Abstract
Polyanionic macromolecules including carboxylate-terminated polymers (polycarboxylates) are capable of inhibiting sexually transmitted viruses such as human immunodeficiency virus (HIV) and herpes simplex virus (HSV). Cellulose acetate phthalate (CAP), a pharmaceutically acceptable pH-sensitive polycarboxylate polymer, showed promising prophylactic activity against HIV and HSV, but the instability of CAP in an aqueous environment prevented its clinical development. Interestingly, several pharmaceutically acceptable polycarboxylates have features similar to CAP with an aqueous stability significantly higher than that of CAP. However, their activity against sexually transmitted viruses remains unexplored. Here, we evaluate the activity of various polycarboxylates such as polyvinyl acetate phthalate (PVAP), various grades of hydroxypropyl methylcellulose phthalate (HPMCP-50, HPMCP-55, and HPMCP-55S), and various grades of methacrylic acid copolymers (Eudragit L100-55, Eudragit L100, Eudragit S100, and Kollicoat MAE 100P) against HSV. We, for the first time, demonstrate that PVAP, HPMCP-55S, and Eudragit S100 have activity and selectivity against HSV-1 and HSV-2. Further, we report that polycarboxylates can be easily transformed into nanoparticles (NPs) and in the nanoparticulate form, they show similar or enhanced activity against HSV. Finally, using PVAP NPs, as a model, we demonstrate using in vitro HSV therapy studies that polycarboxylate NPs are capable of synergizing with antiviral drugs such as acyclovir (ACV), tenofovir, and tenofovir disoproxil fumarate. Thus, pharmaceutically acceptable carboxylic acid-terminated polymers and their NPs have the potential to be developed into topical formulations for the prevention and treatment of HSV infection.
Keywords: enteric coating, methacrylic acid copolymer, poloxamer, genital herpes, polymeric nanoparticles
Graphical Abstract
Natural and synthetic polyanionic macromolecules have been explored for their broad-spectrum antiviral activity for more than 5 decades.1-4 Over the years, several polyanionic macromolecules have been evaluated in preclinical studies for their potential to prevent infection from sexually transmitted viruses such as human immunodeficiency virus (HIV), herpes simplex virus (HSV), and human papillomavirus (HPV).5 More specifically, natural and synthetic polysulfonated anionic macromolecules such as carrageenan, cellulose sulfate, polystyrene sulfonate, and polynaphthalene sulfonate (PRO-2000) were developed into topical (vaginal) formulations and evaluated in clinical trials as a prophylactic modality for the local prevention of sexually transmitted viral infections.5 However, the clinical trials have failed to establish the efficacy of topically applied polysulfonated anionic macromolecules to prevent infection from sexually transmitted viruses.5 Moreover, some of the clinical trials on topically applied polysulfonated anionic macromolecules were halted due to increased acquisition of HIV potentially due to their toxicity to the cervicovaginal barrier.5
The carboxylate-terminated polymers have been shown to be effective against viruses and more tolerable to cells than clinically evaluated polysulfonate polymers almost 5 decades ago.2 In general, polycarboxylates, like other polyanionic molecules, are anticipated to exert an antiviral effect by inhibiting the entry of the virus into the cells. While the mechanism of action of polycarboxylates against HIV has been well-studied in the past,6 their mode of action against HSV remains relatively underexplored. Previous studies on poly(hydroxy)carboxylates suggest that polycarboxylates could interact with cationic domains of HSV glycoproteins gB and gC which will further prevent the interaction of the HSV with heparan sulfate proteoglycans on the cell surface.7
Cellulose acetate phthalate (CAP) is an FDA-approved carboxylate-terminated pH-sensitive polymer mainly used for the enteric coating of oral formulations to prevent the premature release of the drugs in the stomach.8 Interestingly, CAP, being a polycarboxylate, is also capable of inhibiting HIV and HSV.9-11 Importantly, CAP has shown greater safety to human cervical explants compared to polysulfonated macromolecules such as PRO-2000.12 The mechanism of action of CAP against HIV has been well studied, and the preclinical efficacy of CAP to prevent HIV and HSV infections has also been established.10,11 Although preclinical studies on CAP showed considerable promise, the long-term instability of CAP in an aqueous environment limited the development of clinically viable and stable aqua-based topical formulations for the prevention of sexually transmitted viral infections.13
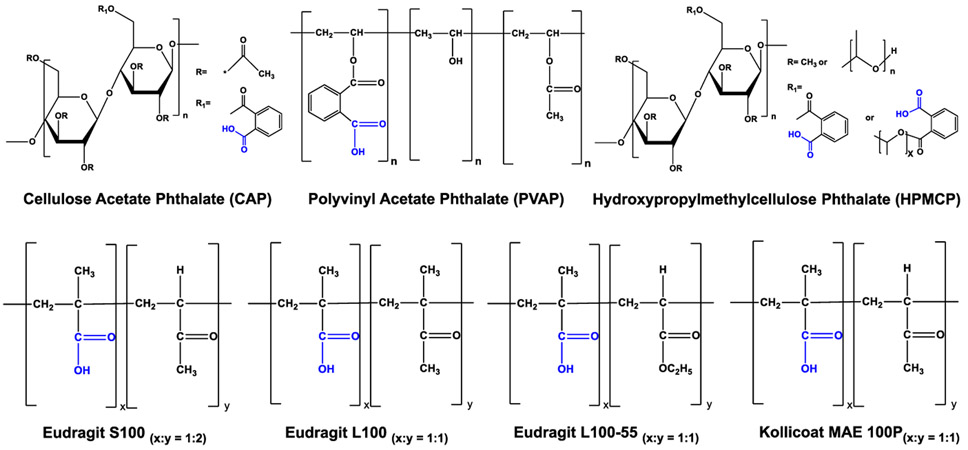
Apart from CAP, several FDA-approved carboxylate-terminated pH-sensitive polymers that have similar features and a higher aqueous stability than that of CAP are available, and their activity against sexually transmitted viruses remains unexplored. In this investigation, we focused on the antiviral activity evaluation of various FDA-approved carboxylate-terminated pH-sensitive polymers such as polyvinyl acetate phthalate (PVAP), various grades of hydroxypropyl methylcellulose phthalate (HPMCP-50, HPMCP-55, and HPMCP-55S), and various methacrylic acid copolymers such as Eudragit L100-55, Eudragit L100, Eudragit S100, and Kollicoat MAE 100P (Figure 1). More specifically, we focused on the exploration of the activity of polycarboxylates against HSV which is responsible for herpes genitalis, a sexually transmitted viral infection that affects more than 500 million individuals globally.14
Figure 1.
Structural backbone of various pharmaceutically acceptable carboxylate-terminated polymers evaluated in this study. CAP and HPMCP are phthalic acid containing polymers. HPMCP is available in three grades: HPMCP-50, HPMCP-55, and HPMCP-55S. Eudragit and Kollicoat are methcrylic acid and alkyl acrylate copolymers with different molecular weights and methacrylic acid (x) and alkyl acrylate (y) ratio.
We, for the first time, demonstrate that PVAP, HPMCP-55S, and Eudragit S100 have activity and selectivity against HSV type-1 (HSV-1) and HSV type-2 (HSV-2). Our HSV neutralization studies show that PVAP is specifically active against HSV-1, Eudragit S100 is specifically active against HSV-2, and HPMCP-55S is active against both HSV-1 and HSV-2. Our studies also demonstrate that PVAP, HPMCP-55S, and Eudragit S100 are capable of treating HSV infection in vitro. To our knowledge, this is the first study that reports the potential of polycarboxylates for HSV treatment. Further, we report that FDA-approved polycarboxylates can be easily transformed into nanoparticles (NPs) and, in the nanoparticulate form, they show similar or enhanced activity against HSV. Finally, using PVAP NPs as a model, we demonstrate that polycarboxylates are capable of synergizing with antiviral drugs such as acyclovir (ACV), tenofovir, and tenofovir disoproxil fumarate (TDF) in in vitro HSV therapy studies. Thus, FDA-approved carboxylic acid-terminated polymers and their NPs have the potential to be developed into topical formulations for the prevention and treatment of HSV infection.
RESULTS
FDA-Approved Polycarboxylate Polymers Have Varied Antiviral Efficacies against HSV-1 and HSV-2.
