Abstract
Objective:
The aim of this study was to determine the best thickness of corneal slices acquired from femtosecond laser surgery—small incision lenticule extraction (SMILE surgery) as patch graft in glaucoma drainage implantation surgery.
Methods:
This study is a prospective randomized study. Patients who received glaucoma drainage implantation from September 2016 to November 2018 were observed. The patients were randomly divided into 3 groups. Group A included 102 cases (104 eyes), receiving 1 layer (120–150 μm) of allogeneic lamellar corneal tissue as the graft. Group B included 117 cases (120 eyes), receiving 2 layers of lamellar corneal tissue from one donor. Group C included 109 cases (111 eyes), using 3 layers of lamellar corneal tissue from 2 donors. The intraocular pressure, corneal graft, conjunctiva stromalysis, drainage tube exposure, and drainage plate were observed.
Results:
Patients were followed up for 6 to 33 months. The intraocular pressure was significantly reduced after surgery in all three groups. Conjunctiva stromalysis and drainage tubes were exposed in 3 eyes (3%) in group A and 1 eye (0.8%, a special case which has nystagmus and the plate was placed infratemporally) in group B, whereas no conjunctiva stromalysis or tube exposure was reported in group C.
Conclusions:
The corneal graft acquired from SMILE surgery can effectively prevent drainage tube exposure and give patients a better cosmetic appearance. Two layers of lamellar corneal tissue (240–300 μm) may be the best suitable thickness because it can effectively reduce tube exposure and rejection. In some special cases, 3 layers of lamellar corneal tissue are needed.
Keywords: Ahmed glaucoma valve implantation surgery, best thickness, lamellar corneal tissue, tube exposure
1. Introduction
Drainage tube exposure is a major complication of glaucoma drainage implant surgery. The exposure rate is 1% to 5% at 5 years of follow-up.[1–3] Tube exposure can occur at any time postoperatively and lead to serious consequences, such as endophthalmitis. To prevent drainage tube exposure, patch grafts, such as autologous lamellar scleral flap, allograft sclera, pericardium, preserved cornea, dura, amniotic membrane, buccal mucous membrane, autologous tragal perichondrium,[4–9] and femoral fascia, were used to cover the anterior part of the tube shunt.[7–10] Each material has its own advantages and disadvantages[11] and there is no consensus on which is the best choice for the prevention of drainage tube exposure.
Donor corneal tissue has been used as a new kind of patch in recent years.[4,12,13] Its tight structure makes the cornea unlikely to melt. In addition, corneal tissue has the advantage of esthetics (especially when it is placed at the bottom of the eye).[4,12] However, corneal tissue used in eye surgery is expensive and scarce, especially in China. Furthermore, corneal tissue cannot meet the demands for keratoplasty, making it difficult to use as a graft material in glaucoma surgery.
Since 2015, we have experimented and used 3 layers of lamellar corneal tissue acquired from SMILE surgery as patch grafts in drainage implant surgery.[14] The diameter of the lamellar corneal tissue is approximately 7 mm, and each layer is 100- to 150-μm thick. Our research provided that 3 layers of corneal graft could effectively prevent drainage tube exposure. Moreover, lamellar corneal tissue is easy to obtain, and is less expensive than other tissues. During our research, no infection or rejection occurred.
In our study, 3 layers of lamellar corneal tissue made the total thickness of the patch approximately 300 to 450 μm. Theoretically speaking, the thicker the patch graft is, the less tube exposure occurs. However, thick grafts may enhance friction against the eyelids and cause discomfort. Moreover, a thick graft can lead to instability of the tear film, resulting in a concave cornea. In addition, one donor only can provide 2 corneal slices and 3 layers of tissue comes from 2 donors, which increases risk of rejection for the recipient.
The purpose of the present study was to determine whether low tube exposure rates can be achieved by reducing the layers of the lamellar corneal tissue. To that end, patches of 1, 2, and 3 layers of lamellar corneal tissue acquired from SMILE surgery were used in glaucoma drainage surgery in our hospital from September 2016 to November 2018. Tube exposure and other complications were observed.
