L, de Ritter F, Tack MJ. The influence of the novel 5-HT1A agonist R137696 on the proximal stomach function in healthy volunteers. Neurogastroenterol Motil 2006;18:919–26. [DOI] [PubMed] [Google Scholar]
- [36].Futagami S, Yamawaki H, Agawa S, et al. New classification Rome IV functional dyspepsia and subtypes. Transl Gastroenterol Hepatol 2018;3:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393–407. e1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


