Abstract
Background:
To study the epidemic features of hand-foot-mouth disease (HFMD) in mainland China through systematic review and meta-analysis so as to provide evidence for the future prevention and control of HFMD.
Methods:
Articles on the epidemic features of HFMD in mainland China, written in English or Chinese and released between January 1, 2015 and January 1, 2020, were searched from English literature databases including Embase, Web of Science, PubMed, Cochrane library, Google academic, and Chinese literature databases including China national knowledge infrastructure (CNKI), Wanfang, and China Biology Medicine (CBM). Papers were selected according to the inclusion and exclusion criteria, and quality scoring was performed. Meta-analysis, sensitivity analysis, and identification of publication bias were finished through STATA version 12.0 software.
Results:
A total of 23 articles were included in this study, the total number of cases was 377,083, of which the total number of male cases was 231,798 and the total number of female cases was 145,285, the sex ratio was about 1.6:1, and the incidence of HFMD in China was 1.61‰ (95% confidence interval [CI]: 1.21‰–1.94‰). The results of the subgroup analysis showed that the incidence of HFMD in mainland China was the highest in South China, in 2014, in 1-year-old group and in other types of enteroviruses, respectively, with the rate of 3.48‰ (95% CI: 1.22‰–5.73‰), 1.81‰ (95% CI: 1.06‰–2.57‰), 15.20‰ (95% CI: 5.00‰–25.30‰), and 1.83‰ (95% CI: 1.32‰–2.33‰), respectively. The differences among the above 4 subgroups were statistically significant (P < .05). There were no publication bias in this study, and the sensitivity analysis results suggested that the meta-analysis results were robust.
Conclusion:
There were differences in the distribution of region, time, population, and etiology of HFMD in mainland China. Health departments should adopt key strategies and measures for key populations in key areas to prevent and control the development of HFMD, and improve the ability of pathogen detection and typing in laboratories.
Keywords: epidemiological characteristics, hand-foot-mouth disease, meta-analysis
1. Introduction
Hand-foot-mouth disease (HFMD) is a common infectious disease caused by coxsackievirus A16 (Cox A16), human enterovirus 71 (EV71), and other human enterovirus infection.[1] HFMD is most common in children under 10 years of age, especially children under 5 years of age. The clinical asymptomatic stage of HFMD is generally maintained at 3 to 5 days, and the course of disease is about 7 to 10 days.[2] The patient usually has an acute onset, accompanied by herpes on the skin or mucous membrane of the hands, feet, mouth, etc, which contains inflammatory exudate and surrounding redness.[2] Most patients have mild symptoms, including fever, fatigue, malaise, and respiratory discomfort. A small number of patients infected with EV71 can cause encephalitis, meningitis, encephalomyelitis, myocarditis, acute delayed paralysis, and even death in severe cases.[3] HFMD has a variety of pathogenic types, with EV71 infection alternating with CoxAl6 infection as the dominant type leading to the epidemic of HFMD. The clinical manifestations of human infection with the 2 viruses are different. The symptoms caused by CoxA16 are mild and self-limited, while EV71 infection leads to rapid progression of the disease and can be life-threatening in severe cases.[4] Worldwide, the proportion of HFMD caused by EV71 infection has increased gradually in recent years, and has become the main pathogen of the disease.[5]
HFMD occurs mainly in children under 3 years of age, and there is a gender difference, with boys slightly higher than girls.[3] The prevalence of HFMD also varies with different countries, regions, seasons, latitudes, weather, and other factors.[6] In China, the peak seasons of HFMD in the north and south are slightly different. The first peak of HFMD in the south is from May to June, and the second peak is from September to October. In the north, the incidence is mostly a single peak, with the season from June to September, and the incidence is less in winter. Studies have shown that latitude can affect the prevalence of HFMD to some extent.[7] The incidence of HFMD is relatively high in low latitudes such as the tropics and temperate zones. In addition, many meteorological factors such as high temperature, low pressure, and abundant precipitation are also closely related to the incidence of HFMD.[8,9]
At present, more articles have been published describing the prevalence characteristics of HFMD in local areas, while fewer articles have described the prevalence characteristics of HFMD in all over the country. In this study, meta-analysis was used to collect literature on the prevalence of HFMD in mainland China published in the past 5 years, and the prevalence of HFMD in mainland China was comprehensively analyzed in order to provide effective basis for disease prevention and control for relevant health departments.
2. Materials and methods
2.1. Literature retrieval
Literatures related to the prevalence of hand, foot, and mouth in mainland China published between January 1, 2015 and January 1, 2020 were searched in English literature databases including Embase, Web of Science, PubMed, Cochrane library, Google academic, and Chinese literature databases including China national knowledge infrastructure (CNKI), Wanfang, and China Biology Medicine (CBM). The keywords searched in the database are “epidemiological characteristics,” “epidemiological study,” “epidemiological features,” “epidemiological status,” “epidemic,” “epidemiology,” “hand foot mouth disease,” “HFMD.” Take “China,” “Chinese,” “mainland” as crowd qualifiers.
2.2. Literature screening
The inclusion criteria of the literature were:
-
(1)
The study disease was hand, foot, and mouth disease;
-
(2)
Original literature using cross-sectional research methods;
-
(3)
The research content includes the 3 distribution of diseases and the distribution of etiology;
-
(4)
The study population is residents of mainland China;
-
(5)
Definite study years, total number of respondents, total incidence, and annual incidence.
The exclusion criteria of literature were:
-
(1)
review, meeting minutes, editorial, etc
-
(2)
Articles whose quality score is less than 4;
-
(3)
Unable to download the full text;
-
(4)
Some data and information in the paper are incomplete, suspicious, or inconsistent.
