Abstract
Objectives
Little has been reported on the yield and characteristics of colorectal neoplasia detected by the two-sample faecal immunochemical test (FIT), particularly the difference between subjects with two-positive results on the two-sample FIT and those with one-positive results. We aimed to assess risk stratification among patients with positive two-sample FIT to prioritise colonoscopy.
Design
A retrospective cross-sectional study.
Setting
A single-centre, representative endoscopy clinic in Japan.
Participants
Consecutive patients who underwent colonoscopy were enrolled. Indications for colonoscopy included two-positive results on the two-sample FIT (FIT (+/+)), one-positive results on the two-sample FIT (FIT (+/−)), and other reasons (non-FIT group, including presence of symptoms, screening or surveillance).
Primary and secondary outcome measures
Primary outcomes were detection rates of colorectal cancers, including in situ (all cancers) and invasive cancers, based on the indications for colonoscopy. Secondary outcomes were cancer features, such as location, size, T stage and histological subtype.
Results
Of the 8724 patients, 264 underwent colonoscopy following FIT (+/+), 1018 following FIT (+/−) and 7442 for reasons other than positive FIT. Detection rates of all (and invasive) cancers in the FIT (+/+), FIT (+/−) and non-FIT groups were 12.1% (8.3%), 1.9% (0.3%) and 0.4% (0.2%), respectively. The cancer detection rates were much higher in the FIT (+/+) group than in the FIT (+/−) group, which in turn had higher rates than the non-FIT group. Moreover, the FIT (+/+) group showed more advanced T stages on tumour, node, metastasis (TNM) classification (Tis/T1/T2/T3/T4: 10/7/4/10/1) than the FIT (+/−) group (16/1/2/0/0, p<0.001).
Conclusions
Two-positive results for two-sample FIT showed a much higher yield for more advanced colorectal cancers than the one-positive result. High priority for diagnostic colonoscopy should be assigned to patients with two-positive-FIT results.
Keywords: gastrointestinal tumours, endoscopy, gastroenterology, preventive medicine, protocols & guidelines
Strengths and limitations of this study.
This study shows real-world data on two-sample faecal immunochemical test (FIT) in Japan, where two-sample FIT-based colorectal screening has been conducted for many years throughout the country.
This study investigated detection rates and features of colorectal cancers in patients with one-positive and two-positive results for the two-sample FIT.
This study also evaluated colorectal cancers in patients aged <50 years with two-positive results for the two-sample FIT.
The retrospective cross-sectional design at a single endoscopy clinic was a limitation.
We could not assess the FIT kit brand, faecal haemoglobin concentration or patients’ symptoms in detail.
Introduction
Colorectal cancer is one of the leading cancers worldwide, with 1.8 million new cases and 860 000 deaths annually, and has a significant impact on public health.1 Screening for colorectal cancer has shown significant effects on reducing the morbidity and mortality, and is also economical.2 There are several options for colorectal cancer screening, such as primary colonoscopy, sigmoidoscopy and stool-based tests.2 Among stool-based tests, the faecal immunochemical test (FIT) is now widely used instead of the guaiac faecal occult blood test, because of its higher accuracy and ease of handling.3 4 Although its accuracy is limited compared with that of primary colonoscopy, FIT is non-invasive and can conserve the resources required for colonoscopy and reduce human contact. Hence, FIT might facilitate the safety and prioritisation of patients during the COVID-19 pandemic.5
In Japan, the population-based annual two-sample FIT has been used for colorectal cancer screening for three decades since 1992.6 For implementation and effectiveness, the number of FIT samples required, the interval between two FITs and the FIT brands have been estimated.4 The two-sample method has been reported to have the best sensitivity and specificity for colorectal cancer.3 7 Some investigators also reported that the sensitivity for advanced neoplasia was higher by using the two-sample method than by the one-sample method.8 9
At least one-positive result is defined as a positive result in the two-sample FIT method.3 7–9 Few studies have investigated the yield and characteristics of neoplasia detected by two-sample FIT.8–10 In particular, little is known about the differences between the subjects with two-positive results in the two-sample FIT and those with one-positive result.11 12 In this study, we investigated the detection rates and features of invasive and in situ colorectal cancers detected by colonoscopy at our institution based on the indication for colonoscopy, focussing on the positivity patterns in the two-sample FIT.
