Abstract
Objective
The goal of treatment in ulcerative colitis (UC) is to induce and maintain remission. The addition of granulocyte and monocyte apheresis (GMA) to conventional therapy may be a promising therapeutic alternative. In this meta-analysis, we aimed to assess the efficacy and safety profile of GMA as an adjunctive therapy.
Design
Systematic review and meta-analysis.
Methods
We searched four databases (MEDLINE, Embase, Web of Science and Cochrane Central Register of Controlled Trials) for randomised or minimised controlled trials which discussed the impact of additional GMA therapy on clinical remission induction and clinical remission maintenance compared with conventional therapy alone. Primary outcomes were clinical remission induction and maintenance, secondary outcomes were adverse events (AEs) and steroid-sparing effect. ORs with 95% CIs were calculated. Trial Sequential Analyses were performed to adjusts for the risk of random errors in meta-analyses.
Results
A total of 11 studies were eligible for meta-analysis. GMA was clearly demonstrated to induce and maintain clinical remission more effectively than conventional therapy alone (598 patients: OR: 1.93, 95% CI 1.28 to 2.91, p=0.002, I2=0.0% for induction; 71 patients: OR: 8.34, 95% CI 2.64 to 26.32, p<0.001, I2=0.0% for maintenance). There was no statistically significant difference in the number of AEs (OR: 0.27, 95% CI 0.05 to 1.50, p=0.135, I2=84.2%).
Conclusion
GMA appears to be more effective as an adjunctive treatment in inducing and maintaining remission in patients with UC than conventional therapy alone.
PROSPERO registration number
CRD42019134050.
Keywords: inflammatory bowel disease, immunology, gastroenterology, haematology
Strengths and limitations of this study.
This is the first meta-analysis assessing the role of granulocyte and monocyte apheresis in clinical remission maintenance in ulcerative colitis.
Grading of Recommendations Assessment, Development and Evaluation approach was applied to appraise the certainty of evidence.
Our results are limited by the relatively low number of patients and the heterogenous reporting of adverse events.
To address the limitation by the number of included patients and to control both type I and type II errors, Trial Sequential Analyses have been performed.
Introduction
Ulcerative colitis (UC) is one of two major types of inflammatory bowel disease. The incidence of this disease varies from 9 to 20 cases per 100 000 person-years.1 UC is a lifelong illness that has a profound impact on patients. The primary goal of treatment is to achieve and maintain remission, thereby preventing colectomy and colorectal neoplasms and ensuring an acceptable quality of life.2 The choice of treatment for patients with UC is tied to the clinical and endoscopic severity of the disease along with the frequency and severity of relapses. Patients with no response to conventional therapies, especially to corticosteroids and immunosuppressive agents, are common candidates for biological treatments and/or surgery. However, both of these options are challenged by the high costs and incidence of side-effects and complications.
Patients with UC usually have a raised level of granulocytes, and in the case of an active disease, the mucosa of the bowel is infiltrated by a large number of granulocytes and macrophages. These leukocytes release degradative enzymes and proinflammatory cytokines, which lead to further inflammation of the bowel. Based on the hypothesis that a reduction of activated granulocytes and monocytes/macrophages may be beneficial, granulocyte and monocyte apheresis (GMA) was proposed as a strategy to promote remission in active UC.3 GMA is a novel non-pharmacological treatment tool for patients with UC, comprising an extra-corporeal absorptive circuit, which decreases inflammatory cytokines and upregulates regulatory T cells. Despite its high cost, GMA seems to have a good safety profile.3
However, data on the efficacy of GMA are still debated. The first studies published in Japan showed remission or response rates of up to 60%–80%.4–6 Sands et al reported a study with a large number of patients comparing GMA to a placebo, and they found no significant difference in terms of clinical response.7 This substantial difference between studies could be explained by the heterogeneity of patients’ characteristics, most probably by the varying severity and extent of the disease.
A large proportion of patients require long-term, high-dose steroid treatment, which often results in severe side-effects impairing patients’ quality of life. If addition of GMA can reduce the dose of corticosteroids, the risk of steroid-induced adverse events (AEs) could be minimised. Therefore, it is also essential to evaluate the steroid-sparing effects of GMA.8 Beyond the induction of remission and the impact on steroid requirement, the role of GMA in maintaining remission is unclear.9 The aim of our study was to assess the role of GMA in the induction and maintenance of clinical remission in UC and to evaluate the potential steroid-sparing effect of the therapy.
Methods
The meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.10 The study protocol was registered on the PROSPERO International Prospective Register of Systematic Reviews and adhered to it completely.
Search strategy
The systematic literature search was conducted by two independent review authors (SK and MF) in MEDLINE (via PubMed), EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science for studies published up to 5 March 2019. The search query in each database was based on the Population, Intervention, Comparison, Outcome (PICO) framework components combined with Boolean operators: (gma OR apheresis OR adsorption OR “cell separation” OR leukapher* OR leukopher* OR leukocytapher* OR leukocytopher* OR lymphapher* OR lymphopher* OR lymphocytopher* OR lymphocytapher*) AND (“inflammatory bowel disease” OR “ulcerative colitis”) AND (random*). Details of our search strategy and terms are presented in online supplemental material.
bmjopen-2020-042374supp001.pdf (714.9KB, pdf)
Eligibility criteria
General criteria
A randomised controlled trial (RCT) or minimised controlled trial (this type of sequence generation is considered to be nearly equivalent to being random)11; only full-text articles were included.
