Abstract
Background
Pituitary dysfunction is a life-threatening immune-related adverse event (irAE) induced by immune checkpoint inhibitors (ICIs). To date, it is not possible to identify patients who may develop pituitary irAEs prior to ICI treatment. The aim of this study was to characterize the predisposition for ICI-induced pituitary irAEs by analyzing anti-pituitary antibodies (APAs) and human leukocyte antigens (HLAs).
Methods
In this case–control study, APAs and HLA alleles were analyzed in 62 patients (17 who developed ICI-induced isolated adrenocorticotropic hormone deficiency (ICI-IAD), 5 who developed ICI-induced hypophysitis (ICI-H) and 40 who did not develop pituitary irAEs) treated with ICIs between November 2, 2015, and March 31, 2020, at Nagoya University Hospital. The main outcome measures in this study were the association between the development of pituitary irAEs with APAs at baseline and after treatment and HLA alleles.
Results
Eleven of 17 (64.7%) patients who developed ICI-IAD had APAs at baseline, whereas APAs were positive only in 1 of 40 (2.5%) control patients. Although APAs were negative at baseline in all patients who developed ICI-H, they had become positive before the onset of ICI-H in 3 of 4 patients several weeks after ipilimumab administration. At the onset of ICI-IAD and ICI-H, APAs were positive in 15 of 17 (88.2%) and 4 of 5 (80%) patients, respectively. The prevalence of HLA-Cw12, HLA-DR15, HLA-DQ7, and HLA-DPw9 was significantly higher in patients with ICI-IAD, whereas that of HLA-Cw12 and HLA-DR15 was significantly higher in patients with ICI-H than in controls.
Conclusions
This study showed distinct and overlapped patterns of APAs and HLA alleles between ICI-IAD and ICI-H. Our findings also showed that positive APAs at baseline and after treatment, together with susceptible HLA alleles, could become predictive biomarkers for ICI-IAD and ICI-H, respectively.
Trial registration number
UMIN000019024.
Keywords: HLA, biomarkers, tumor, immunotherapy
Background
Immune checkpoint inhibitors (ICIs), including anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4), anti-programmed cell death-1 (PD-1), and anti-programmed cell death-1 ligand 1 antibodies, have been applied for several advanced malignancies.1 However, ICIs can cause adverse events, termed immune-related adverse events (irAEs), including pneumonitis, skin toxicities, colitis, and endocrine dysfunction.2 3 There are two different types of pituitary irAEs, that is, isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) without pituitary enlargement and hypophysitis with deficiency of multiple anterior pituitary hormones accompanied by pituitary enlargement.4 5 IAD can be induced by either anti-CTLA-4 or anti-PD-1 antibodies, whereas hypophysitis is mainly induced by anti-CTLA-4 antibodies.4 6 7 Although immune responses to the pituitary glands have been implicated in the pathogenesis of pituitary irAEs,8 9 the specific mechanisms remain unclear.
Anti-pituitary antibodies (APAs), measured by indirect immunofluorescence (IIF), are a surrogate marker of the presence of autoimmunity against pituitary glands10 and are detected with a higher frequency in cases of some pituitary diseases, particularly biopsy proven hypophysitis.11 Although APAs are negative at baseline, one study showed that APAs became positive at the onset of ipilimumab (anti-CTLA-4 antibody)-induced hypophysitis in all patients examined.9 In addition, APAs can be detected at the onset of IAD induced by combination therapy with nivolumab (anti-PD-1 antibody) and ipilimumab.12 Although these data indicate that APAs can become positive after the onset of ICI-induced IAD or hypophysitis, it remains unclear whether APAs are detectable before the onset of pituitary dysfunction.
Specific human leukocyte antigen (HLA) variants can be used as a marker of autoimmune diseases. Recent studies have shown that the frequencies of HLA-DQB1*06:01, HLA-DPB1*09:01, and HLA-DRB5*01:02 are higher in cases of anti-PD-1 antibody-induced IAD compared with those in healthy controls from a database of Japanese individuals.13 Another study reported that the frequencies of HLA-DR15, HLA-B52, and HLA-Cw12 were higher in patients with pituitary irAEs, including both hypophysitis and IAD, compared with those in healthy controls from a database of Japanese individuals.14 However, it is unclear whether the susceptible HLA alleles are different between ICI-induced IAD and hypophysitis.
Because pituitary irAEs are almost always accompanied by ACTH deficiency, which is life-threatening,8 it may be ideal to identify patients with a risk of pituitary irAEs before the initiation of ICI treatment. Accordingly, in this study, we aimed to examine whether APAs were present before the onset of pituitary irAEs and to analyze susceptible HLAs in patients with ICI-induced IAD and hypophysitis.
