Abstract
Background:
Randomizing patients to bladder preservation or radical cystectomy (RC) for the treatment of bladder cancer has not been practical, due to patient and physician preferences. Therefore, continually comparing the 2 treatment modalities is needed, in order to make the proper choice for each patient.
Patients and methods:
The records of T1–4N0M0 bladder cancer patients, who presented to the South Egypt Cancer Institute between 2007 and 2017 and were treated by either bladder preservation or RC were reviewed.
Results:
Out of the 166 included patients, 81 (48.8%) patients were treated by bladder preservation and 85 (51.2%) patients had RC. For the patients treated by bladder preservation and the patients treated by RC, the 5-year overall survival (OS) was 56% and 60% (p = 0.67), the 5-year local recurrence-free survival was 69% and 73% (p = 0.69), and the 5-year disease-free survival was 45% and 53% (p = 0.16), respectively. After propensity matching analysis, the mean 5-year OS was 58% for the bladder preservation patients and 61% for the RC patients (p = 0.51). It is notable that among the bladder preservation group, 8 patients (10%) had squamous cell carcinoma (SCC) pathology and refused RC. Their OS was 56% compared to 53% for the SCC patients treated by RC (p = 0.6).
Conclusion:
Bladder preservation is a safe alternative to cystectomy in transitional cell carcinoma stages T1–4aN0M0, and its use in SCC bladder cancer should be further studied, as it could be feasible to spare them from initial cystectomy.
Keywords: Bladder cancer, Cystectomy, Preservation, Squamous cell carcinoma, Transitional cell carcinoma
1. Introduction
Bladder cancer is the 4th most common cancer in Egypt, [1] and the 9th most common cancer worldwide.2,3 In 2018, there were 9239 new bladder cancer cases in Egypt which were 7.2% of all the cancers and bladder cancer was the 5th cause of cancer deaths. [1] In addition to the fact that bladder cancer is relatively more common in Egypt, more squamous cell carcinoma (SCC) histology exists (41% of the cases) than in the developed countries, with 53% urothelial histology and 6% other histology. [4] The urothelial histology constitutes more than 90% of bladder cancer in other areas of the world. [5]
Muscle-invasive bladder cancer is a fatal disease which leads to death within 2 years if left untreated.2,6 Radical cystectomy (RC) with pelvic node dissection has been considered as the golden standard treatment for a long time. [2] However, surgical morbidity remains significant in a recent series of research, [7] with grade ≥3 toxicity occurring in 13% of the patients, including gastrointestinal, infectious, and wound-related complications. An alternative trimodality treatment which has gained popularity is bladder preservation by maximal transurethral resection of bladder tumor (TURBT), followed by external beam radiotherapy and chemosensitization, with similar outcomes to RC.8,9,10,11 As reported by Fahmy et al. [12] in a meta-analysis, which included 57 studies published between 1990 and 2017, the mean 10-year overall survival (OS) for 1536 patients treated by bladder conservation was 30.9%7,13,14,15,16 and was 35.1% for 5163 patients treated by RC,17,18,19,20 with no statistically significant difference (p = 0.32). Additionally, similar results were reported in other series, as RC has demonstrated 5-year OS rates of 56–66%,21,22,23 and for bladder trimodality treatment, the 5-year OS was 72.0%. [24]
Regarding the quality of life when comparing RC to bladder preservation, patients with initially similar baseline scores, showed better mean global health status and social functioning, after 1 year of treatment with bladder preservation. [25]
Randomized trials comparing the 2 treatment modalities have not been practical, [24] for example, the study conducted in the UK health system, [25] in which non-compliance with assigned treatment interfered with the completion of study, due to strong clinician and patient preferences. Observational data, therefore, remains the principal source of information regarding the efficacy and safety of the 2 treatments, and it continues to support bladder preservation as an acceptable alternative to RC for muscle-invasive bladder cancer. [26] In countries with endemic schistosomiasis like Egypt, differences in incidence pathogenesis and outcome of bladder cancer may exist, when compared to developed countries. This retrospective study is intended to assess the differences in local control, disease-free survival (DFS) and OS with RC and bladder preservation in muscle-invasive bladder cancer with urothelial as well as SCC pathologies.