Specific criteria for clinical remission induction
Patients with active UC (Population1), standard therapy for remission induction and GMA as an adjunctive therapy (Intervention1) and standard therapy for remission induction (Comparison1); Outcomes1: clinical response rate and clinical remission rate (defined either by the Clinical Activity Index (CAI) or full Mayo score) and AEs.
Specific criteria for clinical remission maintenance
Patients with UC in clinical remission induced by GMA (Population2), standard therapy for remission maintenance and GMA as an adjunctive therapy (Intervention2) and standard therapy for remission maintenance (Comparison2); Outcomes2: rate of maintained remission (defined either by the CAI or full Mayo score) and AEs.
Outcome criteria for clinical remission and clinical response were defined individually by the eligible articles. These criteria are presented in table 1. Regarding safety, AEs reported by the individual article were used for the analyses in each case. No preliminary specification was made.
Table 1.
Characteristics of the studies included
| Clinical remission induction | |||||||||||
| Study name and setting | Cycles (n) | Randomisation* | Patients analysed (n) |
Patients achieving response | Patients achieving remission | Time of assessment | Outcome criteria | Concomitant medication | |||
| % | n | % | n | Remission | Response | ||||||
| Bresci et al 200818 single centre study |
5 | GMA | 40 | 92.5 | 37 | 72.5 | 29 | 5 weeks | CAI<6; EI <4 | CAI<6; EI>4 | Oral 5-ASA |
| Steroid | 40 | 65.0 | 26 | 50.0 | 20 | ||||||
| Doménechet al 201819 multi-centre study |
7 | GMA+steroid | 62 1 | 58.1 | 36 | 19.4 | 12 | 12 weeks | Mayo≤2 and no steroid use | Mayo score decrease ≥3 or at least 30% from baseline | Stable dose AZA and steroid were allowed if started before randomisation |
| Steroid | 61 2 | 49.2 | 30 | 18.0 | 11 | ||||||
| Eberhardson et al 201720 single centre study |
5 | GMA | 14 | 57.1 | 8 | 35.7 | 5 | 12 days | Mayo score ≤3 | Mayo score decrease ≥3 or at least 30% from baseline | Stable dose of steroid; 5-ASA and/or thiopurines were allowed |
| Sham | 8 3 | 37.5 | 3 | 12.5 | 1 | ||||||
| Hanai et al 200422 single centre study |
7 | GMA | 46 | 93.5 | 43 | 82.6 | 38 | 12 weeks | CAI≤4 | CAI had fallen, but still 4< | Steroids and/or 5-ASA |
| Steroid | 23 | 78.3 | 18 | 65.2 | 15 | ||||||
| Hanai et al 200821 multi-centre study |
11 | GMA | 35 | 80.0 | 28 | 74.3 | 26 | 12 weeks | CAI≤4 | CAI decreased by ≥5 points, but remained ≥5 | All patients were on salicylates and the majority were on low dose steroid as well |
| Steroid | 35 | 62.9 | 22 | 48.6 | 17 | ||||||
| Nakamura et al 200423 single centre study |
5 | GMA | 10 | N/A | N/A | 80.0 | 8 | 6 weeks | Based on CAI, but not specified | All patients received steroid; 5-ASA was unchanged | |
| No GMA | 10 | N/A | N/A | 20.0 | 2 | ||||||
| Sands et al 20087 A study multi-centre study | 10 | GMA | 31 | 67.7 | 21 | 16.1 | 5 | 12 weeks | Mayo score ≤2; 0–1 endoscopic score | Mayo score decrease ≥3 | One or more of the following: 5-ASA agents, steroid, 6-MP or AZA |
| Sham | 16 | 62.5 | 10 | 18.8 | 3 | ||||||
| Sands et al 20087 B study multi-centre study | 10 | GMA | 112 | 60.7 | 68 | 17.0 | 19 | 12 weeks | Mayo score ≤2; 0–1 endoscopic score | Mayo score decrease ≥3 | One or more of the following: 5-ASA, steroid, 6-MP or AZA |
| Sham | 56 | 50.0 | 28 | 10.7 | 6 | ||||||
| Sawada 200517 multi-centre study |
7 | GMA | 10 | 80.0 | 8 | 20.0 | 2 | 10 weeks | CAI=0 | CAI improved >3 | Except for steroid, other medications remained unchanged |
| Sham | 9 | 33.3 | 3 | 11.1 | 1 | ||||||
| Clinical remission maintenance | |||||||||||
| Study name | Cycles (n) | Randomisation | Patients analysed (n) | Patients in clinical remission at the end of the study | Close-out examination | Outcome criteria for remission | Concomitant medication | ||||
| % | n | ||||||||||
| Emmrich et al 200624 single centre study |
5 | GMA | 8 | 62.5 | 5 | 6 months | CAI≤4 | All patients were on steroid; 5-ASA was allowed; AZA given at baseline remained unchanged | |||
| No GMA | 5 | 20.0 | 1 | ||||||||
| Fukunaga et al 20129 single centre study |
12 | GMA | 10 | 40.0 | 4 | 12 months | CAI≤4 | Stable dose of AZA and steroids were allowed if started before randomisation | |||
| Sham | 11 | 9.1 | 1 | ||||||||
| Maiden et al 200825 single centre study |
5 | GMA | 18 | 77.8 | 14 | 6 months | CAI≤6 | Only 5-ASA or oral steroid | |||
| No GMA | 19 | 26.3 | 5 | ||||||||
*All patients received standard of care added to investigator/comparator. 1: one patient was excluded from analysis because of protocol deviations; 2: one patient was excluded from analysis because of protocol deviations; 3: one patient was excluded due to failure to return blood from the column; 4: minimisation may be implemented without a random element, and this is considered to be equivalent to being random.
