Abstract
Introduction
The WHO recommends human papillomavirus (HPV) cervical self-sampling as an additional screening method and HPV DNA testing as an effective approach for the early detection of cervical cancer for women aged ≥30 years. This systematic review assesses end user’s values and preferences related to HPV self-sampling.
Methods
We searched four electronic databases (PubMed, Cumulative Index to Nursing and Allied Health Literature, Latin American and Caribbean Health Sciences Literature and Embase) using search terms for HPV and self-sampling to identify articles meeting inclusion criteria. A standardised data extraction form was used to capture study setting, population, sample size and results related to values and preferences.
Results
Of 1858 records retrieved, 72 studies among 52 114 participants published between 2002 and 2018 were included in this review. Almost all studies were cross-sectional surveys. Study populations included end users who were mainly adolescent girls and adult women. Ages ranged from 14 to 80 years. Most studies (57%) were conducted in high-income countries. Women generally found HPV self-sampling highly acceptable regardless of age, income or country of residence. Lack of self-confidence with collecting a reliable sample was the most commonly cited reason for preferring clinician-collected samples. Most women preferred home-based self-sampling to self-sampling at a clinic. The cervical swab was the most common and most accepted HPV DNA sampling device.
Conclusions
HPV self-sampling is generally a highly accepted method of cervical cancer screening for end users globally. End user preferences for self-sampling device, method and setting can inform the development of new and expanded interventions to increase HPV screening.
Keywords: systematic review, public health, cancer, other infection, disease, disorder, or injury
Key questions.
What is already known?
Self-sampling for human papillomavirus (HPV) DNA is effective at detecting cervical cancer and generally a highly acceptable form of cervical cancer screening to end users.
What are the new findings?
Self-sampled specimens are seen as acceptable in terms of ease of use, convenience, privacy, and physical and emotional comfort (including decreased embarrassment, anxiety, and pain).
Preferences related to self-sampling including sampling device (eg, swab, brush and tampon), method (eg, cervicovaginal and urine), setting (eg, home, clinic and community-based site) varied by region, subpopulation and age, though most end users found the process of self-sampling acceptable overall.
What do the new findings imply?
End user preferences for self-sampling method, device and setting can inform the development of new and expanded interventions to increase HPV screening.
Understanding end users’ preferences for self-sampling is critical for reaching the WHO global target of 70% HPV screening coverage by 2030.
Introduction
Cervical cancer is the fourth most common cancer among women globally, with the estimated 570 000 new cases yearly mostly affecting women between the ages of 30 and 49 years.1 This preventable cancer causes a woman to die every 2 min, with 90% of 311 000 annual deaths occurring in low-income and middle-income countries.2 Cervical cancer is caused by certain types of human papillomavirus (HPV), with HPV-16 and HPV-18 subtypes causing 70% of cervical cancers and precancerous cervical lesions.3 Evidence-based and cost-effective cervical cancer prevention includes primary prevention with HPV vaccination. Many successful national HPV vaccination programmes have been introduced in high-income and upper middle-income countries, but HPV vaccine introduction in low-income and middle-income countries (LMICs) remains insufficient.4 Therefore, secondary prevention with early, low-cost, high-quality HPV DNA screening is essential to reduce mortality and morbidity from cervical cancer in LMICs. Increasing access to and acceptability of cervical cancer screening is in line with the WHO’s strategy for cervical cancer elimination, which includes targets to achieve a global coverage of 90% vaccination, 70% screening and 90% treatment by 2030.5 6
HPV DNA testing is recommended by WHO, the US Preventive Services Task Force and Australian, US and European national screening programme guidelines as an effective approach for the early detection of cervical cancer for women ≥30 years.7–9 In addition, in areas with high endemic HIV, WHO recommends that sexually active girls and women, regardless of age, should be screened as soon as they have tested positive for HIV.7 Typically, per current recommendations, cervicovaginal samples for HPV DNA testing are collected by a clinician during a pelvic examination.8 9 However, cervicovaginal HPV DNA specimens can also be reliably collected by end users themselves—a process known as self-sampling.10–12 Generally, HPV DNA self-sampling is a highly acceptable method for the purposes of cervical cancer screening13 and has been associated with improved participation in cervical cancer screening studies in low-income and middle-income countries.14
Although previous reviews have found generally high acceptability of HPV DNA self-sampling, end users’ values and preferences regarding self-sampling method, device and setting have not been explored.13 15–17 The purpose of this systematic review is to synthesise the literature on end users’ values and preferences related to HPV DNA self-sampling for the purposes of cervical cancer screening. Values and preferences are defined according to Guyatt et al as the ‘collection of goals, expectations, predispositions, and beliefs that individuals have for certain decisions and their potential outcomes’.18 The values and preferences from this systematic review were used as part of the evidence base for the 2019 WHO Consolidated Guideline on Self-Care Interventions for Health.19 Understanding end users’ values and preferences related to self-sampling is critical because HPV testing must be acceptable to end users, in particular those who are underscreened and most vulnerable to cervical cancer. This review is further intended to support the WHO latest guidance on HPV screening, including to programme managers within the national cervical cancer prevention and control programmes.20
Methods
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.21
Inclusion criteria
We defined HPV self-sampling as a process where a client who wants to know whether they have an HPV infection uses a kit to collect a cervicovaginal or urine sample, which is then sent for analysis by a laboratory. While self-sampled anal specimens can be collected for the detection of HPV DNA, these samples are likely to be indicative of risk for anal cancer rather than cervical cancer. Therefore, we only included articles that focused on cervicovaginal and urine samples given our interest in cervical cancer. Collection devices include lavage, brush, swab, tampon or labial padette and may occur in any setting (eg, home, community and clinic). We defined HPV clinician sampling as any sampling method where a clinician or other healthcare provider obtains the cervicovaginal HPV DNA sample. HPV DNA testing does not provide a positive diagnosis for cervical cancer but rather identifies those end users at risk for developing cervical cancer in the future.
Studies were eligible for inclusion if they met the following criteria: (1) included participants who performed cervicovaginal or urine self-sampling for HPV DNA; (2) measured general acceptability or characteristics of acceptability regarding cervicovaginal or urine self-sampling for cervical cancer or preference for setting for sampling (eg, home, community or clinic setting); and (3) published in a peer-reviewed journal prior to the search date. Both qualitative and quantitative studies were included.
Search strategy and screening process
We searched four electronic databases (PubMed, the Cumulative Index to Nursing and Allied Health Literature, Latin American and Caribbean Health Sciences Literature and Embase) through 19 October 2018 using search terms for HPV and self-sampling. The full search strategy is described in a complementary review of the effectiveness of HPV self-sampling compared with clinician sampling22; the same search strategy was used for both the effectiveness review and this values and preferences review.
Articles were screened based on relevance to each topic. Ongoing randomised controlled trials (RCTs) were hand-searched for through ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform, the Pan African Clinical Trials Registry and the Australian New Zealand Clinical Trials Registry. We conducted secondary searching of included articles and relevant reviews for additional studies that met the inclusion criteria. After initial title–abstract screening, full-text articles were obtained of all potential studies. Two reviewers independently assessed all full-text articles for study inclusion eligibility and resolved differences through consensus.
Data extraction and analysis
Two reviewers independently used a standardised data abstraction form to capture information on location of study, study population, sample size and results related to values and preferences from each study. Differences in data abstraction were resolved through consensus, with a third reviewer as needed.
For studies that presented quantitative data related to values and preferences, two reviewers extracted measures of acceptability and satisfaction and their ratings from the results related to values and preferences.
For qualitative studies, we used an iterative approach to identify salient themes related to end users’ values and preferences. Codes were created based on recurring themes and applied to each article. One member of the study team read through each article and extracted themes inductively related to values and preferences for self-sampling. The study team met throughout the analysis process to discuss themes.
We also stratified findings by end users’ age, location of study, self-sampling device, setting and subpopulation (eg, women living with HIV, sexual and gender minorities) to examine differences in values and preferences by these characteristics.
A coding matrix was modified from the Evidence Project risk of bias tool23 and the Critical Appraisal Skills Program checklist for qualitative studies to facilitate data extraction related to quality assessment. The matrix included fields related measurement bias (eg, representativeness and missingness), sampling or selection bias and generalisability.