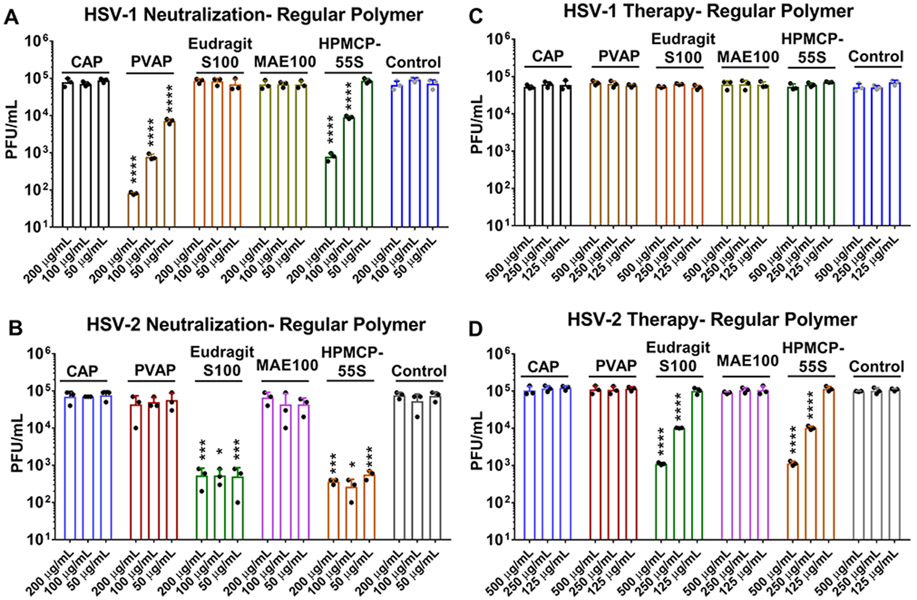
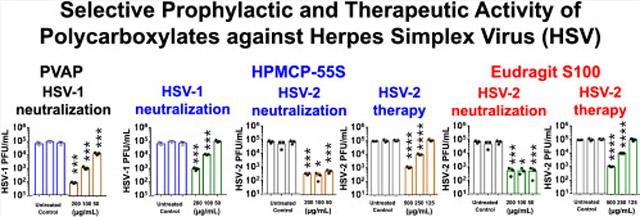
In order to first understand whether the polymers themselves have any antiviral activity associated with them, we performed two key treatments, namely, neutralization and therapy. In the neutralization experiment, the polymers were mixed with the viruses prior to their addition to human corneal epithelial cells (HCEs) or HeLa cells to determine whether the polymers attach to the viruses and curb their entry into the host cell. In the therapeutic treatment assays, viruses were allowed to infect the cells prior to the addition of polymers to the infected cells. At the end of 24 h post-infection (hpi), whole-cell lysates were analyzed via plaque assays conducted on confluent monolayers of Vero cells. Our preliminary studies showed that, at a concentration of ≥250 μg/mL, all polymers including CAP exhibited partial activity against HSV-1 and/or HSV-2 but PVAP, HPMCP-55S, and Eudragit S100 showed a relatively greater effect (data not shown). Our results from the neutralization experiments indicate that, among various FDA-approved carboxylate-terminated polymers, PVAP, HPMCP-55S, and Eudragit S100 have activity against HSV (Figure 2A and B). Interestingly, PVAP was only active against HSV-1 and it showed dose-dependent and significant activity at all tested concentrations (Figure 2A). The HPMCP-55S was significantly effective against HSV-1 at 200 and 100 μg/mL but showed no activity at 50 μg/mL. Interestingly, HPMCP-55S retained its antiviral activity against HSV-2 neutralization with greater efficacy than HSV-1 (Figure 2B). Finally, Eudragit S100 showed a significant reduction in HSV-2 viral load through neutralization assays at all concentrations (Figure 2B); however, it had no discernible activity against HSV-1 (Figure 2A).
Figure 2.
Antiviral efficacy of regular polycarboxylate polymers: (A) HSV-1 and (B) HSV-2 neutralization ability of the polycarboxylate polymers was assessed by incubating the polymers with the virus for a period of 30 min prior to their addition to HCE and HeLa cells, respectively. At 24 hpi, whole cell lysates were titrated for viral load using plaque assays. In a similar experiment, the therapeutic potential of the polymers was tested by adding the polycarboxylate polymers at 2 hpi to (C) HSV-1 infected HCE cells and (D) HSV-2 infected HeLa cells. At 24 hpi, whole cell lysates were titrated for viral load using plaque assays. Asterisks indicate a significant difference by two-way ANOVA with Sidak’s multiple comparison test: *p < 0.05, ***p < 0.001, and ****p < 0.0001.
We also sought to determine the therapeutic potential of polycarboxylate polymers in vitro. Therapeutic treatments using the polymers on HSV-1 infected HCEs showed no discernible antiviral activity (Figure 2C) when compared to the untreated control group. However, Eudragit S100 and HPMCP-55S showed significant therapeutic efficacy against HSV-2 at 500 and 250 μg/mL concentrations (Figure 2D).
Development and Characterization of Polycarboxylate NPs.
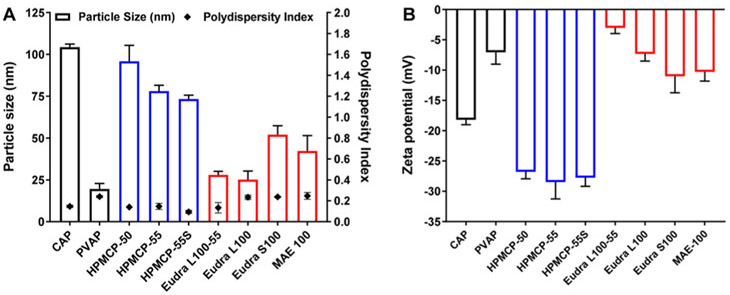
We used a simple and scalable nanoprecipitation method to develop nanoformulations of FDA-approved carboxylate-terminated polymers. We kept the concentration of polymer and stabilizer constant in all nanoformulations to maintain uniformity. The size, polydispersity index, and zeta potential of polymeric NPs considerably changed with the change in the polymer. The size of all of the polymeric NPs was less than 125 nm and the polydispersity index was less than 0.25 (Figure 3), which indicated uniformity of NPs. All NPs exhibited negative zeta potential values due to the presence of carboxylic acid end groups. All of the nanoformulations showed good colloidal stability for at least 2 weeks (data not shown).
Figure 3.
Characterization of polycarboxylate nanoparticles (NPs): (A) particle size and polydispersity index and (B) surface charge or zeta potential of polycarboxylate NPs. The size of the polycarboxylate NPs was dependent on the type of polycarboxylate polymer. The polydispersity index values were <0.3 for all nanoformulations, indicating uniformity of NPs. All polycarboxylate NPs showed a negative zeta potential value due to the presence of the carboxylate end groups (data expressed as mean ± S.D.; n = 3). CAP: Cellulose acetate phthalate; PVAP: polyvinyl acetate phthalate; HPMCP: hydroxypropyl methylcellulose phthalate; Eudra: Eudragit; MAE 100: Kollicoat MAE 100P.
Transformation of the Polycarboxylates into NPs Improves the Antiviral Efficacy.
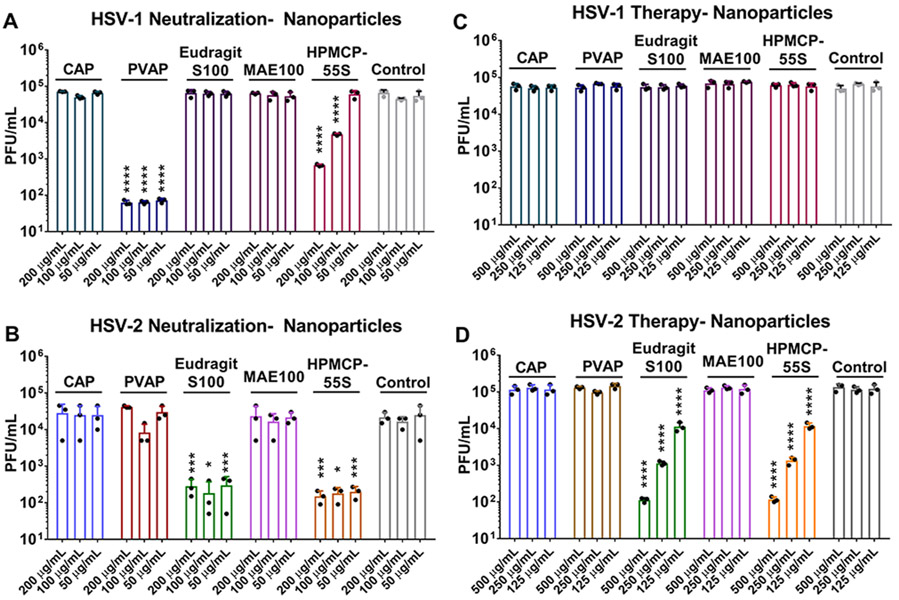
The NPs of the polycarboxylates were tested in a manner similar to their regular polymer counterparts through neutralization and therapeutic assays. As expected, PVAP NPs and HPMCP-55S NPs showed antiviral efficacy against HSV-1 (Figure 4A), while Eudragit S100 NPs and HPMCP-55S NPs showed antiviral efficacy against HSV-2 (Figure 4B and D). The polycarboxylate NPs did not show therapeutic activity against HSV-1 (Figure 4C). While the difference between the regular polymers and their nanoformulations is minor at higher concentrations, PVAP NPs showed a significant reduction in viral load (>3 log10-fold) at 50 μg/mL concentration when compared to its regular polymer counterpart against HSV-1 infection. Eudragit S100 NPs and HPMCP-55S NPs showed half log10-fold reduction across all the concentrations against HSV-2 through neutralization treatment, while therapeutic treatment with the same polymers showed 1 log10-fold decrease in viral load across all concentrations in their nano form compared to regular polymeric counterparts.