5-ASA, 5-aminosalicylic acid; AZA, azathioprine; CAI, Clinical Activity Index; EI, Endoscopic Index; GMA, granulocyte and monocyte apheresis; 6-MP, 6-mercaptopurine; n, number.
The titles of the studies were screened based on predefined criteria, and the relevant studies were selected for abstract review. If the abstract was found to be appropriate, the full text of the article was studied. The decision to include a study in the meta-analysis was based on an independent assessment by the two review authors and eventually by consensus for resolution of any disagreements. Reference lists in included studies and reviews on this topic were searched for additional studies. Publications citing the included studies were also screened in the Google Scholar academic search engine.
Data extraction
The two investigators (SK and MF) reviewed the articles independently and extracted data into a standardised data collection form (discrepancies were resolved based on consensus). For the selected studies, characteristics were extracted, including publication year, country, number of centres, number of patients and study design. In addition, patient characteristics (age, sex and extent of disease), details of therapy (concomitant medication, volume of GMA, number of GMA cycles and duration of treatment) and main outcomes (number of patients with clinical improvement/response, number of patients achieving clinical remission, number of patients with maintained remission and number of AEs) were also extracted.
Risk of bias assessment
The Cochrane Risk of Bias Tool was used by the two independent investigators (SK and MF) to assess the quality of the studies included. Any disagreement was resolved based on consensus.12 Major domains of quality assessment were the following:
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias (early stopping, baseline imbalance, blocked randomisation with unblinded trials and imputation of intention-to-treat (ITT) analysis).
Statistical analysis
The effect measure of dichotomous variables was reported for each outcome as the OR with the related 95% CI.8 All tests were two-sided, and a p value <0.05 was considered statistically significant (except for heterogeneity, for which a p value <0.10 was considered significant). Weighted mean difference (WMD) was calculated for continuous variables. Values of OR, WMD and weights are presented in forest plots. The random-effects model was used to pool effect sizes. Heterogeneity was tested both by performing Cochran’s Q test and calculating Higgins’ I2 indicator.13 14 The Q statistics were computed as the squared deviations from the pooled effect of the weighted sum of individual study effects, with the weights being used in the pooling method. P values were obtained by comparing test statistics with a χ2 with k−1 df (where k was the number of studies). The I2 index corresponds to the percentage of the total variability across studies due to heterogeneity. A rough classification of its value based on the Cochrane Handbook for Systematic Reviews of Interventions is the following: low (0%–40%), moderate (30%–60%), substantial (50%–90%) and considerable (75%–100%).11 Subgroup analysis was performed as described in the study protocol if a sufficient number of studies was available. Funnel plots were used to test the presence of publication bias. A Trial Sequential Analysis (TSA 0.9.5.10.) was also performed for the randomised controlled studies to quantify the statistical reliability and to estimate the optimal information size. This methodology combines an information size with the threshold of statistical significance. All the statistical analyses were performed using Comprehensive Meta-Analysis (V.3, Biostat, Englewood, New Jersey, USA) and StataIC (V.15.1).
Quality of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used by the two independent review authors (SK and MF) to assess the certainty of evidence for each outcome.15 16 Disagreements were resolved by consensus.
Results
Search and selection
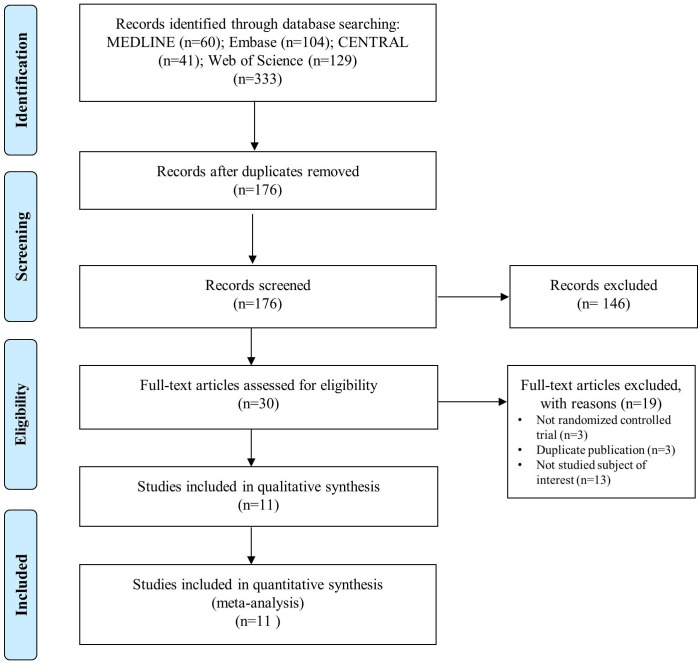
The search process is shown in figure 1. A total of 334 records were identified in the databases. After screening and assessment for eligibility, 11 full-text articles containing 1 minimised controlled trial and 11 RCTs were included for analysis. Eight studies provided data on patients with active UC, and three studies contained data on patients with UC in clinical remission.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart representing the process of the study search and selection. For more information, see www.prisma-statement.org. Source: Moher et al.10
Characteristics of the studies included
The characteristics of the studies included are presented in table 1. In the case of clinical remission induction, all the studies were RCTs, except for the one study with minimisation.17 A total of 598 participants (mean: 77, ranging from 19 to 168) were included in this meta-analysis: 350 patients received GMA, and 248 were in control groups. All the participants had active UC and were treated with Adacolumn.7 17–23 Four of these trials were sham-controlled. All the patients received standard of care added to the intervention/comparator and they did not receive any anti-tumour necrosis factor (anti-TNF) agent.
