Abstract
Background:
To evaluate the value of interleukin (IL)-27 measured in serum and bronchoalveolar lavage fluid (BALF) for the diagnosis of smear-negative pulmonary tuberculosis (TB).
Methods:
This was a prospective study of patients planned to undergo bronchoscopy at Wuxi No.5 People's Hospital between January 2017 and September 2018. The patients were grouped as the TB and control groups. BALF and serum IL-27 were measured by ELISA. Receiver operating characteristic (ROC) curves were used to assess the diagnostic value and calculate the optimal cutoff values.
Results:
There were 40 patients in the control group and 87 in the TB group. In the TB group, 20 had positive sputum smear results and 67 were negative. The area under the ROC curve (AUC) of BALF IL-27 for pulmonary TB was 0.897 (95% CI: 0.830–0.944) (P < .001). The AUC of serum IL-27 for pulmonary TB was 0.703 (95% CI: 0.616–0.781) (P < .001). In patients with negative sputum smear results, the AUCs of BALF IL-27 and serum IL-27 for pulmonary TB was 0.882 (95% confidence interval [CI]: 0.805–0.936) (P < .001) and 0.679 (95% CI: 0.601–0.782) (P < .001), respectively.
Conclusions:
BALF IL-27 can be used for the diagnosis of pulmonary TB, particularly in those with a negative sputum smear result. Serum IL-27 could be an auxiliary method for TB screening.
Keywords: bronchoalveolar lavage fluid, interleukin-27, serum
1. Introduction
Pulmonary tuberculosis (TB) is the infection of lung parenchyma by Mycobacterium tuberculosis. Tuberculosis is found worldwide, but the 6 top high-burden countries (India, Indonesia, China, Nigeria, Pakistan, and South Africa) account for 60% of the active cases of TB in the world. In China, the general incidence of TB is about 64 cases per 100,000 person.[1]
TB is suspected in patients with suggestive symptoms such as productive cough for >2 weeks, dyspnea, chest pain, hemoptysis, loss of appetite, weight loss, night sweats, and malaise. The diagnosis is confirmed by culture and molecular diagnostic, but for countries or hospitals lacking such tests, a sputum smear positive for acid-fast bacilli (AFB) is considered diagnostic.[1] However, sputum smear results may be negative for AFB, especially in patients with pulmonary TB, and diagnosis for such patients is harder. The sensitivity of sputum smear test is 63.6% to 70.2%, and the specificity is 96.9% to 97.8%.[2] Failure to isolate M tuberculosis from appropriately collected samples does not exclude the diagnosis of active TB in patients with clinical symptoms or radiographic findings indicating TB.[3,4] In these cases, other diagnostics procedures such as bronchoalveolar lavage fluid (BALF) should be considered.[3,4]
Interleukin (IL)-27 is one of the cytokines of the IL-12 family, which is a heterodimer composed of Epstein-Barr virus induced gene 3 (EBI3) and p28.[5] As we all know, macrophages have strong bactericidal ability, antigen presentation function, and can secrete various inflammatory cytokines, which play an important role in host anti-tuberculosis immunity. In TB infection, IL-27 mainly inhibits the innate immune response of macrophages, the secretion of macrophage inflammatory factors, the maturation of phagocytes, and the autophagy, thus finally leading to the long-term intracellular condition of TB. In the adaptive immune response of chronic infection, Thl cells and Thl7 cells can be inhibited, and IL-10 secretion can be promoted, leading to the aggravation of infection.[6–10]
Studies have shown that changes in the content of IL-27 in body fluids may indicate the infection status of TB. Clinical studies by Cao et al[11] have found that the content of IL-27 in plasma and sputum of tuberculosis patients is significantly higher than that of healthy people, and the content of IL-27 in sputum is positively correlated with the content of TB in sputum. While in the study by Lu et al,[12] it was found that serum IL-27 levels in patients with active TB were higher than those in the improving and stable stages.