Patient and public involvement
Members of the WHO patient safety working group provided feedback on the review during conceptualisation and protocol development. Patients were involved in a global survey of values and preferences conducted to inform the WHO guideline on self-care interventions; they thus play a significant role in the overall recommendation informed by this review.
Results
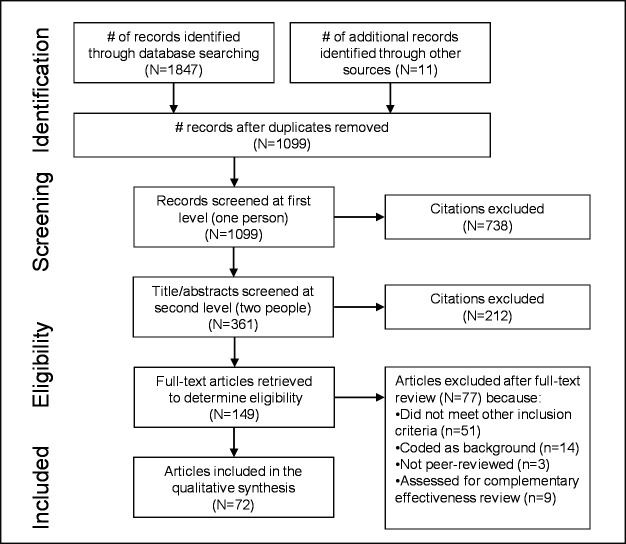
We retrieved 1847 records via electronic databases, with 11 additional citations reviewed from references listed in prior reviews, included studies, and hand-searches. After removing duplicate references and screening citations, we identified 72 unique studies presenting values and preferences data on HPV self-sampling (figure 1).
Figure 1.
PRISMA flow diagram of the different phases of a systematic review. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of included studies
Table 1 presents summary characteristics of the 72 included studies, with more details per included study in table 2. The 72 studies included a total of 52 114 participants; sample sizes for individual studies ranged from 17 to 9484. Articles were published between 2002 and 2018, with 50% published since 2015. Studies were conducted in a variety of countries, including all WHO regions and all World Bank country income classification categories (high, middle and low income). Of the included participants, 57% were from high-income countries. Almost all studies were cross-sectional surveys, though some study designs included in-depth interviews and/or focus group discussions.24–32 Several values and preferences studies were nested within larger studies (often RCTs).31–40
Table 1.
Summary description of included studies
| Characteristic | Articles* |
| Region | |
| Africa: Cameroon, Gambia, Ghana, Kenya, Malawi, Nigeria, South Africa and Uganda | 12 |
| Asia: China, Japan, Laos, Malaysia and Thailand | 12 |
| Europe: France, Germany, Italy, The Netherlands, Portugal, Sweden, Switzerland and UK | 13 |
| Americas: Argentina, Brazil, Canada, Chile, El Salvador, Guatemala, Mexico, Nicaragua, Peru, Puerto Rico and USA | 32 |
| Australia/Pacific | 2 |
| Multiple regions (India, Nicaragua and Uganda) | 1 |
| Populations (not mutually exclusive) | |
| Women from the general population | 63 |
| Never-screened or underscreened | 14 |
| Women living with HIV | 3 |
| Female sex workers | 1 |
| Women experiencing homelessnes | 1 |
| Adolescent girls and young women | 3 |
| Sexual and gender minorities | 2 |
| Study design | |
| Qualitative | 9 |
| Quantitative | 58 |
| Mixed methods or multimethod | 5 |
| Specimen collection device and methods | |
| Swab | 23 |
| Brush | 20 |
| Lavage | 3 |
| Labial padette | 1 |
| Tampon† | 5 |
| Multiple devices | 7 |
| Urine‡ | 2 |
| Unspecified | 11 |
| Setting for self-sampling | |
| Actual use at clinic | 33 |
| Actual use in workplace | 1 |
| Actual use at home | 21 |
| Actual use in community setting | 4 |
| Unspecified | 13 |
| Total | 72 |
*The number of studies within each category is not mutually exclusive.
†All five studies compared tampon with other self-sampling devices.32 34 49–51
‡One study included both urine self-sampling and self-sampling using a cervical swab.66
Table 2.
Characteristics of included studies
| First author, year | Self-sampling device(s) | Hypothetical or actual use | Setting | Location | Population | Sample size | Study design |
| Abuelo,71 2014 | Unspecified | Actual | Community | Peru: Iquitos | Women aged 30–45 years and daughters or other girls aged 10–13 years in the community. | 320 | Quantitative |
| Aiko,72 2017 | Brush | Actual | Home | Japan: Yokohama | Women aged 20–69 with abnormal cervical cytology. | 136 | Quantitative |
| Andersson,66 2018 | Swab; urine | Actual | Clinic | Sweden: Stockholm | Women who had undergone treatment of high-grade CIN2+. | 479 | Quantitative |
| Anhang,57 2005 | Swab | Actual | Clinic | USA: New York, New York | Women aged ≥25 years who have not had a Pap test in the last year from four publicly funded clinics. | 172 | Quantitative |
| Arrossi,69 2016 | Brush | Actual | Home | Argentina: Jujuy | Women aged ≥30 years. | 2616 | MM |
| Bansil,70 2014 | Brush | Actual | Clinic | India, Nicaragua and Uganda | Women enrolled in the Screening Technologies to Advance Rapid Testing Utility and Program Planning project. | 3863 | MM |
| Berner,73 2013 | Swab | Actual | Clinic | Cameroon | Women aged 24–69 years. | 243 | Quantitative |
| Bosgraaf,33 2014 | Brush; lavage | Actual | Home | Netherlands | Women aged 29–63 years who are part of PROHTECT 3B trial. | 9484 | Quantitative |
| Cadman, 67 2015 | Brush; swab | Hypothetical | n/a | UK: London | Hindu women aged 25–64 years. | 185 | MM |
| Castell,74 2014 | Lavage | Actual | Home | Germany: Hamburg, Hanover | Women aged 20–69 years within the German National Cohort. | 108 | Quantitative |
| Chen,75 2014 | Unspecified | Hypothetical | n/a | Taiwan | Women aged 18–65 years. | 500 | Quantitative |
| Crofts,65 2015 | Swab | Actual | Clinic | Cameroon: Tiko, Yaounde | Women aged 30–65 years. | 540 | Quantitative |
| Dareng,76 2015 | Unspecified | Hypothetical | n/a | Nigeria: Ondo, Abuja | Women aged ≥18 years | 600 | Quantitative |
| Dzuba,77 2002 | Swab | Actual | Clinic | Mexico | Women aged ≥20 years who use the Mexican Institute of Social Services, and who were involved in the parent study. | 1069 | Quantitative |
| Esber,41 2017 | Swab | Hypothetical | n/a | Malawi: Lilongwe | Women aged 15–39 years. | 824 | Quantitative |
| Fargnoli 24 2015 | Swab | Hypothetical | n/a | Switzerland: Geneva | Women aged 24–67 years who had not been screened for cervical cancer in the last 3 years. | 125 | Qualitative |
| Flores,78 2003 | Swab | Actual | Clinic | Mexico: Morelos | Women aged 20–80 years using cervical cancer screening services at 23 health units that make up the Mexican National Cervical Cancer Screening Program. | 7872 | Quantitative |
| Gottschlich,58 2017 | Swab | Actual | Home | Guatemala: Santiago Atitlan | Indigenous women aged 18–60 years. | 202 | Quantitative |
| Guan,53 2012 | Brush | Actual | Clinic | China: Xiangyuan County | Women aged 30–58 years from the national cervical cancer screening programme. | 174 | Quantitative |
| Hanley,79 2016 | Brush | Actual | Workplace | Japan: Sapporo | Working women aged 20–49 years attending their annual workplace check-up at Sapporo Industrial Health Management Screening Center. | 203 | Quantitative |
| Harper,34 2002a | Swab; tampon | Actual | Home | USA: New Hampshire | Women age 18–68 years attending Dartmouth teaching clinics who had been referred for a colposcopy for either abnormal Pap smear findings or abnormal cervical cancer examination findings. | 103 | Quantitative |
| Harper,49 2002b | Swab; tampon | Actual | Clinic | USA: New Hampshire | Women aged 18–68 years who attended an affiliated Dartmouth-Hitchcock teaching clinic with abnormal Pap smear findings or abnormal cervical cancer findings. | 103 | Quantitative |
| Howard,25 2009 | Unspecified | Hypothetical | n/a | Canada: Hamilton, Ontario: | Married or previously married women aged 35–65 years who were a part of one of the following: English-speaking Canadian with low SES, and Arabic, Cantonese, Dari, Somali or Latina women who recently immigrated to Canada. | 87 | Qualitative |
