Abstract
Haemophagocytic lymphohistiocytosis has been reported as an uncommon complication of severe COVID-19 disease while thrombotic thrombocytopenic purpura has been rarely reported. Here, we are reporting a 21-year-old man who developed a combination of these complications during the hospital stay in the post-COVID-19 recovery period. He presented with fever and bilateral COVID-19-related pneumonia requiring invasive ventilation. His hospital course was complicated by the development of pneumothorax, ventilator-associated pneumonia, thrombotic thrombocytopenic purpura and haemophagocytic lymphohistiocytosis. He received remdesivir, IVIG, steroid, fresh frozen plasma and supportive care but had a fatal outcome.
Keywords: COVID-19, haematology (incl blood transfusion), pneumonia (infectious disease), mechanical ventilation
Background
This COVID-19 pandemic has made us aware of various haematological and nonhaematological manifestations of the SARS-CoV-2 virus.1 Common haematological presentations include neutrophilia, lymphopenia, secondary immune thrombocytopenia, thrombotic tendency and disseminated intravascular coagulation (DIC).2 Still, many haematological presentations are uncommon and not frequently reported in the literature. Haemophagocytic lymphohistiocytosis (HLH) is a rare phenomenon and occurs due to cytokine storms.3 Thrombotic thrombocytopenic purpura (TTP), a classical type of microangiopathic haemolytic anaemia, is a very rare entity reported in the COVID-19 disease.4 We report the first case of a complicated COVID-19 disease that had features of both HLH and TTP.
Case presentation
A 21-year-old man presented to holding area emergency of King George’s medical university, Lucknow with a history of fever for 2 weeks and breathlessness, dry cough and high-grade fever for 7 days. Fever was not associated with chills and rigour, chest pain, expectoration, haemoptysis or any skin rash. There was no history of addiction, previous illness, tuberculosis, contact with known tuberculosis or significant comorbidities.
On physical examination, he was conscious oriented but severely breathless. He was very thin built, cachexic and his body mass index (BMI) was 15 kg/m2. His vitals were: BP—118/70 mm Hg, pulse rate—104/min, respiratory rate 24/min. Pallor was present and there was no icterus, oedema or lymphadenopathy. Chest examination shows bilateral diffuse crackels. Cardiovascular examination was normal. Abdominal examination shows splenomegaly 3 cm below the left costal margin and neurological examination was normal at presentation. He was not maintaining saturation on noninvasive ventilation and developed multiple episodes of seizure needing intubation and invasive ventilation. Seizures subsided with antiepileptic medications, but he developed altered sensorium with sensorium of E2VTM5 on Glasgow Coma Scale. His COVID-19 test by reverse transcription PCR (RT-PCR) was positive, so he was shifted to COVID-19 intensive care unit (ICU) setup. On day 3, in COVID-19 ICU, he was not maintaining saturation on ventilatory support, and on examination, breath sounds were absent on the right side. Chest X-ray confirmed it to be right-sided pneumothorax possibly due to barotrauma. Intercostal drainage was done on an urgent basis and his vitals were stabilised.