2. Patients and methods
This research was accepted from the ethical committee of the South Egypt Cancer Institute (approval number IORG000656). We reviewed the records of the bladder cancer patients who attended South Egypt Cancer Institute form January 2009 to January 2017. Eligible patients with T1–4N0M0 bladder cancer who were operable and resectable, were treated by RC with or without adjuvant therapy or bladder preservation. The patients were treated by bladder preservation after maximum TURBT followed by external beam radiotherapy and chemosensitization, based on their choice and a multidisciplinary panel decision. None of the patients received neoadjuvant chemotherapy. All pathologies were eligible. We collected data including age, gender, pathological type, pathological grade, stage, type of primary treatment, type of adjuvant treatment if received, type and time of treatment failure if it occurred, date of last follow-up, and their status at that time. All patients were followed-up by physical examination, blood tests, and urine analysis, abdominopelvic computed tomography, chest X-ray followed by computed tomography if needed every 3 months the first year, then every 6 months for the second year and annually thereafter. Cystoscopy was added for the patients on the bladder preservation groups. Bone scan was added if indicated.
2.1. Statistical analysis
The quantitative variables were compared by the independent sample t test and the qualitative variables were compared by the Chi square test. OS was defined from the date of diagnosis till death or last follow-up and DFS was from the date of completion of treatment till local or distant relapse or last follow-up. Local recurrence-free survival (LRFS) was from the date of completion of the bladder preservation protocol till the development of local recurrence or last follow-up. Survival was assessed by the Kaplan–Meier method and compared by the Log rank test. We used propensity score analysis to reduce bias. The SPSS version 22 was used to process the data.
3. Results
We analyzed the data of 166 patients. Patient characteristics are shown in Table 1. There were 81 (48.8%) patients in bladder preservation group and 85 (51.2%) in RC group. Patients and tumor characteristics are shown in Table 1. The median age of all the patients was 57 years (range 30–70 years). The majority of the patients 113 (68%) were males. The most common pathology was transitional cell carcinoma (TCC) in 113 (68%) and SCC in 45 (27%) patients. Stages T2 and T3 were found in 77 (46%) and 62 (37%) patients, respectively. There was a statistically significant difference between the 2 groups in the age at diagnosis and the pathology.
Table 1.
Patients characteristics.
| Bladder preservation (n = 81) | RC (n = 85) | p | |
|---|---|---|---|
| Median age at diagnosis, y | 60 (30–70) | 56 (40–70) | 0.102 |
| Age grouping, n (%) | 0.041 | ||
| <50 years | 13 (16%) | 25 (30%) | |
| ≥50 years | 68 (84%) | 60 (60%) | |
| Sex, n (%) | 0.093 | ||
| Male | 67 (83%) | 61 (72%) | |
| Female | 14 (17%) | 14 (28%) | |
| Pathology, n (%) | 0.000 | ||
| TCC | 72 (89%) | 41 (48%) | |
| Squamous cell | 8 (10%) | 37 (43%) | |
| Adenocarcinoma | 0 (0%) | 4 (5%) | |
| Undifferntiated | 1 (1%) | 3 (3%) | |
| Grade, n (%) | 0.098 | ||
| Low grade | 31 (38%) | 22 (26%) | |
| High grade | 50 (62%) | 63 (74%) | |
| Stage, n (%) | 0.167 | ||
| T1 | 5 (6%) | 3 (3%) | |
| T2 | 43 (53%) | 34 (40%) | |
| T3 | 27 (33%) | 35 (41%) | |
| T4 | 6 (7%) | 13 (15%) |
In bladder preservation group, the TCC pathology was found in majority of the cases, with 72 (89%) out of 81 patients. Most of the TCC patients had T2 and T3 stages (90%) and 65 patients (80%) were treated by conventional irradiation, while 16 (20%) received hypofractionated radiotherapy. A total of 76 patients (94%) in this group received chemosensitization.
In RC group, 55 (64.7%) patients had adjuvant radiotherapy, 19 (22.4%) had adjuvant radiotherapy and chemotherapy, and 11 (12.9%) had no adjuvant therapy. None of the patients had neoadjuvant chemotherapy.