| Igidbashian,80 2011 | Brush; lavage | Actual | Clinic | Italy: Milan | Women aged 19–72 years undergoing an excisional procedure for CIN. | 205 | Quantitative |
| Ilangovan,42 2016 | Brush | Actual | Clinic | USA: Miami, Florida | Haitian and Latina women aged 30–65 years who reported not receiving a Pap test in the last 3 years. | 180 | Quantitative |
| Jones,81 2008 | Lavage | Actual | Home | The Netherlands | Women aged 30–60 years attending 3 obstetrics and gynaecology hospital departments in Veldhoven, Arnhem and Tilburg, in the Netherlands. | 104 | Quantitative |
| Kahn,64 2005 | Swab | Actual | Clinic | USA: Ohio | Sexually active adolescent girls and young women aged 14–21 years attending an urban, hospital-based teen health centre. | 121 | Quantitative |
| Katz,26 2017 | Brush; lavage; swab | Hypothetical | Home | USA: Appalachian Ohio | Rural women aged 30–65 years who had not received a Pap test in the past 3 years. | 15 | Qualitative |
| Ketelaars,82 2017 | Brush | Actual | Home | The Netherlands | Women aged 30–60 years old participating in the Dutch screening programme. | 2460 | Quantitative |
| Kilfoyle,43 2018 | Brush | Actual | Home | USA: North Carolina | Low-income women overdue for cervical cancer screening. | 227 | Quantitative |
| Lack,50 2005 | Swab; tampon | Actual | Community | Gambia | Rural women. | 377 | Quantitative |
| Leniz,44 2013 | Brush | Actual | Clinic | Chile: Santiago | Women aged 30–64 years who reported not receiving a Pap test in the last 3 years. | 1085 | Quantitative |
| Levinson,83 2013 | Unspecified | Actual | Community | Peru: Manchay, Iquitos | Women aged 30–45. | 643 | Quantitative |
| Lindell,45 2012 | Swab | Actual | Home | Sweden | Women aged 50–65 years who had not attended cervical cancer screening in the last 6 years. | 3618 | Quantitative |
| Mahomed,51 2014 | Brush; lavage; tampon | Hypothetical | n/a | South Africa | HIV-positive women aged >18 years from urban and rural HIV clinics. | 106 | Quantitative |
| Mao,35 2017 | Swab | Actual | Home | USA: Seattle, Washington | Women aged 21–65 years attending a University of Washington clinic. | 1769 | Quantitative |
| Ma’som,95 2016 | Brush | Actual | Clinic | Malaysia: Selangor | Women aged 18–60 years attending 5 government-run general practice clinics. | 839 | Quantitative |
| Maza,46 2018 | Unspecified | Actual | Home | El Salvador: Paracentral region | Women aged 30–69 years who reported not receiving a Pap test in the last 3 years or HPV test in the last 5 years and had not undergone procedures associated with cervical intraepithelial neoplasia. | 1867 | Quantitative |
| Mbatha,54 2017 | Brush; swab | Actual | Clinic | South Africa: KwaZulu-Natal | Sexually active adolescent girls and young women aged 16–22 years enrolled in government high school. | 91 | Quantitative |
| McLachlan,47 2018 | Unspecified | Actual | Clinic | Australia: Victoria | Women aged 27–74 years who were underscreened or never screened. | 40 | MM |
| Mitchell,61 2017 | Swab | Actual | Clinic | Uganda | HIV-positive women aged 30–69 years. | 87 | Quantitative |
| Modibbo,36 2017 | Swab | Actual | Home | Nigeria: Abuja | Women aged 30–65 years. | 400 | Quantitative |
| Molokwu,37 2018 | Swab | Actual | Home | USA: El Paso County, Texas | Women aged 30–65 years with no cervical screening in the last 3 years. | 202 | Quantitative |
| Moran,84 2017 | Brush | Actual | Home | Peru: Ventanilla Callao | Women enrolled in the HOPE programme. | 97 | Quantitative |
| Nobbenhuis,85 2002 | Lavage | Actual | Home | Netherlands: Rotterdam and Amsterdam | Women aged 20–63 years. | 56 | Quantitative |
| Obiri-Yeboah,86 2017 | Brush | Actual | Clinic | Ghana: Cape Coast | Every fifth woman aged ≥18 years attending a teaching hospital. | 194 | Quantitative |
| Oranratanaphan 94 2014 | Brush | Actual | Clinic | Thailand: Bangkok | Women aged 30–65 years. | 100 | Quantitative |
| Ortiz,87 2012 | Brush | Actual | Clinic | Puerto Rico | Women aged 18–34 years attending an OBGYN clinic for routine Pap test. | 100 | Quantitative |
| Penaranda,27 2014 | Unspecified | Hypothetical | n/a | US–Mexico border | Women aged 30–65 years. | 21 | Qualitative |
| Phoolcharoen,88 2018 | Brush | Actual | Clinic | Thailand: Bangkok | Women aged 30–70 years. | 250 | Quantitative |
| Pieters,29 2013 | Labial padette | Actual | Clinic | USA: Los Angeles, California | Homeless women aged 32–70 years. | 17 | Qualitative |
| Podolak,68 2017 | Unspecified | Hypothetical | n/a | Kenya: Nairobi | Women in counties near the University of Nairobi. | 107 | MM |
| Quincy,89 2012 | Brush; swab | Actual | Clinic | Nicaragua: Leon | Women aged 25–60 years. | 250 | Quantitative |
| Racey,38 2016 | Swab | Actual | Home | Canada: Ontario | Underscreened rural women aged 30–70 years. | 100 | Quantitative |
| Reisner,62 2018 | Swab | Actual | Clinic | USA: Boston, Massachusetts | Sexually active transgender males aged 21–64 years. | 150 | Quantitative |
| Reiter,63 2015 | Unspecified | Hypothetical | n/a | USA | Lesbian and bisexual women aged 21–26 years. | 418 | Quantitative |
| Rodrigues,60 2018 | Brush | Actual | Clinic | Brazil: Tapájos region | Women aged 17–75 years recruited from health centres; some women HIV+. | 153 | Quantitative |
| Rosenbaum,52 2014 | Brush | Actual | Clinic | El Salvador: Paracentral region | Women aged 30–49 years. | 518 | Quantitative |
| Silva,90 2017 | Unspecified | Actual | Unspecified | Portugal | Women aged 18–66 years. | 313 | Quantitative |
| Sy,30 2017 | Urine | Actual | Clinic | Federated States of Micronesia: Yap State | Women aged 21–65 years. | 217 | Qualitative |
| Szarewski,28 2009 | Lavage; swab | Hypothetical | n/a | UK: London | Muslim women aged 21–65 years recruited from the Noor Ul Islam Trust. | 28 | Qualitative |
| Tisci,55 2003 | Brush | Actual | Clinic | China | Women aged 35–60 years who may not have been screened for cervical neoplasia in the past 10 years. | 1560 | Quantitative |
| Trope,56 2013 | Swab | Actual | Community | Thailand: Roi-it Province | Women aged 25–60 years who had not been screened for cervical cancer in the past 5 years. | 431 | Quantitative |
| van de Wijgert,32 2006 | Swab; tampon | Actual | Clinic | South Africa: Cape Town | Sexually active women >18 years. | 450 | Qualitative |
| Vanderpool,48 2014 | Brush | Actual | Clinic | USA: Appalachian Kentucky | Women aged 30–64 years with no cervical screening in the last 3 years. | 31 | Quantitative |
| Waller,91 2006 | Swab | Actual | Clinic | UK: Central and West London | Women. | 902 | Quantitative |
| Winer,59 2016 | Swab | Actual | Home | USA: Hopi Reservation, Arizona | Hopi women aged 21–65 years. | 353 | Quantitative |
| Wong,39 2016 | Swab | Actual | Clinic | Hong Kong | Women aged 35–65 years. | 392 | Quantitative |
| Wong,40 2018 | Swab | Actual | Clinic | Hong Kong | Female sex workers aged ≥18 years. | 68 | Quantitative |
| Yoshida,92 2013 | Brush | Actual | Home | Laos | Women aged 18–80 years who worked in Khammouane Provincial Hospital, Xebangfay District Health Office or lived in Tung village in Sibounhouane subdistrict. | 290 | Quantitative |
| Zehbe,93 2011 | Swab | Actual | Home | Canada: Ontario | Women aged 25–59 years. | 49 | Quantitative |
| Zehbe,31 2017 | Swab | Hypothetical | n/a | Canada: Ontario | First Nation women aged ≥18 without formal postsecondary education. | 85 | Qualitative |
CIN, Cervical Intraepithelial Neoplasia; HPV, human papillomavirus; SES, socioeconomic status.