IL-27 is also a sensitive and specific biomarker for differentiating tuberculosis from lung cancer and previous tuberculosis. The sensitivity of IL-27 in the diagnosis of tuberculous pleural effusion was 88.0%, and the sensitivity and specificity of IL-27 in the differential diagnosis of pulmonary TB from previous pulmonary TB were 100%.[13] While the sensitivity and specificity of IL-27 in sputum-negative and sputum-positive patients with pulmonary tuberculosis is urgent further study.
Therefore, this study aimed to evaluate the diagnostic value of IL-27 measured in serum and BALF for the diagnosis of TB and assist the diagnosis of phlegm negative patients. The results may provide an innovative means to improve the diagnostic rate of TB and improve the rates of early treatment, thereby improving outcomes.
2. Material and methods
2.1. Study design and patients
This was a prospective study of patients planned to undergo bronchoscopy at the Respiratory and Tuberculosis Departments of Wuxi No.5 People's Hospital between January 2017 and September 2018.
The inclusion criteria are: ≥18 years of age; TB treatment-naïve; suspicious for pulmonary TB (clinically and/or by imaging); and scheduled for a bronchoscopy. The exclusion criteria are as below: serious diseases such as liver and/or kidney dysfunction; autoimmune diseases; or immunodeficiency or use of immunosuppressive agents. The study protocol was approved by the Ethics Committee of Wuxi No.5 People's Hospital. All patients signed the informed consents.
2.2. Grouping
Based on their final diagnoses (including sputum smear results, lavage fluid culture, biopsy, and clinical diagnosis), the patients were allocated into the pulmonary TB group and the control group. The patients in the pulmonary TB group were further divided into the negative and positive groups according to the sputum smear results.
2.3. IL-27 detection
Human IL-27 ELISA kit was used in this experiment, and the content of IL-27 in the samples was detected by the competitive inhibition method. On the day of bronchoscopy, 5 mL of blood and 10 to 15 mL of BALF were obtained from each patient. The supernatants were collected after centrifugation and stored at –80 °C. A commercial ELISA kit (Shanghai Hushang Biological Technology Co., Ltd, Shanghai, China; #11110011) was used for the IL-27 measurement.
2.4. Data collection
Patients’ clinical data including sex, age, clinical symptoms, and imaging features were collected from the charts. The final diagnosis was made based on the sputum smear, lavage fluid culture, biopsy, or clinical diagnosis.
2.5. Statistical analysis
SPSS 25.0 (IBM, Armonk, NY) was used for statistical analysis. Continuous data were tested with the Kolmogorov–Smirnov test for normal distribution. Normally distributed continuous data were expressed as means ± SD and non-normally distributed continuous data were expressed as medians (range or IQR). Categorical data were expressed as n (%). Normally distributed continuous data were tested using the Student t test or ANOVA, while non-normally distributed continuous data were analyzed by the Mann–Whitney U test or Kruskal–Wallis test. Categorical data were analyzed by the chi-square test or Fisher exact test. Area under the ROC curve (AUC) was used to determine the diagnostic value of IL-27 and calculate the optimal cutoff value. Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were calculated based on the optimal cutoff value. P < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
A total of 127 patients were enrolled in the study, with 40 in the control group and 87 in the TB group. Of all patients in the TB group, 20 had positive sputum smear results and 67 had negative sputum smear results. For patients with negative sputum smear results, TB was confirmed in 47, 39, and 1 by sequential clinical diagnosis, lavage fluid culture, and pathological diagnosis, respectively. The control group included patients with tumor, pneumonia, bronchiectasis, chronic obstructive pulmonary disease, and infected patients with old tuberculosis (Fig. 1).
Figure 1.