2. Patients and methods
2.1. Patients
This study was a prospective randomized study. It followed the tenets of the Declaration of Helsinki and was approved by the ethics committee of the Xiamen Eye Center. Patients who received Ahmed glaucoma valve (New World Medical, Inc., Rancho Cucamonga, CA) implantation surgery from September 2016 to November 2018 at the Xiamen Eye Center were observed.
The patients were randomly divided into 3 groups. Group A consisted of 102 patients (104 eyes) who received 1 layer of allogeneic lamellar corneal tissue acquired from SMILE surgery. Group B consisted of 117 patients (120 eyes) who received 2 layers of lamellar corneal tissue. Group C consisted of 109 patients (111 eyes), who received 3 layers of lamellar corneal tissue as allografts.
2.2. Preparation of corneal allografts from SMILE surgery
Written consent to freely offer lamellar corneal tissue was obtained from myopia patients. Bloodwork was conducted to exclude donations from patients with infectious diseases. Corneal tissue obtained from SMILE surgery was immediately placed in sterile anhydrous glycerol and stored at 4°C. Two lamellar corneal tissues from each donor were stored in a single Eppendorf tube. The tissue was removed from the glycerol and washed with a balanced salt solution during glaucoma surgery. After rehydration with tobramycin diluent, the tissue was rinsed again with balanced salt solution and applied to cover the drainage tube. Each layer of the lamellar corneal tissue was approximately 120- to 150-μm thick.
2.3. Surgical technique
All FP7 or FP8 Ahmed glaucoma valve tubes were implanted through a fornix-based conjunctiva incision with the plate secured 8 to 10 mm from the limbus. The tube was inserted into the anterior chamber through a scleral track 1 to 1.5 mm posterior to the limbus. Then, 1, 2, (from 1 donor), or 3 (from 2 donors) layers of lamellar corneal tissue were taken from SMILE surgery to cover the anterior tube and fixed with 10–0 nylonsutures. All surgeries were performed by an experienced physician.
2.4. Image examination
Anterior segment optical coherence tomography (AS-OCT, Tomey, Japan) was used to detect the thickness of the corneal graft. Five scan lines were applied 1.5 to 5.5 mm posterior to the corneal limbus in each examination after surgery. The follow-up appointments were at 1 week, 1 month, 3 months, 6 months, 12 months, 18 months, and 24 months after surgery. Patch graft thinning was defined as a patch graft thickness <50 μm in the 1-layer group, <100 μm in the 2-layer group, and <150 μm in the 3-layer group.
2.5. Statistical analysis
All data were recorded on Microsoft Excel spreadsheets. One-way analysis of variance was used to compare mean age, sex, best corrected visual acuity, intraocular pressure (IOP), and other patient characteristics. The χ2 test was used to compare the type of glaucoma, implantation site, and rate of exposure. Significance was set at the 5% level. Statistical analysis was performed with SPSS software V.22 (SPSS, Inc., Chicago, IL).
3. Results
3.1. Patient Information
There were no statistically significant differences in age, sex, visual acuity, IOP, type of glaucoma, or site of implantation before surgery (P > .05; Table 1). Few children cannot cooperate with visual acuity examinations, so they were excluded from these analyses. Their IOP was measured with an ICARE tonometer. Eyes with traumatic injury that had undergone vitrectomy were classified as vitrectomy, and eyes with neovascular glaucoma after vitrectomy were classified as neovascular glaucoma. Other categories included iridocorneal endothelial (ICE) syndrome, Axenfeld-Rieger (AR) syndrome, congenital aniridia, and so on. Most patients with neovascular glaucoma received anterior chamber and/or intravitreal injection of ranibizumab (Lucentis) 0.05 mL 2 to 3 days before drainage valve implantation. Some patients with neovascular glaucoma had hyphema before surgery.
Table 1.