Study populations typically included adult end users. Participants ranged in age from 14 to 80 years. Fourteen studies specifically targeted women who were under/never screened for cervical cancer.24 26 30 31 37 38 41–48 Others selected participants from specific subgroups or vulnerable populations, including women from rural areas,26 38 41 48–56 racial/ethnic minorities,25 27 28 31 42 57–59 women living with HIV,51 60 61 sexual and gender minorities (lesbian and bisexual women, and transmales),62 63 adolescent girls and young women (AGYW),54 63 64 women of low socioeconomic status,25 43 57 65 and women experiencing homelessness.29
Nine studies employed a qualitative design, specifically in-depth interviews and focus group discussions, to explore women’s preferences related to HPV self-sampling.24–32 Of the nine qualitative studies, seven were conducted in high-income countries in Europe and North America24–29 31; some exclusively focused on vulnerable subpopulations such as ethnic minorities,25 27 28 31 rural women26 or women experiencing homeless.29 Six qualitative studies asked about hypothetical acceptability of HPV self-sampling. Two qualitative studies examined cervicovaginal self-sampling device preferences,26 28 two examined urine self-sampling30 66 and one examined self-sampling with a labial padette.29 Five studies employed a multimethod or mixed methods design.47 67–70 A total of 58 studies employed a quantitative approach: 47 cross-sectional studies,41–46 48 50–63 65 66 71–94 two pre–post surveys,64 95 eight RCTs33–40 and one prospective cohort.49 Quantitative studies examined a wide range of end users, including underscreened and vulnerable subpopulations such as women living HIV,51 60 61 transmales62 and female sex workers.40 Of these studies, the majority included women above age 30 years, but several focused exclusively on the values of preferences of AGYW.54 63 64 Most (n=67) quantitative studies focused on end users in high-income countries, while only 15 quantitative studies were conducted in low-income or lower middle-income countries.
Quality assessment of included studies
Common issues with study quality were sampling/selection bias, lack of validation of survey measures and lack of transferability of values and preferences results. Twenty-seven articles reported sampling or selection bias as a limitation of the study.26 27 29 35–37 39 40 43 46 52 54 57 59 61–64 66 70 83 87 89 91 93 95 96 Furthermore, one study acknowledged that participants self-selected to participate with the knowledge that self-collection would be a critical component of the study, so there may have been some selection bias towards acceptability of the self-test.43 One study assessed test–retest reliability and content validity of acceptability measures,64 but psychometric properties of acceptability were not conducted consistently across studies. Thirty-two articles reported that their results were not transferable to the study population or entire geographic population.25 26 29 35–40 46 53 57 59 62 64–67 70 72 75 77–81 87 88 91 93–95 Other study quality concerns identified in this review included: no consideration of study limitations, preference bias due to patient–physician interaction, recall bias, social desirability bias, low response rate and high loss to follow-up.
Values and preferences related to logistical aspects of self-sampling
Overall acceptability
End users generally found HPV self-sampling acceptable and/or expressed willingness to undergo future HPV testing using self-sampling.24 26 27 30 32 34 36–39 41 42 44 46 47 50 53 54 56 58 60–63 66–69 71 73 74 79–85 87–93 95 Self-sampling was generally highly acceptable regardless of age, income or country of residence.
Self-sampling versus clinician sampling
Across most of the studies asking end users’ preference regarding self-sampling versus clinician sampling for HPV DNA, most end users chose self-sampling.29 35 36 42 43 52 54 56 58 59 62 65 68 69 71 74 77–86 88 89 91–93 95 The most frequently cited reasons for preferring self-sampling over clinician sampling were less pain or physical discomfort,29 31 32 34–37 39 43 44 52 57 58 65 70 71 73 77–80 86 88–91 93–95 ease of use, convenience, ability to perform the test in private28 29 31–33 36 39 42 46 52 57 59 62 74 77 81 89 90 94 and less embarrassment or anxiety.27 28 31 33 35 37 39 40 46 52 59 65 69 70 72 73 77–80 89–91 94 95
In nine studies, end users preferred clinician sampling over self-sampling.25 28 30 53 57 64 73 76 87 Even among end users that preferred self-sampling, many lacked confidence in accuracy of self-sampled specimens.24 25 27–29 33 35–37 39–41 47 49 52–55 57 59 62–66 69 70 73 78–80 85 94 End users expressed greater confidence in the clinician’s ability to collect the specimen properly. These concerns were expressed in both high-income and low-income settings.
Setting for self-sampling
In 21 studies, end users self-sampled from their homes.26 33 35–38 43 45 46 49 58 59 69 72 74 81 82 84 85 92 93 When asked to compare self-sampling at home versus in a clinic, most end users expressed a preference to collect the sample at home.32 33 44 58 66 68 74 91 In high-income countries, samples collected at home were usually sent for processing using the postal service, whereas in some low-income and middle-income countries, self-sampled specimens were collected by community health workers conducting home visits for processing from the homes of participants rather than mail. For example, in the Netherlands, one participant expressed a preference for self-sampling at home because, ‘It can be done whenever you want, at ease and in a familiar environment’.81 In two studies, end users reported a clear preference for clinic-based testing with a physician over self-sampling at home.57 70 Reasons for preferring clinic-based testing were lack of confidence in ability to self-sample57 and ability to receive treatment if needed.70
Instructions and assistance
Other studies suggested strategies to increase end user’s confidence in performing self-sampling correctly.47 55 70 In clinical settings, end users cited the important role of a trusted healthcare provider in fielding questions and providing reassurance that sampling was done correctly.27 30 47 70 In addition, clear, step-by-step instructions with illustrations in the appropriate language were suggested as methods to facilitate self-sampling and improve end users’ confidence.62 68 79 81
Self-sampling devices
Studies examined different HPV self-sampling devices. The cervical swab was by far the most common and well accepted. Other devices tested included the lavage,74 81 85 cervical brush,42–44 48 52 53 55 60 69 70 72 79 82 84 86–88 92 94 95 tampon32 34 49–51 and labial padette.29 Acceptability of the labial padette was assessed among a sample of women experiencing homelessness in the USA. Most women found the labial padette to be an acceptable form of self-sampling and was preferred over clinician sampling using a cervical brush though some women were concerned about reliability of the self-sampled specimens.29
Several studies assessed women’s preferences for different self-sampling devices and acceptability was generally high for all devices.26 28 32–34 49–51 54 67 80 89 Acceptability of self-sampling using a cervical swab and tampon was compared with clinician sampling using a cervical brush in a sample of rural Gambian women.50 Though all three methods were found to acceptable by more than 70% of participants, there was a clear preference for self-sampling using the cervical swab (97.1%) over self-sampling using tampon (84.4%) and clinician sampling using cervical brush (72.4%). When shown examples of different devices, a sample of HIV-positive South African women rated the highest preference for the cervical brush (51%), while fewer women preferred the tampon (31%) or lavage sampler (18%).51
Urine self-sampling
Two studies examined acceptability of an alternative method for cervicovaginal HPV DNA self-sampling, urine self-sampling.30 66 In a sample of Micronesian women, 95% of participants said they were comfortable with the urine test compared with only 82% for the Pap smear. Despite higher ratings for urine self-sampling, more women preferred a clinician to perform a Pap smear (44.2%) over self-sampling (38.8%) because they valued the knowledge and expertise of the clinician.30 In a study of Swedish women with CIN, 85% of participants rated self-sampling procedures (urine and cervical swab) as easy, and 74% said they could see themselves self-sampling at home.66 The study did not compare women’s preferences for the swab versus urine self-sampling.