Methods
Patients
To determine whether APAs and/or HLA haplotypes were associated with ICI-induced pituitary irAEs, we reviewed the clinical records of all patients who had received ICI treatment between November 2, 2015, and March 31, 2020, at Nagoya University Hospital and identified 17 patients with ICI-induced IAD and 5 patients with ICI-induced hypophysitis. The patients were consecutively enrolled in our prospective study population, as reported previously.4 15 16 As controls, 40 patients without pituitary irAEs who had received ICIs from November 2, 2015, to April 30, 2019, were enrolled such that the ratios of malignancy type and drug usage were similar to those in patients with pituitary irAEs.
Clinical assessments
Each endocrine irAE was defined according to the Japan Endocrine Society clinical guidelines for endocrine irAEs.17 At the onset of pituitary irAEs, all patients underwent MRI scans and detailed assessment of anterior pituitary function using loading tests, as described previously.4 IAD was defined based on impaired responses of ACTH to corticotropin-releasing hormone without deficiencies in other anterior pituitary hormones. The cut-off for the diagnosis of ACTH deficiency was a decreased peak serum cortisol level (<18 µg/dL) and an impaired response of ACTH (less than twofold of baseline) in corticotropin-releasing hormone loading tests (intravenous injection of 100 µg human corticorelin)18 performed in the morning. Hypophysitis was defined based on findings of pituitary enlargement and deficiencies in multiple anterior pituitary hormones. IrAEs other than endocrine dysfunction, as reported in the guideline,19 were diagnosed by attending physicians and subjected to analysis if the grade was higher than 1. All irAEs were monitored and graded using Common Terminology Criteria for Adverse Events(CTCAE) V.5.0 criteria. Sixteen patients with pituitary irAEs and 33 patients without pituitary irAEs were included in our previous study, in which we analyzed the clinical characteristics of pituitary irAEs.4
APAs
The presence of serum APAs was tested at baseline in all patients enrolled (n=62) and at the onset of pituitary irAEs in the 22 patients. In four of five patients who developed hypophysitis following ipilimumab administration, serum samples collected 2–3 weeks before onset were also tested for the presence of APAs. As controls, serum samples collected 12 weeks after the initiation of ipilimumab were tested for APAs in six patients with malignant melanoma (MM) without pituitary irAEs. We used IIF of human pituitary gland substrates to assess the presence and cell specificities of serum APAs, as described previously.11 20 Human pituitary glands were obtained from the autopsy laboratory in Nagoya University Hospital. The primary and secondary antibodies used in this study are shown in online supplemental table S1. Images were obtained with a BZ-9000 microscope (Keyence, Osaka, Japan).
jitc-2021-002493supp001.pdf (141.7KB, pdf)
HLA genotyping
HLA alleles in HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQB1, and HLA-DPB1 were genotyped using a Luminex system with WAKFlow HLA typing kits (Wakunaga Pharmaceutical, Hiroshima, Japan) at GenoDive Pharma (Kanagawa, Japan). HLA-Cw12, HLA-Cw14, HLA-DPw9, HLA-DPw13, and HLA-DPw14 are provisionally named antigens that are not serologically recognized by WHO Nomenclature Committee (http://hla.alleles.org/nomenclature/).
Statistical analysis
Continuous variables of patient characteristics are expressed as means±SDs. Differences among continuous variables were tested for significance with two-sample t-tests. Values of nominal variables were compared using Fisher’s exact tests. All statistical tests were two-sided, and significance was defined as a p value less than 0.05. All statistical analyses were performed with IBM SPSS Statistics V.27.
Results
Patient characteristics
The clinical features of 17 patients with ICI-induced IAD, 5 patients with ICI-induced hypophysitis, and 40 control patients without pituitary irAEs are shown in table 1. IAD occurred in nine patients with MM, five patients with non-small cell lung carcinoma, two patients with renal cell carcinoma (RCC), and one patient with head and neck cancer. Among 17 patients with IAD, 13 developed IAD during anti-PD-1 antibody therapy (nivolumab or pembrolizumab), 3 developed IAD during ipilimumab therapy, and 1 developed IAD during combination therapy with ipilimumab and nivolumab. Hypophysitis occurred in four patients with MM and one patient with RCC. Among the five patients with hypophysitis, three developed hypophysitis during ipilimumab therapy and two developed hypophysitis during combination therapy with ipilimumab and nivolumab. Two patients with hypophysitis and four control patients, but no patients with IAD, had a history of prior ICI usage. There were no differences in the prevalence of anti-thyroid antibodies (anti-thyroid peroxidase antibody or anti-thyroglobulin antibody) at baseline or the occurrence rates of thyroid irAEs and non-endocrine irAEs between patients with IAD and controls. There were also no differences in the above variables between patients with hypophysitis and controls.
Table 1.