Demographic and clinical data.
| Group A | Group B | Group C | |
| Cases | 102 (104 eyes) | 117 (120 eyes) | 109 (111 eyes) |
| Sex (male: female) | 54:48 | 59:58 | 52:57 |
| Age, y | 56.2 ± 16.5 | 54.9 ± 17.9 | 58.6 ± 19.4 |
| Range | 5–72 | 4M-69 | 2–75 |
| BCVA | FC-0.4 | NLP-0.5 | 0.05–0.4 |
| IOP, mmHg | 35.5 ± 8.4 | 36.2 ± 7.6 | 34.3 ± 8.6 |
| Glaucoma medications | 3.26 ± 1.55 | 3.15 ± 1.98 | 3.44 ± 1.64 |
| Glaucoma type (eyes) | |||
| NVG | 29 cases (30 eyes) | 34 cases (35 eyes) | 31 cases (31eyes) |
| Intravitreal injection (Lusentis) 24 | 28 | 28 | |
| Hyphema | 5 | 6 | 4 |
| After trauma surgery | 12 | 15 | 13 |
| After vitrectomy | 24 | 28 | 27 |
| After glaucoma surgery | 29 | 32 | 29 |
| other | 9 | 10 | 11 |
| FP7:FP8 | 104:0 | 118:2 | 111:1 |
| Operation site | |||
| (Superotemporal: infratemporal) | 92:12 | 101:19 | 97:14 |
3.2. Clinical efficacy
The patients were followed up for 6 to 33 months. After surgery, the IOP was significantly reduced in the 3 groups compared with IOP before surgery (Group A: 35.5 ± 8.4 mmHg vs 17.5 ± 6.1 mmHg; Group B: 36.2 ± 7.6 mmHg vs 15.6 ± 7.2 mmHg; Group C: 34.3 ± 8.6 mmHg vs 16.3 ± 4.9 mmHg) (Fig. 1/Table 2). However, there were no significant differences among the 3 groups and visual acuity was unchanged. Some patients underwent other surgeries during follow-up, such as cataract removal, vitreous cavity irrigation, or vitrectomy combined with retinal laser irradiation (diabetic retinopathy, vitreous hemorrhage). Some patients received 5-fluorouracil filtering bleb separation or needed glaucoma medicines after drainage implant surgery, and some patients even needed glaucoma surgery again.
Figure 1.

Intraocular pressure pre-operation and post-operation.
Table 2.
Postoperative data.
| Group A | Group B | Group C | |
| Follow-up, mo | 20.8 ± 4.2 | 18.9 ± 5.3 | 19.2 ± 5.5 |
| IOP, mmHg | 17.5 ± 6.1 | 15.6 ± 7.2 | 16.3 ± 4.9 |
| BCVA | 0.05–0.6 | NLP-0.5 | FC-0.5 |
| Glaucoma medications | 0.6 ± 0.4 | 0.5 ± 0.5 | 0.5 ± 0.4 |
| Graft thinner (eyes) | 19 (18%) | 6 (5%) | 3 (2.7%) |
| Tube exposure (eyes) | 3 (3%) | 1 (0.8%) | 0 (0%) |
| Plate exposure (eyes) | 0 (0%) | 0 (0%) | 0 (0%) |
| Repair surgery (eyes) | 2 (1.9%) | 1 (0.8%) | 0 (0%) |
3.3. Image examination
The corneal graft was transparent under the conjunctiva in slit lamp examination and did not affect the cosmetic appearance of patients in the 3 groups (Fig. 2). Each layer of lamellar corneal tissue could be distinguished clearly in AS-OCT images at an early post-surgical stage (Fig. 3). With time, the corneal grafts lost some of their transparency, as seen in the OCT image a year after surgery (Fig. 4). However, the graft was still lucid under slit lamp. A thinner appearance of corneal grafts could be observed in some cases. Corneal tissue could not be seen in some 1-layer grafts 2 years after surgery (Fig. 5). Conjunctiva stromalysis and drainage tube exposure occurred in 3 eyes in Group A (3%) and 1 eye in Group B (0.8%), whereas neither occurred in Group C (0%). In the 1-layer corneal graft group, one of the tube exposing eyes was secondary glaucoma after congenital cataract surgery and having a relatively large eyeball. The tube-exposing eye in the 2-layer group was a Peter syndrome patient with nystagmus after cataract surgery, and the plate was placed infratemporally. Exposure occurred at 7 months in the 1-layer group and 15 months in the 2-layer group. Exposure range was 2 to 3 mm (Fig. 6). All exposed eyes needed surgical repair except for one case in the 1-layer group. Three layers of lamellar corneal tissue and autologous conjunctiva transplant were performed during repair surgery. The drainage tubes did not reexpose.