Values and preferences by end user characteristics
Adolescent girls and young women
Three studies focused solely on the values and preferences of AGYW.54 63 64 In an online survey of lesbian and bisexual AGYW in the USA, 51% participants reported willingness to self-sample at home. Willingness increased with age and concern about getting an HPV-related disease.63 The other two AGYW-focused studies assessed HPV self-sampling acceptability and preferences after participants performed self-sampling.54 64 Among South African students aged 16–22 years, 56% preferred self-sampling (56%) over clinician sampling.54 Among AGYW attending a teen health centre in the USA, acceptability for both self-sampling and clinician sampling was high. However, most participants expressed a preference for clinician sampling.63
Generational differences among end users
Twenty-five studies compared differences in sampling preferences by age group.24 31 33–35 39–41 49 52 55 59 65 67 69 72–74 76 77 79 82 89 91 95 Of these, 14 studies found no differences in preferences for self-sampling versus clinician sampling comparing older versus younger women.35 39 41 52 55 59 65 69 73 76 79 82 89 91 Studies presenting preferences or attitudes adjusted for sociodemographic and/or behavioural characteristics were more likely to find no difference in preference for self-sampling by age group.69 76 89 Five studies reported greater preference for self-sampling among younger age groups compared with older age groups.31 33 34 72 95 In one study, Dutch young women cited reasons of decreased embarrassment, time/effort investment and a ‘do-it-yourself’ attitude more often than older women when describing why they preferred HPV self-sampling over clinician sampling.33 Other studies found that older women were more likely to prefer self-sampling over clinician sampling.24 40 42 63 A qualitative study of women in Switzerland found that younger women were used to visiting a gynaecologist and did not see the necessity of changing this practice, while some older women, less used to regular gynaecological appointments, were more in favour of self-sampling, especially if they previously had had a negative experience with pelvic examinations.24
Geographic region
Only one study compared end users’ preferences across different regions and countries.70 The study found that women in rural Uttar Pradesh (93.1%), Hyderabad (95.5%), Uganda (64.5%) and Nicaragua (50.0%) preferred self-sampling over clinician sampling. More than half of surveyed respondents in each site reported self-sampling as ‘easy’ with the lowest percentage in Hyderabad (53.6%) and the highest percentage in Uganda (89.7%). Women across all study sites preferred to conduct self-sampling in a clinic setting rather than at home so that a provider could clarify questions or do the test if needed.
Women living with HIV
Three studies examined HPV sampling preferences among end users living with HIV.51 60 61 One study surveyed a sample of HIV-positive and HIV-negative women from northern Brazil, reporting that 87% of women found cervicovaginal sampling for HPV DNA acceptable. A greater proportion of HIV-positive women reported acceptability (97%) than HIV-negative women (84%).60 A study of HIV-positive urban and rural women in South Africa compared preferences for cervical brush, tampon and lavage sampling devices.51 HIV-positive rural women preferred the cervical brush over other sampling devices, whereas urban women preferred the tampon over other devices.
Sexual and gender minorities
Two studies conducted in the USA examined preferences among sexual and gender minorities. As mentioned previously, more than half of lesbian and bisexual AGYW surveyed reported willingness to self-sample at home.63 Among transmales who had undergone both self-sampling and clinician sampling for HPV DNA, 90% expressed a preference for self-sampling citing privacy, ease and self-empowerment as reasons for their preference. Some participants cited feelings of gender dysphoria and difficulty positioning the body or swab for self-sampling as challenges of self-sampling.62
Discussion
In this systematic review, we found general consensus that self-sampling is a highly acceptable method for HPV testing, regardless of study location and sampling method, device, setting or participant demographics. Our findings align with previous reviews, such as one by Nelson et al, which reported a pooled estimate prevalence of women preferring self-sampling of 59% (95% CI 48% to 69%).13 Similarly, high levels of acceptability were consistent among vulnerable and underscreened subpopulations. Women generally preferred self-sampling over clinician sampling, citing ease of use, privacy, convenience and physical and emotional comfort as major reasons for their preference. Women who preferred clinician sampling expressed concerns about the reliability of self-sampled specimens, which was also the most common reason women reported for disliking self-sampling in the review by Nelson et al.13 Another common reason identified uniquely in this review is end user concern that they would not get face time with a clinician if needed. Counselling prior to the invitation to self-sample as well as clear instructions and availability of trustworthy clinical staff to assist with self-sampling could remedy some of these concerns.
A number of studies examined acceptability of self-sampling in different settings, such as the clinic, home or community. More than half of home-based self-sampling studies took place in high-income countries, since a quick and reliable postal system was necessary for mailing the samples for processing. In some low-income and middle-income countries, community health workers collected specimens self-sampled by participants for processing during home-visits. Across studies, the home was regarded as a highly acceptable and convenient setting for self-sampling. However, women expressed that they would prefer clinic-based sampling if home-based sampling meant they would not have access to a healthcare provider. Ensuring that women have access to a healthcare provider to answer questions about screening or a potential positive result is an important consideration for the expansion of home-based testing programmes.
The most common device for self-sampling was the cervical swab. Among studies comparing acceptability of different self-sampling devices, end users preferred the cervical swab over other devices such as the lavage or cervical brush, but this preference could be due to greater familiarity with cervical swab. Studies examining less commonly used devices (eg, tampon and labial padette) and methods (eg, urine sampling) generally found high acceptability; however, only 15 studies examined these alternative self-sampling devices and methods. Therefore, preference for self-sampling device and method is an important area for further study.
This review identifies a need for further research examining women’s preferences in low-income countries, which bear a disproportionate burden of new cervical cancer cases. Only 3 of the 72 studies included in this review were conducted in low-income countries, leaving a critical gap in understanding of end users’ preferences in these settings. HPV DNA self-sampling is a promising screening method that, when offered to women in low-resource settings, can address barriers that create inequalities in access to cervical cancer screening.97
Strengths and limitations
This is one of the first studies to systematically review both qualitative and quantitative literature on values and preferences for HPV self-sampling for cervical cancer screening. We synthesised end users’ values and preferences related to the setting of sample collection and the device used to collect the sample. We also examined values and preferences for HPV self-sampling among vulnerable populations.
Findings from this review should be viewed in light of its limitations. We did not include conference abstracts or grey literature in this review, so our findings may not fully represent of the full body of literature on values and preferences for HPV self-sampling. Given the diversity of measures used to assess values and preferences, it is difficult to make comparisons across studies and therefore difficult to determine the specific aspects of self-sampling that clients find acceptable and whether these hold true for clients in different age groups, geographic regions and socioeconomic status.
Conclusion
WHO strongly supports inclusion of self-sampling for HPV testing as an additional approach to sampling in cervical cancer screening programmes where HPV tests are used. HPV self-sampling is generally a highly accepted method of cervical cancer screening for women across the world. This systematic review of values and preferences builds on previous reviews on self-sampling acceptability. Understanding women’s preferences for HPV self-sampling is a critical factor for expanding choice, coverage and uptake of cervical cancer screening. This will be critical to reach WHO’s target of 70% cervical cancer screening coverage by 2030.6 Screening with high uptake will expedite reductions and will be necessary to eliminate cervical cancer in countries with the highest burden.98
Acknowledgments
We would like to thank Laura Ferguson for her contribution to conceptualising this review and Patricia Garcia, Gina Ogilvie, and Nandi Siegfried for their thoughtful comments on the protocol. We would also like to thank our Johns Hopkins Bloomberg School of Public Health graduate students (Melissa Camila Alamo, Chergai Gao Rittenberg, Priyanka Mysore and Laura Graf) for their assistance in searching trial registries, screening citations and extracting data. We gratefully acknowledge the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP).
Footnotes
Handling editor: Stephanie M Topp
Contributors: MN conceptualised the study. CEK and PTY designed the protocol. PTY ran the search and oversaw screening, data extraction and assessment of bias and quality of reporting. HN drafted the manuscript. All authors reviewed the draft, provided critical review, and read and approved the final manuscript. The corresponding author, as guarantor, accepts full responsibility for the finished article, has access to any data and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
The named authors alone are responsible for the views expressed in this publication and do not necessarily represent the decisions or the policies of the World Health Organization (WHO) nor the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP).