Methods
Study design
This cross-sectional study included consecutive patients who underwent colonoscopy at the Toyoshima Endoscopy Clinic from April 2017 to August 2019. The indications for colonoscopy included a positive FIT result, evaluation of symptoms, screening, surveillance and treatment. Samples for FIT measurements were collected from two consecutive bowel movements. FITs were conducted at our clinic or at referral medical institutions. The FIT kits included both qualitative and quantitative types. The FIT kit brand and cut-off values for positivity were chosen by the institutes conducting the FIT. At our institute, FIT was performed using OC-Auto Sampling Bottle 3 (Eiken Chemical Co, Tokyo, Japan) with the threshold of 32 µg haemoglobin/g faeces. We divided the patients who were FIT positive into two categories: FIT (+/+) and FIT (+/−). We defined two-positive results for two samples as FIT (+/+) and one-positive result for two samples as FIT (+/−). Patients with a one-positive result for the one-sample FIT and positive FIT results with unknown number of positivity were excluded from this study; these findings are summarised in online supplemental table 1. The symptoms included abnormal bowel habits, haematochezia and abdominal pain. The surveillance included patients with a medical history of colorectal cancer, colorectal polyps or inflammatory bowel diseases. Treatment involved polypectomy and haemostasis. We excluded colonoscopies performed for treatment from this study. All indications other than positive FIT were divided into two categories: symptoms and screening+surveillance (asymptomatic).
bmjopen-2020-046055supp001.pdf (67.6KB, pdf)
Colonoscopy
Colonoscopies were performed by certified gastroenterologists. Olympus Elite 290 endoscope series (Olympus, Tokyo, Japan) was used.13 14 The clinical data were recorded on an electronic endoscopy reporting system, T-File System (STS Medic, Tokyo, Japan). The data included the patients’ baseline characteristics (age, sex and indication for colonoscopy) and tumour characteristics (location and size).
All colonoscopists were instructed to observe the entire colorectum, with a withdrawal time of 6 min or longer.15 16 Polyps 15 mm in size or smaller were resected at the time of the examination, and if the polyp was larger than 15 mm, or if invasive cancer was suspected, the patient was referred to the hospital.17
Colorectal cancer
Colorectal cancer was treated by endoscopic resection, surgery, chemotherapy and/or best supportive care. The patients received treatment at our clinic or at the hospital they were referred to. Colorectal cancer was diagnosed by histopathology. The location of cancer was determined by colonoscopy, surgery or CT. The location from the caecum to the transverse colon was defined as proximal colon. The size of the cancer was measured by colonoscopy, pathology or CT. The extent of tumour invasion was determined by pathology in combination with colonoscopy and CT findings. Tumours were classified according to the T stage of the Union for International Cancer Control (UICC) tumour, node, metastasis (TNM) classification.18 We included non-invasive carcinoma (carcinoma in situ) as a cancer.19 The histological subtype of the cancer was determined by histopathological evaluation of the resected or biopsy specimens. Four histological subtypes of adenocarcinomas (ie, well-differentiated, moderately differentiated, poorly differentiated and mucinous adenocarcinoma) were classified into two categories: well-differentiated+moderately differentiated and poorly differentiated+mucinous adenocarcinoma based on the prognosis of the subtypes.20
Outcomes
The main outcomes were detection rates of all colorectal cancers (including carcinomas in situ) and those of invasive colorectal cancers, based on the indication for colonoscopy. The secondary outcomes were the features of the cancers, such as location, size, T stage and histological subtype. We also divided the patients into two groups according to age: <50 years and ≥50 years, and analysed the detection rates and features of the cancers.
Statistical analysis
The detection rates were compared using the χ2 test or Fisher’s exact test. The characteristics of the cancer were compared using the Mann-Whitney U test, χ2 test or Fisher’s exact test. The association between the T stages of colorectal cancers and the number of positive results of FIT was analysed using Spearman’s rank correlation test. A two-sided p value <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS V.21.0 (IBM SPSS).
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or report, or dissemination plans of this research.