Patient characteristics
| Control | IAD | Hypophysitis | |
| Total number | 40 | 17 | 5 |
| Malignancy | |||
| MM | 24 | 9 | 4 |
| NSCLC | 10 | 5 | 0 |
| RCC | 4 | 2 | 1 |
| HN | 2 | 1 | 0 |
| Drugs | |||
| Ipi | 10 | 5 | 3 |
| Niv | 21 | 9 | 2 |
| Pem | 15 | 11 | 1 |
| Ipi+Niv | 5 | 1 | 2 |
| Ate | 0 | 1 | 0 |
| History of prior ICIs | 4 | 0 | 2 |
| Positive TPOAb or TgAb at baseline | 13 | 3 | 1 |
| Thyroid irAEs | 6 | 1 | 4 |
| Non-endocrine irAEs (≥G2) | 14 | 6 | 4 |
ACTH, adrenocorticotropic hormone; Ate, atezolizumab; HN, head and neck cancer; IAD, isolated ACTH deficiency; ICIs, immune checkpoint inhibitors; Ipi, ipilimumab; irAE, immune-related adverse event; MM, malignant melanoma; Niv, nivolumab; NSCLC, non-small cell lung carcinoma; Pem, pembrolizumab; RCC, renal cell carcinoma; TgAb, anti-thyroglobulin antibody; TPOAb, anti-thyroid peroxidase antibody.
Prevalence of serum APAs at baseline in patients with and without ICI-induced IAD
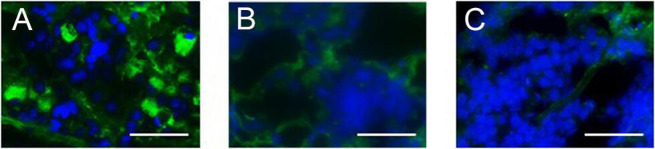
APAs were positive at baseline in 11 of 17 patients with IAD, but in only 1 of 40 control patients and in none of the patients with hypophysitis (table 2 and online supplemental table S2). Representative images of IIF analyses in patients with IAD, hypophysitis, and no pituitary irAEs are shown in figure 1A–C. The prevalence of APAs at baseline was significantly higher in patients with IAD than in control patients (64.7% (11/17) in patients with IAD vs 2.5% (1/40) in controls, p<0.05, table 2). Positive APAs at baseline had high specificity (97.5%, 39/40) and positive predictive value (91.7%, 11/12) for the diagnosis of ICI-induced IAD.
Table 2.
Anti-pituitary antibodies by IIF on human pituitary substrate
| Control N=40 |
IAD N=17 |
Hypophysitis N=5 |
|
| Pre APA (+) | 1 | 11* | 0 |
| Type of cell | |||
| ACTH | 1/1 | 11/11 | |
| TSH | 0/1 | 4/11 | |
| FSH | 1/1 | 11/11 | |
| LH | 0/1 | 1/11 | |
| GH | 0/1 | 0/11 | |
| PRL | 0/1 | 0/11 | |
| Post APA (+) | – | 15 | 4 |
| Type of cell | |||
| ACTH | 15/15 | 4/4 | |
| TSH | 13/15 | 4/4 | |
| FSH | 15/15 | 4/4 | |
| LH | 12/15 | 3/4 | |
| GH | 7/15 | 3/4 | |
| PRL | 1/15 | 2/4 |
*P<0.05 compared with the control.
ACTH, adrenocorticotropic hormone; Post APA, anti-pituitary antibodies at onset; Pre APA, anti-pituitary antibodies at baseline; FSH, follicle stimulating hormone; GH, growth hormone; IAD, isolated ACTH deficiency; IIF, indirect immunofluorescence; LH, luteinizing hormone; PRL, prolactin; TSH, thyroid stimulating hormone.
Figure 1.
Indirect immunofluorescence (IIF) analysis using human anterior pituitary gland tissue. Representative images of IIF staining of anti-pituitary antibodies (APAs) in a patient who developed pembrolizumab-induced isolated adrenocorticotropic hormone deficiency (Pem092; APA-positive) (A), a patient who developed ipilimumab-induced hypophysitis (Ipi005; APA-negative) (B), and a patient who did not develop pituitary irAEs (Niv070; APA-negative) (C). Scale bars: 50 μm. Pem, pembrolizumab; Ipi, ipilimumab; Niv, nivolumab.
APAs before the onset of hypophysitis
APAs were examined in sera collected from four patients before the onset of hypophysitis and from six patients with no pituitary irAEs 12 weeks after the initiation of ipilimumab as a control. APAs became positive 6–9 weeks after initiation of ipilimumab (2–3 weeks before onset) in patients with ICI-induced hypophysitis but not in controls (75% (3/4) vs 0% (0/6), p<0.05, table 3), suggesting that positive changes in APAs preceded the onset of hypophysitis.
Table 3.
Development of anti-pituitary antibodies (APAs) in patients treated with ipilimumab (Ipi)
| 12 weeks after Ipi N=6 |
Hypo N=4 |
P value | |
| Developed APAs N (%) |
0 (0) |
3 (75) |
p<0.05 |
12 weeks after Ipi, group in which APAs were evaluated in sera collected 12 weeks after the initiation of Ipi in patients who did not develop pituitary immune-related adverse events; Hypo, group in which APAs were evaluated in sera collected at 2–3 weeks before the clinical onset of hypophysitis.