Funding: This work was funded by the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP).
Disclaimer: The funder was involved in the study design but played no part in the decision to submit the article for publication, nor in the collection, analysis, and interpretation of data. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on request. All data relevant to the study are included in the article or uploaded as supplementary information. Extracted data are available on request to the corresponding author.
References
- 1.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020;8:e191–203. 10.1016/S2214-109X(19)30482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS/WHO . Cervical cancer and HIV – addressing linkages and common inequalities to save women’s lives. PCB thematic session, 2020. [Google Scholar]
- 3.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 4.Gallagher KE, LaMontagne DS, Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine 2018;36:4761–7. 10.1016/j.vaccine.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 5.Canfell K, Kim JJ, Brisson M, et al. Mortality impact of achieving who cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020;395:591–603. 10.1016/S0140-6736(20)30157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . Draft: global strategy towards the elimination of cervical cancer as a public health problem, 2020. Available: https://www.who.int/activities/a-global-strategy-for-elimination-of-cervical-cancer
- 7.World Health Organization . Guidelines for screening and treatment of precancerous lesions for cervical cancer prevention, 2013. Available: https://www.who.int/reproductivehealth/publications/cancers/screening_and_treatment_of_precancerous_lesions/en/ [PubMed]
- 8.von Karsa L, Arbyn M, De Vuyst H, et al. European guidelines for quality assurance in cervical cancer screening. summary of the supplements on HPV screening and vaccination. Papillomavirus Res 2015;1:22–31. 10.1016/j.pvr.2015.06.006 [DOI] [Google Scholar]
- 9.Moyer VA. Screening for cervical cancer: U.S. preventive services Task force recommendation statement. Ann Intern Med 2012;156:W312:880–91. 10.7326/0003-4819-156-12-201206190-00424 [DOI] [PubMed] [Google Scholar]
- 10.Snijders PJF, Verhoef VMJ, Arbyn M, et al. High-Risk HPV testing on self-sampled versus clinician-collected specimens: a review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer 2013;132:2223–36. 10.1002/ijc.27790 [DOI] [PubMed] [Google Scholar]
- 11.Petignat P, Faltin DL, Bruchim I, et al. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol 2007;105:530–5. 10.1016/j.ygyno.2007.01.023 [DOI] [PubMed] [Google Scholar]
- 12.Arbyn M, Verdoodt F, Snijders PJF, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol 2014;15:172–83. 10.1016/S1470-2045(13)70570-9 [DOI] [PubMed] [Google Scholar]
- 13.Nelson EJ, Maynard BR, Loux T, et al. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect 2017;93:56–61. 10.1136/sextrans-2016-052609 [DOI] [PubMed] [Google Scholar]
- 14.Racey CS, Withrow DR, Gesink D. Self-Collected HPV testing improves participation in cervical cancer screening: a systematic review and meta-analysis. Can J Public Health 2013;104:e159–66. 10.1007/BF03405681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong JPH, Vahabi M, Miholjcic J, et al. Knowledge of HPV/cervical cancer and acceptability of HPV self-sampling among women living with HIV: a scoping review. Curr Oncol 2018;25:73–82. 10.3747/co.25.3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S, Palmer C, Bik EM, et al. Self-Sampling for human papillomavirus testing: increased cervical cancer screening participation and incorporation in international screening programs. Front Public Health 2018;6:77. 10.3389/fpubh.2018.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: focused literature review. Can Fam Physician 2017;63:597–601. [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt G, Jaeschke R, Wilson MC. What is evidence-based medicine? : Guyatt G, Rennie D, Meade MO, . Users' guides to the medical literature: a manual for evidence-based clinical practice. 3rd Ed. New York, NY: McGraw Hill Education, 2015: 7–14. [Google Scholar]
- 19.WHO . Consolidated guidelines on self-care interventions for health: sexual and reproductive health and rights, 2019. Available: https://www.who.int/reproductivehealth/publications/self-care-interventions/en/ [PubMed]
- 20.World Health Organization . Introducing and scaling up testing for human papillomavirus as part of a comprehensive programme for evention and control of cervical cancer: a step-by-step-guide. Geneva, 2020. [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh PT, Kennedy CE, de Vuyst H, et al. Self-Sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Glob Health 2019;4:e001351. 10.1136/bmjgh-2018-001351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy CE, Fonner VA, Armstrong KA, et al. The evidence project risk of bias tool: assessing study rigor for both randomized and non-randomized intervention studies. Syst Rev 2019;8:3. 10.1186/s13643-018-0925-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fargnoli V, Petignat P, Burton-Jeangros C. To what extent will women accept HPV self-sampling for cervical cancer screening? A qualitative study conducted in Switzerland. Int J Womens Health 2015;7:883–8. 10.2147/IJWH.S90772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard M, Lytwyn A, Lohfeld L, et al. Barriers to acceptance of self-sampling for human papillomavirus across ethnolinguistic groups of women. Can J Public Health 2009;100:365–9. 10.1007/BF03405272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz ML, Zimmermann BJ, Moore D, et al. Perspectives from health-care providers and women about completing human papillomavirus (HPV) self-testing at home. Women Health 2017;57:1161–77. 10.1080/03630242.2016.1243608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penaranda E, Molokwu J, Hernandez I, et al. Attitudes toward self-sampling for cervical cancer screening among primary care Attendees living on the US-Mexico border. South Med J 2014;107:426–32. 10.14423/SMJ.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 28.Szarewski A, Cadman L, Ashdown-Barr L, et al. Exploring the acceptability of two self-sampling devices for human papillomavirus testing in the cervical screening context: a qualitative study of Muslim women in London. J Med Screen 2009;16:193–8. 10.1258/jms.2009.009069 [DOI] [PubMed] [Google Scholar]
- 29.Pieters HC, Wiley DJ. Decision-Making about cervical cancer screening methods by homeless women. J Natl Black Nurses Assoc 2013;24:9–15. [PubMed] [Google Scholar]
- 30.Sy AU, Hernandez BY, Tareg A, et al. Acceptability and feasibility of a community based participatory research project comparing cytology and urine HPV DNA testing for cervical cancer screening in YAP, Federated states of Micronesia. Cancer Epidemiol 2017;50:283–8. 10.1016/j.canep.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zehbe I, Wakewich P, King A-D, et al. Self-Administered versus provider-directed sampling in the Anishinaabek cervical cancer screening study (ACCSS): a qualitative investigation with Canadian first nations women. BMJ Open 2017;7:e017384. 10.1136/bmjopen-2017-017384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Wijgert J, Altini L, Jones H, et al. Two methods of self-sampling compared to clinician sampling to detect reproductive tract infections in Gugulethu, South Africa. Sex Transm Dis 2006;33:516–23. 10.1097/01.olq.0000204671.62529.1f [DOI] [PubMed] [Google Scholar]
- 33.Bosgraaf RP, Ketelaars PJW, Verhoef VMJ, et al. Reasons for non-attendance to cervical screening and preferences for HPV self-sampling in Dutch women. Prev Med 2014;64:108–13. 10.1016/j.ypmed.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 34.Harper DM, Noll WW, Belloni DR, et al. Randomized clinical trial of PCR-determined human papillomavirus detection methods: self-sampling versus clinician-directed--biologic concordance and women's preferences. Am J Obstet Gynecol 2002;186:365–73. 10.1067/mob.2002.121076 [DOI] [PubMed] [Google Scholar]