The treatment response for the patients who had bladder preservation, a majority of 66 (81.5%) achieved complete response (CR), 11 (13%) with partial response and 4 (5%) with disease progression. There were 54 patients (67%) who ultimately did not have salvage cystectomy. The treatment outcomes for all patients in the 2 treatment groups were detailed in Table 2. There were no statistically significant differences in the outcome between the 2 treatment groups. For patients treated by bladder preservation and by RC, the 5-year LRFS was 69% and 73% (p = 0.69) (Fig. 1), the 5-year DFS was 45% and 53% (p = 0.16) (Fig. 2), and the 5-year OS was 56% and 60% (p = 0.67) (Fig. 3), respectively. After propensity matching analysis, the mean 5-year OS was 58% for bladder preservation patients and 61% for RC patients (p = 0.51). It is noteworthy that in bladder preservation group, 8 patients (10%) had SCC pathology and refused RC. Their OS was 56% compared to 53% for the SCC patients treated by RC (p = 0.6). Although, these are a few number of patients, this could be encouraging to randomize willing SCC bladder cancer patients for preservation versus initial cystectomy.
Table 2.
Treatment outcome for all the patients.
| Bladder preservation group | RC group | |
|---|---|---|
| Local recurrence, n (%) | 22 (27%) | 22 (25.9%) |
| Distant metastasis, n (%) | 35 (43%) | 31 (36.5%) |
| Free from disease, n (%) | 37 (45.7%) | 45 (25.9% |
| Living at the end of the study, n (%) | 37 (45.7%) | 50 (58.8%) |
Figure 1.
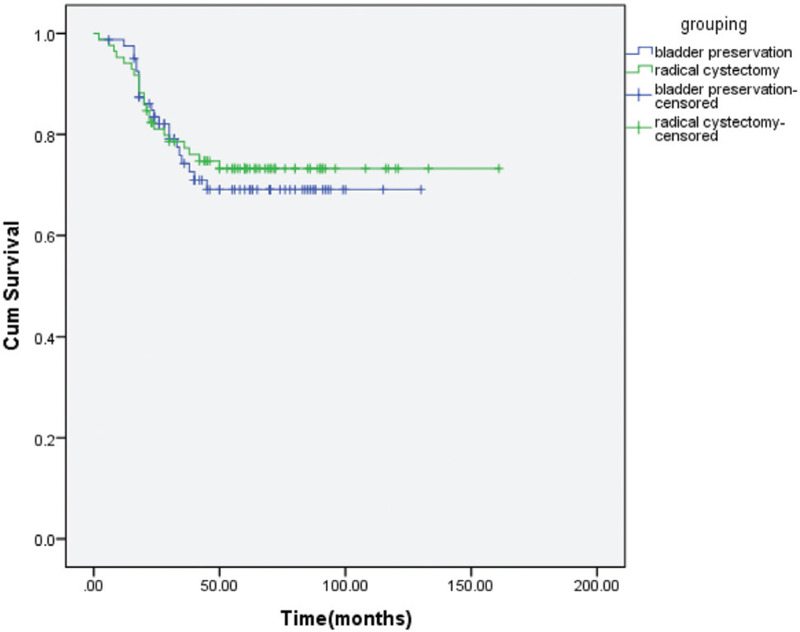
LRFS for bladder preservation versus RC groups.
Figure 2.
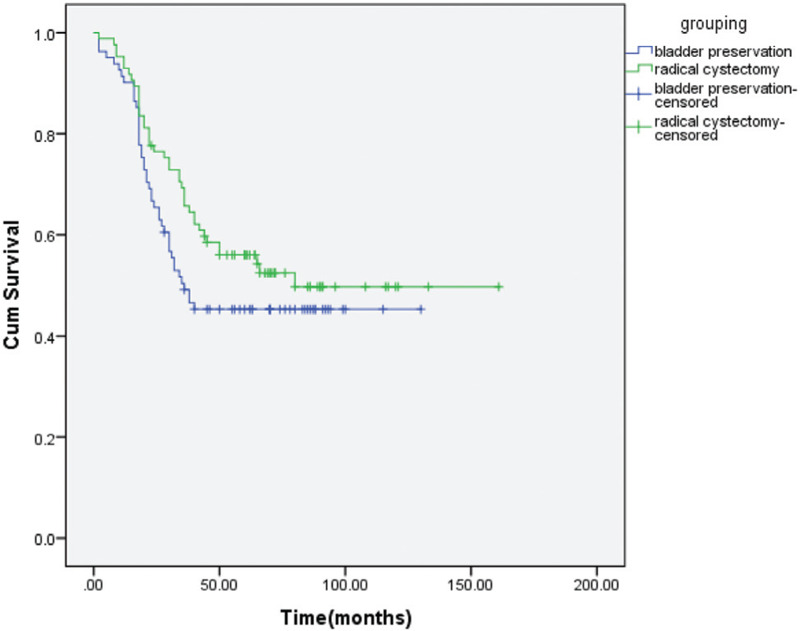
DFS for bladder preservation versus RC groups.