APAs at the onset of pituitary irAEs
APAs were positive in 15 of 17 patents who developed IAD at the onset of pituitary irAEs: 11 who had positive APAs at baseline remained positive, while 4 became positive after the onset of treatment. In the four patients who showed a positive change in APAs at the onset of pituitary irAEs, the causative drugs were anti-PD-1 antibodies (one nivolumab and three pembrolizumab) (table 2 and online supplemental table S3). APAs were also positive at the onset of pituitary irAEs in four of five patients who developed hypophysitis (table 2 and online supplemental table S3).
APAs at baseline mainly targeted ACTH-secreting and FSH-secreting cells
To identify the pituitary cell types recognized by APAs, we performed double IIF using each patient’s serum and polyclonal antibodies against each anterior pituitary hormone as the primary antibodies. Representative images of double IIF are shown in online supplemental figure S1. Among patients with IAD with positive APAs at baseline (n=11), APAs recognized ACTH-secreting and follicle stimulating hormone (FSH)-secreting cells in all patients, whereas in some patients, APAs also recognized thyroid stimulating hormone (TSH)-secreting (4/11) and luteinizing hormone (LH)-secreting cells (1/11) (table 2 and online supplemental table S2). In the control patient who had APAs at baseline, APAs recognized ACTH-secreting and FSH-secreting cells (Pem049; table 2 and online supplemental table S2).
Recognition of APAs was broad at the onset of pituitary irAEs
Among the 15 patients with IAD with positive APAs at onset, the numbers of patients whose APAs recognized ACTH-secreting, TSH-secreting, FSH-secreting, LH-secreting, growth hormone (GH)-secreting, and prolactin (PRL)-secreting cells were 15, 13, 15, 12, 7, and 1, respectively (table 2 and online supplemental table S3). Among the four patients with hypophysitis with positive APAs at onset, APAs recognized ACTH-secreting, TSH-secreting, and FSH-secreting cells in all patients (4/4), LH-secreting and GH-secreting cells in three patients, and PRL-secreting cells in two patients (table 2 and online supplemental table S3).
HLA alleles susceptible to pituitary irAEs
HLA alleles in patients with IAD or hypophysitis and control patients are shown in table 4 (HLA class I: HLA-A, HLA-B, HLA-C and class II: HLA-DRB1, HLA-DQB1, HLA-DPB1). There were significant differences between patients with IAD and control patients in the frequencies of HLA-Cw12 (7/17 (41.2%) vs 6/40 (15%), p<0.05, table 4), HLA-DR15 (10/17 (58.8%) vs 10/40 (25%), p<0.05, table 4), HLA-DQ7 (8/17 (47.1%) vs 7/40 (17.5%), p<0.05, table 4), and HLA-DPw9 (7/17 (41.2%) vs 5/40 (12.5%), p<0.05, table 4). The frequencies of HLA-Cw12 (4/5 (80%) vs 6/40 (15%), p<0.05, table 4) and HLA-DR15 (4/5 (80%) vs 10/40 (25%), p<0.05, table 4) were significantly higher in patients with hypophysitis than in controls.
Table 4.
Comparison of HLA class Ⅰ and class Ⅱ allele frequencies
| Control N=40 |
IAD N=17 |
Hypophysitis N=5 |
|
| HLA-A | |||
| A2 | 13 (32.5%) | 6 (35.3%) | 1 (20%) |
| A11 | 7 (17.5%) | 2 (11.8%) | 1 (20%) |
| A24 | 26 (65%) | 11 (64.7%) | 5 (100%) |
| A26 | 5 (12.5%) | 3 (17.6%) | 0 (0%) |
| A31 | 12 (30%) | 4 (23.5%) | 0 (0%) |
| A33 | 6 (15%) | 2 (11.8%) | 1 (20%) |
| HLA-B | |||
| B7 | 3 (7.5%) | 1 (5.9%) | 0 (0%) |
| B13 | 1 (2.5%) | 1 (5.9%) | 0 (0%) |
| B35 | 10 (25%) | 4 (23.5%) | 1 (20%) |
| B3902 | 1 (2.5%) | 1 (5.9%) | 0 (0%) |
| B44 | 8 (20%) | 2 (11.8%) | 1 (20%) |
| B48 | 1 (2.5%) | 1 (5.9%) | 1 (20%) |
| B51 | 8 (20%) | 6 (35.3%) | 0 (0%) |
| B52 | 7 (17.5%) | 7 (41.2%) | 3 (60%) |
| B54 | 6 (15%) | 3 (17.6%) | 2 (40%) |
| B55 | 2 (5%) | 3 (17.6%) | 1 (20%) |
| B56 | 1 (2.5%) | 1 (5.9%) | 0 (0%) |
| B60 | 4 (10%) | 0 (0%) | 1 (20%) |
| B62 | 3 (7.5%) | 4 (23.5%) | 0 (0%) |
| HLA-C | |||
| Cw1 | 11 (27.5%) | 9 (52.9%) | 2 (40%) |
| Cw4 | 3 (7.5%) | 1 (5.9%) | 1 (20%) |
| Cw7 | 11 (27.5%) | 2 (11.8%) | 0 (0%) |
| Cw8 | 4 (10%) | 1 (5.9%) | 1 (20%) |
| Cw9 | 11 (27.5%) | 5 (29.4%) | 1 (20%) |
| Cw10 | 8 (20%) | 1 (5.9%) | 0 (0%) |
| Cw12 | 6 (15%) | 7* (41.2%) | 4* (80%) |