- 35.Mao C, Kulasingam SL, Whitham HK, et al. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health 2017;26:609–15. 10.1089/jwh.2016.5965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modibbo F, Iregbu KC, Okuma J, et al. Randomized trial evaluating self-sampling for HPV DNA based tests for cervical cancer screening in Nigeria. Infect Agent Cancer 2017;12:11. 10.1186/s13027-017-0123-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molokwu JC, Penaranda E, Dwivedi A, et al. Effect of educational intervention on self-sampling acceptability and follow-up PAPS in border Dwelling Hispanic females. J Low Genit Tract Dis 2018;22:295–301. 10.1097/LGT.0000000000000424 [DOI] [PubMed] [Google Scholar]
- 38.Racey CS, Gesink DC, Burchell AN, et al. Randomized intervention of self-collected sampling for human papillomavirus testing in Under-Screened rural women: uptake of screening and acceptability. J Womens Health 2016;25:489–97. 10.1089/jwh.2015.5348 [DOI] [PubMed] [Google Scholar]
- 39.Wong ELY, Chan PKS, Chor JSY, et al. Evaluation of the impact of human papillomavirus DNA self-sampling on the uptake of cervical cancer screening. Cancer Nurs 2016;39:E1–11. 10.1097/NCC.0000000000000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong ELY, Cheung AWL, Huang F, et al. Can human papillomavirus DNA self-sampling be an acceptable and reliable option for cervical cancer screening in female sex workers? Cancer Nurs 2018;41:45–52. 10.1097/NCC.0000000000000462 [DOI] [PubMed] [Google Scholar]
- 41.Esber A, McRee A-L, Norris Turner A, et al. Factors influencing Malawian women's willingness to self-collect samples for human papillomavirus testing. J Fam Plann Reprod Health Care 2017;43:135–41. 10.1136/jfprhc-2015-101305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilangovan K, Kobetz E, Koru-Sengul T, et al. Acceptability and feasibility of human papilloma virus self-sampling for cervical cancer screening. J Womens Health 2016;25:944–51. 10.1089/jwh.2015.5469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilfoyle KA, Des Marais AC, Ngo MA, et al. Preference for human papillomavirus self-collection and Papanicolaou: survey of underscreened women in North Carolina. J Low Genit Tract Dis 2018;22:302–10. 10.1097/LGT.0000000000000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Léniz J, Barriga MI, Lagos M, et al. Hpv vaginal self-sampling among women non-adherent to Papanicolaou screening in Chile. Salud Publica Mex 2013;55:162–9. 10.1590/S0036-36342013000200007 [DOI] [PubMed] [Google Scholar]
- 45.Lindell M, Sanner K, Wikström I, et al. Self-sampling of vaginal fluid and high-risk human papillomavirus testing in women aged 50 years or older not attending Papanicolaou smear screening. Bjog-Int J Obstet Gy 2012;119:245–8. 10.1111/j.1471-0528.2011.03147.x [DOI] [PubMed] [Google Scholar]
- 46.Maza M, Melendez M, Masch R, et al. Acceptability of self-sampling and human papillomavirus testing among non-attenders of cervical cancer screening programs in El Salvador. Prev Med 2018;114:149–55. 10.1016/j.ypmed.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 47.McLachlan E, Anderson S, Hawkes D, et al. Completing the cervical screening pathway: factors that facilitate the increase of self-collection uptake among under-screened and never-screened women, an Australian pilot study. Curr Oncol 2018;25:17–26. 10.3747/co.25.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanderpool RC, Jones MG, Stradtman LR, et al. Self-collecting a cervico-vaginal specimen for cervical cancer screening: an exploratory study of acceptability among medically underserved women in rural Appalachia. Gynecol Oncol 2014;132 Suppl 1:S21–5. 10.1016/j.ygyno.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harper DM, Raymond M, Noll WW, et al. Tampon samplings with longer cervicovaginal cell exposures are equivalent to two consecutive swabs for the detection of high-risk human papillomavirus. Sex Transm Dis 2002;29:628–36. 10.1097/00007435-200211000-00003 [DOI] [PubMed] [Google Scholar]
- 50.Lack N, West B, Jeffries D, et al. Comparison of non-invasive sampling methods for detection of HPV in rural African women. Sex Transm Infect 2005;81:239–41. 10.1136/sti.2004.010413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahomed K, Evans D, Sauls C, et al. Human papillomavirus (HPV) testing on self-collected specimens: perceptions among HIV positive women attending rural and urban clinics in South Africa. Pan Afr Med J 2014;17:189. 10.11604/pamj.2014.17.189.3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbaum AJ, Gage JC, Alfaro KM, et al. Acceptability of self-collected versus provider-collected sampling for HPV DNA testing among women in rural El Salvador. Int J Gynaecol Obstet 2014;126:156–60. 10.1016/j.ijgo.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 53.Guan Y, Castle PE, Wang S, et al. A cross-sectional study on the acceptability of self-collection for HPV testing among women in rural China. Sex Transm Infect 2012;88:490–4. 10.1136/sextrans-2012-050477 [DOI] [PubMed] [Google Scholar]
- 54.Mbatha JN, Galappaththi-Arachchige HN, Mtshali A, et al. Self-Sampling for human papillomavirus testing among rural young women of KwaZulu-Natal, South Africa. BMC Res Notes 2017;10:702. 10.1186/s13104-017-3045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tisci S, Shen YH, Fife D, et al. Patient acceptance of self-sampling for human papillomavirus in rural China. J Low Genit Tract Dis 2003;7:107–16. 10.1097/00128360-200304000-00007 [DOI] [PubMed] [Google Scholar]
- 56.Trope LA, Chumworathayi B, Blumenthal PD. Feasibility of community-based careHPV for cervical cancer prevention in rural Thailand. J Low Genit Tract Dis 2013;17:315–9. 10.1097/LGT.0b013e31826b7b70 [DOI] [PubMed] [Google Scholar]
- 57.Anhang R, Nelson JA, Telerant R, et al. Acceptability of self-collection of specimens for HPV DNA testing in an urban population. J Womens Health 2005;14:721–8. 10.1089/jwh.2005.14.721 [DOI] [PubMed] [Google Scholar]
- 58.Gottschlich A, Rivera-Andrade A, Grajeda E, et al. Acceptability of human papillomavirus self-sampling for cervical cancer screening in an Indigenous community in Guatemala. J Glob Oncol 2017;3:444–54. 10.1200/JGO.2016.005629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winer RL, Gonzales AA, Noonan CJ, et al. Assessing acceptability of self-sampling kits, prevalence, and risk factors for human papillomavirus infection in American Indian women. J Community Health 2016;41:1049–61. 10.1007/s10900-016-0189-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodrigues LLS, Morgado MG, Sahasrabuddhe VV, et al. Cervico-Vaginal self-collection in HIV-infected and uninfected women from Tapajós region, Amazon, Brazil: high acceptability, hrHPV diversity and risk factors. Gynecol Oncol 2018;151:102–10. 10.1016/j.ygyno.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell SM, Pedersen HN, Eng Stime E, Stime EE, et al. Self-Collection based HPV testing for cervical cancer screening among women living with HIV in Uganda: a descriptive analysis of knowledge, intentions to screen and factors associated with HPV positivity. BMC Womens Health 2017;17:4. 10.1186/s12905-016-0360-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reisner SL, Deutsch MB, Peitzmeier SM, et al. Test performance and acceptability of self- versus provider-collected swabs for high-risk HPV DNA testing in female-to-male trans masculine patients. PLoS One 2018;13:e0190172. 10.1371/journal.pone.0190172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reiter PL, McRee A-L. Cervical cancer screening (PAP testing) behaviours and acceptability of human papillomavirus self-testing among lesbian and bisexual women aged 21-26 years in the USA. J Fam Plann Reprod Health Care 2015;41:259–64. 10.1136/jfprhc-2014-101004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kahn JA, Bernstein DI, Rosenthal SL, et al. Acceptability of human papillomavirus self testing in female adolescents. Sex Transm Infect 2005;81:408–14. 10.1136/sti.2004.012047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crofts V, Flahault E, Tebeu P-M, et al. Education efforts may contribute to wider acceptance of human papillomavirus self-sampling. Int J Womens Health 2015;7:149–54. 10.2147/IJWH.S56307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersson S, Belkić K, Mints M, et al. Is self-sampling to test for high-risk papillomavirus an acceptable option among women who have been treated for high-grade cervical intraepithelial neoplasia? PLoS One 2018;13:e0199038–18. 