Figure 3.
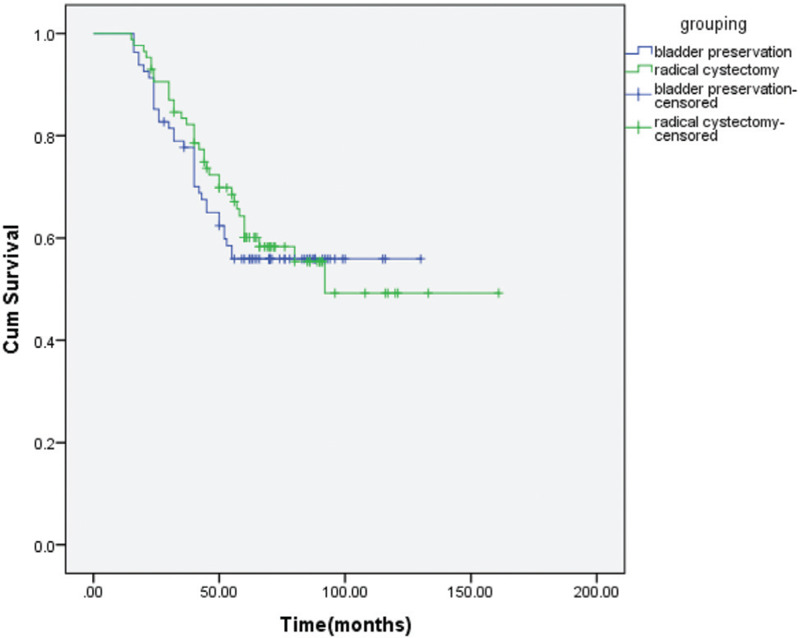
OS of the bladder preservation versus RC groups.
4. Discussion
RC has been the golden standard for bladder cancer treatment for decades. Bladder preservation has emerged as an alternative treatment which could give a better quality of life. There have been no randomized trials completed to compare the 2 treatment modalities. There has only been a part of a randomized trial, comparing the treatments, which was stopped as a feasibility study concluding that randomization was not possible due to patient and doctor preferences. [25] In this study, we compared the LRFS, DFS, and OS. Among most of the previous studies, the difference in this comparison is that SCC is a more common pathology found in 45 patients (27.1%), probably due to endemic schistosomiasis in Egypt.
The median age of diagnosis in the current study was 60 years in preservation group and 56 in RC group, respectively. The median ages reported by Zhong et al. [26] were 65 and 66 years for cystectomy and preservation, respectively. In some other studies, preservation was more often the treatment of older patients, for example, the reported ages were 75 years for preservation and 67 for cystectomy, [27] and older patients were frankly selected for cystectomy with median age 82 years old in one study. [28] Our population may by slightly younger than that reported in some studies due to endemic schistosomiases.
Men had a higher incidence of bladder cancer in our study, with 83% of bladder preservation group and 72% of RC group, which is in agreement with the systemic review and meta-analysis, [12] who reported 80% in bladder preservation and 71% in the RC group. Another study [26] reported that male accounted for 73% of bladder preservation and 75% of RC groups.
Regarding the pathology, the urothelial variant commonly constitutes 80%–90% of bladder cancer. [29] Pure SCC is about 3%–5% of all bladder cancer [30] and it is less responsive to chemotherapy. [31] However, it is more common in countries with endemic schistosomiasis like Egypt. [29] Due to lack of evidence, patients with SCC bladder cancer have RC followed by radiotherapy to decrease the local recurrence risk. [32] Only SCC bladder cancer patients who refuse surgery, are treated by bladder preservation. [33] In our study, there were 8 SCC patients who were treated by preservation.
Stages T2 and T3 are the majority in both treatment groups. Stage T2 is 53 and 33% of the RC group and bladder preservation group, respectively, while T3 is 40% and 41% of the RC and preservation groups respectively in our study. This is comparable to the stages reported in a systematic review, [27] in which 48% and 40% of T2 and 33% and 63% of T3 in preservation and RC groups respectively.