| Cw14 | 11 (27.5%) | 6 (35.3%) | 1 (20%) |
| HLA-DRB1 | |||
| DR4 | 16 (40%) | 4 (23.5%) | 2 (40%) |
| DR8 | 12 (30%) | 2 (11.8%) | 1 (20%) |
| DR9 | 8 (20%) | 5 (29.4%) | 0 (0%) |
| DR11 | 3 (7.5%) | 1 (5.9%) | 0 (0%) |
| DR12 | 5 (12.5%) | 4 (23.5%) | 0 (0%) |
| DR13 | 8 (20%) | 1 (5.9%) | 1 (20%) |
| DR14 | 4 (10%) | 3 (17.6%) | 1 (20%) |
| DR1403 | 1 (2.5%) | 3 (17.6%) | 0 (0%) |
| DR15 | 10 (25%) | 10* (58.8%) | 4* (80%) |
| HLA-DQB1 | |||
| DQ4 | 13 (32.5%) | 5 (29.4%) | 2 (40%) |
| DQ5 | 10 (25%) | 2 (11.8%) | 1 (20%) |
| DQ6 | 24 (60%) | 11 (64.7%) | 4 (80%) |
| DQ7 | 7 (17.5%) | 8* (47.1%) | 0 (0%) |
| DQ8 | 9 (22.5%) | 1 (5.9%) | 1 (20%) |
| DQ9 | 11 (27.5%) | 6 (35.3%) | 0 (0%) |
| HLA-DPB1 | |||
| DPw2 | 21 (52.5%) | 8 (47.1%) | 2 (40%) |
| DPw3 | 3 (7.5%) | 2 (11.8%) | 1 (20%) |
| DPw4 | 11 (27.5%) | 1 (5.9%) | 2 (40%) |
| DPw5 | 29 (72.5%) | 10 (58.8%) | 2 (40%) |
| DPw9 | 5 (12.5%) | 7* (41.2%) | 2 (40%) |
| DPw13 | 0 (0%) | 2 (11.8%) | 0 (0%) |
| DPw14 | 0 (0%) | 2 (11.8%) | 0 (0%) |
| DPB1*2901 | 0 (0%) | 1 (5.9%) | 0 (0%) |
*P<0.05 compared with the control group.
ACTH, adrenocorticotropic hormone; HLA, human leukocyte antigen; IAD, isolated ACTH deficiency.
Discussion
In this study, we showed that APAs were present at baseline in patients who developed ICI-induced IAD and that APAs became positive during ICI treatment in patients who developed hypophysitis. Our data also showed that susceptible HLA alleles overlapped but were not the same between patients with ICI-induced IAD and hypophysitis.
Anti-PD-1 antibodies, the main causative drugs of ICI-induced IAD (13 of 17) in this study, function mainly in the effector phase in which pre-existing autoreactive T cells are activated.21 22 This may explain the high prevalence of positive APAs at baseline in patients who developed ICI-induced IAD. We previously reported that the incidence of thyroid irAEs was significantly higher in patients who had anti-thyroid antibodies prior to ICI treatment than in those who did not.15 16 23 Similarly, our findings indicated that patients who had positive APAs at baseline were prone to develop ICI-induced IAD. In addition, positive conversion of APAs at the onset of pituitary irAEs was also observed in four cases who developed IAD during anti-PD-1 antibody treatment, suggesting that the humoral or cellular immune system was activated by ICI treatment. While there is no report showing histological features of pituitary glands in patients with ICI-induced IAD, lymphocytic infiltration in the anterior pituitary gland has been reported in patients with idiopathic IAD.24–26 Therefore, cytotoxic T cells specific to ACTH secreting cells may also be involved in the pathogenesis of ICI-induced IAD.
Interestingly, APAs had become positive in three of four patients who developed hypophysitis during ipilimumab administration and in whom sera were collected 2–3 weeks before the onset of hypophysitis. Because we could not detect APAs in sera collected from patients without pituitary irAEs at 12 weeks after the initiation of ipilimumab, our findings suggested that positive conversion of APAs occurred during ipilimumab treatment in patients with ipilimumab-induced hypophysitis. Anti-CTLA-4 antibodies, including ipilimumab, function in the priming phase, in which T cells are activated to recognize new antigens22; thus, this class of ICIs may provoke de novo development of autoimmune reactions against pituitary antigens in some patients, resulting in a positive conversion of APAs in patients with ICI-induced hypophysitis. Moreover, because T-cell infiltration has been observed as an irAE in involved organs, such as the skin, colon, and liver,27 cytotoxic T cells may contribute to the development of pituitary dysfunction. In addition to T-cell infiltration, an autopsy study also reported B-cell infiltration in the pituitary gland from a patient who developed hypophysitis induced by tremelimumab (an anti-CTLA-4 antibody).8 Taken together, it is likely that positive conversion of APAs may reflect the new development of autoimmunity in the pituitary glands.