Figure 4.
Antiviral efficacy of polycarboxylate nanoparticles (NPs): (A) HSV-1 and (B) HSV-2 neutralization ability was assessed by incubating polycarboxylate NPs with the virus for a period of 30 min prior to their addition to HCE and HeLa cells, respectively. At 24 hpi, whole cell lysates were titrated for viral load using plaque assays. In a similar experiment, the therapeutic potential of the nanopolymers was evaluated by adding them at 2 hpi to (C) HSV-1 infected HCE cells and (D) HSV-2 infected HeLa cells. At 24 hpi, whole cell lysates were titrated for viral load using plaque assays. Asterisks indicate significant difference by two-way ANOVA with Sidak’s multiple comparison test: *p < 0.05, ***p < 0.001, and ****p < 0.0001.
Polymeric NPs Are Highly Tolerable in Vitro.
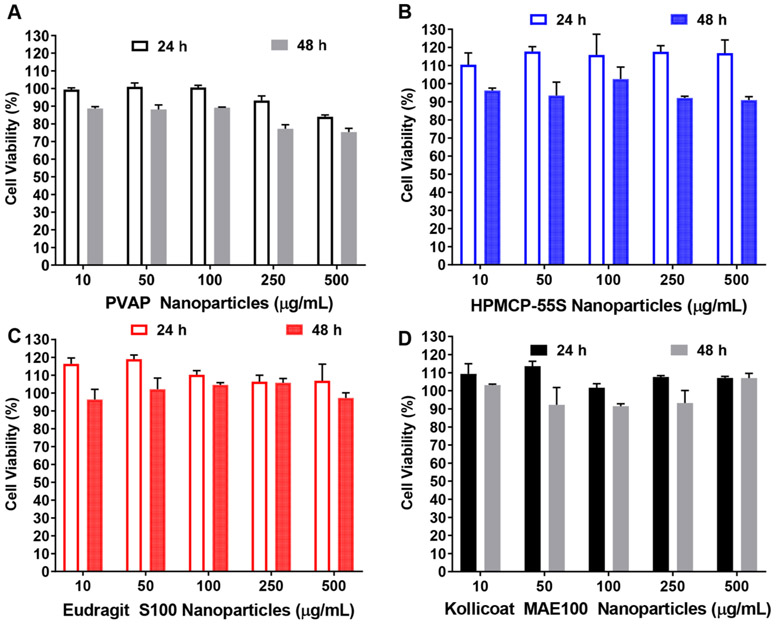
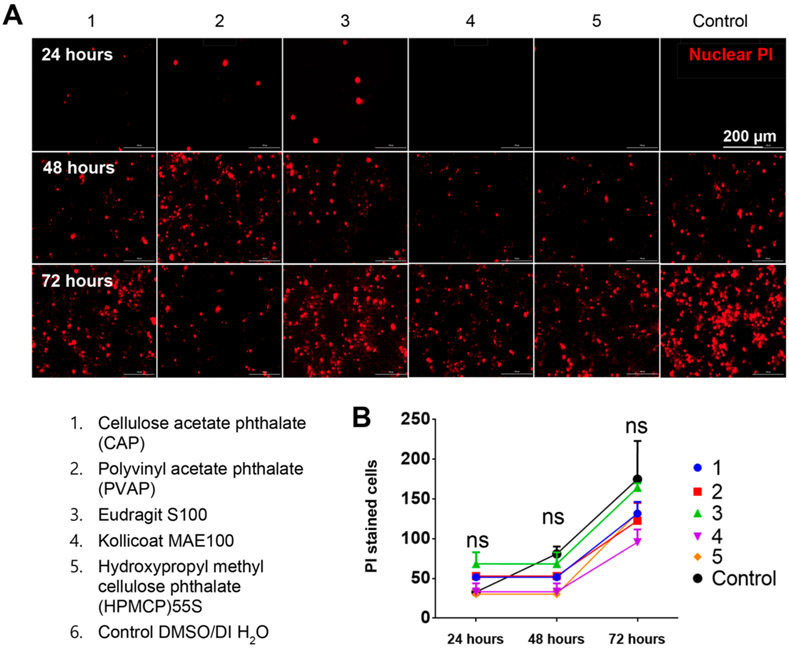
Given the variability in antiviral efficacy between the polymers, we sought to understand whether any of them caused significant toxicity which would indirectly be responsible for lower viral load. To test this hypothesis, we first tested the tolerability of various concentrations of PVAP NPs, HPMCP-55S NPs, Eudragit S100 NPs, and Kollicoat MAE 100 NPs on HeLa cells. PVAP NPs showed a slight reduction in cell viability at 250 μg/mL or higher concentration especially after 48 h of treatment (Figure 5A) whereas NPs of HPMCP-55S, Eudragit S100, and Kollicoat MAE 100P were very well tolerated by HeLa cells even at a concentration of 500 μg/mL up to 48 h. We also tested the tolerability of the highest concentration (500 μg/mL) of the aforementioned NPs to HCEs for 72 h. The cells were imaged every 24 h to visualize and quantify the total number of PI positive (dead) cells. Interestingly, we observed no difference between mock-treated and NPs-treated samples in all of our replicate experiments (Figure 6A). Plotting the number of PI positive cells per frame in a graph over time showed no significant differences (Figure 6B). These results indicate little or no toxicity associated with the polycarboxylate NPs to the target cells.
Figure 5.
Polycarboxylate nanoparticles (NPs) are well-tolerated by HeLa cells. HeLa cells were treated with different concentrations of (A) HPMCP-55S NPs, (B) Eudragit S100 NPs, (C) Kollicoat MAE 100 NPs, and (D) PVAP NPs for 24 and 48 h. The viability of NP-treated HeLa cells was compared to untreated controls using MTT assay and to obtain % cell viability (data expressed as means ± SEM.; n = 3).
Figure 6.
Polycarboxylate nanoparticles (NPs) are well-tolerated by human corneal epithelial (HCE) cells. (A) HCEs were stained with propidium iodide (PI-cell death marker) prior to the addition of either mock DMSO/water or 200 μg/mL polycarboxylate nanopolymers. The cells were imaged at shown time points to assess the extent of PI taken up by cells (a mark of necrotic death). (B) The total number of cells with PI stain in four frames was counted and averaged over three replicates. The graph represents the number of dead cells (stained with PI) counted over a period of 72 h. Two-way ANOVA with Dunnett’s multiple comparison test was conducted (n = 3 replicates)
Confocal Microscopy Studies Demonstrate the Interaction of Polycarboxylate NPs with GFP-Labeled HSV.
To confirm the interaction between polycarboxylates and HSV, we first synthesized fluorescent polycarboxylate NPs. We treated cells with either fluorescent NPs (mock control) or fluorescent NP–fluorescent HSV mixture and tracked the intracellular uptake of fluorescent NPs. Interestingly, the mock control showed significant intracellular uptake of fluorescent NPs in the HCEs, whereas HCEs treated with a mixture of fluorescent NPs and GFP-labeled HSV-1 showed considerably lower intracellular fluorescence and the presence of neutralized viral particles (Figure S1). This clearly demonstrated the interaction between polycarboxylate NPs and HSV.
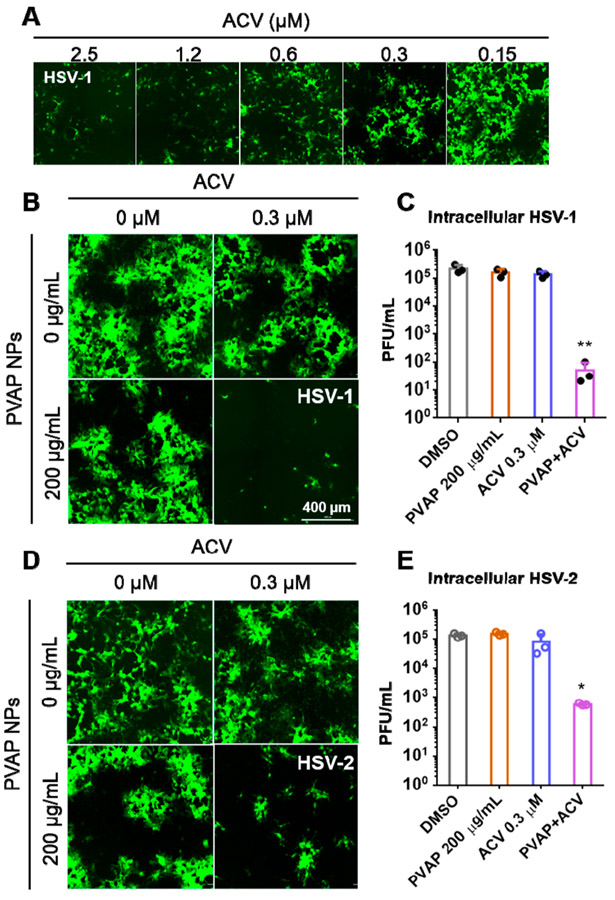
PVAP NPs Have Synergistic Antiviral Efficacy with Common Anti-Herpetic ACV.