In this study, 81.5% of the patients treated by trimodality bladder preservation had CR. Giacalone et al. [23] reported 84% CR for those who had completed TURBT. They had slightly higher CR probably because of complete TURBT, which was not ascertained in our study. They also had 90% of the patients staged T2 and T3a, which are earlier stages than those included in the present study. Some reported slightly lower rates of CR34,35 and Fahmy et al. [12] stated that 75.3% of the patients achieved CR in their meta-analysis. [12] Mostly, the results have been improving over time, as stated by Giacalone et al., [23] after long-term evaluation bladder cancer, CR for the patients treated by trimodality therapy increased from 66% to 88% between 1986 and 2013.
Salvage cystectomy in our study was conducted in 27 patients (33%), the salvage cystectomy rate in the study conducted by Mitin et al. [36] was 30%, which is slightly higher than our study. It may be due to the lower radiation dose used in their study of 46 Gy. Reported salvage cystectomy rates ranged from 0% to 55%.37,38,39,40
In our study, the local recurrence rates were 27% and 26% for bladder preservation and RC after 2 years. The 5-year LRFS was 69% and 73%. Huddart et al. [25] reported a local recurrence rate of 15.3% in RC versus 68.9% in bladder preservation. However, they had a lower number of patients, the target volume of the radiotherapy given for their patients, included only the bladder with a safety margin, and many of the patients received no radiosensitizers. Kim et al. [41] also reported a 5-year local recurrence rate of 74% for the patients treated by bladder preservation. However, 28% of the patients in their study only received radiotherapy and 24% only received neoadjuvant chemotherapy. Hong et al. [40] reported a similar 5-year LRFS to us, of 57% in both the bladder preservation and the RC groups and there was no statistical significance in both groups.
The 5-year OS was 56% and 60% without a statistically significant difference for bladder preservation and cystectomy groups, respectively. After matching by propensity score analysis, the OS was 58% and 62% for preservation and cystectomy, respectively, and the difference remained insignificant. Our results are similar to those reported by Kim et al., [41] who reported 5-year OS 56% and 57% for preservation and cystectomy, respectively (p = 0.13). Based on a meta-analysis of 57 studies for the long-term oncological outcomes for bladder preservation and RC treatment protocols, Fahmy et al. [12] reported 10-year OS of 30.9% versus 35.1% (p = 0.32) with no statistically significant difference.
Zhong et al. [26] and Seisen et al. [42] reported an initial OS advantage for RC over bladder preservation, but they finally concluded that the potential long-term benefit of RC may be attenuated by the risk of operative mortality. Propensity-matched analysis that the statistical significance may have been due to that older patients with comorbidities are assigned to have bladder preservation.
In conclusion, bladder preservation gives similar survival with a better quality of life for TCC bladder cancer stages T1–4aN0M0. Currently, the bladder cancer patients, with SCC pathology tumors, are denied the chance of preservation due to lack of data for its safety. The limited number of patients with SCC, who refused cystectomy in this study, shows that it may be feasible to offer them bladder preservation. Larger trials are needed to confirm this possibility.
Acknowledgments
None.
Statement of ethics
The research was approved by the Institutional Review Board of the South Egypt Cancer Institute and conducted in accordance with the principles of the Declaration of Helsinki. Patients gave their written, informed consent to participate in this research.
Conflict of interest statement
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Funding source
None.
Author contributions
Conceived and designed the experiments: Dalia O Mohamed and Mona M Sayed. Collected the data: Dalia O Mohamed, Islam F Abdelkawi, Mahmoud H Elshoieby, Salah M Khallaf. Analyzed the data: Dalia O Mohamed and Mona M Sayed. Wrote the first draft of the article: Dalia Osaam. Contributed to the writing of the article: Mona M Sayed. Agreed with the article results and conclusions: Lamia M Khallaf and Doaa M Fouad. Jointly developed the structure and arguments for the article: Dalia O Mohamed and Mona M Sayed. Made critical revisions and approved the final version: Mona M Sayed. All authors reviewed and approved the final article.