Our data showed that ACTH-secreting cells were recognized by APAs in all patients with positive APAs not only at baseline but also at the onset of ICI-induced IAD. However, pituitary cells secreting other hormones (eg, TSH, FSH, LH, GH, and/or PRL) were also recognized by APAs at the onset of ICI-induced IAD in some patients. Because the functions of pituitary hormones other than ACTH were not clinically impaired in ICI-induced IAD, our findings may suggest that cytotoxic T cells rather than humoral immunity disrupted the ACTH-secreting cells.
Several autoantigen candidates have been reported by analyses using APAs as probes in pituitary diseases28–31; therefore, it is important to identify pituitary autoantigens targeted by APAs in patients with pituitary irAEs. These studies may lead to the establishment of a system to evaluate cytotoxic T-cell responses and an ELISA system to quantitatively measure APAs. Because it is not possible in current clinical practice to identify patients with a high risk of pituitary irAEs at baseline or after the initiation of ICI treatment, or alternatively when adrenal insufficiency is suspected, it is necessary to regularly measure ACTH and cortisol levels. Biomarkers for pituitary irAEs, once established, will allow identification of patients who had a high risk of pituitary irAEs before the onset of pituitary irAEs. While ACTH deficiency is life-threatening, we and other researchers have shown that the development of pituitary irAEs is associated with better overall survival if patients are treated appropriately with hormone substitution therapy.4 32 Therefore, a biomarker of pituitary irAEs could not only contribute to prediction of pituitary dysfunction but also lead to improved management of patients with cancer and a better prognosis following ICI treatment.
In addition to the presence of APAs, the association of specific HLA alleles with pituitary irAEs also strongly suggested the involvement of autoimmunity in the pathogenesis of pituitary irAEs. In this study, we found that susceptible HLA alleles overlapped but were not exactly the same between patients with ICI-induced IAD and hypophysitis. Among the susceptible HLA alleles evaluated in this study (HLA-Cw12, HLA-DR15, HLA-DQ7, and HLA-DPw9 for ICI-induced IAD and HLA-Cw12 and HLA-DR15 for ICI-induced hypophysitis), HLA-Cw12, HLA-DR15,14 and HLA-DPw913 were previously reported to be associated with pituitary irAEs. By analyzing the association of HLAs with the development of ICI-induced IAD and hypophysitis separately, we demonstrated that HLA-DQ7 was associated with the development of ICI-induced IAD, but not with hypophysitis. Notably, analyses from the previous two studies used a database of HLAs from a Japanese population as controls. Because some HLA alleles have been shown to be associated with the development of malignancies,33 the distribution of HLA frequencies may not be the same between ICI-treated patients with malignancies and healthy controls in the Japanese database. This study evaluated HLA frequencies in patients with malignancies who were treated with ICIs.
Clinical characteristics of ICI-induced hypophysitis are similar to those of lymphocytic hypophysitis (autoimmune hypophysitis).34 However, associations of different HLA alleles (HLA-DQ8 and HLA-DR53) with lymphocytic hypophysitis have been reported,35 suggesting that the pathogenesis may also be different between lymphocytic hypophysitis and ICI-induced hypophysitis.
This study had some limitations. First, the sample size of patients with ICI-induced hypophysitis was small. However, there was minimal selection bias because all patients who developed pituitary irAEs during the observation period were enrolled in the study, while patients in the control group were selected so that the ratios of malignancy type and drug usage were similar to those of the patients with pituitary irAEs. Second, the method for measuring APAs in this study is only available in a few laboratories owing to the requirement for normal human pituitary glands as the substrate. Accordingly, it is important to identify the autoantigens related to ICI-induced pituitary dysfunction in order to establish an ELISA system that quantitatively assesses APAs, thereby allowing measurement of APAs in a larger patient cohort in future studies.
Conclusions
Pituitary irAEs are not only life-threatening but also associated with better overall survival.4 32 Therefore, it is important to identify patients who may develop pituitary irAEs before initiation of treatment with ICIs. Our data showed distinct and overlapped patterns of APAs and HLA alleles between patients with ICI-induced IAD and hypophysitis and demonstrated that positive APAs at baseline and after treatment, together with susceptible HLA alleles, could be a predictive biomarker for ICI-induced IAD and hypophysitis, respectively.
Acknowledgments
We thank Ms E Makinose, M Esaki, and Y Hata for their technical assistance.