Both GMA and control were added to conventional treatment. In terms of main outcomes, the studies investigated the rate of clinical remission and clinical response. Investigators assessed the activity of UC with either the Mayo score or CAI. One study required steroid-free remission to regard cases as being in clinical remission.
In the case of clinical remission maintenance, all the studies were RCTs. A total of 71 participants (mean: 24, ranging from 13 to 37) were included in this meta-analysis: 36 patients received GMA, and 35 were in control groups. All the participants had UC in remission and were treated with Adacolumn or Cellsorba. One trial evaluated GMA versus sham control24 and two trials assessed GMA compared with standard therapy alone.9 25 Both GMA and sham control were added to conventional treatment. In terms of main outcome, the studies investigated the rate of clinical relapse.
Three studies also reported on the steroid-sparing effect of GMA.9 17 22
Risk of bias assessment
A summary of risk of bias assessment is shown in online supplemental figures 1 and 2. Three unblinded studies were at high risk of performance bias.19 22 25 Because of the nature of the intervention, four studies which lacked a description of the blinding process were interpreted as having a high risk of bias.18 21 23 24 As regards assessment blinding, two unblinded studies were judged to be at high risk of bias.19 25 Two studies were deemed as having a high risk of other bias; although they used ITT analysis, they considered subjects who left the study as a treatment failure that may lead to bias.7
Efficacy and safety of GMA in clinical remission induction
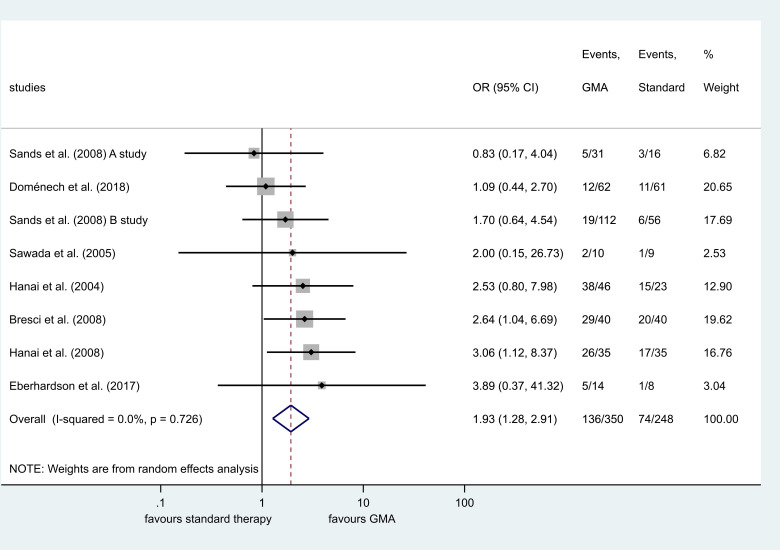
Seven randomised and one minimised controlled trial evaluated clinical remission induction. GMA therapy was associated with a better clinical response rate compared with the control group (OR=2.03, 95% CI 1.36 to 3.01, p<0.001, I2=8.4%) (online supplemental figure 3). Subgroup analysis of studies with assessment at 12 weeks also showed benefit (OR=1.67, 95% CI 1.12 to 2.49, p=0.012, I2=0.0%) (online supplemental figure 4). Patients undergoing GMA therapy had a higher remission rate compared with standard therapy without GMA (OR=1.93, 95% CI 1.28 to 2.91, p=0.002, I2=0.0%) (figure 2). Subgroup analyses were performed based on activity indices and number of GMA cycles. No difference was found between the two groups in studies assessing UC with the Mayo score (OR=1.34, 95% CI 0.74 to 2.43, p=0.334, I2=0.0%), but the remission induction was more successful in studies using CAI for assessment (OR=2.70, 95% CI 1.52 to 4.79, p=0.001, I2=0.0%) (online supplemental figure 5). A significant difference was found in studies using five cycles compared with the control (OR=2.78, 95% CI1.17 to 6.60, p=0.021, I2=0.0%) and more than five cycles compared with standard therapy alone (OR=1.73, 95% CI 1.08 to 2.77, p=0.022, I2=0.0%). There was no statistically significant difference in the number of AEs (p=0.135) (online supplemental figure 6). No statistically significant steroid-sparing effect was detected among patients with active UC (p=0.080). A list of reported AEs is presented in online supplemental table 1.
Figure 2.
Forest plot of studies comparing clinical remission induction between patients with and without granulocyte and monocyte apheresis (GMA) as adjunctive therapy. Black diamonds represent the individual studies effect and vertical lines show the corresponding 95% CIs. Size of the grey squares reflect on the weight of a particular study. The blue diamond is the overall or summary effect. The outer edges of the diamonds represent the CIs.