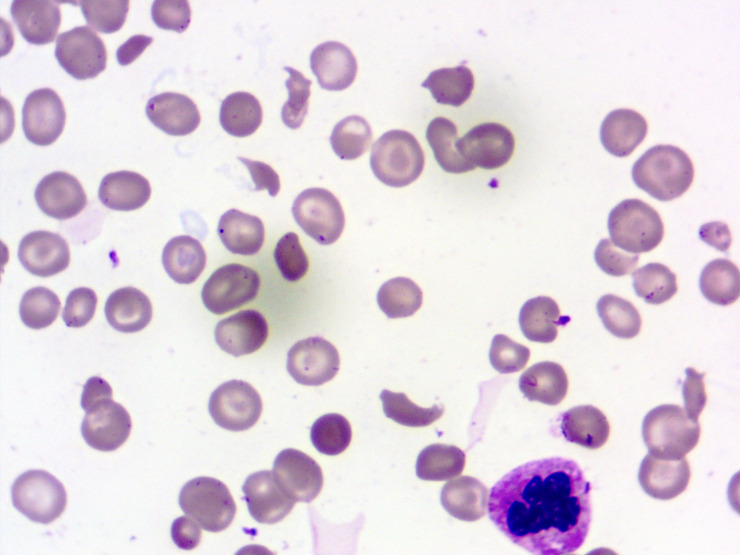
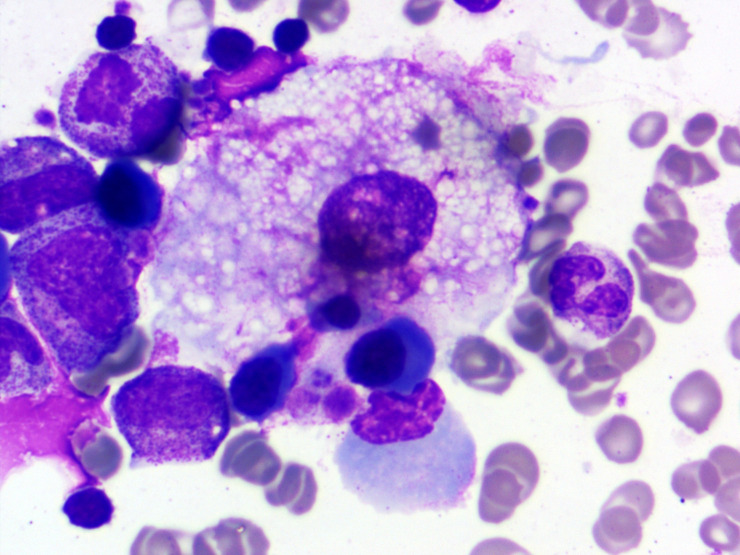
Initial complete blood count showed white blood cells of 15.8×109/L (normal range 4.5–11×109/L) with neutrophils 80%, severe anaemia with haemoglobin 59 g/L (normal range 130–160 g/L) and very low platelet count of 10×109/L (normal range 150–400×109/L). Peripheral smear examination showed a schistocyte of 4% per high power field (figure 1). His coagulation profile including prothrombin time (PT) and activated partial thromboplastin time (APTT) was normal and there was no renal involvement. In view of anaemia, thrombocytopenia and schistocytes in peripheral smear possibility of TTP were considered. His PLASMIC score was 7 supporting a very high possibility of underlying high risk of severe ADAMTS-13 deficiency. ADAMTS-13 activity, ADAMTS-13 antibody and complement levels were not assessed due to the poor affordability of patients. Along with the above investigations, his serum ferritin was 2233 ng/mL, bone marrow examination showed increased number of histiocytes and presence of haemophagocytosis in form of engulfment of platelets, red blood cell, normoblasts and polymorphs (figures 2–4). A diagnosis of HLH was also considered as he had five out of eight criteria according to HLH-2004 diagnostic criteria. These were ferritin >500, fever, bicytopenia, splenomegaly and evidence of HLH in the bone marrow. All relevant investigations are given in table 1.
Figure 1.
Peripheral blood smear showing the presence of schistocytes (Leishman stain, 1000x).
Figure 2.
Bone marrow aspirate smear showing haemophagocytosis with engulfment of multiple erythroid precursor and platelets (Leishman stain, 1000x).
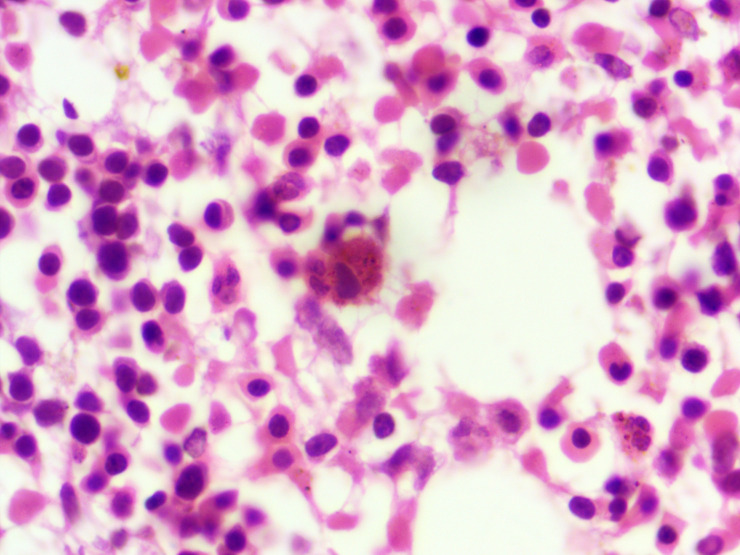
Figure 3.
