Asfari et al reported that non-alcoholic fatty liver disease (NAFLD) is fourfold more prevalent in women with than without PCOS, and concluded that ‘further studies are needed to assess if specific PCOS treatments can affect NAFLD progression’.1
In the meantime, an international consensus has replaced NAFLD by metabolic dysfunction-associated fatty liver disease (MAFLD).2 In the absence of overweight and obesity, the definition of MAFLD requires not only that steatosis be present, but also that at least two markers point to metabolic dysfunction, for example, a circulating concentration of C reactive protein (CRP) >2.0 mg/L, and an Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) value ≥2.5.
Adolescent polycystic ovary syndrome (PCOS) is a prevalent condition (≈10% of girls and women between 2 and 8 years postmenarche) that is characterised by androgen excess and menstrual irregularity (as a proxy of oligoanovulation)3 but seems to be driven by ectopic lipid accumulation in the liver, essentially resulting from a mismatch between (reduced) prenatal weight gain and (augmented) postnatal weight gain.4 5 Adolescent PCOS is increasingly viewed as a state of metabolic dysfunction, often with low-grade inflammation (by CRP) and/or insulin resistance (by HOMA-IR); a polycystic appearance of the ovaries is no longer a diagnostic criterion of PCOS in adolescence.3 4 6
The new definitions of MAFLD and adolescent PCOS have led to the insight that there may be an overlap between both entities, and that interventions for PCOS in late adolescence could indeed influence the prevalence of MAFLD in early adulthood. We investigated the latter possibility by taking advantage of recent data from randomised pilot studies in non-obese adolescents with PCOS.6 These studies compared the effects of a widely recommended treatment (targeting the ovaries) to those of a new treatment (targeting ectopic fat), each on top of lifestyle measures.6 The recommended treatment was an oestroprogestagen contraceptive (OC; 20 µg ethinylestradiol plus 100 mg levonorgestrel for 21/28 days, and placebo for 7/28 days) that silences the gonadotropic axis, thereby reducing the androgen excess and ensuring anovulation.7 8 The new treatment was a low-dose combination of three generics (SPIOMET),6 namely spironolactone 50 mg/day (to activate brown adipose tissue),9 pioglitazone 7.5 mg/day (to double high-molecular-weight adiponectinaemia,10 and to prioritise subcutaneous adipogenesis)11 and metformin 850 mg/day (to triple the circulating concentrations of appetite-attenuating GDF15).12 SPIOMET does not elicit a loss of body weight, but redistributes body fat from ectopic to subcutaneous depots, thereby conferring more broadly normalising benefits than OC, in particular on liver fat (by MRI) and on post-treatment androgen excess and ovulation rate6 13 (online supplemental table 1).
bmjgast-2020-000574supp001.pdf (82.8KB, pdf)
Here, we highlight the effects of OC versus SPIOMET intervention on a key MAFLD ensemble in non-obese young women with PCOS, namely the triad of hepatic fat, HOMA-IR and circulating CRP.6 Figure 1 shows that the randomised interventions were accompanied by opposing influences on these MAFLD components, so that the prevalence of the MAFLD triad increased from 13% to 35% during OC treatment, and decreased from 13% to 0% during SPIOMET treatment (p<0.001 for between-group difference at 6 and 12 months, by χ2 test). Mean body mass index increased over 12 months on OC (from 24.2 to 24.9 kg/m2; p≤0.01) but did not change detectably on SPIOMET (from 24.2 to 23.9 kg/m2).6
Figure 1.
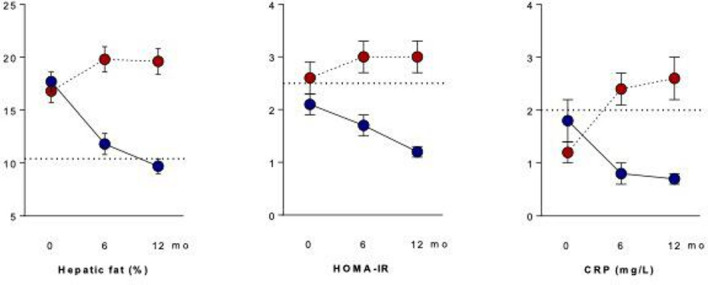
Opposing effects of randomised interventions on the MAFLD triad of hepatic fat, HOMA-IR and circulating CRP in adolescent girls with polycystic ovary syndrome (PCOS). Adolescents with PCOS (mean age 16 years) were randomised to receive an oestro-progestagen contraceptive (OC; red) or a low-dose combination of spironolactone-pioglitazone-metformin (SPIOMET; blue). On-treatment (0–12 months) results are shown as mean and SEM; n=31 in each treatment group. The dotted line in the hepatic-fat panel refers to the average fraction in healthy controls.6 The dotted lines in the HOMA-IR and CRP panels refer to the cut-off levels for these MAFLD criteria.2 Original data are from reference 6. CRP, C reactive protein; MAFLD, metabolic dysfunction-associated fatty liver disease.