Footnotes
Contributors: SIw and HA designed the study. TK, SIw, and HA performed the clinical study. TK, SIw, DS, SIt, and HN performed the experiments. TK, SIw, and HA analyzed all the data. TK, SIw, YY, TOk, MI, MS, TOn, HT, DH, YI, HS, RB, and HA treated the enrolled patients and collected and discussed the clinical data. All authors discussed the data. TK, SIw, and HA wrote the manuscript. All authors were involved in revision of the manuscript.
Funding: This work was supported, in part, by JSPS KAKENHI (grant number 16K19552 to SIw). Part of this research was also funded by Ono Pharmaceutical as an academia-initiated sponsored research study at Nagoya University (HA).
Competing interests: SIw received fees from Ono Pharmaceutical Company, Bristol Myers Squibb, MSD KK, and Chugai Pharmaceutical. TOn received personal fees from MSD KK. YI received grants from Sanwa Kagaku Kenkyusho, Kowa Pharmaceutical, MSD KK, Dainippon Sumitomo, Kyowa Kirin, Chugai Pharmaceutical, Boehringer Ingelheim, and Nihon Medi-Physics, and personal fees from Astellas Pharma, Daiichi Sankyo, and Ono Pharmaceutical Company. HN received honoraria and research funding from Ono Pharmaceutical, Chugai Pharmaceutical, MSD, and Bristol Myers Squibb, and research funding from Taiho Pharmaceutical, Daiichi-Sankyo, Kyowa Kirin, Zenyaku Kogyo, Oncolys BioPharma, Debiopharma, Asahi-Kasei, Sysmex, Fujifilm, SRL, Astellas Pharmaceutical, Sumitomo Dainippon Pharma, and BD Japan outside of this study. HA received grants from Ono Pharmaceutical Company, MSD KK, Chugai Pharmaceutical, and personal fees from Ono Pharmaceutical Company, Bristol Myers Squibb, and MSD KK.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was approved by the Ethical Committee of Nagoya University Hospital, No. 2015-0273. Written informed consent was obtained from all patients prior to enrollment in the study.
References
- 1.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. JCO 2015;33:1974–82. 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott LJ. Nivolumab: a review in advanced melanoma. Drugs 2015;75:1413–24. 10.1007/s40265-015-0442-6 [DOI] [PubMed] [Google Scholar]
- 3.Graziani G, Tentori L, Navarra P. Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacological Research 2012;65:9–22. 10.1016/j.phrs.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T, Iwama S, Yasuda Y, et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: a prospective study. J Immunother Cancer 2020;8:e000779. 10.1136/jitc-2020-000779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Percik R, Shlomai G, Tirosh A, et al. Isolated autoimmune adrenocorticotropic hormone deficiency: from a rare disease to the dominant cause of adrenal insufficiency related to check point inhibitors. Autoimmun Rev 2020;19:102454. 10.1016/j.autrev.2019.102454 [DOI] [PubMed] [Google Scholar]
- 6.Faje A, Reynolds K, Zubiri L, et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol 2019;181:211–9. 10.1530/EJE-19-0238 [DOI] [PubMed] [Google Scholar]
- 7.Ariyasu R, Horiike A, Yoshizawa T, et al. Adrenal insufficiency related to Anti-Programmed death-1 therapy. Anticancer Res 2017;37:4229–32. 10.21873/anticanres.11814 [DOI] [PubMed] [Google Scholar]
- 8.Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-Lymphocyte-Associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol 2016;186:3225–35. 10.1016/j.ajpath.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwama S, De Remigis A, Callahan MK, et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014;6:230ra45. 10.1126/scitranslmed.3008002 [DOI] [PubMed] [Google Scholar]
- 10.Iwama S, Arima H. Anti-pituitary antibodies as a marker of autoimmunity in pituitary glands. Endocr J 2020;67:1077–83. 10.1507/endocrj.EJ20-0436 [DOI] [PubMed] [Google Scholar]
- 11.Ricciuti A, De Remigis A, Landek-Salgado MA, et al. Detection of pituitary antibodies by immunofluorescence: approach and results in patients with pituitary diseases. J Clin Endocrinol Metab 2014;99:1758–66. 10.1210/jc.2014-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupi I, Brancatella A, Cosottini M, et al. Clinical heterogeneity of hypophysitis secondary to PD-1/PD-L1 blockade: insights from four cases. Endocrinol Diabetes Metab Case Rep 2019;2019. 10.1530/EDM-19-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inaba H, Ariyasu H, Iwakura H, et al. Comparative analysis of human leucocyte antigen between idiopathic and anti‐PD‐1 antibody induced isolated adrenocorticotropic hormone deficiency: a pilot study. Clin Endocrinol 2019;91:786–92. 10.1111/cen.14082 [DOI] [PubMed] [Google Scholar]