Results
Characteristics of the study patients
During the study period, 9321 patients underwent colonoscopy. Of them, we excluded 174 patients for undergoing colonoscopy for treatment, 136 patients for a one-positive result for the one-sample FIT and 287 patients for positive FIT results with unknown number of positivity. Finally, 8724 patients (age, mean±SD: 53.7±12.5 years; male sex: 49.9%) were eligible for this study. In all, 1282 colonoscopies were performed following positive FIT results. Of the positive FIT results, 264 were FIT (+/+) and 1018 were FIT (+/−). The remaining 7442 colonoscopies performed for indications other than positive FIT included 1826 for evaluation of the symptoms, and 5616 for screening (2394)+surveillance (3222), all these were performed without FIT investigation (table 1).
Table 1.
Study patients
| Total | Positive FIT | Other than positive FIT | |||||
| Total | FIT (+/+) | FIT (+/−) | Total | Symptom | Screening+surveillance | ||
| No. | 8724 | 1282 | 264 | 1018 | 7442 | 1826 | 5616 |
| Age, mean (SD), years | 53.7 (12.5) | 50.8 (12.4) | 52.7 (13.7) | 50.3 (11.9) | 54.2 (12.4) | 49.2 (13.4) | 55.8 (11.6) |
| Male, n (%) | 4350 (49.9) | 613 (47.8) | 144 (54.5) | 469 (46.1) | 3737 (50.2) | 722 (39.5) | 3015 (53.7) |
FIT (+/+) indicates two-positive results for the two-sample FIT. FIT (+/−) indicates a one-positive result for the two-sample FIT.
FIT, faecal immunochemical test.
Cancer detection rates based on the indication for colonoscopy
The detection rates of colorectal cancer based on the indication for colonoscopy are shown in table 2. All colorectal cancers (including carcinoma in situ) and invasive cancers were detected in 0.9% (79/8724 cases) and 0.4% (37 cases) of patients included in the study. The detection rates of all cancers and invasive cancers in FIT-positive patients were 4.0% (51/1282 cases) and 2.0% (25 cases), which were significantly higher than those detected patients who did not undergo FIT (0.4% for all cancers, 28/7442, p<0.001; 0.2% for invasive cancers, 12 cases, p<0.001).
Table 2.
Detection rates of colorectal cancer based on the indication for colonoscopy
| Total | Positive FIT | Other than positive FIT | |||||
| Total | FIT (+/+) | FIT (+/−) | Total | Symptom | Screening+surveillance | ||
| No. | 8724 | 1282 | 264 | 1018 | 7442 | 1826 | 5616 |
| All cancers (including in situ), n | 79 | 51 | 32 | 19 | 28 | 15 | 13 |
| Detection rate (%) | 0.9 | 4.0* | 12.1† | 1.9‡ | 0.4 | 0.8§ | 0.2 |
| Invasive cancers, n | 37 | 25 | 22 | 3 | 12 | 7 | 5 |
| Detection rate (%) | 0.4 | 2.0* | 8.3† | 0.3 | 0.2 | 0.4¶ | 0.1 |
FIT (+/+) indicates two-positive results for the two-sample FIT. FIT (+/−) indicates a one-positive result for the two-sample FIT
*P<0.001, positive FIT vs other than positive FIT.
†P<0.001, FIT (+/+) vs FIT (+/−).
‡P=0.02, FIT (+/−) vs symptom.
§P<0.001, symptom vs screening+surveillance.
¶P=0.01, symptom vs screening+surveillance.
FIT, faecal immunochemical test.
Among FIT-positive patients, the detection rate of all cancers in the FIT (+/+) group was very high at 12.1% (32/264 patients) and that in the FIT (+/−) group was 1.9% (19/1018 patients). Invasive cancers accounted for 8.3% (22 cases) in the FIT (+/+) group and 0.3% (3 cases) in the FIT (+/−) group. FIT (+/+) had significantly higher detection rates than FIT (+/−) (p<0.001 and p<0.001, respectively).
Among patients who did not undergo FIT, the cancer detection rates in symptomatic patients were significantly higher than in asymptomatic patients, in whom colonoscopy was performed for screening and surveillance (0.8% and 0.2% for all cancers, p<0.001; 0.4% and 0.1% for invasive cancers, p=0.01).