Bone marrow biopsy section showing erythroid hyperplasia along with presence of many haemophagocytes (H&E,1000×).
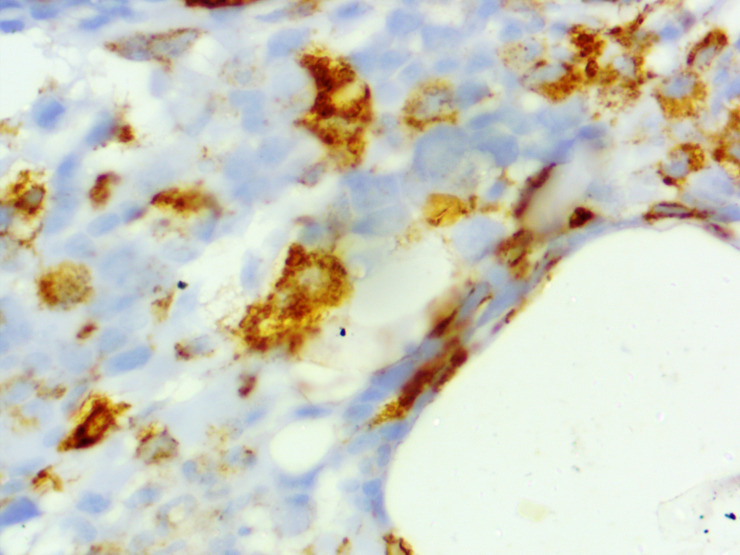
Figure 4.
CD68 immunohistochemical staining on bone marrow biopsy highlighting the increased number of histiocytes (1000x).
Table 1.
Showing relevant investigations during a hospital stay
| Complete blood count and peripheral smear | Haemoglobin—59 g/L (normal range 130–160 g/L), white cell counts—15.8×109/L (normal range 45–110×109/L), MCV −86.6fL (normal range 83–100 fL) RDW—17.6% (normal range11%–14%), platelets—10×109/L (normal range 150–410×109/L) The smear shows anisopoikilocytosis, a fair number of microcytes, macrocytes, and polychromophilic cells. Marked thrombocytopenia and 3–4 schistocytes/high power field are also seen (figure 1) |
| Renal function and urine analysis | Serum urea 37.3 mg/dL (normal range, 10–45 mg/dL), serum creatinine, 0.69 mg/dL (normal range, 0.6–1.5 mg/dL), urine analysis—normal |
| Liver function test and investigation related to haemolysis | Total bilirubin, 2.12 mg/dL (normal range 0.3–1.4 mg/dL), indirect bilirubin 1.86 mg/dL (normal range 0–0.8 mg/dL), aspartate aminotransferase 137 IU/L, (normal range 0–40 IU/L), alanine transaminase, 118 IU/L (normal range 0–40 IU/L) serum alkaline phosphatase 763 IU/L, (normal range 50–270 IU/L) LDH 1193 U/L, (normal range 240–480 U/L), reticulocyte count 4% (normal range 0.3%–1.5%), negative direct Coombs test |
| Coagulation profile | PT, APTT and fibrinogen level: normal, D-dimer—3.93 µg/ml (<0.4 µg/mL) |
| Autoimmune workup | ANA by Hep-2 negative, CRP—227 mg/L (normal range 0–6 mg/L) |
| Work up for underlying possible infection | Nasopharyngeal and oropharyngeal swab for SARS-CoV-2 virus by RT-PCR: positive. Blood culture and urine culture—negative. Viral serology anti-HBV, anti-HCV and anti-HIV negative. Serum ferritin—2233 ng/mL (normal range18-270 ng/mL), CSF study—normal, IgM and IgG antibody for cytomegalovirus, Epstein-Barr virus and Parvovirus B19—negative, real-time PCR for HSV-1, Anti-Japanese encephalitis virus IgM antibody—negative. Endotracheal tube aspirate culture showed growth of Klebsiella pneumonia. |
| Bone marrow examination | Bone marrow aspirate smear: particulate and normocellular for age, myeloid: erythroid ratio=7.1:1, histiocytes- increased number and show the presence of haemophagocytosis in form of engulfment of platelets, red cells, normoblasts and occasional polymorphs (figures 2–4) |
| Radiological investigations | CECT thorax—right-sided moderate to severe hydropneumothorax with enhancing overlying pleura with ICD in situ. Cetrilobular nodular opacities with the tree in bud appearance in the visualised right lung and left lower lobe. Most of them are coalescing to form consolidation patches in the right upper lobe and right middle lobe. Patchy fibrosis is noted in the left upper lobe. Left-sided moderate pleural effusion with the sub-segmental collapse of underlying lung. CT brain normal study, no evidence of bleeding or ischaemia |
ANA, Anti nuclear antibody; APTT, Activated partial thromboplastin time; CECT, Contrast enhanced computed tomography; CRP, C reactive protein; CSF, Cerebrospinal fluid; HSV, Herpes simplex virus; ICD, Intercostal drainage; LDH, Lactate dehydrogenase; MCV, Mean corpuscular volume; PT, Prothrombin time; RDW, Red cell distribution width.