2.3. Literature quality evaluation
The Agency for Healthcare Research and Quality scale was used to score the selected original literatures in this study.[10] There are 11 items in the scale, with a full mark of 11. Each item that meets the requirements is denoted as 1 point, while those that do not meet, are unclear or are not involved in the study are denoted as 0 points. 0-3 points, 4-7 points, and 8-11 points are judged as low, medium, and high quality literature, respectively.
2.4. Data extraction
Two researchers completed a literature screening and data extraction, respectively, and then compared the consistency of the selected literature and data extraction. After 2 researchers conducted a data extraction of the included literature respectively, the third researcher compared the data extraction results of the first 2 researchers, and made the final selection after careful consideration of some data with different opinions, so as to complete the data extraction work and develop the best data extraction table. After the opinions of the 3 researchers were unified, an information extraction table was established in Excel software to extract the following information from qualified literature:
-
(1)
First author, quality score results, study region, study province, and study year;
-
(2)
The number of cases, the number of people investigated, and the annual incidence;
-
(3)
Type of prevailing dominant virus.
2.5. Statistical analysis
The point assessment of the incidence rate and the 95% confidence interval of each study were combined for the pooled rates. Cochran Q test and I2 value were used for qualitative and quantitative analysis of the heterogeneity existing in the studies.[11] The P-value obtained from the Cochran Q test was used to identify whether there was heterogeneity between different studies. If P ≤ .1, it was considered that there was heterogeneity, the random effect model was used for meta-analysis; if P > .1, it was considered that all studies were homogeneous, the fixed effect model was used for meta-analysis. I2 value is used to represent the heterogeneity between different studies, 0 < I2 < 50%, 50% ≤ I2 ≤ 75%, I2 > 75% means low, medium, and high degree of heterogeneity, respectively.
Subgroup analysis was carried out according to study area, epidemic trend, age, and dominant strains. Differences in sample size, quality score, and other characteristics among different studies may affect meta results. In order to identify potential influencing factors, we conducted a sensitive analysis by removing study with small sample size one by one. If there is no significant difference in the combined effect before and after the exclusion of the literature, it means that the results of this meta-analysis are reliable, on the contrary, it means that certain characteristics of an article are potential influencing factors of the research results. Publication bias was assessed using the Begg funnel plot.[12] Egger linear regression was used to evaluate the symmetry of the funnel plot.[13] All statistical analyses were performed using STATA version 12.0 software (STATA Corporation, College Station, TX). A P-value less than .05 was considered statistically significant.
3. Results
3.1. Literature search results
A total of 2605 articles were retrieved at initial literature searching, and 23 qualified literature were finally included in this meta-analysis.[14–36] The literature screening flow chart is shown in Figure 1. Of the 23 references included, 1 study received a score of 8, 12 studies received a score of 7, and 10 studies received a score of 6. The basic characteristics of the included literature are shown in Table 1.
Figure 1.

Selection process of studies about the epidemiological studies of HFMD in China. HFMD = hand-foot-mouth disease.
Table 1.
The basic characteristics of the included literature in the meta-analysis.
| First author | AHRQ scores | Region | Province | Size of the population | HFMD cases | Incidence (per 100,000) | Superiority strains |
| Chang et al | 6 | Northwest China | Jilin | 1,231,238 | 3175 | 25.79 | Other HEVs |
| Liu et al | 6 | Northwest China | Shaanxi | 4,310,000 | 3675 | 83.98 | CA16 |
| Liu et al | 6 | Northwest China | Shaanxi | 26,430,342 | 40,827 | 154.47 | EV71 |
| Ma et al | 7 | Northwest China | Xinjiang | 1,817,533 | 810 | 44.57 | Other HEVs |
| Dai et al | 7 | Northwest China | Liaoning | 49,501,034 | 41,043 | 82.91 | Other HEVs |
| Cui et al | 7 | Northwest China | Liaoning | 21,633,909 | 5366 | 24.80 | CA16 |
| Wang et al | 7 | North China | Hebei | 4,424,053 | 1715 | 38.77 | EV71 |
| Li et al | 6 | North China | Hebei | 2,253,074 | 742 | 32.93 | Other HEVs |
| Wei et al | 6 | Central China | Henan | 43,358,350 | 25,764 | 118.84 | Other HEVs |
| Zhao et al | 7 | Central China | Hubei | 11,117,900 | 13,403 | 120.55 | Other HEVs |
| Zhang et al | 7 | Central China | Jiangxi | 7,527,093 | 23,516 | 312.37 | Other HEVs |
| Liao et al | 8 | Southwest China | Chongqing | 6,358,800 | 7922 | 124.58 | Other HEVs |
| Luo et al | 7 | Southwest China | Yunnan | 27,264,341 | 21,047 | 77.20 | CA16 |
| He et al | 7 | Southwest China | Yunnan | 22,745,339 | 23,403 | 102.89 | Other HEVs |
| Lu et al | 6 | Southwest China | Guizhou | 13,445,840 | 7672 | 57.06 | Other HEVs |
| Yan et al | 6 | South China | Guangxi | 4,666,431 | 30,484 | 653.26 | Other HEVs |
| Deng et al | 7 | South China | Guangxi | 37,829,818 | 57,136 | 151.03 | EV71 |
| Xiong et al | 7 | South China | Guangxi | 4,120,300 | 25,900 | 652.87 | Other HEVs |
| Xia et al | 6 | South China | Guangxi | 1,276,670 | 1146 | 89.76 | EV71 |
| Yuan et al | 6 | South China | Guangxi | 3,484,358 | 6666 | 191.31 | EV71 |
| Yu et al | 7 | East China | Shandong | 9,584,479 | 4271 | 44.56 | Other HEVs |
| Qian et al | 7 | East China | Zhejiang | 7,202,876 | 29,413 | 408.40 | EV71 |
| Hu et al | 6 | East China | Shandong | 936,780 | 987 | 105.40 | CA16 |
3.2. The overall incidence of HFMD in China
In this meta-analysis, the total number of respondents was 312,520,558, and the total number of cases was 377,083. Among them, the total number of male cases was 231,798, and the total number of female cases was 145,285, with a sex ratio of about 1.6:1. The heterogeneity test results showed that P < .1, I2 > 50%, so the random effect model is used to combine the study results. Meta-analysis results showed that the average incidence of HFMD in all regions of China was (1.68‰, 95% CI: 1.36‰–2.01‰) (Table 2), and the forest plot is shown in Figure 2.