Figure 2.

Transparent corneal graft under slit lamp examination.
Figure 3.
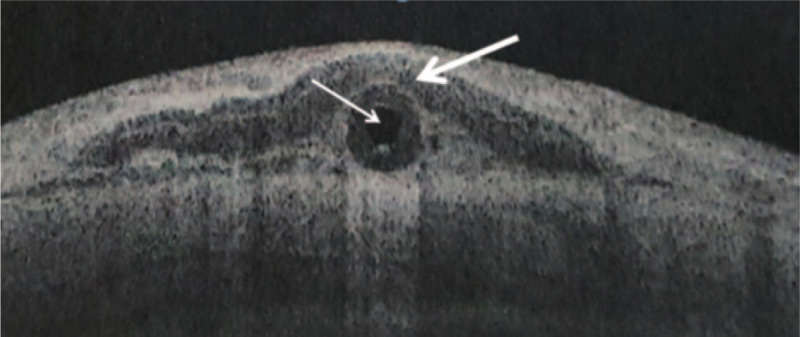
Anterior segment optical coherence tomography showed the cornea patch on the tube. The thick arrow shows the cornea, and the thin arrow shows the tube.
Figure 4.

One year after surgery, the corneal graft was not transparent as it was.
Figure 5.

Two years after surgery, the corneal patch could not be seen in single-layer graft.
Figure 6.

The patch graft and conjunctiva melted. The tube was exposed.
3.4. Recurrence and other complications (Table 3)
Table 3.
Recurrence and other complications.
| Goup A | Group B | Group C | |
| During surgery (eyes) | |||
| Hyphema | 3 (3%) | 2 (1.7%) | 3 (2.7%) |
| Post surgery (eyes) | |||
| Explosive suprachoroidal hemorrhage 0 (0%) | 1 (0.8%) | 0 (0%) | |
| Shallow anterior chamber | 4 (3.8%) | 3 (2.5%) | 3 (2.7%) |
| Choroid detachment | 10 (9.6%) | 11 (8.33%) | 12 (10.8%) |
| Endophthalmitis | 0 (0%) | 1 (0.8%) | 0 (0%) |
| Further glaucoma surgery (eyes) | 2 (1.9%) | 1 (0.8%) | 1 (0.9%) |
During surgery, hyphema was observed in 3 eyes (3%) in the 1-layer corneal graft group, 2 eyes (1.7%) in the 2-layer corneal graft group, and 3 eyes (2.7%) in the 3-layer corneal graft group. Hyphema was mild because many patients received Lucentis intravitreal injection before drainage implant surgery. Post-surgery complications included shallow anterior chamber, choroid detachment, endophthalmitis, and explosive suprachoroidal hemorrhage. Shallow anterior chamber occurred in 4 eyes (3.8%) in the 1-layer corneal graft group, three eyes (2.5%) in the 2-layer group, and 3 eyes (2.7%) in 3-layer group. Choroid detachment occurred in 10 eyes (9.6%) in the 1-layer group, 11 eyes (8.3%) in the 2-layer group, and 12 eyes (10.8%) in the 3-layer group. There were no significant differences between the 3 groups (P > .05). Endophthalmitis occurred in 1 eye in the 2-layer group. The patient was diagnosed with diabetic retinopathy and underwent vitrectomy. The source of infection was from the vitreous body, not the drainage device. One eye in the 2-layer group experienced expulsive suprachoroidal hemorrhage. This eye was an aphakic eye and underwent vitrectomy. There was a little hemorrhage in the suprachoroidal space before surgery (Table 3).