Differential diagnosis
In view of fragmented red blood cells in peripheral blood, severe thrombocytopenia, anaemia and fever a possibility of sepsis with DIC was also considered at one point of time. Normal PT, APTT, serum fibrinogen and weakly raised D-dimer ruled out the possibility of acute DIC.
Treatment
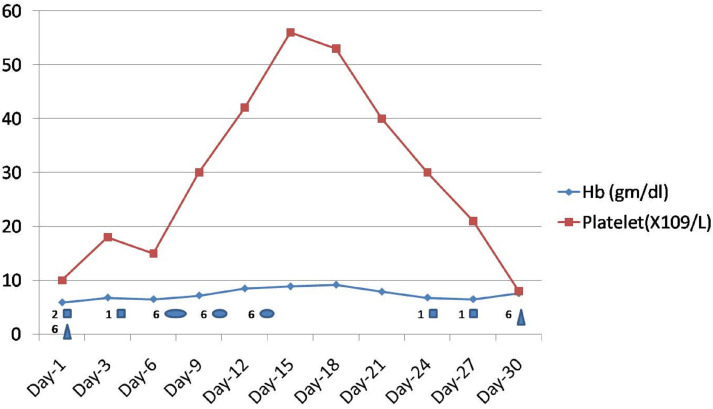
During the hospital stay, the patient received invasive ventilator support, broad-spectrum antibiotics, antiepileptic medications, remdisvir for COVID-19 disease along other supportive treatment. In view of severe COVID-19-related lung complications and haemophagocytic syndrome, he received dexamethasone 10 mg/m2 for 2 weeks followed by tapering. He also received a single dose of IVIG for HLH at doses of 1 g/kg body weight. Ciclosporin and etoposide were not started in view of significantly elevated liver enzymes. He was planned for plasma exchange therapy but did not tolerate it and developed hypotension during the procedure, so it was withheld. The patient received fresh frozen plasma at 15 mL/kg body weight on three occasions, 2–3 days apart with partial improvement in clinical or lab parameters. He received 7 units of packed red blood cell transfusion during his hospital stay. He received 6 units of random donor platelets prophylactically on two occasions for platelet count <10×109/L without any efficacy or any detrimental effect (figure 5). He received physiotherapy as required during his hospital stay.
Figure 5.
Showing Hb and platelet trends during hospital stay along with blood component transfusion details. Hb, haemoglobin.
Outcome and follow-up
The patient showed improvement as his clinical and haematological parameters improved initially. There was an improvement in platelet count along with a decrease in white cell count, C reactive protein (CRP), lactate dehydrogenase (LDH), ferritin and D-dimer. He became COVID-19 negative after 2 weeks of ICU admission. His ventilator parameters improved and he was extubated and kept on noninvasive ventilation. He was shifted to a non-COVID ICU setup in the department of medicine for convalescence. He required 2–3 L of oxygen and intercostal drainage (ICD) closure was also done. The patient again developed right-sided pneumothorax on the 10th day after the closure of the ICD. He also developed high-grade fever, sepsis and rapid clinical deterioration. Pneumothorax with a secondary lung infection, progressive cachexia and the possible progression of TTP led to the rapid clinical deterioration of the patient. Patient died after 2 months of prolonged hospital stay despite all possible measures.