Table 2.
Meta-analysis results of the incidence rate of HFMD in mainland China.
| Group | Incidence rate | 95% CI | Cohran Q test for heterogeneity (P-value) | I2 (%) | Model | Begg P-value | Egger P-value |
| Overall | 1.68‰ | 1.36‰–2.01‰ | <.001 | 98.4 | Random | .011 | .250 |
| Regional distribution | |||||||
| Northwest China | 1.36‰ | 0.73‰–1.98‰ | <.001 | 94.6 | Random | .402 | .301 |
| Northeast China | 0.54‰ | 0‰–1.11‰ | <.001 | 90.4 | Random | .611 | .621 |
| North China | 0.36‰ | 0.30‰–0.42‰ | <.001 | 85.8 | Random | .414 | .418 |
| Central China | 1.64‰ | 0.53‰–2.76‰ | <.001 | 94.9 | Random | .320 | .221 |
| Southwest China | 0.90‰ | 0.67‰–1.14‰ | <.001 | 84.8 | Random | .121 | .118 |
| Southern China | 3.48‰ | 1.22‰–5.73‰ | <.001 | 94.2 | Random | .112 | .101 |
| East China | 1.86‰ | 0‰–4.39‰ | <.001 | 84.1 | Random | .224 | .218 |
| Age | |||||||
| 0 years old | 3.10‰ | 1.20‰–5.00‰ | <.001 | 95.7 | Random | .303 | .201 |
| 1 year old | 15.20‰ | 5.00‰–25.30‰ | <.001 | 87.3 | Random | .201 | .109 |
| 2 years old | 10.00‰ | 4.10‰–15.90‰ | <.001 | 75.7 | Random | .124 | .098 |
| 3 years old | 11.40‰ | 4.90‰–17.90‰ | <.001 | 80.3 | Random | .080 | .091 |
| 4 years old | 9.00‰ | 4.30‰–13.70‰ | <.001 | 92.7 | Random | .074 | .068 |
| Year | |||||||
| 2014 | 1.81‰ | 1.06‰–2.57‰ | <.001 | 99.7 | Random | .124 | .108 |
| 2015 | 1.62‰ | 1.01‰–2.23‰ | <.001 | 97.3 | Random | .110 | .101 |
| 2016 | 1.60‰ | 1.25‰–1.95‰ | <.001 | 95.7 | Random | .324 | .208 |
| 2017 | 0.66‰ | 0.46‰–0.86‰ | <.001 | 90.3 | Random | .110 | .101 |
| 2018 | 0.54‰ | 0.22‰–0.85‰ | <.001 | 92.7 | Random | .124 | .108 |
| Superiority strains | |||||||
| Other HEVs | 1.96‰, | 1.47‰–2.44‰ | <.001 | 96.7 | Random | .241 | .080 |
| EV71 | 1.72‰ | 1.03‰–2.41‰ | <.001 | 98.3 | Random | .110 | .201 |
| CA16 | 0.73‰ | 0.37‰–1.10‰ | <.001 | 96.7 | Random | .074 | .080 |
Figure 2.
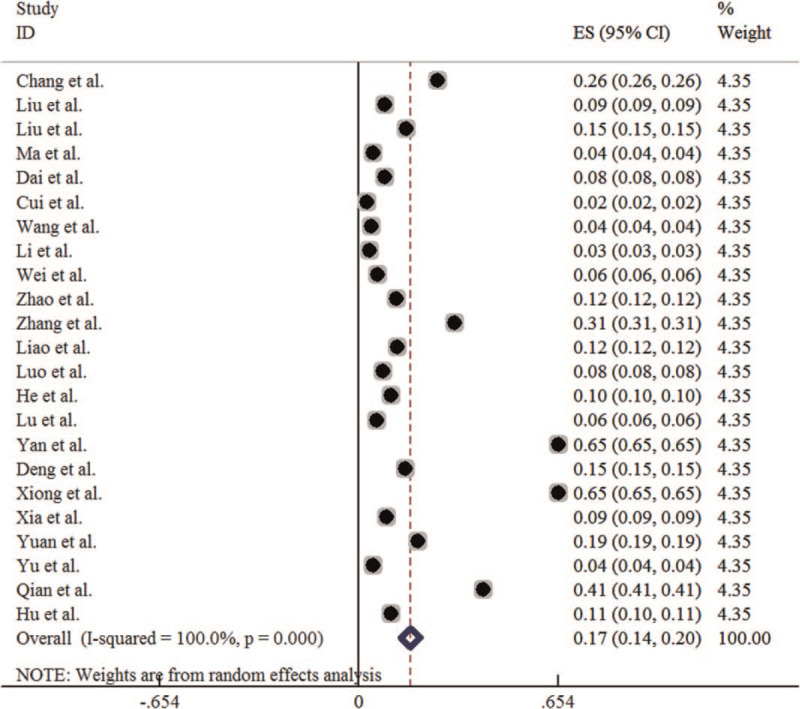
Forest plot for the annual incidence rate of HFMD in China. HFMD = hand-foot-mouth disease.