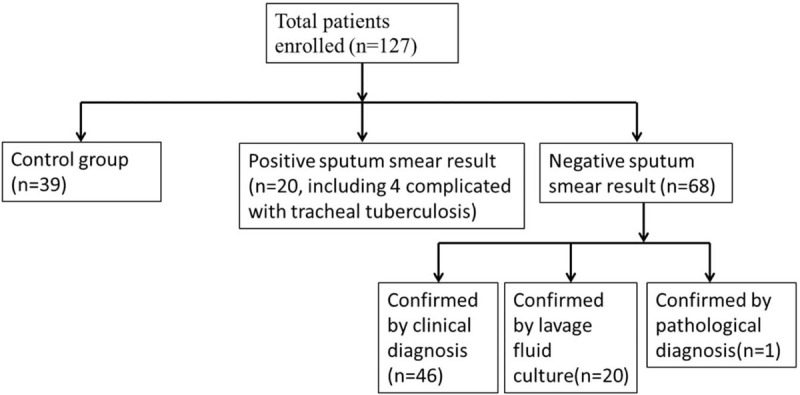
Patient flowchart.
Patients’ median age was 51 (16.0–81.0) years old, and 68 (53.5%) were men. The main clinical symptoms were cough and expectoration (87.4%), and fever (30.7%). The major CT features included spots and patches (95.3%), and cavitation (14.2%) (Table 1).
Table 1.
Characteristics of the patients.
| Total (n = 127) | |
| Age, y (median, range) | 51.0 (16.0–81.0) |
| Male (n, %) | 68 (53.5) |
| Symptoms (n, %) | |
| Cough and expectoration | 111 (87.4) |
| Fever | 39 (30.7) |
| Hemoptysis | 6 (4.7) |
| Chest pain | 4 (3.1) |
| Night sweats | 6 (4.7) |
| Emaciation | 2 (1.6) |
| PE finding | 9 (7.1) |
| Computed tomography features | |
| Spot and patch (n, %) | 121 (95.3) |
| Cord | 12 (9.4) |
| Nodule | 7 (5.5) |
| Cavitation | 18 (14.2) |
| Plural effusion | 2 (1.6) |
| Final diagnosis (n, %) | |
| Pulmonary tuberculosis (n = 4 with tracheal tuberculosis) | 87 (68.5) |
| Pneumonia | 13 (10.2) |
| Bronchiectasis with infection | 5 (3.9) |
| Lung abscess | 5 (3.9) |
| Lung cancer | 2 (1.6) |
| Old tuberculosis with infection | 9 (7.1) |
| AECOPD | 6 (4.7) |
3.2. BALF and serum IL-27 level
The BALF IL-27 levels were 73.69 ± 25.28, 120.78 ± 36.03, and 136.61 ± 31.83 ng/L in patients from the control, negative, and positive groups, respectively. BALF IL-27 levels in the control group were significantly different from that in the negative and positive groups (both P < .001). BALF IL-27 levels were not significantly different between the negative and positive groups (P = .172).
The serum IL-27 levels were 47.59 ± 17.90, 62.69 ± 21.33, and 64.63 ± 18.32 ng/L in patients from the control, negative, and positive groups, respectively. Serum IL-27 levels in the control group were significantly different from that in the negative and positive groups (both P < .05). However there was no statistic difference between the negative and positive groups in serum IL-27 levels (P = 1.000) (Fig. 2).
Figure 2.
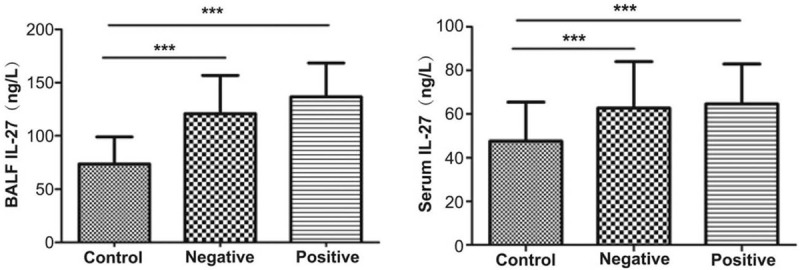
Bronchoalveolar lavage fluid and serum IL-27 levels in the 3 groups. IL = interleukin.