Additionally, the rate of cancer detection was significantly higher in patients with FIT (+/−) than in those with symptoms (1.9% and 0.8%, p=0.02, respectively).
The detection rate of benign adenomas was significantly higher in the FIT (+/+) group than in the FIT (+/−) group (61.4% vs 47.7%, p<0.001). The difference in the detection rates of adenomas between the FIT (+/+) group and the FIT (+/−) group was less remarkable than those of cancers.
Cancer detection rates based on age and FIT positivity groups
Table 3 shows the rates of detection for colorectal cancer based on age group and FIT positivity patterns.
Table 3.
Colorectal cancer detection rates based on age and FIT positivity groups
| Age ≥50 years | Age <50 years | |||||
| Total | FIT (+/+) | FIT (+/−) | Total | FIT (+/+) | FIT (+/−) | |
| No. | 630 | 140 | 490 | 652 | 124 | 528 |
| All cancers (including in situ), n | 35 | 18 | 17 | 16 | 14 | 2 |
| Detection rate (%) | 5.6* | 12.9† | 3.5‡ | 2.5 | 11.3† | 0.4 |
| Invasive cancers, n | 15 | 12 | 3 | 10 | 10 | 0 |
| Detection rate (%) | 2.4 | 8.6† | 0.6 | 1.5 | 8.1† | 0 |
FIT (+/+) indicates two-positive results for the two-sample FIT. FIT (+/−) indicates a one-positive result for the two-sample FIT.
*P=0.006, age ≥50 years vs age <50 years.
†P<0.001, FIT (+/+) vs FIT (+/−).
‡P<0.001, age ≥50 years vs age <50 years.
FIT, faecal immunochemical test.
In patients aged ≥50 years, the FIT (+/+) group showed the highest rate of cancer detection (12.9% for all cancers and 8.6% for invasive cancers). For the FIT (+/−) group, the respective rates were 3.5% and 0.6%, which were significantly lower than those in the FIT (+/+) group (p<0.001 and p<0.001), suggesting that early stage cancers are more predominant.
In the <50 years age group as well, the rate of cancer detection was higher in the FIT (+/+) group (11.3% for all cancers and 8.1% for invasive cancers). They were comparable to those in the ≥50 age group. However, the detection rate in the FIT (+/−) group was low (0.4% for all cancers and 0.0% for invasive cancers, respectively); moreover, the detection rate for all cancers was lower than that in the same FIT (+/−) group at age ≥50 years (p<0.001).
Features of the colorectal cancers
Table 4 shows the features of colorectal cancers based on the indication for colonoscopy. The colorectal cancers in patients with FIT (+/+) were larger than those in the FIT (+/−) patients (31.2 mm and 17.4 mm, p=0.004). The T stage of FIT (+/+) colorectal cancer was more advanced than that of the FIT (+/−) cancers (p<0.001). Although cancers were generally likely to be located in the distal colon or rectum, the cancers detected during screening and surveillance colonoscopies were predominantly in the proximal colon (proximal colon/distal colon and rectum: 7/6 for screening and surveillance vs 16/50 for the others, p=0.046).
Table 4.
Features of colorectal cancers based on indication for colonoscopy
| Total | Positive FIT | Other than positive FIT | |||
| FIT (+/+) | FIT (+/−) | Symptom | Screening+surveillance | ||
| No. | 79 | 32 | 19 | 15 | 13 |
| Location (proximal/distal), n | 23/56 | 10/22 | 5/14 | 1/14 | 7/6* |
| Size (SD), mm | 26.1 (19.9) | 31.2 (22.7)† | 17.4 (9.7) | 30.0 (20.4) | 22.0 (19.8) |
| T stage (Tis/T1/T2/T3/T4), n | 42/12/8/15/2 | 10/7/4/10/1‡ | 16/1/2/0/0 | 8/1/1/4/1 | 8/3/1/1/0 |
| Histological subtype (Well+Mod/Por+Muc), n | 74/4 | 30/2 | 19/0 | 13/1 | 12/1 |
FIT (+/+) indicates two-positive results for the two-sample FIT. FIT (+/−) indicates a one-positive result for the two-sample FIT. ‘Proximal’ indicates from the caecum to the transverse colon and ‘Distal’ indicates from the descending colon to the rectum. ‘T stage’ of the tumour was based on the Union for International Cancer Control (UICC) tumour, node, metastasis (TNM) Classification. ‘Well+Mod’ indicates well-differentiated and moderately-differentiated adenocarcinoma. ‘Por+Muc’ indicates poorly-differentiated and mucinous adenocarcinoma. One squamous cell carcinoma was excluded from this analysis.