3.3. Regional distribution
Of the literature included in this meta-analysis, there are 4 study groups from the Northwest China, 2 from the Northeast China, 2 from the North China, 3 from the central China, 4 from the Southwest China, 5 from the southern China, 3 from the East China. The heterogeneity test results show that the P < .1, I2 > 50%, so the random effect model is adopted for meta-analysis. The meta-analysis results showed that the incidence of HFMD in the 7 regions ranged from high to low in South China (3.48‰, 95% CI: 1.22‰–5.73‰), East China (1.86‰, 95% CI: 0‰–4.39‰), Central China (1.84‰, 95% CI: 1.00‰–2.68‰), Southwest China (0.90‰, 95% CI: 0.67‰–1.14‰), Northwest China (0.77‰, 95% CI: 0.11‰–1.43‰), and Northeast China (0.54‰, 95% CI: 0‰–1.11‰), and North China (0.36‰, 95% CI: 0.30‰–0.42‰), among which South China has the highest and North China the lowest. The difference in incidence of HFMD among 7 regions was statistically significant (χ2 = 99480.348, P < .001) (Table 2). The forest plot is shown in Figure 3.
Figure 3.
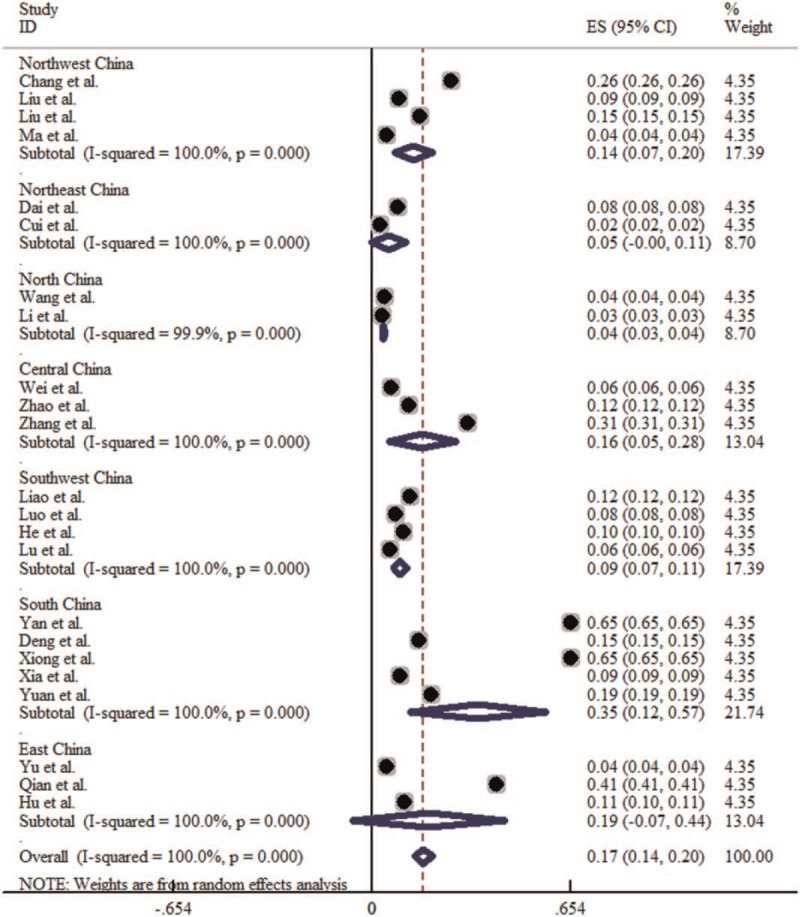
Forest plot for the regional distribution of HFMD incidence in China. HFMD = hand-foot-mouth disease.
3.4. Trends from 2014 to 2018
Of the literature included in this meta-analysis, there are 15 articles studied the incidence in year of 2014, 17 articles in year of 2015, 15 articles in year of 2016, 6 articles in year of 2017, and 3 articles in year of 2018. The heterogeneity test results showed that P < .1, I2 > 50%, so the random effect model is adopted for meta-analysis. Meta-analysis results showed that the incidence of HFMD in the 5 years ranged from high to low in 2014 (1.81‰, 95% CI: 1.06‰–2.57‰), 2015 (1.62‰, 95% CI: 1.01‰–2.23‰), 2016 (1.60‰, 95% CI: 1.25‰–1.95‰), 2017 (0.66‰, 95% CI: 0.46‰–0.86‰), 2018 (0.54‰, 95% CI: 0.22‰–0.85‰). The difference of incidence of HFMD in 5 years was statistically significant (χ2trend = 25367.102, P < .001) (Table 2). It was highest in 2014 and lowest in 2018, and showed a decreasing trend of incidence during 2014 to 2018. The forest plot is shown in Figure 4.
Figure 4.