4. Discussion
With the increase of refractory glaucoma, drainage implant surgery has been increasingly applied in clinical work. Although patch grafts are more often used to cover the anterior tube, tube exposure cannot be completely avoided. Regina believed that graft thinning was a critical step in the development of tube exposure, and that if patch graft thinning could be eliminated, tube exposure was unlikely to occur. He also found that corneal patch grafts did not always retain their integrity after drainage implantation; the longer the time after surgery, the thinner the graft became. The rate of corneal graft thinning is 60 μm per year.[15]
However, few studies have examined the thickness of patch grafts. In an effort to determine whether patch graft thickness affected tube erosion rates, Lankaranian compared the effect of single thickness versus double thickness pericardium as a patch graft in glaucoma drainage implant surgery. The authors found that the erosion rate was 16% (5 eyes) in the single thickness patch versus 0% in the double thickness patches in the study. Five eyes in the single thickness group underwent repair with double thickness pericardium. Interestingly, after an average follow-up of 8.6 months, none of the 5 eyes had experienced erosion again. Mitomycin C was also used during tube shunt implant surgery in some of the study's subjects.[11] Muir et al[10] also found no erosion in 14 patients patched with double-layer Tutoplast pericardium, and 2 erosions occurred in 39 patients patched with single-layer Tutoplast pericardium. Partial thickness (300 μm) tissue was studied by Spierer et al and Anand et al. In his study, Spierer et al[8] found that one of the 44 eyes patched with corneal grafts needed revision after an average follow-up of 27.6 months. Anand et al[9] used 300-μm amniotic membrane as the patch graft material. Similarly, 1 erosion of 41 patients occurred at a mean follow-up of 2 months. Higher rates of tube erosion were also been reported. Nagi found a 10% erosion rate in patients who had a lamellar porcine small intestinal submucosa graft called Kerasys (IOP Ophthalmics). The study's average follow-up time was 15.2 months. Across kinds of patch materials used in drainage tube implant surgery, the tube exposure rate varied between 0% and 10%. It seems that the thicker the patch, the less exposure occurred. Our previous research proved that 3 layers of corneal grafts from SMILE surgery can effectively prevent tube exposure; however, grafts that are too thick may cause other problems, such as increased friction, foreign body sensation, cornea dry spots, and ulceration. In our research, 3 layers of corneal tissue from 2 donors theoretically indicated an increasing rate of rejection. Therefore, we aim to reduce the thickness of the patch graft and try to find the optimal thickness.
In this study, we observed the effect of different thicknesses of lamellar corneal tissue acquired from SMILE surgery in the prevention of drainage tube exposure. AS-OCT was used to detect the thickness of the corneal graft. Over time, the corneal patch became thinner in many cases. Finally, corneal tissue could not be detected in some of the 1-layer group members. This result is consistent with Regina A's conclusion. Conjunctiva melted, and drainage tube exposure occurred in 3 eyes in Group A (3%) and 1 eye in Group B (0.8%), whereas neither was reported in Group C (0%). Although there was no significant difference in conjunctiva stromalysis and tube exposure, conjunctiva stromalysis and tube exposure seemed more likely to occur in the single layer group.
There was 1 case of tube exposure in the 2-layer corneal graft group. However, the patient who is Peter's anomaly and has nystagmus is a special case. Nystagmus increases friction. In addition, the plate was implanted in the inferior hemisphere. Inferior tube location has been suggested as a risk factor for erosions of Ahmed implants. Pakravan et al found that implants in the inferior hemisphere had an 8.3% chance of explanations, whereas only 1.7% of devices placed in the superior hemisphere developed erosion. Donor scleral patch grafts were used in this study.[13] Geffen et al[12] also found that a high conjunctival dehiscence rate occurred when the plate was located in the inferior hemisphere compared with the superior hemisphere. His study also reported differences in erosion rates when the tube was placed in the inferior hemisphere versus the superior hemisphere.
In conclusion, although there was 1 case of tube exposure in the 2-layer group, we still concluded that 2 layers of lamellar corneal tissue (approximately 300 μm) were sufficient for the prevention of conjunctiva stromalysis and drainage tube exposure. In some special cases, 3 layers of lamellar corneal tissue (approximately 450 μm) may be needed. One layer of lamellar corneal tissue (approximately 150 μm) is not recommended.
Author contributions
Data curation: Weiyi Huang, Yazhang Xu.
Methodology: Weiyi Huang, Yazhang Xu, Meizhu Cheng, Zhengwei Shen.
Resources: Zhengwei Shen.
Supervision: Yuhong Wang.
Writing – original draft: Yuhong Wang, Jinkun Liu.