*P=0.046, screening+surveillance vs the others.
†P=0.004, FIT (+/+) vs FIT (+/−).
‡P<0.001, FIT (+/+) vs FIT (+/−).
FIT, faecal immunochemical test.
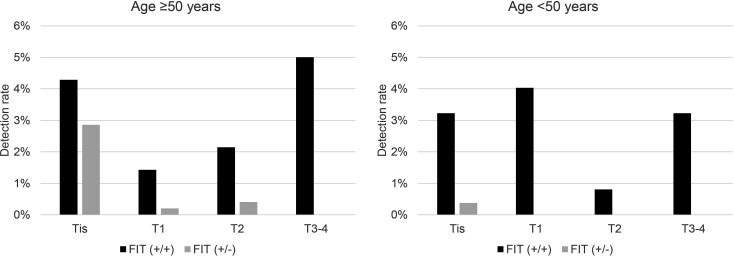
The cancer detection rates stratified by T stage based on age (≥50 and <50 years) and FIT positivity patterns (FIT (+/+) and FIT (+/−)) are shown in figure 1. FIT (+/+) patients had predominantly more advanced cancers than the FIT (+/−) patients (Tis/T1/T2/T3/T4 for FIT (+/+) vs FIT (+/−): 6/2/3/7/0 vs 14/1/2/0/0 in ≥50 years: p<0.001; 4/5/1/3/1 vs 2/0/0/0/0 in <50 years: p=0.10).
Figure 1.
Cancer detection rates stratified by T stages based on age and FIT positivity groups. T stage was classified according to the Union for International Cancer Control (UICC) tumour, node, metastasis (TNM) Classification. FIT (+/+) had a higher percentage of invasive cancers than in FIT (+/−). FIT, faecal immunochemical test. FIT (+/+) indicates two-positive results for the two-sample FIT. FIT (+/−) indicates a one-positive result for the two-sample FIT.
Patients with positive FIT overlapping symptoms or history of colorectal lesions
Because FIT was conducted annually as part of colorectal cancer screening system, independent of symptoms or history of colorectal lesions, the FIT groups included patients with accompanying symptoms or history of polypectomy. In the positive FIT groups, 31 patients were symptomatic and 19 had a history of colorectal lesions. In situ cancers were found in three patients with two-positive FIT results and haematochezia. No cancer was detected in patients with positive FIT results and history of colorectal lesions.
Discussion
This study found that cases with two-positive FIT results in two samples had significantly high rates of more advanced-stage colorectal cancers among all cases with positive FIT results. Although FIT has been an important screening tool for colorectal cancer and can help in selecting candidates for diagnostic colonoscopy, patients with two-positive results were shown to be at the highest risk for life-threatening cancer. In the face of the COVID-19 pandemic, when resources for colonoscopy are limited, FIT can stratify the patients’ risk. The study outcomes indicate that those with FIT (2+) should be given the highest priority for colonoscopy.
Although the sensitivity of FIT is superior to that of the guaiac test,3 4 21 22 it decreases considerably for early-stage cancer or high-grade dysplasia compared with direct colonoscopy. Morikawa et al23 compared the results of one-sample FIT and total colonoscopy in asymptomatic Japanese patients and reported that the sensitivity for invasive cancer was 78.3% (18/23) for Dukes’ stages C or D, 70.0% (7/10) for Dukes’ stage B and 52.8% (19/36) for Dukes’ stage A, and that for high-grade dysplasia was 32.7% (39/119). A similar study from Taiwan reported that the one-sample FIT showed a sensitivity of 100% (5/5) for cancers in T2-4 stages and 66.7% (12/18) for those of Tis or T1.24 The two-sample method was adopted to improve the sensitivity of FIT.3 7–9
A simulation analysis based on the results of colonoscopic screening in the Japanese subjects predicted markedly higher positive predictive values (PPVs) for invasive cancers in patients with two-positive-FIT results.25 The PPVs were estimated to be 1.7% and 26% for male subjects in their 50s with one-positive and two-positive FITs, respectively. PPVs could increase because of lower rates of false positivity in cases with two-positive FITs. The effect of improving the sensitivity could be higher for early stage lesions than for advanced cancers, for which the sensitivity is already high in single-sample FIT. One-positive result in two samples was predicted to detect predominantly earlier-stage lesions. Few investigators have reported the actual findings of colonoscopy comparing one-positive and two-positive FITs in two-sample FIT screening. Our result is compatible with a previous Canadian study, which reported PPVs for colorectal cancer to be 1% and 8%, in patients with one-positive and two-positive FITs, respectively.11 A recent study from the Netherlands suggested that two-positive FITs from two samples of the same bowel movement also have high cancer detection rates.12 The present study showed more advanced stages of colorectal cancers were predominant in patients with two-positive FITs.