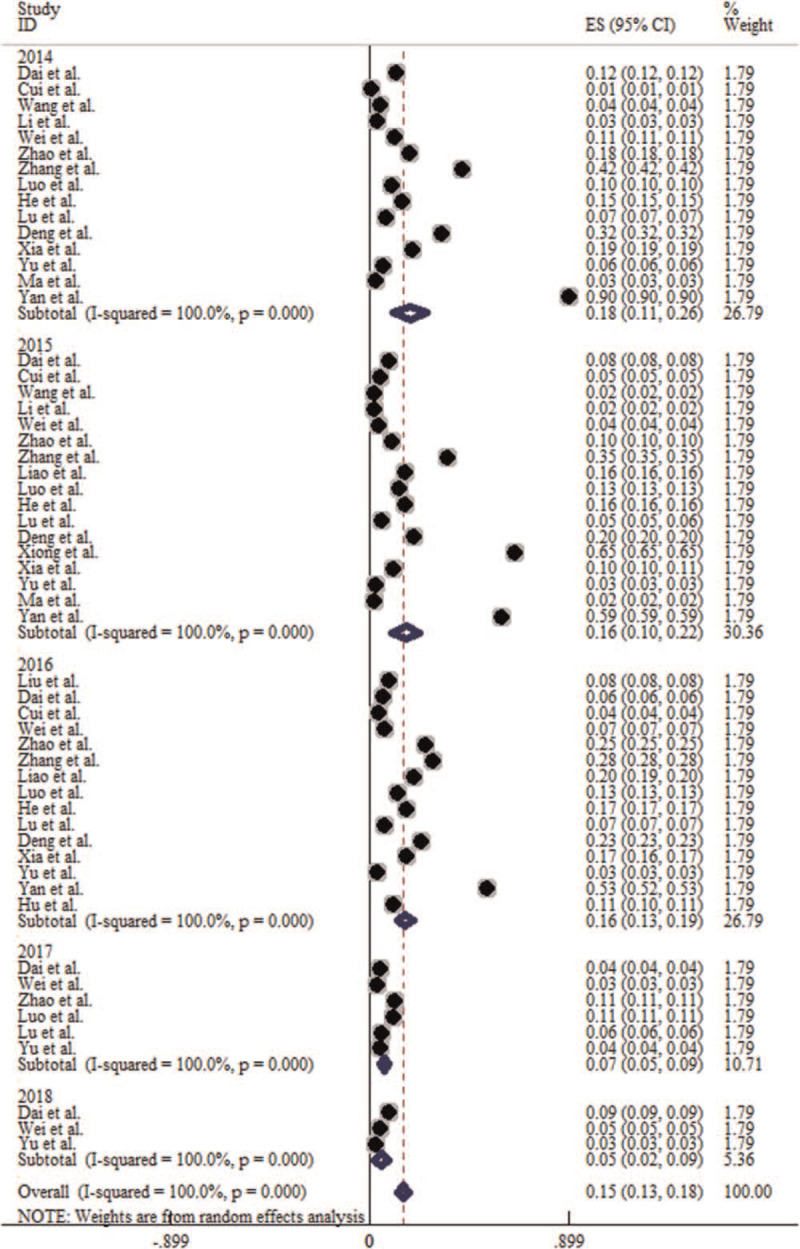
Forest plot for the prevalence trends of HFMD in China from 2014 to 2018. HFMD = hand-foot-mouth disease.
3.5. Age distribution
Of the literature included in this meta-analysis, there are 3 literature provided complete data on the age distribution of 0 to 5 years old population, but these 3 literature did not provide complete on the age distribution of over 5 years old, so this meta-analysis only analyzed the age distribution of HFMD of preschoolers under 5 years old. The heterogeneity test results showed that P < .1, I2 > 50%, so the random effect model is adopted for meta-analysis. Meta-analysis results showed that the incidence of HFMD in the 5 age groups ranged from high to low was in 1-year old (15.20‰, 95% CI: 5.00‰–25.30‰), 3-years old (11.40‰, 95% CI: 4.90‰–17.90‰), 2-years old (10.00‰, 95% CI: 4.10‰–15.90‰), 4-years old (9.00‰, 95% CI: 4.30‰–13.70‰), and 0-years old (3.10‰, 95% CI: 1.20‰–5.00‰) (Table 2). Among them, 1-year old is the highest, 0-years old is the lowest. The incidence of HFMD in children under 5 years old was statistically significant (χ2 = 667.079, P < .001).
3.6. Etiological distribution
Of the literature included in this meta-analysis, there are 13 literature with other types of enterovirus as the dominant epidemic strain, 4 literature with Cox A16 (CA16) as the dominant epidemic strain, and 6 literature with EV71 as the dominant epidemic strain. The heterogeneity test results showed that P < .1, I2 > 50%, so the random effect model is adopted for meta-analysis. The results showed that the incidence of HFMD associated with the 3 dominant epidemic strains was followed by other intestinal viruses (1.83‰, 95% CI: 1.32‰–2.33‰), EV71 (1.72‰, 95% CI: 1.03‰–2.41‰), and Cox A16 (0.73‰, 95% CI: 0.36‰–1.09‰) from high to low (Table 2). When the dominant strains were different, the incidence of HFMD was statistically significant (χ2 = 33277.301, P < .001). The forest plot is shown in Figure 5.
Figure 5.
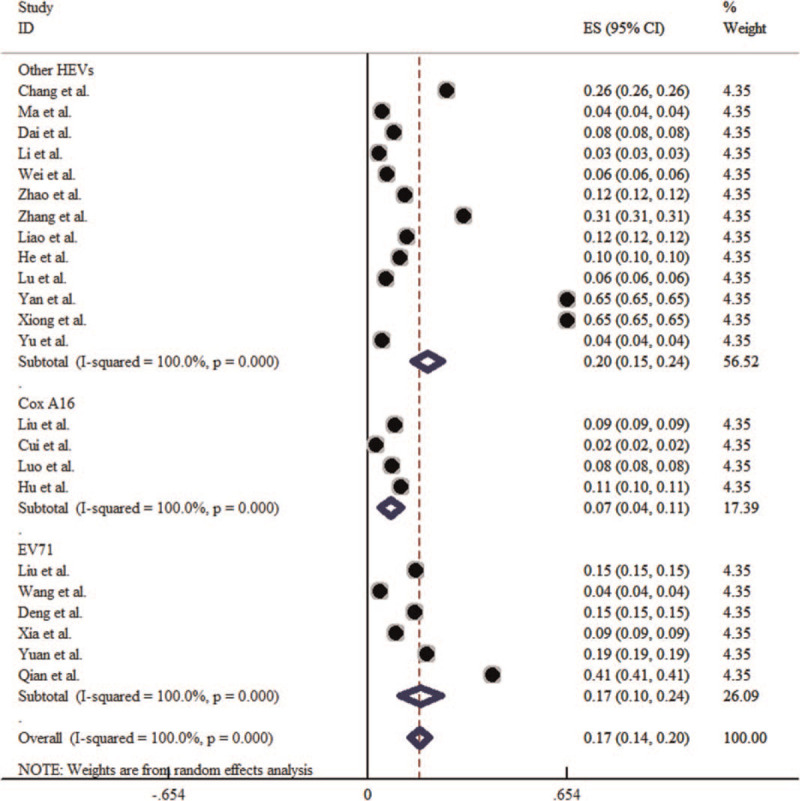
Forest plot for the etiological distribution of HFMD in China. HFMD = hand-foot-mouth disease.