3.3. ROC curve
Figure 3 and Table 2 present the results of ROC curves analyses of IL-27 for the diagnosis of TB. For BALF IL-27 levels, the optimal cut-off of 86.26 ng/L resulted in AUC of 0.897 (0.830–0.944), sensitivity 92.0%, specificity 71.8%, and accuracy 85.8%. For serum IL-27 levels, the optimal cut-off of 62.55 ng/L resulted in AUC of 0.703 (0.616–0.781), sensitivity 45.5%, specificity 89.7%, and accuracy 59.1%. The AUC of sputum smear was 0.614 (0.523–0.699) with sensitivity 22.7%, specificity100.0%, and accuracy 46.4%.
Figure 3.
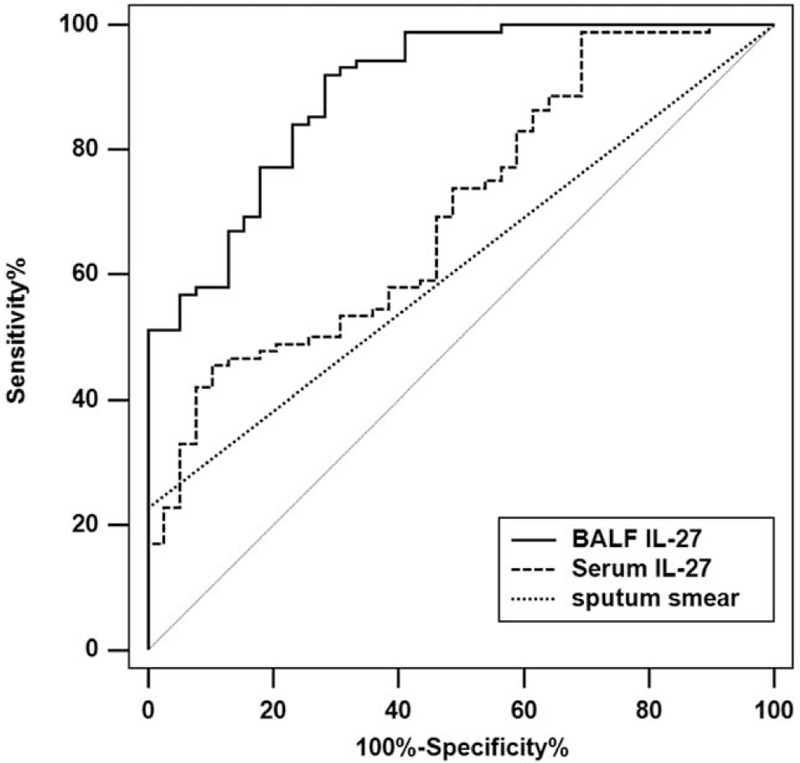
Received operating characteristics curve (ROC) of IL-27 for the diagnosis of pulmonary tuberculosis. Optimal cutoff values of BALF IL-27, serum IL-27, and sputum smear for the diagnostic value calculated using ROC curves. BALF = bronchoalveolar lavage fluid, IL = interleukin.
Table 2.
Optimal cutoff values of bronchoalveolar lavage fluid, serum interleukin-27, and sputum smear for the diagnostic value calculated using ROC curves, using the final diagnosis as the gold standard.