The FIT (+/−) group showed higher rates of cancer detection than those in whom the colonoscopy was performed for evaluation of symptoms, screening or surveillance. This might be partly because the patients’ symptoms at our clinic were generally mild. Although further evaluations are necessary, FIT might be helpful in making decisions about performing colonoscopy in symptomatic patients26 or at the time of surveillance for patients after polypectomy.27
Even in patients <50 years of age, those with FIT (+/+) showed negligible rates of colorectal cancers, and in those with FIT (+/−), the rates were very low. Our results suggest that patients under 50 years of age with two-positive FITs might need to receive a higher priority for colonoscopy than those over 50 years with one-positive FIT. There is some discussion as to whether colorectal cancer screening should be started for subjects under 50 years of age, in whom the incidence of colorectal cancer is quite low but is increasing.28 If they were screened by two-positive results from two-sample FITs, the cost–benefit balance might be acceptable.
The present study cannot answer whether the two-sample FIT is superior to the one-sample quantitative FIT as a tool for organised colorectal cancer screening programme. The one-sample FIT is simpler and less expensive at the primary screening step. Careful and wide-range evaluations are necessary to select the best method, which should depend on the various conditions of the population. An advantage of the two-sample FIT is based on the considerable discordance in FIT results between samples collected even from the same person. The result can sometimes change from 1 ng/mL of the first sample to 1000 ng/mL of the second sample on the next day (cut-off: 100 ng/mL=20 µg Hb/g faeces, in the case of the OC Sensor method, Eiken Chemical). The two-sample FIT may have advantages over the one-sample FIT, even after adjusting the threshold, under some circumstances. On the other hand, for risk stratification, the appropriate secondary cut-off values for the one-sample quantitative FIT need to be decided for each FIT kit. The two-sample FIT, using the established threshold for each FIT kit, has two possible results: two-positive or one-positive result.
We propose that patients with two-positive results should be prioritised for colonoscopy, especially when resources are limited. In addition, given the COVID-19 pandemic, patients are likely to hesitate to undergo colonoscopy. In such cases, they should be strongly encouraged to receive colonoscopy with high priority. It may be useful to stratify patients with symptoms in a primary care setting. In the setting of one-sample FIT screening, our results suggest that secondary FIT administered to patients with a positive primary FIT result can help identify patients at higher risk for whom colonoscopy should not be delayed.
Limitations and strengths
This study has several limitations. First, it was conducted at a single endoscopy unit; hence, the results cannot be generalised. However, the indications and quality of colonoscopy as well as the criteria for diagnosis were well controlled. Two-sample FIT-based colorectal screening has been conducted for many years throughout Japan. Our results could well represent the regular practice of colorectal screening in Japan. Second, the FIT kit brands and cut-off values for positivity were various and unknown in many cases that were referred from other medical institutions for colonoscopy. The guidelines for colorectal cancer screening in Japan only recommend the two-sample FIT as standard, with no specific kits or cut-off values. As differences in FIT kit features and thresholds have been known to affect screening performance,29 these variations are certainly a limitation of our study. However, a notable difference in the results between two-positive and one-positive FIT groups shown in our study suggests a common trend irrespective of kits brand. Third, we did not assess the patients’ symptoms in detail, as cancer detection rates were low in symptomatic patients without FIT evaluation. However, the symptoms in our patients were generally mild. In populations with more serious symptoms, they could also be useful to urge early colonoscopy.30 Fourth, PPVs are highly associated with the expected prevalence of lesions in the study population. Our results are susceptible to bias due to heterogeneity among our patients, which is a limitation of our study design. However, based on our results, detection rates of more advanced tumours were excellent in patients with two-positive results, whereas they were generally quite low in the other positive groups. Further, this trend was observed irrespective of age groups. Although the results could change according to the study population, we assume that higher risk for advanced-stage lesions in two-positive FIT results is generally true for various populations.