3.7. Sensitivity analysis results
After the exclusion of the small sample study by Hu et al,[36] the overall incidence of HFMD in mainland China was (1.63‰, 95% CI: 1.29‰–1.98‰), which was not significantly different from the overall incidence of HFMD before the exclusion (1.68‰, 95% CI: 1.36‰–2.01‰). Therefore, the meta-analysis results of this study were stable, and the sample size difference was not a potential influencing factor in this study.
3.8. Publication bias
The P-values of Begg test and Egger test were both greater than .05, suggesting that there was no publication bias in this study and that the funnel plot had symmetry. The graphs of the results of Begg test is shown in Figure 6.
Figure 6.
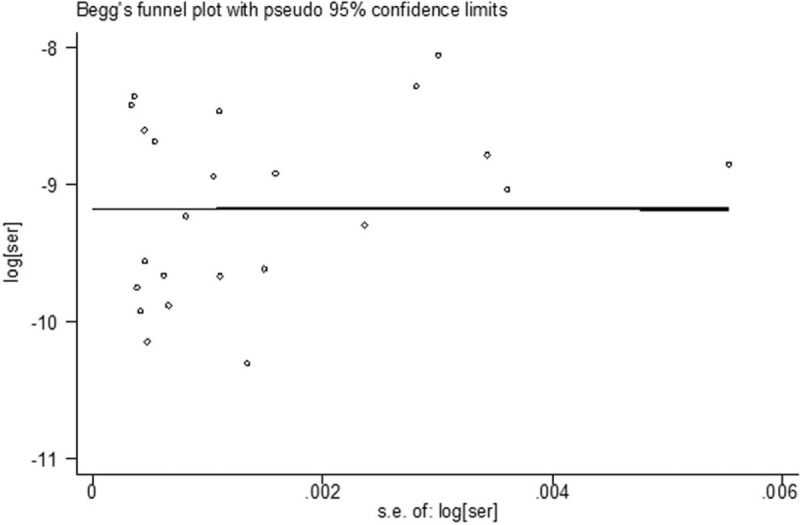
Begg funnel plot for the annual incidence rate of HFMD in China. HFMD = hand-foot-mouth disease.
4. Discussion
Previous study showed that in the 7 administrative regions of China, Hainan province in the central and southern part of China had the highest average incidence of HFMD, while Xinjiang Province in the northwest had the lowest average incidence of HFMD.[37] Same as the conclusion of this study that the incidence of HFMD is the highest in South China, but different from the conclusion of this study that the incidence of HFMD is the lowest in North China. Temperature, humidity, rainfall, and other climatic conditions can promote the reproduction, survival, and transmission of HFMD pathogens to a certain extent, leading to the prevalence and outbreak of HFMD in the population.[8,9] The coastal tropical and subtropical monsoon climate is the main climate type in South China, which is under the climatic conditions of high temperature, high humidity, and high rainfall. South China is more likely to cause HFMD epidemic compared with North China. Therefore, compared with the north areas, the southeast coastal areas should be better implemented in the prevention and control of HFMD.
Zhao et al[38] showed that from 2010 to 2015, the incidence trend of HFMD in China showed a peak every 2 years. The research of Xiao et al[39] showed that the incidence of HFMD in China had an obvious rising trend from 2008 to 2017. This study concluded that the incidence of HFMD declined year by year from 2014 to 2018 in China. The improvement of the ability to diagnose and distinguish HFMD, the improvement of laboratory pathogen detection ability, the effective implementation of HFMD protection knowledge publicity, and the attention paid by schools, families, and communities to the prevention of HFMD can all contribute to the decrease of the incidence of HFMD year by year.
Ji T et al[40] showed that people under the age of 5 years old are the key patients of HFMD in China, and some adults can also have HFMD under certain conditions. The conclusion that children aged 1 to 3 years old have the highest incidence level is consistent with this study. Children under 5 years of age have incomplete immune system function and thus becoming the key population of HFMD. Children in the 0-year group have a certain level of antibodies in their mothers, and children in the 4 to 5 year group can have a certain degree of good hygiene habits, so the 1 to 3 year group is the main population with high incidence of HFMD.
Xu et al[41] showed that EV71 was the dominant epidemic strain in China from 2008 to 2017, which was different from the conclusion drawn in this study that the dominant epidemic strain was other types of enterovirus. The alternate existence of dominant epidemic strains in different years and the mutation and recombination of viruses over time may lead to different distribution of etiology in different study years. The long-term prevalence and low variability of EV71 and Cox A16 as well as the introduction of EV71 vaccine in China since 2016 have enhanced people's immunity against these 2 viruses and reduced people's susceptibility, thus reducing EV71 and Cox A16 infection to some extent. The relevant health departments should take the initiative to strengthen disease surveillance in the areas with high incidence, such as the coastal areas in the southeast, pay special attention to the population with high incidence, improve the disease surveillance capacity in economically backward areas and improve their disease surveillance equipment and facilities, which will help prevent disease epidemics and outbreaks caused by inadequate disease surveillance.