After establishing that the polycarboxylate NPs have antiviral efficacy and no potential toxicity, we sought to understand whether they would have a synergistic effect with a well-known antiviral drug ACV when treated therapeutically. To conduct this experiment, we first needed to determine the concentration of ACV at which no antiviral effect is seen. We observed that, at a concentration of 0.3 μM, ACV showed minimal or no antiviral activity (Figure 7A), and hence, our synergy experiments were performed at this concentration of ACV. Subsequently, therapeutic assays were conducted where the PVAP NPs and ACV mixture was added at 2 hpi, where the concentration of ACV remained the same (0.3 μM) while the PVAP NPs concentration varied from 500 to 125 μg/mL (data not shown). We hypothesized that PVAP, Eudragit S100, and HPMCP-55S NPs would show increased antiviral efficacy with ACV. However, to our surprise, our pilot studies revealed no synergistic effects between Eudragit S100/HPMCP-55S NPs and ACV (data not shown). Only PVAP NPs at 200 μg/mL showed synergistic therapeutic antiviral efficacy against both HSV-1 and HSV-2 (Figure 7B-E). It should be noted that PVAP NPs were not active against HSV- or HSV-2 at 200 μg/mL. We repeated our experiments with GFP-reporter virus (both HSV-1 and HSV-2) infected HCE and HeLa cells treated with mock (DMSO), ACV (0.3 μM), PVAP NPs (200 μg/mL), and ACV + PVAP NPs (0.3 μM, 200 μg/mL). At 24 hpi, cells were imaged using a fluorescent microscope to quantify the extent of viral spread (green) and the whole-cell lysates were quantified using a plaque assay to estimate the viral load. We observed a significant reduction in viral load in the ACV and PVAP NPs mixture for both HSV-1 and HSV-2 infected samples when compared to ACV, PVAP NPs, or mock-treated control samples (Figure 7B-E).
Figure 7.
Antiviral synergy evaluation of PVAP nanoparticles (NPs) with ACV. (A) HCEs were infected with 0.1 MOI GFP-expressing HSV-1 and then treated with shown concentrations of ACV. At 24 hpi, fluorescent images were recorded to evaluate the extent of infection (GFP spread). (B) Using the ACV (0.3 μM), 200 μg/mL PVAP NPs, and both of them in combination, HSV-1 infected HCE cells were therapeutically treated for a period of 24 h. At 24 hpi, fluorescent images were captured to evaluate the extent of viral spread. (C) Whole cell lysates were titrated for viral load using plaque assays. (D) In a similar experiment, HSV-2 infected HeLa cells were therapeutically treated with ACV (0.3 μM), 200 μg/mL PVAP NPs, and both of them in combination. At 24 hpi, fluorescent images were captured to evaluate the extent of viral spread. (E) Whole cell lysates were titrated for viral load using plaque assays. Asterisks indicate significance by multiple Student’s t test: *p < 0.05, **p < 0.01.
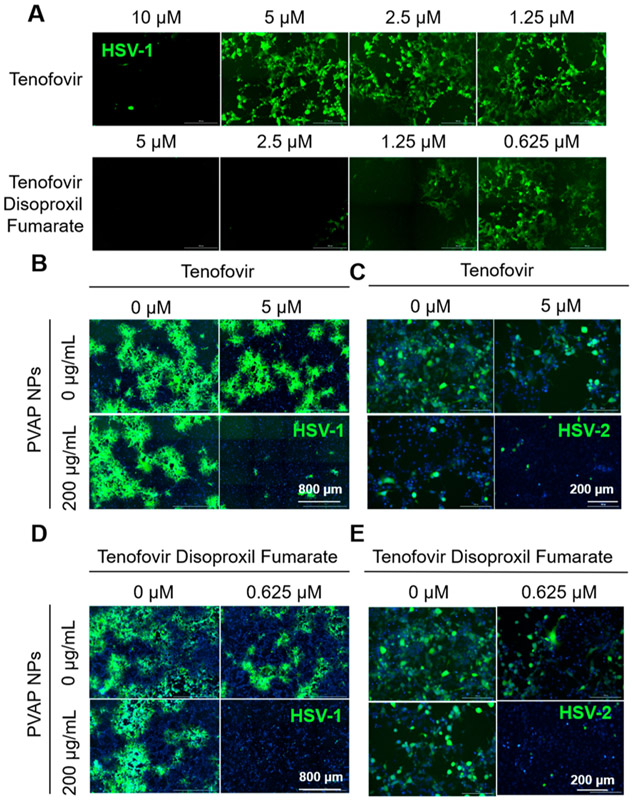
PVAP NPs Synergize with Tenofovir and Tenofovir Disoproxil Fumarate (TDF).
The synergistic efficacy of PVAP NPs with ACV was very encouraging, and we wanted to understand whether this synergy could be translated to clinically tested vaginal microbicides such as tenofovir or its FDA-approved prodrug, TDF. Our studies showed that tenofovir and TDF did not show any antiviral activity at a concentration of 5 and 0.625 μM, respectively (Figure 8A). The higher activity of TDF is due to its higher cell permeability compared to tenofovir. The ineffective concentrations of tenofovir and TDF were mixed with 200 μg/mL of PVAP NPs and added to GFP-reporter HSV-1 infected HCEs or HSV-2 infected HeLa cells. At 24 hpi, the cells were imaged using a fluorescent microscope to estimate the extent of viral spread (green) and quantified using flow cytometry and/or their whole-cell lysates were quantified for intracellular viral load using plaque assays. Interestingly, PVAP NPs showed synergistic interaction with tenofovir and TDF, which resulted in significantly higher anti-HSV activity of PVAP NPs and tenofovir/TDF combination compared to PVAP NPs, tenofovir, and TDF alone (Figure 8B-E; Figures S2 and S3).
Figure 8.
Antiviral synergy of PVAP nanoparticles (NPs) with tenofovir and tenofovir disoproxil fumarate (TDF). (A) HCEs were infected with 0.1 MOI GFP-expressing HSV-1 and then treated with shown concentrations of tenofovir or TDF. At 24 hpi, fluorescent images were recorded to evaluate the extent of infection (GFP spread). Using 200 μg/mL PVAP NPs and tenofovir (5 μM) individually or in combination, HSV-1 infected HCE cells (B) or HSV-2 infected HeLa cells (C) were therapeutically treated for a period of 24 h. At 24 hpi, fluorescent images were captured to evaluate the extent of viral spread. In a similar experiment, 200 μg/mL PVAP NPs were synergized with TDF (0.625 μM) and tested on HSV-1 infected HCE cells (D) or HSV-2 infected HeLa cells (E). Experiments exploring the synergistic interaction among PVAP NPs and tenofovir/TDF against HSV-2 represented in 8 (C) and 8 (E) were carried out using same “untreated control” (“0 μM” in tenofovir, “0 μg/mL” PVAP NPs; “0 μM” tenofovir disoproxil fumarate, “0 μg/mL” PVAP NPs). Hence, the same representative image has been used for the untreated control.
DISCUSSION
The CAP garnered a lot of interest as a prophylactic modality for local prevention of sexually transmitted viral infections such as HIV and HSV due to several reasons such as (1) appreciable activity against HIV and HSV, (2) safety to the vaginal microflora and vaginal epithelium, (3) better tolerability than polysulfonated polymers, (4) stability at the acidic pH of vaginal milieu, and (5) ease of availability and relatively low cost.9-12,15,16 Neurath and co-workers, in a series of investigations, reported the in vivo efficacy and safety of topically applied CAP in the prevention of infection from HIV and HSV in mice and macaques.9-11,17 Despite the promising preclinical data, the instability of CAP in an aqueous environment impeded the development of stable aqua-based topical formulations of CAP. Although Neurath and co-workers developed and tested a nonaqueous topical formulation containing 13% w/v micronized CAP,10-13 the early phase clinical trials were halted due to the vehicle osmolarity associated intolerability of the topical CAP formulation.18 The development of polycarboxylates as a prophylactic modality against sexually transmitted viral infections has been dormant after the CAP clinical study was halted.
It is noteworthy that, other than CAP, several carboxylate-terminated pH-sensitive polymers are commonly used in the pharmaceutical field as an enteric coating material for oral formulations or as a matrix former and/or drug delivery carrier for oral and topical formulations.19 The pharmaceutically acceptable pH-sensitive polymers are broadly categorized into 1) phthalate-terminated polymers which include CAP, phthalavin (PVAP), HPMCP-50, HPMCP-55, and HPMCP-55S and 2) methacrylic acid copolymers which include Eudragit L100-55, Eudragit L100, Eudragit S100, and Kollicoat MAE 100P. These polymers greatly differ from each other with respect to the structural backbone, phthalate or carboxylate content, molecular weight, and pH sensitivity. Interestingly, except for CAP, the activity of the other pharmaceutically acceptable enteric polycarboxylates against sexually transmitted viruses has not been explored. Neurath and co-workers did show that CAP was more active than HPMCP against HIV-1 in vitro.9 However, they did not specify the type of HPMCP used.