Writing – review & editing: Jinkun Liu.
Footnotes
Abbreviations: AR syndrome = Axenfeld-Rieger syndrome, AS-OCT = anterior segment optical coherence tomography, ICE syndrome = iridocorneal endothelia syndrome, IOP = intraocular pressure, NVG = neovascular glaucoma, SMILE = small incision lenticule extraction.
How to cite this article: Wang Y, Liu J, Huang W, Xu Y, Cheng M, Shen Z. The best thickness of cornea graft from SMILE surgery as patch graft in glaucoma drainage implant surgery. Medicine. 2021;100:20(e25828).
The authors report no conflicts of interest.
Funding/Support: Supported by Key laboratory of myopia, National Health Commission/Chinese Academy of Medical Sciences (Fudan University).
YW and JL contributed equally to this work.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
BCVA = best-corrected visual acuity, IOP = intraocular pressure, NVG = neovascular glaucoma. All P > .05.
BCVA = best corrected visual acuity, IOP = intraocular pressure. All P > .05.
All P > .05.
References
- [1].Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol 2012;153:804–14. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Budenz DL, Feuer WJ, Barton K, et al. Postoperative complications in the Ahmed Baerveldt comparison study during five years of follow-up. Am J Ophthalmol 2016;163:75–82. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Christakis PG, Kalenak JW, Tsai JC, et al. The Ahmed versus Baerveldt study: five-year treatment outcomes. Ophthalmology 2016;123:2093–102. [DOI] [PubMed] [Google Scholar]
- [4].Chun YS, Kim KW, Kim JC. Autologous tragal perichondrium patch graft for ahmed glaucoma valve tube exposure. J Glaucoma 2013;22:e27–30. [DOI] [PubMed] [Google Scholar]
- [5].Tanji TM, Lundy DC, Minckler DS, et al. Fascia lata patch graft in glaucoma tube surgery. Ophthalmology 1996;103:1309–12. [DOI] [PubMed] [Google Scholar]
- [6].Rootman DB, Trope GE, Rootman DS. Glaucoma aqueous drainage device erosion repair with buccal mucous membrane grafts. J Glaucoma 2009;18:618–22. [DOI] [PubMed] [Google Scholar]
- [7].Nagi KS, Cumba RJ, Bell NP, et al. Short-term outcomes of KeraSys patch graft for glaucoma drainage devices: a case series. J Ophthalmol 2013;2013:784709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Spierer O, Waisbourd M, Golan Y, et al. Partial thickness corneal tissue as a patch graft material for prevention of glaucoma drainage device exposure. BMC Ophthalmol 2016;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anand A, Sheha H, Teng CC, et al. Use of amniotic membrane graft in glaucoma shunt surgery. Ophthalmic Surg Lasers Imaging 2011;42:184–9. [DOI] [PubMed] [Google Scholar]
- [10].Muir KW, Lim A, Stinnett S, et al. Risk factors for exposure of glaucoma drainage devices: a retrospective observational study. BMJ Open 2014;4:e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lankaranian D, Reis R, Henderer JD, et al. Comparison of single thickness and double thickness processed pericardium patch graft in glaucoma drainage device surgery: a single surgeon comparison of outcome. J Glaucoma 2008;17:48–51. [DOI] [PubMed] [Google Scholar]
- [12].Geffen N, Buys YM, Smith M, et al. Conjunctival complications related to Ahmed glaucoma valve insertion. J Glaucoma 2014;23:109–14. [DOI] [PubMed] [Google Scholar]
- [13].Pakravan M, Yazdani S, Shahabi C, et al. Superior versus inferior Ahmed glaucoma valve implantation. Ophthalmology 2009;116:208–13. [DOI] [PubMed] [Google Scholar]
- [14].Wang Y, Li X, Huang W, et al. Partial thickness cornea tissue from small incision lenticule extraction: a novel patch graft in glaucoma drainage implant surgery. Medicine (Baltimore) 2019;98:e14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].de Luna RA, Moledina A, Wang J, et al. Measurement of gamma-irradiated corneal patch graft thickness after aqueous drainage device surgery. JAMA Ophthalmol 2017;135:941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