There are some limitations in this meta-analysis: First, differences in sampling methods, specimen types, sampling detection time interval and detection methods, differences in the ability to detect and report disease cases in rural and urban areas, and differences in the level of economic development may be sources of heterogeneity in this study. In addition, because there are not enough references in the included literature to provide sample size and incidence data of HFMD such as gender distribution, occupational distribution, or age distribution of people over 5 years old in China, this study is unable to provide a complete description of the prevalence of HFMD.
Due to the differences in natural and social factors, the incidence of HFMD in different regions of China is quite different, with the highest incidence in South China and the lowest in Northeast China. The incidence of HFMD decreased year by year from 2014 to 2018. In the under-5 age group, the incidence was highest in the 1-year group and lowest in the 0-year group. Compared with Cox A16 and EV71, regions with other types of enteroviruses as the dominant strains had a higher annual incidence. Based on the available information, predicting the epidemic trend of the disease in the future and strengthening the surveillance of HFMD in different regions are conducive to the effective prevention and control of HFMD. Although this meta-analysis has some defects, it is still helpful for the follow-up epidemiological research and etiological research of HFMD.
Author contributions
Conceptualization: Bo Chen, Ying Yang, Ya-Li Zhang, Yongquan Chen.
Data curation: Bo Chen, Ying Yang, xufeng Xu, Ya-Li Zhang, Yi Li, Shi Yin, Yongquan Chen.
Formal analysis: xufeng Xu, Haixia Zhao, Ya-Li Zhang, Yi Li, Shi Yin.
Investigation: xufeng Xu.
Methodology: Ying Yang, Yi Li.
Validation: Bo Chen, Haixia Zhao.
Writing – original draft: Yi Li, Shi Yin.
Writing – review & editing: Bo Chen, Yongquan Chen.
Footnotes
Abbreviations: Cox A16 = coxsackievirus A16, EV71 = human enterovirus 71, HFMD = hand-foot-mouth disease.
How to cite this article: Chen B, Yang Y, Xu X, Zhao H, Li Y, Yin S, Chen YQ. Epidemiological characteristics of hand, foot, and mouth disease in China: a meta-analysis. Medicine. 2021;100:20(e25930).
BC and YY contributed equally to this work.
This study is a meta-analysis and does not involve patient and animal experiments so the ethical approval is not necessary.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
AHRQ = Agency for Healthcare Research and Quality, EV71 = human enterovirus 71, HFMD = hand-foot-mouth disease.
CI = confidence interval, Cox A16 = coxsackievirus A16, EV71 = human enterovirus 71, HFMD = hand-foot-mouth disease.
References
- [1].Li L, Zhu F, Shi Z, et al. Analysis on the epidemiological characteristics of hand, foot and mouth disease etiology in Jiangsu Province. Chin J Prev Med 2010;44:676–7. [Google Scholar]
- [2].Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010;10:778–90. [DOI] [PubMed] [Google Scholar]
- [3].Esposito S, Principi N. Hand foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis 2018;37:391–8. [DOI] [PubMed] [Google Scholar]
- [4].Yang F, Ren L, Xiong Z, et al. Enterovirus 71 outbreak in the People's Republic of China in 2008. J Clin Microbiol 2009;47:2351–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med 1999;341:929–35. [DOI] [PubMed] [Google Scholar]
- [6].Sun BJ, Chen HJ, Chen Y, An XD, Zhou BS. The risk factors of acquiring severe hand, foot, and mouth disease: a meta-analysis. Can J Infect Dis Med Microbiol 2018;2018:2751457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tang JH, Chan TC, Shigematsu M, Hwang JS. Latitude-based approach for detecting aberrations of hand, foot, and mouth disease epidemics. BMC Med Inform Decis Mak 2015;15:01–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu Z, Hu W, Jiao K, Ren C, Jiang B, Ma W. The effect of temperature on childhood hand, foot and mouth disease in Guangdong Province, China, 2010-2013: a multicity study. BMC Infect Dis 2019;19:01–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee CC, Tang JH, Hwang JS, Shigematsu M, Chan TC. Effect of meteorological and geographical factors on the epidemics of hand, foot, and mouth disease in island-type territory, East Asia. Biomed Res Int 2015;2015:805039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:02–10. [DOI] [PubMed] [Google Scholar]
- [11].Cohen JF, Chalumeau M, Cohen R, Korevaar DA, Khoshnood B, Bossuyt PM. Cochran's Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J Clin Epidemiol 2015;68:299–306. [DOI] [PubMed] [Google Scholar]
- [12].Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Ins 1989;81:107–15. [DOI] [PubMed] [Google Scholar]
- [13].Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chang YX, Yuan WP, Zhang YX. Analysis of prevalence characteristics of HFMD in Zhangye City from 2009 to 2018. Dis Prevent Control Bull 2019;2019:68–70. [Google Scholar]
- [15].Liu QL, Shi W. Epidemiological analysis of hand, foot and mouth disease in Xianyang city, Shaanxi Province. Stat Manag 2016;2018:91–4. [Google Scholar]