In conclusion, two-positive results for two samples of FIT showed a much higher yield of advanced colorectal cancers than the one-positive result, which also showed a higher yield than colonoscopy performed in patients with symptoms or with an associated history. The highest priority for diagnostic colonoscopy should be assigned to patients with two-positive-FIT results.
STROBE statement
The authors have read the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist of items, and the manuscript was prepared and revised according to the STROBE statement checklist of items.
Supplementary Material
Footnotes
Contributors: Study concept and design: OT, YY and KKoike. Acquisition, analysis or interpretation of data: OT, YY, TN, SY, TY, KKurokawa, MO, RK and MT. Writing of the manuscript: OT. Critical revision of the manuscript for important intellectual content: YY, TN, SY, TY, KKurokawa, MO, RK, MT and KKoike. Statistical analysis: OT and YY. Supervision: KKoike. All authors approved the submitted version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement statement: Patients and/or the public were not involved in the design, or conduct, or report, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was conducted in accordance with ethical guidelines for medical studies in Japan. Written informed consent was obtained from patients at the time of colonoscopy to use their data for research purposes. The study design was described in a protocol prepared by Toyoshima Endoscopy Clinic and was approved by the Certificated Review Board, Hattori Clinic (certification no. CRB3180027) on 6 September 2019 (approval no. S1909-U06, registration no. UMIN000018541). We published this study’s protocol on our institute’s website (http://www.ichou.com), so that patients can opt out of the study. All clinical investigations were conducted according to the ethical guidelines of the Declaration of Helsinki.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Segnan N, Patnick J, von Karsa L, . European guidelines for quality assurance in colorectal cancer screening and diagnosis. 1st edn. Luxembourg: Publications Office of the European Union, 2010. [Google Scholar]
- 3.Park DI, Ryu S, Kim Y-H, et al. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol 2010;105:2017–25. 10.1038/ajg.2010.179 [DOI] [PubMed] [Google Scholar]
- 4.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task force on colorectal cancer. Gastroenterol 2017;152:1217–37. 10.1053/j.gastro.2016.08.053 [DOI] [PubMed] [Google Scholar]
- 5.Issaka RB, Somsouk M. Colorectal cancer screening and prevention in the COVID-19 Era. JAMA Health Forum 2020;1:e200588. 10.1001/jamahealthforum.2020.0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64:1637–49. 10.1136/gutjnl-2014-309086 [DOI] [PubMed] [Google Scholar]
- 7.Nakama H, Yamamoto M, Kamijo N, et al. Colonoscopic evaluation of immunochemical fecal occult blood test for detection of colorectal neoplasia. Hepatogastroenterol 1999;46:228–31. [PubMed] [Google Scholar]
- 8.Grazzini G, Visioli CB, Zorzi M, et al. Immunochemical faecal occult blood test: number of samples and positivity cutoff. What is the best strategy for colorectal cancer screening? Br J Cancer 2009;100:259–65. 10.1038/sj.bjc.6604864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Roon AHC, Wilschut JA, Hol L, et al. Diagnostic yield improves with collection of 2 samples in fecal immunochemical test screening without affecting attendance. Clin Gastroenterol Hepatol 2011;9:333–9. 10.1016/j.cgh.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 10.Cai S-R, Zhu H-H, Huang Y-Q, et al. Cost-effectiveness between double and single fecal immunochemical test(s) in a mass colorectal cancer screening. Biomed Res Int 2016;2016:6830713 10.1155/2016/6830713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moosavi S, Enns R, Gentile L, et al. Comparison of one versus two fecal immunochemical tests in the detection of colorectal neoplasia in a population-based colorectal cancer screening program. Can J Gastroenterol Hepatol 2016;2016:1–5. 10.1155/2016/5914048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wieten E, de Klerk CM, Lansdorp-Vogelaar I, et al. A quarter of participants with advanced neoplasia have discordant results from 2-sample fecal immunochemical tests for colorectal cancer screening. Clin Gastroenterol Hepatol 2020;18:1805–11. 10.1016/j.cgh.2019.09.024 [DOI] [PubMed] [Google Scholar]