| AUC (95% CI) | P value | Cutoff | Sensitivity (%) | Specificity (%) | Accuracy (%) | +LR | –LR | PPV (%) | NPV (%) | |
| All populations (n = 127) | ||||||||||
| Sputum smear | 0.614 (0.523–0.699) | <.001 | N/A | 22.7 | 100 | 46.4 | — | 0.77 | 100 | 36.4 |
| Serum IL-27 | 0.703 (0.616–0.781) | <.001 | 65.55 | 45.5 | 89.7 | 59.1 | 4.43 | 0.61 | 90.9 | 42.2 |
| BALF IL-27 | 0.897 (0.830–0.944) | <.001 | 86.26 | 92 | 71.8 | 85.8 | 3.26 | 0.11 | 88 | 80 |
| Population with a negative sputum smear result (n = 68) | ||||||||||
| Serum IL-27 | 0.679 (0.601–0.782) | <.001 | 65.55 | 47.1 | 89.7 | 62.6 | 4.59 | 0.59 | 88.9 | 49.3 |
| BALF IL-27 | 0.882 (0.805–0.936) | <.001 | 86.26 | 91.2 | 71.8 | 84.1 | 3.23 | 0.12 | 84.9 | 82.4 |
Among the patients with negative sputum smear results, the optimal cut-off of 86.26 ng/L of IL-27 in the BALF resulted in AUC of 0.882 (0.805–0.936), sensitivity 91.2%, specificity 71.8%, and accuracy 84.1%. Regarding serum IL-27, the optimal cut-off of 65.55 ng/L resulted in AUC of 0.679 (0.601–0.782), sensitivity 47.1%, specificity 89.7%, and accuracy 62.6%.
4. Discussion
The diagnostic value of IL-27 for pulmonary TB, especially in those with negative sputum smear result, is unclear. Therefore, this study aimed to evaluate the diagnostic value of IL-27 measured in serum and BALF for the diagnosis of TB. The results strongly suggest that BALF IL-27 could be used for the diagnosis of pulmonary TB, particularly in those with a negative sputum smear result. Serum IL-27 could be an auxiliary method for TB screening. The advantages of BALF IL-27 testing compared with culture and molecular diagnostic are the rapidity of obtaining the results after sampling and the low cost.
The diagnosis of TB is usually confirmed by culture and molecular diagnostic, but many countries and hospitals do not have access to those expensive tests and the sputum smear AFB test is considered diagnostic when done properly. Nevertheless, the sensitivity of sputum smear is low, ranging from 63.6% to 70.2%[2] and a negative result cannot exclude the diagnosis of TB, posing an important dilemma in countries and hospitals where more sensitive tests are not available. Of course, empiric antibiotics can be tried,[3,4] but there is a risk of missing a differential diagnosis such as cancer, thus delaying the proper treatment.
Hence, a variety of easy-to-measure markers of TB are being sought for the confirmation of TB diagnosis in patients with clinical symptoms and suspect imaging.[14–16] IL-27 is involved in the granulation process of M tuberculosis, making it an appropriate TB marker.[17,18] IL-27 is secreted by macrophages and dentritic cells.[19] IL-27 activates cytotoxic T lymphocytes, natural killer cells, and natural killer T cells, but antagonizes the inflammatory response mediated by Th1, Th2, and Th17 cells.[15] In the presence of TB, IL-27 secretion modulates macrophage response.[20,21] TB can decrease the bactericidal properties of macrophages by inducing IL-27 secretion, but the exact mechanism of increased secretion of IL-27 remains unclear.[17] Nevertheless, the levels of IL-27 correlate with the bacterial burden in the granulomas[22] and the increased IL-27 secretion results in the suppression of phagosomal acidification, contributing to the survival of the bacteria.
IL-27 is of particularly good value for the diagnosis of TB in patients with pleural effusion and can discriminate TB effusion from malignant effusion.[6–10,23] However, many patients with TB don’t develop pleural effusion and it is necessary to examine the diagnostic value of IL-27 in other fluids. Only the study, by Cao et al,[11] examined the IL-27 levels in the sputum and plasma. They showed that sputum levels of IL-27, but not plasma levels of IL-27, were associated with the mycobacterial load in the sputum.