Efficacy and safety of GMA in clinical remission maintenance
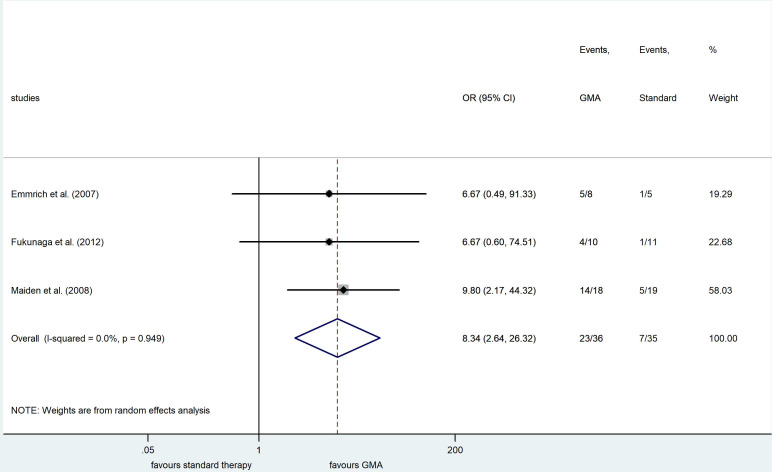
Three randomised clinical trials evaluated the clinical remission rate in remitting UC induced by GMA. Patients receiving GMA had a higher rate of clinical remission maintenance (OR=8.34, 95% CI 2.64 to 26.32, p<0.001, I2=0.0%) (figure 3). Due to lack of data, the rate of AEs could not be assessed in this population.
Figure 3.
Forest plot of studies comparing clinical remission maintenance between patients with and without granulocyte and monocyte apheresis (GMA) as adjunctive therapy. Black diamonds represent the individual studies effect and vertical lines show the corresponding 95% CIs. Size of the grey squares reflect on the weight of a particular study. The blue diamond is the overall or summary effect. The outer edges of the diamonds represent the CIs.
Trial Sequential Analysis
Based on the TSAs, the cumulative Z curve crossed the trial sequential significance boundary as regards clinical remission induction and clinical remission maintenance (power=80.0%; alpha=5.0%) (online supplemental figure 7). Moreover, clinical remission maintenance exceeded the required meta-analysis sample size, possibly suggesting that further clinical trials are not required. A TSA for AEs and steroid-sparing effects could not be carried out due to insufficient information size.
Certainty of evidence
The GRADE analysis rated the certainty of evidence for primary and secondary outcomes at a very low to low level. GRADE evidence profile is shown in online supplemental table 2.
Discussion
The main goal of care is to achieve and maintain remission of UC. This condition is usually treated by a step-up approach, during which treatments are switched or additional treatment is administered to optimise current therapy. There are several therapeutic agents to slow down the clinical activity of UC. Corticosteroids, 5-aminosalicylates, immunosuppressive agents and tumour necrosis alpha-inhibitors are commonly used, and new therapeutic targets, such as anti-adhesion molecules and anti-interleukins, are emerging. Despite these multiple therapeutic options, there is still a need to expand the scope of treatment methods due to possible development of intolerance or resistance to current treatments. After running out of treatment options, surgical therapy is frequently the last remaining option for patients. GMA is a novel non-pharmacological treatment option for active and remitting UC, by which activated granulocytes and monocytes are removed from the circulation. These cells may contribute to the pathogenesis of UC.
Guidelines describing the role of GMA in UC are in agreement on the potential beneficial effect and favourable safety profile. They also agree that there is insufficient evidence in this field of practice.26 27
To our knowledge, the first report on the efficacy of GMA in UC was published in Japan in 2001.28 This study found a considerably high remission rate with only five sessions of GMA in patients refractory to conventional drug therapy. Subsequent studies from the early 21st century had similar results.29–31 In 2008, Sands et al failed to prove a significant difference in clinical remission rate between GMA and a placebo on a relatively large population.7 However, this study was not free of attrition bias; a high proportion of patients were lost to follow-up. Three systematic reviews and meta-analyses have been conducted in this field so far.32–34 All of them have agreed on the benefit of GMA in clinical remission induction, and they pointed out the necessity for more trials with a rigorous and clear design to further narrow the focus on specific patient groups. These studies used one to three databases for a systematic search and selection.
In our current meta-analysis, a broader literature search was carried out, and the role of GMA in clinical remission maintenance was assessed. Our work supported the hypothesis that GMA improves the rates of clinical response and clinical remission in patients with UC. It should be noted that response and remission rates defined by symptom scores should be cautiously interpreted because they also include subjective elements, such as overall physician judgement on disease activity. A few recent retrospective and prospective studies have suggested certain prognostic factors in the therapeutic response.35–37 It seems that younger patients respond better to GMA therapy, whereas gender and smoking status showed no difference in response to treatment.35 Yokoyama et al found that shorter duration of UC and lower cumulative corticosteroid dose are associated with a higher efficacy rate.36 In their study, patients who received GMA treatment immediately after relapse were the best responders. It would be advisable to conduct further research to identify subgroups of UC where patients benefit the most from GMA.38
In the eligible studies, clinical remission induction was achieved in 29.8% without adjunctive GMA therapy. Based on our analysis, addition of GMA may be more effective for induction of remission in UC compared with conventional therapy alone (very low certainty). This result (OR=1.93, 95% CI 1.28 to 2.91, p=0.002, I2=0.0%) implies that patients receiving GMA have higher odds of achieving clinical remission by between 28% and 191%. To date, there is no uniformly accepted GMA regimen. There are RCTs to compare a ten-cycle and a five-cycle GMA regimen. Dignass et al and Ricart et al found similar remission rates between ten and five cycles (46% vs 36%, p=0.479; 35.7% vs 45.5%, p>0.05, respectively).38 39 The latter study also showed a steroid-sparing effect in the group receiving ten cycles of GMA. Sakuraba et al found that an improved remission rate is associated with intensive GMA (54.0% vs 71.2%, p=0.029 in five-cycle and ten-cycle regimens, respectively).40 In our meta-analysis, the number of GMA cycles varied among studies as well. We assessed the efficacy of GMA based on the two main regimens in previous trials. Both groups showed a benefit of adding GMA to the therapy compared with standard treatment alone.