- 13.Toyoshima O, Hata K, Yoshida S, et al. New-generation chromoendoscopy may increase confidence in the DISCARD2 study. Gut 2018;67:1742–3. 10.1136/gutjnl-2017-314999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyoshima O, Yoshida S, Nishizawa T, et al. CF290 for pancolonic chromoendoscopy improved sessile serrated polyp detection and procedure time: a propensity score-matching study. Endosc Int Open 2019;7:E987–93. 10.1055/a-0953-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533–41. 10.1056/NEJMoa055498 [DOI] [PubMed] [Google Scholar]
- 16.Toyoshima O, Nishizawa T, Sakitani K, et al. Colonoscopy using back brace support belt: a randomized, prospective trial. JGH Open 2020;4:441–5. 10.1002/jgh3.12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyoshima O, Nishizawa T, Yoshida S, et al. Expert endoscopists with high adenoma detection rates frequently detect diminutive adenomas in proximal colon. Endosc Int Open 2020;8:E775–82. 10.1055/a-1136-9971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brierley JD, Gospodarowicz MK, Wittekind C. UICC TNM classification of malignant tumours. 8th edn. Oxford, UK; Hoboken, NJ: John Wiley & Sons, Inc, 2017. [Google Scholar]
- 19.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47:251–5. 10.1136/gut.47.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara S, Watanabe T, Akahane T, et al. Tumor location is a prognostic factor in poorly differentiated adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma of the colon. Int J Colorectal Dis 2012;27:371–9. 10.1007/s00384-011-1343-0 [DOI] [PubMed] [Google Scholar]
- 21.Moss S, Mathews C, Day TJ, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut 2017;66:1631–44. 10.1136/gutjnl-2015-310691 [DOI] [PubMed] [Google Scholar]
- 22.Clark G, Strachan JA, Carey FA, et al. Transition to quantitative faecal immunochemical testing from guaiac faecal occult blood testing in a fully rolled-out population-based national bowel screening programme. Gut 2021;70:106–13. 10.1136/gutjnl-2019-320297 [DOI] [PubMed] [Google Scholar]
- 23.Morikawa T, Kato J, Yamaji Y, et al. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterol 2005;129:422–8. 10.1016/j.gastro.2005.05.056 [DOI] [PubMed] [Google Scholar]
- 24.Chiu H-M, Lee Y-C, Tu C-H, et al. Association between early stage colon neoplasms and false-negative results from the fecal immunochemical test. Clin Gastroenterol Hepatol 2013;11:832–8. 10.1016/j.cgh.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 25.Yamaji Y, Majima K, Wada R. Simulating positive predictive values in two-sample fecal immunochemical test screening. J Gastrointestinal Cancer Screen 2017;55:666–75. [Google Scholar]
- 26.Loveday C, Sud A, Jones ME, et al. Prioritisation by FIT to mitigate the impact of delays in the 2-week wait colorectal cancer referral pathway during the COVID-19 pandemic: a UK modelling study. Gut 2021;70:1053–60. 10.1136/gutjnl-2020-321650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task force on colorectal cancer. Gastroenterol 2012;143:844–57. 10.1053/j.gastro.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 28.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American cancer Society. CA Cancer J Clin 2018;68:250–81. 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- 29.Chapman CJ, Banerjea A, Humes DJ, et al. Choice of faecal immunochemical test matters: comparison of OC-Sensor and HM-JACKarc, in the assessment of patients at high risk of colorectal cancer. Clin Chem Lab Med 2020:cclm-2020-1170. 10.1515/cclm-2020-1170 [DOI] [PubMed] [Google Scholar]
- 30.NICE: National Institute for Health and Care Excellence . Suspected cancer: recognition and referral. NICE guideline [NG12] 2017. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-046055supp001.pdf (67.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request.