The hydrolytic instability of CAP was one of the roadblocks for its clinical development of CAP as a prophylactic modality. However, it is well-known that HPMCP, PVAP, and methacrylic acid copolymers exhibit greater hydrolytic stability than CAP.20,21 Furthermore, some of these polymers are commercially available as stable aqueous dispersions for pharmaceutical coating applications. In view of this, the evaluation of pharmaceutically acceptable polycarboxylates other than CAP was highly warranted. We envisaged that hydrolytically stable and pharmaceutically acceptable polycarboxylates, if successful in demonstrating the acceptable antiviral activity, could be developed further as an aqua-stable topical formulation or polymeric film for the prevention and/or treatment of sexually transmitted viral infections. We sought to explore the antiviral activity of polycarboxylates against HSV, as it is responsible for herpes genitalis, a sexually transmitted viral infection of global prevalence. To date, no effective HSV vaccine is commercially available, and the available drug therapies for the treatment of HSV infection are very limited.
Using fluorescent HSV strains, we carried out preliminary dose-ranging studies on various pH-sensitive polycarboxylates including CAP to identify the most active polymer(s) against HSV-1 and HSV-2 (data not shown). Our preliminary studies showed that, at a concentration of ≥250 μg/mL, all polymers including CAP exhibited partial activity against HSV-1 and/or HSV-2 (data not shown). Our studies and previous data show that polycarboxylates are highly tolerated by cells even at a concentration of 500 μg/mL.15,16,22-24 Hence, the reduction in the fluorescence did not emanate from the cell death and it was indicative of the antiviral activity of polycarboxylate polymers. Our preliminary dose-ranging studies identified PVAP, HPMCP-55S, and Eudragit S100 as lead polycarboxylates with activity against HSV-1 and/or HSV-2 at lower concentrations (data not shown). Hence, we focused our studies on the antiviral activity of PVAP, HPMCP-55S, and Eudragit S100.
As stated in the introduction, the prevention of interaction between HSV and cell surface heparan sulfate proteoglycans is the proposed mechanism of poly(hydroxy)carboxylates.23 It is likely that the polycarboxylates in the current investigation exert their activity against HSV in a similar fashion, although detailed studies are required. Previous studies on poly(hydroxy)carboxylates did demonstrate that the antiviral effects of polymers are dependent on their molecular weight and the number of carboxylic acid groups present in the polymer.7,25 Our results are in congruence with these reports. Among phthalate-terminated polymers, PVAP showed the highest activity against HSV-1 that was significant even at a concentration of 50 μg/mL. PVAP has a molecular weight similar to that of HPMCP-55S, but it has higher phthalyl content (~55–62%) compared to HPMCP-55S (~31%).26-28 This could be the reason for the greater activity of PVAP against HSV-1 when compared to HPMCP-55S. Among different grades of HPMCP, only HPMCP-55S showed anti-HSV activity. HPMCP-50 has a lower molecular weight and lower phthalyl content (~24%) than HPMCP-55S,28 whereas HPMCP-55 has a lower molecular weight than HPMCP-55S but it has similar phthalyl content.28 It is possible that only HPMCP-55S has the right balance of molecular weight and phthalyl content to exhibit antiviral activity.
Recently, Schandock et al. explored the activity of linear poly(acrylic acid) polymers with different structural backbones against various viruses including HSV-2.29 They observed that linear polymethacrylic acid showed considerable activity against HSV-2. While Eudragit and Kollicoat polymers have a quite different structural backbone and molecular weights compared to polymethacrylic acid explored by Schandock et al., they still contain methacrylic acid in the backbone.29,30 Hence, the anti-herpetic activity of Eudragit S100 can be attributed to the presence of methacrylic acid in the backbone. Among various grades of Eudragits, only Eudragit S100 showed significant activity against HSV-2 at a concentration of 50 μg/mL. Eudragit S100 is a methacrylic acid and methyl methacrylate copolymer (ratio of methacrylic acid to methyl methacrylate 1:2). Eudragit L100 is a methacrylic acid and methyl methacrylate copolymer with a methacrylic acid to methyl methacrylate ratio of 1:1, whereas Eudragit L100-55 is a methacrylic acid and ethyl acrylate copolymer with a methacrylic acid to ethyl acrylate ratio of 1:1.30,31 Kollicoat MAE 100P has properties similar to Eudragit L100-55.32 Eudragit L100 and S100 have a similar molecular weight (~125,000 Da), and Eudragit L100-55 has a molecular weight of ~320,000 Da.33 Surprisingly, Eudragit S100, despite its lower carboxylic acid content compared to the other Eudragits showed a higher antiviral effect. This can be attributed to the relatively higher hydrophobicity of Eudragit S100 compared to Eudragit L100-55 and Eudragit L100. It is well-known that polymers with higher hydrophobicity have higher antiviral activity.29
Our studies showed that PVAP is only active against HSV-1 and Eudragit S100 is only active against HSV-2, whereas HPMCP-55S was active against both HSV-1 and HSV-2. Our studies also showed that HPMCP-55S was more active against HSV-2 than HSV-1, and our data is in agreement with the data on CAP reported by Neurath and co-workers.9 However, to our knowledge, the selectivity of pharmaceutically acceptable polycarboxylates against HSV-1 or HSV-2 has been described for the first time in this investigation. Studies have shown that purified gB and gC proteins from HSV-1 and HSV-2 interact differently with heparan sulfate proteoglycans on the cell surface.34,35 It is possible that PVAP, HPMCP-55S, and Eudragit S100 have differential binding to gB, gC, or other glycoproteins on the surface of HSV-1 and HSV-2, which may be responsible for their differential and/or selective activity against HSV-1 and HSV-2. Our future studies would focus on elucidating this interesting behavior of polycarboxylates.
Generally, polyanionic macromolecules are believed to be viral entry inhibitors that have applicability as prophylactic modalities. However, it was also interesting to see that HPMCP-55S and Eudragit S100 showed therapeutic activity against only HSV-2. This selective behavior corroborates that polycarboxylates have differential interaction with HSV-1 and HSV-2. Previous studies have shown that polyanionic molecules can get cointernalized by the cell along with the virus into the endosomes, and after cointernalization, they may inhibit an intermediate step in viral replication between virus release and late protein synthesis.35 Previously, polyacrylates have been shown to induce production of interferon in vivo, which is reflected in the protection of animals from intranasal HSV-1 challenge.36 It has been shown that interferon can inhibit HSV protein synthesis by interfering with the viral replication cycle. These reports may explain the therapeutic efficacy of polycarboxylates against HSV-2, although detailed studies are warranted.
Nanotechnology has demonstrated great potential for the prevention and treatment of infectious diseases. Nanotechnology-enabled topical formulation (VivaGel) was explored in the clinical trials for the prevention of sexually transmitted viral infections.37 The dramatically high surface area of the NPs allows for greater interaction with the infectious microorganisms and greater uptake into cells.37 We envisaged that the transformation of polycarboxylates into NPs will allow for greater interaction with HSV and greater uptake in the cells, which will eventually lead to an increase in the antiviral activity. Further, the transformation of polycarboxylates into NPs will facilitate the development of aqua-based topical formulations. We have previously shown that CAP NPs can be successfully incorporated into the thermosensitive vaginal gel.15 Finally, the transformation of polycarboxylates into NPs will also allow for the incorporation of antiviral drugs into NPs for synergistic activity, which will be explored in the future. Interestingly, we did observe that PVAP NPs and Eudragit S100 NPs have greater antiviral efficacy compared to PVAP and Eudragit S100 in DMSO solution especially at lower concentration, indicating the advantage associated with NPs.
Polycarboxylates are also of interest due to their excellent cytocompatibility. Previously, we have shown that CAP NPs are well tolerated by HeLa cells up to 500 μg/mL.15 Other investigators have also shown that polycarboxylates such as Eudragit S100 can be tolerated by vaginal epithelial cells at 500 μg/mL or higher concentration.22 Our data is in congruence with previous reports. Our studies showed that polycarboxylate NPs are well-tolerated by HeLa and HCE cells even at a concentration of 500 μg/mL (Figures 5 and 6).