In the present study, the diagnostic value (as represented by the AUC) of BALF IL-27 was higher than that of serum IL-27 when considering all patients, and sputum smear-negative TB patients. These results could be explained by the fact that IL-27 is produced and released by antigen-presenting cells at the site of infection. Therefore, the level of lung IL-27 is higher than that of the blood because IL-27 has to diffuse in the nodes and lymphatic circulation before entering the blood.[17,18] These results are partially supported by Cao et al[11] in that sputum originates in part from the bronchi and therefore contains lung IL-27. A study in HIV-infected individuals showed that plasma IL-27 levels were elevated in patients with HIV and TB.[24] The present study suggests that BALF IL-27 could be used to diagnose TB in patients with suspect clinical and imaging profiles but negative sputum smear test, but that serum IL-27 could be used as an auxiliary test because of the lower diagnostic value. Of course, venous puncture is less invasive than bronchoscopy, and the exact value, patient acceptability, and cost-effectiveness remain to be determined and weighed.
The present study has limitations. First, the number of patients was relatively small and from a single center. Second, the extent of TB infection was not taken into account when assessing the diagnostic value of IL-27. Third, there was no follow-up to examine the effects of treatments on BALF and serum IL-27 levels. Fourth, no patient with immunodeficiency was included, limiting the generalizability of the results since immunodeficiency is a major risk factor for TB infection. Finally, there were no healthy controls recruited in this study. Indeed, healthy controls recruitment would increase the generalizability of the results, however, it is difficult to implement in clinical setting. Additional studies are necessary to address these issues.
5. Conclusions
In conclusion, BALF IL-27 detection can be used for the diagnosis of pulmonary TB, particularly those with a negative sputum smear result. Serum IL-27 detection can serve as one of the auxiliary methods for TB screening. Clinical studies with larger sample size are required to further validate the diagnostic value.
Acknowledgments
The authors acknowledge the help of Youth Foundation Program of Nanjing Medical University (No. 2016NJMU170).
Author contributions
Conceptualization: Feng Zhu, Xiufeng Jiang.
Data curation: Feng Zhu, Qinfang Ou, Jian Zheng, Min Zhou.
Formal analysis: Feng Zhu, Qinfang Ou, Jian Zheng, Min Zhou, Huaxin Chen, Xiufeng Jiang.
Funding acquisition: Xiufeng Jiang.
Investigation: Qinfang Ou.
Methodology: Jian Zheng, Min Zhou, Huaxin Chen.
Project administration: Xiufeng Jiang.
Software: Qinfang Ou, Min Zhou, Huaxin Chen.
Supervision: Jian Zheng.
Validation: Feng Zhu, Qinfang Ou, Huaxin Chen, Xiufeng Jiang.
Writing – original draft: Feng Zhu, Xiufeng Jiang.
Writing – review & editing: Feng Zhu, Xiufeng Jiang.
Footnotes
Abbreviations: AFB = acid-fast bacilli, AUC = area under the ROC curve, BALF = bronchoalveolar lavage fluid, IL = interleukin, TB = tuberculosis.
How to cite this article: Zhu F, Ou Q, Zheng J, Zhou M, Chen H, Jiang X. Role of bronchoalveolar lavage fluid and serum interleukin-27 in the diagnosis of smear-negative pulmonary tuberculosis. Medicine. 2021;100:20(e25821).
Ethics approval and consent to participate: The study protocol was approved by the Ethics Committee of Wuxi No.5 People's Hospital. All patients signed the informed consents.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
All authors declare that they have no competing interests.
This study was funded by Youth Foundation Program of Nanjing Medical University (No. 2016NJMU170). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUC = area under the ROC curve, BALF = bronchoalveolar lavage fluid, IL = interleukin.