References
- [1]. Bray F, Ferlay J, Soermjomatarum I, et al. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality of 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2]. Pentyala S, Nerli R, Ghagane S, Hiremath M. Bladder preservation protocols in the management of muscle-invasive bladder cancer: a systematic review. J Sci Soc 2018;45 (2):84–89. [Google Scholar]
- [3]. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017;71 (1):96–108. [DOI] [PubMed] [Google Scholar]
- [4]. Zaghloul MS, Christodouleas JP, Smith A, et al. A randomized clinical trial comparing adjuvant radiation versus chemo-RT versus chemotherapy alone after radical cystectomy for locally advanced bladder cancer. J Clin Oncol 2016;34 (2_suppl):356. [Google Scholar]
- [5]. el-Mekresh M, Akl A, Mosbah A, Abdel-Latif M, Abol-Enein H, Ghoneim MA. Prediction of survival after radical cystectomy for invasive bladder carcinoma: risk group stratification, nomograms or artificial neural networks? J Urol 2009;182 (2):466–472. [DOI] [PubMed] [Google Scholar]
- [6]. Prout GR, Marshall VF. The prognosis with untreated bladder tumors. Cancer 1956;9:551–558. [DOI] [PubMed] [Google Scholar]
- [7]. Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55 (1):164–174. [DOI] [PubMed] [Google Scholar]
- [8]. Pham A, Ballas LK. Trimodality therapy for bladder cancer: modern management and future directions. Curr Opin Urol 2019;29 (3):210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Johnson SB, Yu JB. Bladder preserving trimodality therapy for muscle-invasive bladder cancer. Curr Oncol Rep 2018;20 (9):66. [DOI] [PubMed] [Google Scholar]
- [10]. George L, Bladou F, Bardou VJ, et al. Clinical outcome in patients with locally advanced bladder carcinoma treated with conservative multimodality therapy. Urology 2004;64 (3):488–493. [DOI] [PubMed] [Google Scholar]
- [11]. Sabaa MA, El-Gamal OM, Abo-Elenen M, Khanam A. Combined modality treatment with bladder preservation for muscle invasive bladder cancer. Urol Oncol 2010;28 (1):14–20. [DOI] [PubMed] [Google Scholar]
- [12]. Fahmy O, Khairul-Asri MG, Schubert T, et al. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol Oncol 2018;36 (2):43–53. [DOI] [PubMed] [Google Scholar]
- [13]. Zapatero A, Martin De Vidales C, Arellano R, et al. Long-term results of two prospective bladder-sparing trimodality approaches for invasive bladder cancer: neoadjuvant chemotherapy and concurrent radio-chemotherapy. Urology 2012;80 (5):1056–1062. [DOI] [PubMed] [Google Scholar]
- [14]. Suer E, Hamidi N, Gokce MI, et al. Significance of second transurethral resection on patient outcomes in muscle-invasive bladder cancer patients treated with bladder-preserving multimodal therapy. World J Urol 2016;34 (6):847–851. [DOI] [PubMed] [Google Scholar]
- [15]. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group Protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32 (34):3801–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Shipley WU, Winter KA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol 1998;16 (11):3576–3583. [DOI] [PubMed] [Google Scholar]
- [17]. Bekelman JE, Handorf EA, Guzzo T, et al. Radical cystectomy versus bladder-preserving therapy for muscle-invasive urothelial carcinoma: examining confounding and misclassification bias in cancer observational comparative effectiveness research. Value Health 2013;16 (4):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Gofrit ON, Nof R, Meirovitz A, et al. Radical cystectomy vs. chemoradiation in T2-4aN0M0 bladder cancer: a case-control study. Urol Oncol 2015;33 (1):19.e1–19.e5. [DOI] [PubMed] [Google Scholar]
- [19]. Kang M, Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. Prognostic factors for conditional survival in patients with muscle-invasive urothelial carcinoma of the bladder treated with radical cystectomy. Sci Rep 2015;5:12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Moschini M, Sharma V, Dell’oglio P, et al. Comparing long-term outcomes of primary and progressive carcinoma invading bladder muscle after radical cystectomy. BJU Int 2016;117 (4):604–610. [DOI] [PubMed] [Google Scholar]
- [21]. Zehnder P, Studer UE, Skinner EC, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J Urol 2011;186 (4):1261–1268. [DOI] [PubMed] [Google Scholar]
- [22]. Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19 (3):666–675. [DOI] [PubMed] [Google Scholar]
- [23]. Giacalone NJ, Shipley WU, Clayman RH, et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: an updated analysis of the Massachusetts General Hospital Experience. Eur Urol 2017;71 (6):952–960. [DOI] [PubMed] [Google Scholar]