While the anti-herpetic effect of polycarboxylates observed in this investigation is quite promising, they are certainly not as potent as classic anti-HSV drugs such as ACV or vaginal microbicides such as tenofovir or TDF. Over the years, polyanionic macromolecules, despite their promising in vitro and preclinical efficacy and safety, have failed to show clinically significant prophylactic activity against sexually transmitted viral infections in several trials. Hence, it may not be prudent to develop polyanionic molecules as a stand-alone prophylactic and therapeutic modality. Instead, it is pragmatic to use polyanionic molecules in conjunction with existing or emerging antiviral drugs to develop a robust prophylactic and/or therapeutic modality against sexually transmitted viral infections. Previously, we and others have shown that polycarboxylates can synergize with various antiretroviral drugs such as efavirenz, UC-781, TMC-125, and TDF.15,16,38 Hence, we chose to carry out synergistic studies on polycarboxylate NPs and antiviral drugs such as ACV, tenofovir, and TDF. While ACV is a well-known anti-herpetic drug, tenofovir and TDF were chosen because of their ability to inhibit HSV DNA polymerase.39 TDF, due to its higher permeability, is more active against HSV compared to tenofovir, and our results are in agreement with the previous reports.40 We primarily focused on PVAP NPs due to their inactivity against HSV-1 and HSV-2, as shown by our in vitro HSV therapy studies. It should be noted that we mixed PVAP NPs at an inactive concentration with an inactive concentration of antiviral drugs for our therapy studies. Interestingly, we observed significant synergy between PVAP NPs and ACV, tenofovir, and TDF even after simple mixing (Figures 7, 8, S2, and S3). The mechanism responsible for the synergy will be investigated in our future studies. However, the results of our synergistic studies showed the advantage of combining polycarboxylate NPs with standard anti-HSV drugs such as ACV or vaginal microbicides such as tenofovir or TDF. Our future studies would focus on the encapsulation of anti-HSV drugs or vaginal microbicides (tenofovir/TDF) into polycarboxylate NPs to develop topical nanomedicine for the prevention and treatment of genital herpes. We anticipate that topical application of polycarboxylate NPs containing anti-HSV drug/vaginal microbicide will improve the delivery of anti-HSV drug/vaginal microbicide and offer enhanced efficacy due to synergy between the polycarboxylate and the drug, which could be useful for the treatment of acute and recurrent genital herpes.
CONCLUSION
Pharmaceutically acceptable pH-sensitive polycarboxylates such as PVAP, HPMCP-55S, and Eudragit S100 have potential for the prevention and treatment of HSV infection. The transformation of polycarboxylates into NPs can improve their antiviral activity. Furthermore, these polymers are also capable of synergizing with the standard antiviral drugs which clearly support the development of a topical combination product containing polycarboxylate and antiviral drugs for prophylactic and/or therapeutic application. Finally, such a topical combination product could be explored for the prevention and/or treatment of herpes genitalis, herpes labialis, and herpes simplex keratitis.
METHODS
Materials.
Cellulose acetate phthalate (Eastman C-A-P, Eastman Chemical Company, Longview, TX), polyvinyl acetate phthalate (Phthalavin, Colorcon, Inc., Harleysville, PA), Eudragit L100-55, Eudragit L100, and Eudragit S100 (Evonik Corporation, Los Angeles, CA), Poloxamer 407 (Kolliphor P407), and Kollicoat MAE 100P (BASF Corporation, Florham Park, NJ), and hydroxypropyl methylcellulose phthalate (HPMCP-50, HPMPCP-55, and HPMCP-55S, Shin-Etsu Chemical Co. Ltd., Tokyo, Japan) were received as a gift sample. Acetone (AR grade) and dimethyl sulfoxide (DMSO) were purchased from VWR International (Radnor, PA). Absolute ethanol (200-proof) was purchased from Fisher Scientific (Waltham, MA).
Drugs.
ACV was purchased from Selleck Chemicals LLC (Houston, TX). Tenofovir (TFV) and tenofovir disoproxil fumarate (TDF) were purchased from Biosynth-Carbosynth LLC (San Diego, CA). Drugs were dissolved in dimethyl sulfoxide (DMSO) to obtain a stock concentration of 50 mM. Aliquots (20 μL) of the drug stock were prepared and stored at −80 °C.
Cells and Virus.
In this study, human corneal epithelial (HCE) and Henrietta lacks (HeLa) cell lines were used for infection against HSV-1 (17GFP) and HSV-2 (333-GFP), respectively. Both of the viruses used have a green fluorescence protein-based reporter system on a CMV (cytomegalovirus) promoter. African green monkey kidney (Vero) cells were used for plaque assays. HCEs were grown in minimum essential medium (MEM), while HeLa and Vero cells were grown in Dulbecco’s modified Eagle’s medium (DMEM). Both media were supplemented with 10% fetal bovine serum (Sigma US Origin) and 1% penicillin and streptomycin (Gibco).
Treatment Protocols.
Two different types of previously reported treatment protocols,41,42 namely, neutralization and therapy, were conducted in this study. Polycarboxylates were dissolved in DMSO to obtain a concentration of 25–50 mg/mL. In the neutralization assay, the virus and the polycarboxylates (at different concentrations) were mixed and incubated in a microcentrifuge tube for 30 min before adding the mixture to a confluent monolayer of the target cell line. In therapy, target cells were first infected with the respective virus for a period of 2 h to ensure viral entry into the cells. At 2 h post-infection, cells were washed and fresh media containing the antiviral compound was added. In both treatment protocols, cells were incubated for 24 h at 37 °C, 5% CO2 in a humidified chamber before collection or sampling. In both treatment protocols, 0.1 MOI (multiplicity of infection) virus was used for infecting the cells.
Plaque Assays.
Viral titration was conducted using whole cell lysates of infected cells using a previously established protocol.43 Briefly infected cells were collected either using a cell lifter (Fisher Scientific) or Hank’s cell dissociation buffer (Gibco). The cells were washed once with phosphate buffer saline (PBS) and resuspended in 1 mL of OptiMEM (Gibco). The resuspended cells were sonicated using a probe sonication system for 30 s (5 s pulses at 20% amplitude). After sonication, 10 μL of cell lysate was mixed with 990 μL of OptiMEM to make a 10−2 dilution of the viral solution. This solution was then serially diluted to reach a dilution of 10−8 in a total volume of 1 mL. From this, 250 μL of each dilution (dilution factor: 4) was overlaid on a confluent monolayer of Vero cells in a 24-well plate to start the infection. At 2 h post-infection, cells were washed with PBS and overlaid with 5% methylcellulose (Sigma-Aldrich) laden DMEM solution. The plates were incubated at 37 °C in a 5% CO2 chamber for a period of 3 days or until visual confirmation of plaques was possible. To each well, 250 μL of 100% methanol was added for 10 min to fix the cells. The solution was aspirated, and 250 μL of crystal violet solution was added to each well to stain the cells. After 30 min of staining, the solution was aspirated and dried. The plaques were manually counted and multiplied with the dilution factor to reveal plaque-forming units per mL (PFU/mL) for each infected sample.
Nanoparticle Synthesis and Characterization.
NPs of various polycarboxylate polymers were prepared using the previously described nanoprecipitation method with suitable modifications.15 Briefly, polycarboxylate polymer (50 mg) and Kolliphor P407 (Poloxamer 407, 200 mg) were transferred to a 20 mL scintillation vial. To this mixture, 10 mL of acetone was added and the contents in the scintillation vial were vigorously vortexed to obtain a clear solution (organic phase). To dissolve PVAP, a mixture of acetone (7 mL) and ethanol (3 mL) was required. The aqueous phase consisted of 10 mL of ultrapure water. The aqueous phase was transferred to a clean 50 mL beaker. The beaker containing aqueous phase was placed on a multipoint magnetic stirrer (IKA Works), and the stirrer was set at 600 rpm. The organic phase was slowly added to the aqueous phase to avoid any splashing and to allow the formation of NPs. The stirring was continued for at least 3 h in a fume hood to allow for complete evaporation of the organic solvent. The synthesis of NPs was carried out in triplicate. The size, polydispersity index, and surface charge (zeta potential) of NPs were evaluated using a Litesizer 500 particle analyzer (Anton-Paar USA, Inc., Torrance, CA).
Preparation of Rhodamine-6G Encapsulated Polymeric NPs.
Briefly, polycarboxylate polymer (PVAP, HPMCP-55S or Eudragit S100; 50 mg) and Kolliphor P407 (200 mg) were transferred to a 20 mL scintillation vial. To this mixture, 9.5 mL of acetone (or 6.5 mL of acetone and 3 mL of ethanol for PVAP) was added and the contents in the scintillation vial were vigorously vortexed to obtain a clear solution (organic phase). Rhodamine-6G (Acros Organics) was dissolved in acetone at a concentration of 0.01 mg/mL and added to the organic phase containing polymers to obtain a final concentration of 0.001 mg/mL. The polymeric NPs were prepared as described earlier. The unencapsulated rhodamine-6G in the NPs was removed using Amicon Ultra-0.5 CentrifUgal Filter Devices (Amicon Ultra 10K device). Briefly, a nanoparticle dispersion (0.5 mL) was transferred to the Amicon-Ultra-0.5 device and centrifuged for 15 min at 14000 rpm (Thermo Scientific, Legend Micro 17R centrifuge). The NPs were washed twice with ultrapure distilled water to get rid of free rhodamine-6G. The filtrate was discarded, and the concentrated rhodamine-6G encapsulated polymeric NPs (~100 μL) were collected in a separate tube by inverting and centrifuging the Amicon Ultra filter for 2 min at 1000g. The concentrated NPs were then diluted to their original volume by adding ~400 μL of distilled water; the tube was covered with the aluminum foil and stored until further use.
Confocal Microscopy to Assess the Interaction between Rhodamine-6G Encapsulated NPs and GFP-Labeled HSV.