Regarding the induction and maintenance of remission, our results relate to clinical remission. In 2015, based on insights from various clinical trials, a new consensus was made on appropriate evidence-based treatment targets.41 From then on, in addition to controlling symptoms, more objective markers came to the fore and endoscopic remission came to the spotlight. Only three of the articles analysed reported a comparison of endoscopic remission. Nakamura et al found that the improvement in endoscopic score was significantly higher in the group receiving GMA as well.23 Another study showed that the Rachmilewitz’s endoscopic index was significantly improved in patients treated with GMA compared with the control group.17 The third study reported similar endoscopic remission rate in the two groups (12% vs 11% in GMA and sham group, respectively; p=1.00).7 Data on objective inflammatory markers are also contradictory and insufficient.18 20 25 In light of this, there is a need for additional, high-quality RCTs that focus on current therapeutic targets.
We found no significant difference between the two groups as regards AEs (very low certainty). Further studies are called for to provide a higher level of evidence on this topic. They would be particularly important for specific subgroups where the safety profile is of paramount importance, such as in cytomegalovirus infection, adolescence and pregnancy. Clinical trials should also target these populations because fewer therapeutic options are available for them and the safety profile of GMA seems favourable compared with other treatments.
As with any therapeutic option, cost-effectiveness should also be considered. The cost of GMA is much higher compared with regular medication, such as corticosteroids, but GMA could be cost-effective in the long term. The use of GMA may reduce the cost of medical services, hospitalisation and surgery in the long term. Nevertheless, GMA’s safety profile is in sharp contrast to multiple severe AEs associated with conventional pharmacologicals and biologicals. According to recommendations, if UC flares up, treatment is usually escalated to biologicals. As GMA and biologicals are also likely to differ in terms of invasiveness, safety and efficacy, the question arises: which one may be more beneficial? However, there is currently no evidence of this. In this regard, limited data are available from recent studies suggesting that GMA may be beneficial in patients who no longer respond to biologicals.42–44
To our knowledge, this is the first meta-analysis to assess the role of GMA in UC remission maintenance. Our study showed that the addition of GMA enhances the proportion of patients who can maintain their remission (low certainty). Fukunaga et al and Emmrich et al enrolled clinically active patients with UC based on CAI.9 24 After successful induction therapy with the inclusion of GMA, patients achieving clinical remission were allocated to groups with and without GMA treatment for remission maintenance. Maiden et al enrolled patients with UC with a high level of faecal calprotectin, which is considered as a risk factor of relapse.25 Their results showed that faecal calprotectin level significantly decreases following five treatment session. This study differs from the previous two in the fact that they enrolled an asymptomatic population regardless of how patients achieved remission. The two studies recruiting patients with active UC detected no statistically significant difference between study arms in time to first relapse; however, it must be noted that in one of these studies, all the patients became steroid-free in the GMA group.9 Maiden et al found that time to first relapse was significantly higher in patients receiving GMA (99±73 days vs 161±44 days, p=0.0004). Despite our very promising results, these findings are limited by the amount of available data. More RCTs are necessary in this area to strengthen our results. This study has some potential limitations. Allowed concomitant therapies have differed among included studies; therefore, our estimates may have been subject to bias, as reflected by the grade of evidence (online supplemental table 2). Moreover, our funnel plots showed symmetry by visual assessment, but publication bias still cannot be ruled out because of the low number of included studies. Side-effects and safety data were not uniformly reported in most of the publications under analysis, according to the International Conference on Harmonisation-Good Clinical Practice guidelines.15 Therefore, our second main objective, the safety assessment of GMA, was only achieved to a limited extent. Furthermore, this result is strongly limited by the high heterogeneity of studies. The most likely source of this is the heterogeneous nature of concomitant treatment. All in all, GMA seems to be a reasonable therapeutic option, but finding its exact place to treat UC demands further research. A particularly promising area could be remission maintenance.
Conclusion
Implications for practice
The results support the hypothesis that patients with active UC have a better chance of clinical remission if GMA is administered as an adjunctive therapy. As regards the frequency of AEs, we found no statistically significant difference between the two groups. With regard to remission maintenance, GMA was identified as an effective alternative therapeutic option.
Implications for research
Further studies are required to select patients who may benefit the most from GMA therapy. Nevertheless, more randomised controlled studies are necessary to justify its role in remission induction. There is currently evidence available about induction and maintenance of clinical remission; however, the role of GMA concerning endoscopic and histological remission is currently unclear. If GMA is proven to be safe and effective, cost-effectiveness studies will also be worthwhile in the future.