10.1371/journal.pone.0199038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cadman L, Ashdown-Barr L, Waller J, et al. Attitudes towards cytology and human papillomavirus self-sample collection for cervical screening among Hindu women in London, UK: a mixed methods study. J Fam Plann Reprod Health Care 2015;41:38–47. 10.1136/jfprhc-2013-100705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Podolak I, Kisia C, Omosa-Manyonyi G, et al. Using a multimethod approach to develop implementation strategies for a cervical self-sampling program in Kenya. BMC Health Serv Res 2017;17:222. 10.1186/s12913-017-2160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arrossi S, Ramos S, Straw C, et al. Hpv testing: a mixed-method approach to understand why women prefer self-collection in a middle-income country. BMC Public Health 2016;16:832. 10.1186/s12889-016-3474-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bansil P, Wittet S, Lim JL, et al. Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health 2014;14:596. 10.1186/1471-2458-14-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abuelo CE, Levinson KL, Salmeron J, et al. The Peru cervical cancer screening study (PERCAPS): the design and implementation of a mother/daughter screen, treat, and vaccinate program in the Peruvian jungle. J Community Health 2014;39:409–15. 10.1007/s10900-013-9786-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aiko K-Y, Yoko M, Saito OM, et al. Accuracy of self-collected human papillomavirus samples from Japanese women with abnormal cervical cytology. J Obstet Gynaecol Res 2017;43:710–7. 10.1111/jog.13258 [DOI] [PubMed] [Google Scholar]
- 73.Berner A, Hassel SB, Tebeu P-M, et al. Human papillomavirus self-sampling in Cameroon: women's uncertainties over the reliability of the method are barriers to acceptance. J Low Genit Tract Dis 2013;17:235–41. 10.1097/LGT.0b013e31826b7b51 [DOI] [PubMed] [Google Scholar]
- 74.Castell S, Krause G, Schmitt M, et al. Feasibility and acceptance of cervicovaginal self-sampling within the German national cohort (pretest 2). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2014;57:1270–6. 10.1007/s00103-014-2054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen S-L, Hsieh P-C, Chou C-H, et al. Determinants of women's likelihood of vaginal self-sampling for human papillomavirus to screen for cervical cancer in Taiwan: a cross-sectional study. BMC Womens Health 2014;14:139. 10.1186/PREACCEPT-4942375021314755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dareng E, Jedy-Agba E, Bamisaye P, et al. P-B25 Influence of spirituality and modesty on acceptance of self sampling for cervical cancer screening. J Acquir Immune Defic Syndr 2016;71:84. 10.1097/01.qai.0000479597.35968.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dzuba IG, Díaz EY, Allen B, et al. The acceptability of self-collected samples for HPV testing vs. the Pap test as alternatives in cervical cancer screening. J Womens Health Gend Based Med 2002;11:265–75. 10.1089/152460902753668466 [DOI] [PubMed] [Google Scholar]
- 78.Flores Y, Bishai D, Lazcano E, et al. Improving cervical cancer screening in Mexico: results from the Morelos HPV study. Salud Publica Mex 2003;45 Suppl 3:388–98. 10.1590/S0036-36342003000900013 [DOI] [PubMed] [Google Scholar]
- 79.Hanley SJ, Fujita H, Yokoyama S, et al. Hpv self-sampling in Japanese women: a feasibility study in a population with limited experience of tampon use. J Med Screen 2016;23:164–70. 10.1177/0969141315625702 [DOI] [PubMed] [Google Scholar]
- 80.Igidbashian S, Boveri S, Spolti N, et al. Self-Collected human papillomavirus testing acceptability: comparison of two self-sampling modalities. J Womens Health 2011;20:397–402. 10.1089/jwh.2010.2189 [DOI] [PubMed] [Google Scholar]
- 81.Jones HE, Wiegerinck MAHM, Nieboer TE, et al. Women in the Netherlands prefer self-sampling with a novel lavaging device to clinician collection of specimens for cervical cancer screening. Sex Transm Dis 2008;35:916–7. 10.1097/OLQ.0b013e3181812cf0 [DOI] [PubMed] [Google Scholar]
- 82.Ketelaars PJW, Bosgraaf RP, Siebers AG, et al. High-Risk human papillomavirus detection in self-sampling compared to physician-taken smear in a Responder population of the Dutch cervical screening: results of the vera study. Prev Med 2017;101:96–101. 10.1016/j.ypmed.2017.05.021 [DOI] [PubMed] [Google Scholar]
- 83.Levinson KL, Abuelo C, Salmeron J, et al. The Peru cervical cancer prevention study (PERCAPS): the technology to make screening accessible. Gynecol Oncol 2013;129:318–23. 10.1016/j.ygyno.2013.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morán F, Cárcamo C, Valderrama M, et al. [Preferences and satisfaction towards a screening program with self-administered human papilloma virus detection tests]. Rev Peru Med Exp Salud Publica 2017;34:228–32. 10.17843/rpmesp.2017.342.2453 [DOI] [PubMed] [Google Scholar]
- 85.Nobbenhuis MAE, Helmerhorst TJM, van den Brule AJC, et al. Primary screening for high risk HPV by home obtained cervicovaginal lavage is an alternative screening tool for unscreened women. J Clin Pathol 2002;55:435–9. 10.1136/jcp.55.6.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obiri-Yeboah D, Adu-Sarkodie Y, Djigma F, et al. Self-Collected vaginal sampling for the detection of genital human papillomavirus (HPV) using careHPV among Ghanaian women. BMC Womens Health 2017;17:86. 10.1186/s12905-017-0448-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ortiz AP, Alejandro N, Pérez CM, et al. Acceptability of cervical and anal HPV self-sampling in a sample of Hispanic women in Puerto Rico. P R Health Sci J 2012;31:205–12. [PMC free article] [PubMed] [Google Scholar]
- 88.Phoolcharoen N, Kantathavorn N, Krisorakun W, et al. Acceptability of self-sample human papillomavirus testing among Thai women visiting a colposcopy clinic. J Community Health 2018;43:611–5. 10.1007/s10900-017-0460-2 [DOI] [PubMed] [Google Scholar]
- 89.Quincy BL, Turbow DJ, Dabinett LN. Acceptability of self-collected human papillomavirus specimens as a primary screen for cervical cancer. J Obstet Gynaecol 2012;32:87–91. 10.3109/01443615.2011.625456 [DOI] [PubMed] [Google Scholar]
- 90.Silva J, Cerqueira F, Medeiros R. Acceptability of self-sampling in Portuguese women: the good, the bad or the ugly? Sex Health 2017;14:298–300. 10.1071/SH16077 [DOI] [PubMed] [Google Scholar]
- 91.Waller J, McCaffery K, Forrest S, et al. Acceptability of unsupervised HPV self-sampling using written instructions. J Med Screen 2006;13:208–13. 10.1177/096914130601300409 [DOI] [PubMed] [Google Scholar]
- 92.Yoshida T, Nishijima Y, Hando K, et al. Primary study on providing a basic system for uterine cervical screening in a developing country: analysis of acceptability of self-sampling in Lao PDR. Asian Pac J Cancer Prev 2013;14:3029–35. 10.7314/APJCP.2013.14.5.3029 [DOI] [PubMed] [Google Scholar]
- 93.Zehbe I, Moeller H, Severini A, et al. Feasibility of self-sampling and human papillomavirus testing for cervical cancer screening in first nation women from northwest Ontario, Canada: a pilot study. BMJ Open 2011;1:e000030. 10.1136/bmjopen-2010-000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oranratanaphan S, Termrungruanglert W, Khemapech N. Acceptability of self-sampling HPV testing among Thai women for cervical cancer screening. Asian Pac J Cancer Prev 2014;15:7437–41. 10.7314/APJCP.2014.15.17.7437 [DOI] [PubMed] [Google Scholar]
- 95.Ma'som M, Bhoo-Pathy N, Nasir NH, et al. Attitudes and factors affecting acceptability of self-administered cervicovaginal sampling for human papillomavirus (HPV) genotyping as an alternative to Pap testing among multiethnic Malaysian women. BMJ Open 2016;6:e011022-e. 10.1136/bmjopen-2015-011022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giorgi Rossi P, Pezzarossi A. Hpv self-sampling. Int J Gynecol Cancer 2013;23:43–4. [Google Scholar]
- 97.Defo VF, JFJAjoe D. Why consider self-sampling for cervical cancer screening in Low-and middle-income countries? 2020;22:116–25. [DOI] [PubMed] [Google Scholar]
- 98.Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. The Lancet 2020;395:575–90. 10.1016/S0140-6736(20)30068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request. All data relevant to the study are included in the article or uploaded as supplementary information. Extracted data are available on request to the corresponding author.