The intracellular distribution of rhodamine-labeled polymeric NPs (PVAP, Eudragit S100 and HPMCP-55S) in HCE cells was determined using confocal laser scanning microscopy (CLSM) in both the presence and absence of HSV-1 K26-GFP virus. The HCE cells were seeded in an eight-chamber confocal microscopy slide (Nunc Lab-Tek, Thermo Fisher Scientific, Waltham, MA, USA) with 200 μL of complete MEM media. This was followed by addition of 10 μL of various treatment samples which included control (no treatment and virus only) into each chamber of the eight-chamber plate. Further, the treatment groups were incubated for 2 h before the cells were fixed using 4% paraformaldehyde for 10 min. Nuc-blue (Thermo Fisher Scientific) DAPI stain was added to each well (2 drops per mL of solution) for 5 min before the imaging was performed. Initial screening was performed on polymer only and virus only samples to separate the excitation wavelengths of rhodamine and GFP to ensure no leakage occurred. Images were taken at 488–505 nm for GFP and 525–581 nm for rhodamine stains. Three separate wells were dedicated to each sample group, and three images were taken per sample. The representative images have been shown in this article.
Fluorescent Imaging.
All images were taken on a Lionheart LX (BioTek) automated imaging system. Cells were stained with NucBlue Live reagent (Hoechst 33342 dye) in addition to inherent viral GFP produced from infected cells. All images were captured using a 10× lens, and multiple images were taken together and stitched automatically by the BioTeK software.
HeLa Cell Viability Studies on Polycarboxylate NPs.
The tolerability of polycarboxylate NPs to HeLa cells was evaluated using the MTT assay. Briefly, HeLa cells were seeded in flat-bottom 96-well plates (Greiner Bio, NC, USA) at a density of 1.0 × 104 cells/well and kept at 30 °C in a humidified atmosphere of 5% CO2 for 24 h to allow for the attachment of cells to the bottom of the plate. The cells were then treated with different concentrations of polycarboxylate NPs for 24 or 48 h. After 24 or 48 h treatment, the cell media was removed, and cells were washed twice with phosphate buffer saline. MTT solution (5 mg/mL) was diluted with fresh media to a final concentration of 0.5 mg/mL. Then, 100 μL of DMEM, containing the MTT reagent (VWR Life Science, PA, USA), was added to the cells and incubated at 37 °C for 2 h. The blue formazan crystals were dissolved in DMSO after discarding the media. The absorbance of untreated cells (control) and NP-treated cells was measured at 570 nm using a microplate reader (Synergy H1, BioTek, Vermont, USA), and the percent cell viability of NP-treated HeLa cells in comparison to untreated control was calculated. All experiments were performed in triplicate.
Human Corneal Epithelial (HCE) Cell Viability via Nuclear PI Staining.
This assay is based on the principle that cells that are dead or about to die take up propidium iodide (PI) stain as a result of increased membrane perfusion.44 In this regard, HCE cells were incubated with polymeric NPs dispersed in MEM containing 1% PI solution. The cells were imaged at various times post-addition of the polymeric NPs to analyze the number of PI stained cells per frame. All experiments were performed in triplicate, and the results were graphed for representation.
Evaluating Synergistic Activity between Polycarboxylate NPs and Antiviral Drugs to treat HSV Infection in Vitro.
To understand whether polymeric NPs can work in tandem with anti-HSV drugs, we used a noneffective concentration of PVAP NPs with a noneffective concentration of acyclovir (ACV), tenofovir, or tenofovir disoproxil fumarate (TDF). PVAP NPs did not show a therapeutic effect against HSV-1 and HSV-2 in vitro at 200 μg/mL (refer to Figure 4). Hence, we used PVAP NPs at a concentration of 200 μg/mL. To identify the noneffective concentration of anti-HSV drugs, we used a GFP-reporter HSV-1 virus to infect HCE cells at 0.1 MOI and 2 hpi; ACV, tenofovir, and TDF were serially diluted starting at 10 μM concentration. The noneffective concentration of ACV was 0.3 μM, that of tenofovir was 5 μM, and that of TDF was 0.625 μM. The TDF, due to its high cell permeability compared to tenofovir, exhibited greater activity against HSV. To test the synergy between the polymeric NPs and the anti-HSV drugs, we infected HCEs with HSV-1 17-GFP or HeLa cells with HSV-2 333 GFP virus at 0.1 MOI. At 2 hpi, cells were washed with PBS once and fresh media containing a combination of PVAP NPs at 200 μg/mL and a noneffective concentration of the anti-HSV drug. In addition to a no-treatment control, we used PVAP NPs alone at 200 μg/mL or the drug alone at a noneffective concentration. The efficacy was evaluated qualitatively using fluorescent imaging for the GFP-reporter virus and quantitatively using plaque assay or flow cytometry analysis.
Statistical Analysis.
All of the statistical analyses were performed using GraphPad Prism software. Two-way ANOVA was conducted for the analysis of plaque assay data comparing the antiviral efficacy of regular and nanopolymeric compounds in Figures 2 and 4. One-way ANOVA was used for cell viability analysis in Figure 6 and plaque assay data in Figures 7 and 8. All experiments were performed at least in triplicates and repeated three times using three different passages of the cell lines. Significance was proven only with a p-value <0.05, while all other values were considered nonsignificant. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Eastman Chemical Company, Shin-Etsu Chemical Co. Ltd., Evonik Corporation, BASF Corporation, and Colorcon, Inc., for providing the gift samples of various polycarboxylates. A.A.D. would like to acknowledge the support from John A. Burns School of Medicine, University of Hawaii Manoa Pilot Project Grants, viz., Ola HAWAII Pilot Project Grant (NIMHD Grant Number U54MD007601) and Diabetes COBRE Pilot Project Grant (NIGMS Grant Number P20GM113134), Hawaii Community Foundation Grant (Grant Number 19ADVC-95449), and INBRE IV Junior Investigator Award (NIGMS Grant Number P20GM103466). D.S. acknowledges support from NIH grants R01 EY024710, R01 AI139768, and P30 EY001792.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.0c00368.
Figure S1, understanding the interaction between fluorescent polycarboxylate nanoparticles and fluorescent HSV-1; Figure S2, synergy between PVAP nanoparticles and tenofovir/tenofovir disoproxil fumarate against HSV-1; Figure S3, synergy between PVAP nanoparticles and tenofovir/tenofovir disoproxil fumarate against HSV-2 (PDF)
The authors declare no competing financial interest.
Contributor Information
Tejabhiram Yadavalli, Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago, Chicago, Illinois 60612, United States.
Sudipta Mallick, Department of Pharmaceutical Sciences, The Daniel K. Inouye College of Pharmacy, University of Hawaii Hilo, Hilo, Hawaii 96720, United States.
Pratikkumar Patel, Department of Pharmaceutical Sciences, The Daniel K. Inouye College of Pharmacy, University of Hawaii Hilo, Hilo, Hawaii 96720, United States.
Raghuram Koganti, Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago, Chicago, Illinois 60612, United States.
Deepak Shukla, Department of Ophthalmology and Visual Sciences, Department of Microbiology and Immunology, and Department of Bioengineering, University of Illinois at Chicago, Chicago, Illinois 60612, United States.
Abhijit A. Date, Department of Pharmaceutical Sciences, The Daniel K. Inouye College of Pharmacy, University of Hawaii Hilo, Hilo, Hawaii 96720, United States.