- 14.Yano S, Ashida K, Sakamoto R, et al. Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor–induced secondary adrenal insufficiency. Eur J Cancer 2020;130:198–203. 10.1016/j.ejca.2020.02.049 [DOI] [PubMed] [Google Scholar]
- 15.Okada N, Iwama S, Okuji T, et al. Anti-Thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer 2020;122:771–7. 10.1038/s41416-020-0736-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi T, Iwama S, Yasuda Y, et al. Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: a prospective study. J Endocr Soc 2018;2:241–51. 10.1210/js.2017-00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arima H, Iwama S, Inaba H, et al. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan endocrine Society. Endocr J 2019;66:581–6. 10.1507/endocrj.EJ19-0163 [DOI] [PubMed] [Google Scholar]
- 18.Yanase T, Tajima T, Katabami T, et al. Diagnosis and treatment of adrenal insufficiency including adrenal crisis: a Japan Endocrine Society clinical practice guideline [Opinion]. Endocr J 2016;63:765–84. 10.1507/endocrj.EJ16-0242 [DOI] [PubMed] [Google Scholar]
- 19.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwama S, Welt CK, Romero CJ, et al. Isolated prolactin deficiency associated with serum autoantibodies against prolactin-secreting cells. J Clin Endocrinol Metab 2013;98:3920–5. 10.1210/jc.2013-2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wing YK, Chen CN, Ho CK. Hla DR2 and DQ1 frequency among narcoleptic patients in Hong Kong Chinese. Psychiatry Clin Neurosci 1998;52:523–7. 10.1046/j.1440-1819.1998.00423.x [DOI] [PubMed] [Google Scholar]
- 22.Chen DS, Mellman I. Oncology meets immunology: the Cancer-Immunity cycle. Immunity 2013;39:1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 23.Kimbara S, Fujiwara Y, Iwama S, et al. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci 2018;109:3583–90. 10.1111/cas.13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen MD, et al. Lymphocytic hypophysitis with isolated corticotropin deficiency. Ann Intern Med 1986;105:200–3. 10.7326/0003-4819-105-2-200 [DOI] [PubMed] [Google Scholar]
- 25.Richtsmeier AJ, et al. Lymphoid hypophysitis with selective adrenocorticotropic hormone deficiency. Arch Intern Med 1980;140:1243–5. 10.1001/archinte.1980.00330200119034 [DOI] [PubMed] [Google Scholar]
- 26.Kubo S-ichi, Kitamura O, Orihara Y, et al. Isolated adrenocorticotropic hormone deficiency: an autopsy case of adrenal crisis. Am J Forensic Med Pathol 1997;18:202–5. 10.1097/00000433-199706000-00020 [DOI] [PubMed] [Google Scholar]
- 27.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003;100:8372–7. 10.1073/pnas.1533209100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landek-Salgado MA, Leporati P, Lupi I, et al. Growth hormone and proopiomelanocortin are targeted by autoantibodies in a patient with biopsy-proven IgG4-related hypophysitis. Pituitary 2012;15:412–9. 10.1007/s11102-011-0338-8 [DOI] [PubMed] [Google Scholar]
- 29.Iwata N, Iwama S, Sugimura Y, et al. Anti-pituitary antibodies against corticotrophs in IgG4-related hypophysitis. Pituitary 2017;20:301–10. 10.1007/s11102-016-0780-8 [DOI] [PubMed] [Google Scholar]
- 30.Iwama S, Sugimura Y, Kiyota A, et al. Rabphilin-3A as a targeted autoantigen in lymphocytic Infundibulo-neurohypophysitis. J Clin Endocrinol Metab 2015;100:E946–54. 10.1210/jc.2014-4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bensing S, Hulting A-L, Höög A, et al. Lymphocytic hypophysitis: report of two biopsy-proven cases and one suspected case with pituitary autoantibodies. J Endocrinol Invest 2007;30:153–62. 10.1007/BF03347415 [DOI] [PubMed] [Google Scholar]
- 32.Faje AT, Lawrence D, Flaherty K, et al. High-Dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–14. 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 33.Kageshila T, Naruse T, Hiral S, et al. Molecular genetic analysis of HLA class II alleles in Japanese patients with melanoma. Tissue Antigens 1997;49:466–70. 10.1111/j.1399-0039.1997.tb02780.x [DOI] [PubMed] [Google Scholar]
- 34.Takagi H, Iwama S, Sugimura Y, et al. Diagnosis and treatment of autoimmune and IgG4-related hypophysitis: clinical guidelines of the Japan endocrine Society. Endocr J 2020;67:373–8. 10.1507/endocrj.EJ19-0569 [DOI] [PubMed] [Google Scholar]
- 35.Heaney AP, Sumerel B, Rajalingam R, et al. Hla markers DQ8 and DR53 are associated with lymphocytic hypophysitis and may aid in differential diagnosis. J Clin Endocrinol Metab 2015;100:4092–7. 10.1210/jc.2015-2702 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002493supp001.pdf (141.7KB, pdf)
Data Availability Statement
Data are available on reasonable request.