- [24]. Moran GW, Li G, Robins DJ, Matulay JT, McKiernan JM, Anderson CB. Systematic review and meta-analysis on the efficacy of chemotherapy with transurethral resection of bladder tumors as definitive therapy for muscle invasive bladder cancer. Bladder Cancer 2017;3 (4):245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Huddart RA, Birtle A, Maynard L, et al. Clinical and patient-reported outcomes of SPARE—a randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int 2017;120 (5):639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Zhong J, Switchenko J, Jegadeesh NK, et al. Comparison of outcomes in patients with muscle-invasive bladder cancer treated with radical cystectomy versus bladder preservation. Am J Clin Oncol 2019;42 (1):36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Arcangeli G, Strigari L, Arcangeli S. Radical cystectomy versus organ-sparing trimodality treatment in muscle-invasive bladder cancer: a systematic review of clinical trials. Crit Rev Oncol Hematol 2015;95 (3):387–396. [DOI] [PubMed] [Google Scholar]
- [28]. Boustani J, Bertaut A, Galsky MD, et al. Radical cystectomy or bladder preservation with radiochemotherapy in elderly patients with muscle-invasive bladder cancer: Retrospective International Study of Cancers of the Urothelial Tract (RISC) Investigators. Acta Oncol 2017;57 (4):491–497. [DOI] [PubMed] [Google Scholar]
- [29]. Chalasani V, Chin JL, Izawa JI. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can Urol Assoc J 2009;3 (6 Suppl 4):S193–S198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Ge P, Wang ZC, Yu X, Lin J, He Q. Sensitivity of initial biopsy or transurethral resection of bladder tumor(s) for detecting histological variants on radical cystectomy. BMC Urol 2015;15 (1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Bertz S, Hartmann A, Knüchel-Clarke R, Gaisa NT. Specific types of bladder cancer. Pathologe 2016;37 (1):40–51. [DOI] [PubMed] [Google Scholar]
- [32]. Black PC, Brown GA, Dinney CP. The impact of variant histology on the outcome of bladder cancer treated with curative intent. Urol Oncol 2009;27 (1):3–7. [DOI] [PubMed] [Google Scholar]
- [33]. Zaghloul MS, Christodouleas JP, Smith A, et al. Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy: a randomized phase 2 trial. JAMA Surg 2018;153 (1):e174591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Baxter E, Dennis K, Kollmannsberger C, et al. Radical trimodality therapy for patients with locally advanced bladder cancer: The British Columbia Cancer Agency experience. Urol Oncol 2015;33 (2). 66.e13-19. [DOI] [PubMed] [Google Scholar]
- [35]. Tunio MA, Hashmi A, Qayyum A, Mohsin R, Zaeem A. Whole-pelvis or bladder-only chemoradiation for lymph node-negative invasive bladder cancer: single-institution experience. Int J Radiat Oncol Biol Phys 2012;82 (3):e457–e462. [DOI] [PubMed] [Google Scholar]
- [36]. Mitin T, Shipley WU, Efstathiou JA, et al. Trimodality therapy for bladder conservation in treatment of invasive bladder cancer. Curr Urol Rep 2013;14 (2):109–115. [DOI] [PubMed] [Google Scholar]
- [37]. Ploussard G, Daneshmand S, Efstathiou JA, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol 2014;66 (1):120–137. [DOI] [PubMed] [Google Scholar]
- [38]. Arias F, Domínguez MA, Martínez E, et al. Chemoradiotherapy for muscle invading bladder carcinoma. Final report of a single institutional organ-sparing program. Int J Radiat Oncol Biol Phys 2000;47 (2):373–378. [DOI] [PubMed] [Google Scholar]
- [39]. Inoue M, Koga F, Yoshida S, et al. Significance of ERBB2 overexpression in therapeutic resistance and cancer-specific survival in muscle-invasive bladder cancer patients treated with chemoradiation-based selective bladder-sparing approach. Int J Radiat Oncol Biol Phys 2014;90 (2):303–311. [DOI] [PubMed] [Google Scholar]
- [40]. Hong JH, Lin YH, Lu YC, et al. Comparative analysis between radical cystectomy and trimodality therapy for clinical stage II bladder cancer—experience from a tertiary referral center. Urol Sci 2018;29 (1):25–32. [Google Scholar]
- [41]. Kim YJ, Byun SJ, Ahn H, et al. Comparison of outcomes between trimodal therapy and radical cystectomy in muscle-invasive bladder cancer: a propensity score matching analysis. Oncotarget 2017;8 (40):68996–69004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Seisen T, Sun M, Lipsitz SR, et al. Comparative effectiveness of trimodal therapy versus radical cystectomy for localized muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2017;72 (4):483–487. [DOI] [PubMed] [Google Scholar]