Discussion
COVID-19 is a global pandemic. It has affected approximately one billion people worldwide and leads to the mortality of two million people.5 COVID-19 can present with various haematological manifestations and some of them have predictive value for the outcome and some are associated with high mortality.1 2 We report this case of severe COVID-19 who presented with COVID-19 pneumonia and further got complicated by the development of pneumothorax, TTP and HLH and had mortality after a prolonged hospital stay. All secondary causes of TTP were excluded in this case and his COVID-19 report by RT-PCR was positive, so the SARS-CoV-2 virus was the most probable cause of TTP and HLH in this case.
It is well known that both primary and secondary HLH can be triggered by many viral infections like the Epstein-Barr virus and others.6 The mechanism behind SARS-CoV-2 and the development of HLH were not clearly understood. Underlying extensive cytokine release in severe COVID-19 is probably responsible for the occurrence of HLH. Tholin et al reported a case of a 71-year-old man, having COVID-19 disease and HLH suspected by marked elevation in his inflammatory markers. His CRP was 334 mg/dL, lactate dehydrogenase was 1074 U/L and ferritin was 36 023 µg/L. He also met five out of eight criteria for HLH according to HLH-2004 diagnostic criteria along with evidence of bone marrow haemophagocytosis. The patient was treated with tocilizumab (anti-IL-6 antibody) with other supportive care and his inflammatory parameter improved gradually.7
The mechanism of TTP in COVID-19 disease is even poorly understood. Altowyan E et al have reported a 39-year-old man, with COVID-19 disease and TTP, which was suspected by peripheral smear examination showing evidence of thrombocytopenia and microangiopathic haemolytic anaemia. This patient developed an ischaemic stroke. He was treated by plasma exchange therapy along with supportive care and showed excellent recovery.4 In the non-COVID setup, there is another case report by Daniel et al who had overlapping features of autoimmune haemolytic anaemia, TTP and haemophgocytic lymphohistiocytosis.8 She was a 26-year-old woman presented with features of Autoimmune hemolytic anemia (AIHA), HLH and TTP and complicated by multiorgan failure, DIC and gastrointestinal bleeding. She was managed by IVIG, steroid, splenectomy, haemodialysis, broad-spectrum antibiotics and vasopressor support.
CONCLUSION(S)
COVID-19 can present with a myriad of clinical manifestations. Haematological manifestations can vary from very benign to life-threatening conditions. The occurrence of haemophagocytic syndrome or TTP in COVID- 19 is very rare and has a poor outcome. The co-occurrence of both these entities has not been reported in the literature. The involvement of expert haematologists and haematopathologists in the COVID-19 management team is much needed for timely diagnosis and management of such cases.
Learning points.
COVID-19 disease may present with various haematological manifestations.
COVID-19 disease can also present with features of thrombotic thrombocytopenic purpura and haemophagocytic lymphohistiocytosis simultaneously, so there is a need for high suspicion and awareness of these conditions especially in patients with severe COVID-19.
Haematologists and haematopathologists should be part of the COVID-19 management team with physicians and intensive care experts for better management of these cases.
Footnotes
Contributors: DPV and SPV have written the case and discussion. GY has provided laboratory support and bone marrow evaluations. HD has helped in preparation of the manuscript and discussion.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med Overseas Ed 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol 2020;95:e131. 10.1002/ajh.25774 [DOI] [PubMed] [Google Scholar]
- 3.Lima R, Filho CC, Ferreira Filho CM, et al. Hemophagocytic syndrome and COVID-19. Respir Med Case Rep 2020;31:101162. 10.1016/j.rmcr.2020.101162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altowyan E, Alnujeidi O, Alhujilan A, et al. COVID-19 presenting as thrombotic thrombocytopenic purpura (TTP). BMJ Case Rep 2020;13. 10.1136/bcr-2020-238026. [Epub ahead of print: 17 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worldometers . Corona virus. Available: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1?
- 6.Filipovich A, McClain K, Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant 2010;16:S82–9. 10.1016/j.bbmt.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 7.Tholin B, Hauge MT, Aukrust P, et al. Hemophagocytic lymphohistiocytosis in a patient with COVID-19 treated with tocilizumab: a case report. J Med Case Rep 2020;14:187. 10.1186/s13256-020-02503-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aruch DB, Aledort L. Fatal hematologic alphabet soup: a complicated case with overlapping features of TTP, AIHA, and HLH. J Hematol Thrombo Dis 2015. 10.4172/2329-8790.1000202 [DOI] [Google Scholar]