References
- [1].Boatin AA, Schlotheuber A, Betran AP, et al. Within country inequalities in caesarean section rates: observational study of 72 low and middle income countries. BMJ 2018;360:k55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cuevas LE, Yassin MA, Al-Sonboli N, et al. A multi-country non-inferiority cluster randomized trial of frontloaded smear microscopy for the diagnosis of pulmonary tuberculosis. PLoS Med 2011;8:e1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nahid P, Dorman SE, Alipanah N, et al. Executive summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016;63:853–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].International Standards for Tuberculosis Care. 3rd ed. The Hague: TB CARE I; 2014. [Google Scholar]
- [5].Yoshida H, Hunter CA. The immunobiology of interleukin-27. Ann Rev Immunol 2015;33:417–43. [DOI] [PubMed] [Google Scholar]
- [6].Valdes L, San Jose E, Ferreiro L, et al. Interleukin 27 could be useful in the diagnosis of tuberculous pleural effusions. Respir Care 2014;59:399–405. [DOI] [PubMed] [Google Scholar]
- [7].Liu Q, Yu YX, Wang XJ, et al. Diagnostic accuracy of interleukin-27 between tuberculous pleural effusion and malignant pleural effusion: a meta-analysis. Respiration 2018;95:469–77. [DOI] [PubMed] [Google Scholar]
- [8].Wu YB, Ye ZJ, Qin SM, et al. Combined detections of interleukin 27, interferon-gamma, and adenosine deaminase in pleural effusion for diagnosis of tuberculous pleurisy. Chin Med J 2013;126:3215–21. [PubMed] [Google Scholar]
- [9].Zeng N, Wan C, Qin J, et al. Diagnostic value of interleukins for tuberculous pleural effusion: a systematic review and meta-analysis. BMC Pulm Med 2017;17:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang W, Zhou Q, Zhai K, et al. Diagnostic accuracy of interleukin 27 for tuberculous pleural effusion: two prospective studies and one meta-analysis. Thorax 2018;73:240–7. [DOI] [PubMed] [Google Scholar]
- [11].Cao J, Zhang L, Li D, et al. IL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL10. Chest 2012;141:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lu X, Fu Y, Yi Z, et al. Expression of interleukin-27 and interleukin-27 against Mycobacterium tuberculosis (Mycobacterium tuberculosis). Chin J Cell Mol Immunol 2016;32:406–9. [Google Scholar]
- [13].Lin S, Wang Y, Li Y, et al. Diagnostic accuracy of interleukin-27 in bronchoalveolar lavage fluids for pulmonary tuberculosis. Infec Drug Resist 2019;12:3755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goletti D, Petruccioli E, Joosten SA, et al. Tuberculosis biomarkers: from diagnosis to protection. Infect Dis Rep 2016;8:6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sigal GB, Segal MR, Mathew A, et al. Biomarkers of tuberculosis severity and treatment effect: a directed screen of 70 host markers in a randomized clinical trial. EBioMedicine 2017;25:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang C, Wei LL, Shi LY, et al. Screening and identification of five serum proteins as novel potential biomarkers for cured pulmonary tuberculosis. Sci Rep 2015;5:15615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abdalla AE, Li Q, Xie L, et al. Biology of IL-27 and its role in the host immunity against Mycobacterium tuberculosis. Int J Biol Sci 2015;11:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Torrado E, Fountain JJ, Liao M, et al. Interleukin 27R regulates CD4+ T cell phenotype and impacts protective immunity during Mycobacterium tuberculosis infection. J Exp Med 2015;212:1449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang J, Qian X, Ning H, et al. Activation of IL-27 p28 gene transcription by interferon regulatory factor 8 in cooperation with interferon regulatory factor 1. J Biol Chem 2010;285:21269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Robinson CM, Nau GJ. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J Infect Dis 2008;198:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Robinson CM, O’Dee D, Hamilton T, et al. Cytokines involved in interferon-gamma production by human macrophages. J Innate Immun 2010;2:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gideon HP, Flynn JL. Characterization of immunomodulatory cytokine IL-27 in tuberculosis infection. J Immunol 2016;196: 1 suppl: 206.5. [Google Scholar]
- [23].Li M, Zhu W, Khan RSU, et al. Accuracy of interleukin-27 assay for the diagnosis of tuberculous pleurisy: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 2017;96:e9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Olsson O, Bjorkman P, Jansson M, et al. Plasma profiles of inflammatory markers associated with active tuberculosis in antiretroviral therapy-naive human immunodeficiency virus-positive individuals. Open Forum Infect Dis 2019;6:ofz015. [DOI] [PMC free article] [PubMed] [Google Scholar]