In conclusion, the new MAFLD concept unmasks that the widely recommended OC therapy for PCOS does not attenuate the underpinning problem of metabolic dysfunction, and that approximately one-third of OC-treated non-obese adolescents with PCOS become young women with MAFLD. SPIOMET treatment represents a more pathophysiological approach: it is a weight-loss-mimicking intervention that normalises liver fat, HOMA-IR and circulating CRP, and that may be particularly preferable in adolescent settings of sexual abstinence, where contraception is not at stake. PCOS in adolescent girls and young women is, in essence, a postmenarcheal or pregestational central obesity syndrome (PCOS) that should not only be treated from a gynaecological but also from a hepatological perspective. OCs should be prescribed with more caution, and metabolic corrections should receive more attention. It is a shared responsibility to prevent that millions of non-obese adolescents with PCOS continue to become young women with both PCOS and MAFLD.
Footnotes
Contributors: FdZ wrote the Communication; MD researched data and reviewed/edited the text; LI contributed to data interpretation and reviewed/edited the text.
Funding: This study was supported by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, and by the Fondo Europeo de Desarrollo Regional (FEDER) (PI15/01078). This work is also supported by a grant PERIS-SLT006/17/00140 from the AQU, Generalitat de Catalunya, Spain.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Asfari MM, Sarmini MT, Baidoun F, et al. Association of non-alcoholic fatty liver disease and polycystic ovarian syndrome. BMJ Open Gastroenterol 2020;7:e000352. 10.1136/bmjgast-2019-000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202–9. 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 3.Ibáñez L, Oberfield SE, Witchel S, et al. An international Consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr 2017;88:371–95. 10.1159/000479371 [DOI] [PubMed] [Google Scholar]
- 4.de Zegher F, López-Bermejo A, Ibáñez L. Central obesity, faster maturation, and 'PCOS' in girls. Trends Endocrinol Metab 2018;29:815–8. 10.1016/j.tem.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 5.de Zegher F, Reinehr T, Malpique R, et al. Reduced prenatal weight gain and/or augmented postnatal weight gain precedes polycystic ovary syndrome in adolescent girls. Obesity 2017;25:1486–9. 10.1002/oby.21935 [DOI] [PubMed] [Google Scholar]
- 6.Ibáñez L, Díaz M, García-Beltrán C, et al. Toward a treatment normalizing ovulation rate in adolescent girls with polycystic ovary syndrome. J Endocr Soc 2020;4:bvaa032. 10.1210/jendso/bvaa032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peña AS, Witchel SF, Hoeger KM, et al. Adolescent polycystic ovary syndrome according to the International evidence-based guideline. BMC Med 2020;18:72. 10.1186/s12916-020-01516-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565–92. 10.1210/jc.2013-2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Beltran C, Cereijo R, Quesada-López T, et al. Reduced circulating levels of chemokine CXCL14 in adolescent girls with polycystic ovary syndrome: normalization after insulin sensitization. BMJ Open Diabetes Res Care 2020;8:e001035. 10.1136/bmjdrc-2019-001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibáñez L, Del Río L, Díaz M, et al. Normalizing ovulation rate by preferential reduction of hepato-visceral fat in adolescent girls with polycystic ovary syndrome. J Adolesc Health 2017;61:446–53. 10.1016/j.jadohealth.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 11.White U, Fitch MD, Beyl RA, et al. Adipose depot-specific effects of 16 weeks of pioglitazone on in vivo adipogenesis in women with obesity: a randomised controlled trial. Diabetologia 2021;64:159–67. 10.1007/s00125-020-05281-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Zegher F, Díaz M, Villarroya J, et al. The relative deficit of GDF15 in adolescent girls with PCOS can be changed into an abundance that reduces liver fat. Sci Rep 2021;11:7018. 10.1038/s41598-021-86317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Zegher F, Ibáñez L. Tackling NAFLD in adolescent PCOS: reducing liver fat to mimick weight loss. Hepatology 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2020-000574supp001.pdf (82.8KB, pdf)