- [16].Liu G, Ma N, Lei DC, et al. Epidemiological characteristics of hand, foot and mouth disease in Weinan city from 2009 to 2013. South China Prev Med 2016;42:454–6. [Google Scholar]
- [17].Ma AG, Zhang J. Analysis of prevalence characteristics of hand, foot and mouth disease in Qitai county, Xinjiang from 2008 to 2015. Dis Prevent Control Bull 2016;6:50–3. [Google Scholar]
- [18].Dai MY. Analysis of epidemic characteristics of hand, foot and mouth disease in Shenyang from 2013 to 2018. Prev Med Forum 2019;25:690–1. [Google Scholar]
- [19].Cui RM, Teng YZ. Epidemiological and etiological characteristics of hand, foot and mouth disease in Dandong city from 2008 to 2016. South China Prev Med 2008;44:138–43. [Google Scholar]
- [20].Wang SH, Xie WL, Sun J, et al. Analysis of prevalence characteristics of hand, foot and mouth disease in Qinglong Manchu autonomous county, Hebei province from 2008 to 2015. Medical Animal Control 2017;33:343–5. [Google Scholar]
- [21].Li Y, Yang R. Analysis of epidemiological characteristics of hand-foot-mouth disease in Changli county, Hebei province from 2012 to 2015. Medical Animal Control 2017;33:782–6. [Google Scholar]
- [22].Wei WY. Epidemiological characteristics of hand, foot and mouth disease in Nanyang city from 2014 to 2018. Prev Med Forum 2019;25:849–54. [Google Scholar]
- [23].Zhao QP. Epidemic Characteristics of Hand Foot and Mouth Disease and its Influence on Temperature in Xiantao City from 2009 to 2017. Chinese Center for Disease Control and Prevention, 2018: 1–85. [Google Scholar]
- [24].Zhang PF, Xu L, Feng X, et al. Epidemiological characteristics of hand, foot and mouth disease in Pingxiang city from 2013 to 2016. Appl Prev Med 2016;24:25–7. [Google Scholar]
- [25].Liao QD, Luo T, Wang Y, et al. Epidemiological characteristics and etiological characteristics of hand, foot and mouth disease in Jiangjin district, Chongqing from 2012 to 2016. Mod Med Health 2019;35:2875–7. [Google Scholar]
- [26].Luo QM, Wu XL, Hu QL, Hu QL. Epidemiological characteristics of Hand, foot and mouth disease in Chuxiong Prefecture from 2008 to 2017. Mod Prev Med 2015;45:2898–901. [Google Scholar]
- [27].He K, Huang DS, An L, et al. Epidemiological characteristics of hand, foot and mouth disease in Baoshan city from 2008 to 2016. Prev Med Forum 2008;24:188–92. [Google Scholar]
- [28].Lu JX, Jiang YQ, Sun ZY, et al. Epidemiological characteristics of hand, foot and mouth disease in Anshun, Guizhou province from 2012 to 2017. Medical Animal Control 2019;35:1021–4. [Google Scholar]
- [29].Yan P, Huang TY, Wei FQ. Epidemiological and pathogenic characteristics of hand, foot and mouth disease in Xingbin district, Laibin city, 2012-2016. Appl Prev Med 2017;23:462–4. [Google Scholar]
- [30].Deng XC, Qin XL, He JZ, et al. Analysis of prevalence characteristics of Hand, foot and mouth disease in Guigang city, Guangxi from 2008 to 2016. Medical Animal Control 2008;34:1060–3. [Google Scholar]
- [31].Xiong GL, Liu J, Yang L, et al. Analysis of prevalence characteristics of Hand, foot and mouth disease in Baise, Guangxi in 2015. World Latest Medical Information Digest 2016;16:05–8. [Google Scholar]
- [32].Xia GX, Hu CQ, Lan XY. Epidemiological and pathogenic characteristics of hand, foot and mouth disease in Liannan Yao autonomous county from 2008 to 2016. Henan J Prev Med 2019;30:395–9. [Google Scholar]
- [33].Yuan LF, Ou S, Xu PL, et al. Epidemiological analysis of hand, foot and mouth disease in Teng county from 2010 to 2013. South China Prev Med 2016;42:46–9. [Google Scholar]
- [34].Yu CL, Li FY, Wang DP, et al. Epidemiological characteristics of hand, foot and mouth disease in Anqiu from 2009 to 2018. Prev Med Forum 2019;25:779–81. [Google Scholar]
- [35].Qian XF. Epidemiological characteristics of hand-foot-mouth disease from 2008 to 2013 in Rui’an city. China Rural Med 2015;22:69–70. [Google Scholar]
- [36].Hu CT, Hu PF. Analysis of HFMD in Donggang District, Rizhao city, 2016. J Community Med 2018;16:29–32. [Google Scholar]
- [37].Cui A, Zhu Z, Hu Y, et al. Mumps Epidemiology and mumps virus genotypes circulating in mainland China during 2013-2015. PLoS One 2017;12:e0169561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhao J, Jiang F, Zhong L, Sun J, Ding J. Age patterns and transmission characteristics of hand, foot and mouth disease in China. BMC Infect Dis 2016;16:01–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xiao Y, Zhou J, Zhang H, Ding C, Shi P. Epidemiological and aetiological characteristics of hand, foot and mouth disease cases 2011-2017 in Yixing, China. Infect Dis (Lond) 2018;50:859–61. [DOI] [PubMed] [Google Scholar]
- [40].Ji T, Han T, Tan X, et al. Surveillance, epidemiology, and pathogen spectrum of hand, foot, and mouth disease in mainland of China from 2008 to 2017. Biosafety and Health 2019;1:32–40. [Google Scholar]
- [41].Xu Y, Li S, Cai C, et al. Characterization of inflammatory cytokine profiles in cerebrospinal fluid of hand, foot, and mouth disease children with enterovirus 71-related encephalitis in Hangzhou, Zhejiang, China. Medicine (Baltimore) 2019;98:e18464. [DOI] [PMC free article] [PubMed] [Google Scholar]