Supplementary Material
Footnotes
Contributors: All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript. SK: drafting the manuscript, selection of studies, data extraction, risk of bias assessment; DN: statistical analysis, preparation of the standardised data collection sheet, drafting the manuscript; PH: substantial contribution in study design, critical revision of the content; MF: selection of studies, data extraction, risk of bias assessment, drafting the manuscript; ZS: participation in the design of the study and its coordination, critical revision of the manuscript; BE: provided revisions to the scientific content of the manuscript, substantial contribution in design of the work; BT: substantial contribution in study design, drafting the manuscript; PJH: preparation of the standardised data collection sheet, stylistic and grammatical revision of the manuscript, substantial contribution in study design; PS: expert in the field of gastroenterology, substantial contribution in study design and interpretation of data, preparation of study protocol and the first draft of the manuscript; HA: expert in the field of haematology, substantial contribution in study design and interpretation of data, preparation of study protocol and the first draft of the manuscript.
Funding: This research was not a company-initiated study. All costs were covered by the Economic Development and Innovation Operative Programme Grant (GINOP 2.3.2-15-2016-00048), the Grant of the Hungarian Science Foundation (FK 132834) and by Human Resources Development Operational Programme Grants (EFOP-3.6.2-16-2017-00006). Sponsors were not involved in the design, data collection, analysis, interpretation or preparation of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request (alizadeh.hussain@pte.hu).
References
- 1.Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–19. 10.1016/S0140-6736(12)60150-0 [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756–70. 10.1016/S0140-6736(16)32126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saniabadi AR, Tanaka T, Ohmori T, et al. Treating inflammatory bowel disease by adsorptive leucocytapheresis: a desire to treat without drugs. World J Gastroenterol 2014;20:9699–715. 10.3748/wjg.v20.i29.9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto T, Fukunaga K, Kamikozuru K, et al. Cytapheresis as a non-pharmacological therapy for inflammatory bowel disease. Transfus Med Hemother 2008;35:18–23. 10.1159/000111763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto T. The role of granulocyte and monocyte apheresis in inflammatory bowel disease. J Crohns Colitis 2013;7:e114. 10.1016/j.crohns.2012.07.016 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T, Umegae S, Matsumoto K. Long-term clinical impact of early introduction of granulocyte and monocyte adsorptive apheresis in new onset, moderately active, extensive ulcerative colitis. J Crohns Colitis 2012;6:750–5. 10.1016/j.crohns.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 7.Sands BE, Sandborn WJ, Feagan B, et al. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology 2008;135:400–9. 10.1053/j.gastro.2008.04.023 [DOI] [PubMed] [Google Scholar]
- 8.Bresci G, Parisi G, Mazzoni A, et al. Treatment of patients with acute ulcerative colitis: conventional corticosteroid therapy (MP) versus granulocytapheresis (GMA): a pilot study. Dig Liver Dis 2007;39:430–4. 10.1016/j.dld.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 9.Fukunaga K, Yokoyama Y, Kamokozuru K, et al. Adsorptive granulocyte/monocyte apheresis for the maintenance of remission in patients with ulcerative colitis: a prospective randomized, double blind, sham-controlled clinical trial. Gut Liver 2012;6:427–33. 10.5009/gnl.2012.6.4.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J, Green S. Cochrane Handbook for systematic reviews of interventions. Hoboken: Wiley, 2008. [Google Scholar]
- 12.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. 10.2307/3001666 [DOI] [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt A. International Council for Harmonisation E6(R2) addendum: Challenges of implementation. Perspect Clin Res 2017;8:162–6. 10.4103/picr.PICR_124_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schünemann H, Brożek J, Guyatt G AO. Grade Handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013, 2013. [Google Scholar]
- 17.Sawada K, Kusugami K, Suzuki Y, et al. Leukocytapheresis in ulcerative colitis: results of a multicenter double-blind prospective case-control study with sham apheresis as placebo treatment. Am J Gastroenterol 2005;100:1362–9. 10.1111/j.1572-0241.2005.41089.x [DOI] [PubMed] [Google Scholar]
- 18.Bresci G, Parisi G, Mazzoni A, et al. Granulocytapheresis versus methylprednisolone in patients with acute ulcerative colitis: 12-month follow up. J Gastroenterol Hepatol 2008;23:1678–82. 10.1111/j.1440-1746.2008.05595.x [DOI] [PubMed] [Google Scholar]
- 19.Domènech E, Panés J, Hinojosa J, et al. Addition of granulocyte/monocyte apheresis to oral prednisone for steroid-dependent ulcerative colitis: a randomized multicentre clinical trial. J Crohns Colitis 2018;12:687–94. 10.1093/ecco-jcc/jjy023 [DOI] [PubMed] [Google Scholar]
- 20.Eberhardson M, Karlén P, Linton L, et al. Randomised, double-blind, placebo-controlled trial of CCR9-targeted leukapheresis treatment of ulcerative colitis patients. J Crohns Colitis 2017;11:534–42. 10.1093/ecco-jcc/jjw196 [DOI] [PubMed] [Google Scholar]