REFERENCES
- (1).Nahmias AJ, and Kibrick S (1964) Inhibitory effect of heparin on herpes simplex virus. J. Bacteriol 87, 1060–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).De Somer P, De Clercq E, Billiau A, Schonne E, and Claesen M (1968) Antiviral activity of polyacrylic and polymethacrylic acids. I. Mode of action in vitro. J. Virol 2, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).De Somer P, De Clercq E, Billiau A, Schonne E, and Claesen M (1968) Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J. Virol 2, 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Claes P, Billiau A, De Clercq E, Desmyter J, Schonne E, Vanderhaeghe H, and De Somer P (1970) Polyacetal carboxylic acids: a new group of antiviral polyanions. J. Virol 5, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pirrone V, Wigdahl B, and Krebs FC (2011) The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res. 90, 168–182. [DOI] [PubMed] [Google Scholar]
- (6).Luscher-Mattii M (2000) Polyanions–a lost chance in the fight against HIV and other virus diseases? Antivir. Chem. Chemother 11, 249–259. [DOI] [PubMed] [Google Scholar]
- (7).Reymen D, Witvrouw M, Esté JA, Neyts J, Schols D, Andrei G, Snoeck R, Cushman M, Hejchman E, and De Clercq E (1996) Mechanism of the Antiviral Activity of New Aurintricarboxylic Acid Analogues. Antivir Chem. Chemother 7, 142–152. [Google Scholar]
- (8).Lin SY, and Kawashima Y (1987) Drug release from tablets containing cellulose acetate phthalate as an additive or enteric-coating material. Pharm. Res 4, 70–74. [DOI] [PubMed] [Google Scholar]
- (9).Neurath AR, Strick N, Li YY, Lin K, and Jiang S (1999) Design of a “microbicide” for prevention of sexually transmitted diseases using “inactive” pharmaceutical excipients. Biologicals 27, 11–21. [DOI] [PubMed] [Google Scholar]
- (10).Gyotoku T, Aurelian L, and Neurath AR (1999) Cellulose acetate phthalate (CAP): an ‘inactive’ pharmaceutical excipient with antiviral activity in the mouse model of genital herpesvirus infection. Antivir. Chem. Chemother 10, 327–332. [DOI] [PubMed] [Google Scholar]
- (11).Manson KH, Wyand MS, Miller C, and Neurath AR (2000) Effect of a cellulose acetate phthalate topical cream on vaginal transmission of simian immunodeficiency virus in rhesus monkeys. Antimicrob. Agents Chemother 44, 3199–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Cummins JE, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, Grohskopf LA, Paxton L, and Dezzutti CS (2007) Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob. Agents Chemother 51, 1770–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Mayhew JW, Gideon LT, Ericksen B, Hlavaty JJ, Yeh SM, Chavdarian CG, Strick N, and Neurath AR (2009) Development of a gel permeation chromatographic assay to achieve mass balance in cellulose acetate phthalate stability studies. J. Pharm. Biomed. Anal 49, 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Jaishankar D, and Shukla D (2016) Genital Herpes: Insights into Sexually Transmitted Infectious Disease. Microb Cell 3, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Date AA, Shibata A, McMullen E, La Bruzzo K, Bruck P, Belshan M, Zhou Y, and Destache CJ (2015) Thermosensitive Gel Containing Cellulose Acetate Phthalate-Efavirenz Combination Nanoparticles for Prevention of HIV-1 Infection. J. Biomed. Nanotechnol 11, 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Huang C, Soenen SJ, van Gulck E, Vanham G, Rejman J, Van Calenbergh S, Vervaet C, Coenye T, Verstraelen H, Temmerman M, Demeester J, and De Smedt SC (2012) Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials 33, 962–969. [DOI] [PubMed] [Google Scholar]
- (17).Ratterree M, Gettie A, Williams V, Malenbaum S, Neurath AR, Cheng-Mayer C, and Blanchard J (2005) Safety and distribution of cellulose acetate 1,2-benzenedicarboxylate (CAP), a candidate anti-HIV microbicide in rhesus macaques. AIDS 19, 1595–1599. [DOI] [PubMed] [Google Scholar]
- (18).Lacey CJ, Woodhall S, Qi Z, Sawant S, Cowen M, McCormack S, and Jiang S (2010) Unacceptable side-effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial. Int. J. STD AIDS 21, 714–717. [DOI] [PubMed] [Google Scholar]
- (19).Maderuelo C, Lanao JM, and Zarzuelo A (2019) Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur. J. Pharm. Sci 138, 105019. [DOI] [PubMed] [Google Scholar]
- (20).Thoma K, and Bechtold K (1999) Influence of aqueous coatings on the stability of enteric coated pellets and tablets. Eur. J. Pharm. Biopharm 47, 39–50. [DOI] [PubMed] [Google Scholar]
- (21).Shoaib MH, Yousuf RI, Ahmed FR, Ali FR, Qazi F, Ahmed K, and Zafar F (2020) in Polymers Coatings: Technology and Applications (Inamuddin I, Boddula R, Ahamed MI, and Asiri AM, Eds.) 1st ed., pp 275–311, John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- (22).Yoo J, Giri N, and Lee CH (2011) pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int. J. Pharm 403, 262–267. [DOI] [PubMed] [Google Scholar]
- (23).Neyts J, Snoeck R, Wutzler P, Cushman M, Klöcking R, Helbig B, Wang P, and Clercq ED (1992) Poly(Hydroxy)-Carboxylates as selective inhibitors of Cytomegalovirus and Herpes Simplex Virus replication. Antivir Chem. Chemother 3, 215–222. [Google Scholar]
- (24).Mandal S, Khandalavala K, Pham R, Bruck P, Varghese M, Kochvar A, Monaco A, Prathipati PK, Destache C, and Shibata A (2017) Cellulose acetate phthalate and antiretroviral nanoparticle fabrications for HIV pre-exposure prophylaxis. Polymers 9 (9), 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Cushman M, Wang PL, Chang SH, Wild C, De Clercq E, Schols D, Goldman ME, and Bowen JA (1991) Preparation and anti-HIV activities of aurintricarboxylic acid fractions and analogues: direct correlation of antiviral potency with molecular weight. J. Med. Chem 34, 329–337. [DOI] [PubMed] [Google Scholar]
- (26).Davis M, Ichikawa I, Williams EJ, and Banker GS (1986) Comparison and evaluation of enteric polymer properties in aqueous solutions. Int. J. Pharm 28, 157–166. [Google Scholar]
- (27).Nesbitt RU, Goodhart FW, and Gordon RH (1985) Evaluation of polyvinyl acetate phthalate as an enteric coating material. Int. J. Pharm 26, 215–226. [Google Scholar]
- (28).Fukasawa M, and Obara S (2003) Molecular weight determination of hypromellose phthalate (HPMCP) using size exclusion chromatography with a multi-angle laser light scattering detector. Chem. Pharm. Bull 51, 1304–1306. [DOI] [PubMed] [Google Scholar]
- (29).Schandock F, Riber CF, Röcker A, Müller JA, Harms M, Gajda P, Zuwala K, Andersen AHF, Løvschall KB, Tolstrup M, Kreppel F, Münch J, and Zelikin AN (2017) Macromolecular Antiviral Agents against Zika, Ebola, SARS, and Other Pathogenic Viruses. Adv. Healthcare Mater 6, 1700748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Thakral S, Thakral NK, and Majumdar DK (2013) Eudragit: a technology evaluation. Expert Opin. Drug Delivery 10, 131–149. [DOI] [PubMed] [Google Scholar]
- (31).Nguyen MNU, Tran PHL, and Tran TTD (2019) A single-layer film coating for colon-targeted oral delivery. Int. J. Pharm 559, 402–409. [DOI] [PubMed] [Google Scholar]
- (32).Barbosa JAC, Abdelsadig MSE, Conway BR, and Merchant HA (2019) Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their pKa. International Journal of Pharmaceutics: X 1, 100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gupta SS, Meena A, Parikh T, and Serajuddin ATM (2014) Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion - I: Polyvinylpyrrolidone and related polymers. J. Excipients Food. Chem 5, 32–45. [Google Scholar]
- (34).Trybala E, Liljeqvist J, Svennerholm B, and Bergström T (2000) Herpes Simplex Virus Types 1 and 2 Differ in Their Interaction with Heparan Sulfate. J. Virol 74, 9106–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).González ME, Alarcón B, and Carrasco L (1987) Polysaccharides as antiviral agents: antiviral activity of carrageenan. Antimicrob. Agents Chemother 31, 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).de Clercq E, and Luczak M (1976) Antiviral activity of Carbopol, a cross-linked polycarboxylate. Arch. Virol 52, 151–158. [DOI] [PubMed] [Google Scholar]
- (37).Date AA, and Destache CJ (2013) A review of nanotechnological approaches for the prophylaxis of HIV/AIDS. Biomaterials 34, 6202–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Liu S, Lu H, Neurath AR, and Jiang S (2005) Combination of candidate microbicides cellulose acetate 1,2-benzenedicarboxylate and UC781 has synergistic and complementary effects against human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother 49, 1830–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Andrei G, Gillemot S, Topalis D, and Snoeck R (2018) The Anti-Human Immunodeficiency Virus Drug Tenofovir, a Reverse Transcriptase Inhibitor, Also Targets the Herpes Simplex Virus DNA Polymerase. J. Infect. Dis 217, 790–801. [DOI] [PubMed] [Google Scholar]
- (40).Nixon B, Jandl T, Teller RS, Taneva E, Wang Y, Nagaraja U, Kiser PF, and Herold BC (2014) Vaginally delivered tenofovir disoproxil fumarate provides greater protection than tenofovir against genital herpes in a murine model of efficacy and safety. Antimicrob. Agents Chemother 58, 1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Yadavalli T, Agelidis A, Jaishankar D, Mangano K, Thakkar N, Penmetcha K, and Shukla D (2017) Targeting herpes simplex virus-1 gD by a DNA aptamer can be an effective new strategy to curb viral infection. Mol. Ther.–Nucleic Acids 9, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Yadavalli T, Ames J, Agelidis A, Suryawanshi R, Jaishankar D, Hopkins J, Thakkar N, Koujah L, and Shukla D (2019) Drug-encapsulated carbon (DECON): A novel platform for enhanced drug delivery. Sci. Adv 5, eaax0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Jaishankar D, Yakoub AM, Yadavalli T, Agelidis A, Thakkar N, Hadigal S, Ames J, and Shukla D (2018) An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye. Sci. Transl. Med 10, eaan5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Yadavalli T, Suryawanshi R, Ali M, Iqbal A, Koganti R, Ames J, Aakalu VK, and Shukla D (2020) Prior inhibition of AKT phosphorylation by BX795 can define a safer strategy to prevent herpes simplex virus-1 infection of the eye. Ocul Surf. 18, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.