- 21.Hanai H, Iida T, Takeuchi K, et al. Intensive granulocyte and monocyte adsorption versus intravenous prednisolone in patients with severe ulcerative colitis: an unblinded randomised multi-centre controlled study. Dig Liver Dis 2008;40:433–40. 10.1016/j.dld.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 22.Hanai H, Watanabe F, Yamada M, et al. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion 2004;70:36–44. 10.1159/000080079 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Kawagoe Y, Matsuda T, et al. Effect of granulocyte and monocyte adsorption apheresis on urinary albumin excretion and plasma endothelin-1 concentration in patients with active ulcerative colitis. Blood Purif 2004;22:499–504. 10.1159/000081896 [DOI] [PubMed] [Google Scholar]
- 24.Emmrich J, Petermann S, Nowak D, et al. Leukocytapheresis (LCAP) in the management of chronic active ulcerative colitis--results of a randomized pilot trial. Dig Dis Sci 2007;52:2044–53. 10.1007/s10620-006-9696-x [DOI] [PubMed] [Google Scholar]
- 25.Maiden L, Takeuchi K, Baur R, et al. Selective white cell apheresis reduces relapse rates in patients with IBD at significant risk of clinical relapse. Inflamm Bowel Dis 2008;14:1413–8. 10.1002/ibd.20505 [DOI] [PubMed] [Google Scholar]
- 26.Leukapheresis for inflammatory bowel disease: interventional procedures guidance, 2005. Available: https://www.nice.org.uk/guidance/ipg126
- 27.Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol 2018;53:305–53. 10.1007/s00535-018-1439-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimoyama T, Sawada K, Hiwatashi N, et al. Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher 2001;16:1–9. 10.1002/jca.1000 [DOI] [PubMed] [Google Scholar]
- 29.Kanke K, Nakano M, Hiraishi H, et al. Clinical evaluation of granulocyte/monocyte apheresis therapy for active ulcerative colitis. Dig Liver Dis 2004;36:811–7. 10.1016/j.dld.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Kim JS, Han DS, et al. [Granulocyte and monocyte adsorption apheresis in Korean conventional treatment-refractory patients with active ulcerative colitis: a prospective open-label multicenter study]. Korean J Gastroenterol 2005;45:34–44. [PubMed] [Google Scholar]
- 31.Shimoyama T, Sawada K, Hiwatashi N, et al. Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher 2001;16:1–9. 10.1002/jca.1000 [DOI] [PubMed] [Google Scholar]
- 32.Habermalz B, Sauerland S. Clinical effectiveness of selective granulocyte, monocyte adsorptive apheresis with the Adacolumn device in ulcerative colitis. Dig Dis Sci 2010;55:1421–8. 10.1007/s10620-009-0845-x [DOI] [PubMed] [Google Scholar]
- 33.Thanaraj S, Hamlin PJ, Ford AC. Systematic review: granulocyte/monocyte adsorptive apheresis for ulcerative colitis. Aliment Pharmacol Ther 2010;32:1297–306. 10.1111/j.1365-2036.2010.04490.x [DOI] [PubMed] [Google Scholar]
- 34.Yoshino T, Nakase H, Minami N, et al. Efficacy and safety of granulocyte and monocyte adsorption apheresis for ulcerative colitis: a meta-analysis. Dig Liver Dis 2014;46:219–26. 10.1016/j.dld.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 35.Imperiali G, Amato A, Terpin MM, et al. Granulocyte-Monocyte apheresis in steroid-dependent, Azathioprine-Intolerant/Resistant moderate ulcerative colitis: a prospective multicenter study. Gastroenterol Res Pract 2017;2017:9728324. 10.1155/2017/9728324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama Y, Kawai M, Fukunaga K, et al. Looking for predictive factors of clinical response to adsorptive granulocyte and monocyte apheresis in patients with ulcerative colitis: markers of response to GMA. BMC Gastroenterol 2013;13:27. 10.1186/1471-230X-13-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T, Sugiyama S, Goishi H, et al. Treatment of children and adolescents with ulcerative colitis by adsorptive depletion of myeloid lineage leucocytes as monotherapy or in combination with low dose prednisolone after failure of first-line medications. BMC Gastroenterol 2013;13:130. 10.1186/1471-230X-13-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dignass AU, Eriksson A, Kilander A, et al. Clinical trial: five or ten cycles of granulocyte-monocyte apheresis show equivalent efficacy and safety in ulcerative colitis. Aliment Pharmacol Ther 2010;31:1286–95. 10.1111/j.1365-2036.2010.04295.x [DOI] [PubMed] [Google Scholar]
- 39.Ricart E, Esteve M, Andreu M, et al. Evaluation of 5 versus 10 granulocyteaphaeresis sessions in steroid-dependent ulcerative colitis: a pilot, prospective, multicenter, randomized study. World J Gastroenterol 2007;13:2193–7. 10.3748/wjg.v13.i15.2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakuraba A, Motoya S, Watanabe K, et al. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol 2009;104:2990–5. 10.1038/ajg.2009.453 [DOI] [PubMed] [Google Scholar]
- 41.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for Treat-to-Target. Am J Gastroenterol 2015;110:1324–38. 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama Y, Sawada K, Aoyama N, et al. Efficacy of granulocyte and monocyte adsorptive apheresis in patients with inflammatory bowel disease showing lost response to infliximab. J Crohns Colitis 2020;14:1264–73. 10.1093/ecco-jcc/jjaa051 [DOI] [PubMed] [Google Scholar]
- 43.Nakamura M, Yamamura T, Maeda K, et al. Refractory ulcerative colitis improved by scheduled combination therapy of Vedolizumab and granulocyte and monocyte adsorptive apheresis. Intern Med 2020;59:3009–14. 10.2169/internalmedicine.5302-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez-Lago I, Sempere L, Gutiérrez A, et al. Granulocyte-monocyte apheresis: an alternative combination therapy after loss of response to anti-TNF agents in ulcerative colitis. Scand J Gastroenterol 2019;54:459–64. 10.1080/00365521.2019.1600715 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-042374supp001.pdf (714.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request (alizadeh.hussain@pte.hu).