Abstract
Objective
To investigate serum protein expression in participants with psoriatic arthritis (PsA) and changes after guselkumab treatment.
Methods
Participants with PsA were treated with guselkumab or placebo in the DISCOVER-1 and DISCOVER-2 studies. Serum levels of acute phase reactants C reactive protein (CRP) and serum amyloid A (SAA) and inflammatory cytokines/chemokines were measured at weeks 0, 4 and 24 in 300 study participants and 34 healthy controls (HCs). The PSUMMIT studies measured serum interleukin (IL)-17A, IL-17F and CRP after ustekinumab treatment and levels with ustekinumab versus guselkumab treatment were compared.
Results
Baseline serum levels of CRP, SAA, IL-6, IL-17A and IL-17F were elevated in participants with active PsA vs HCs (p<0.05, geometric mean (GM) ≥40% higher). Baseline T-helper cell 17 (Th17) effector cytokines were significantly associated with baseline psoriasis but not joint disease activity. Compared with placebo, guselkumab treatment resulted in decreases in serum CRP, SAA, IL-6, IL-17A, IL-17F and IL-22 as early as week 4 and continued to decrease through week 24 (p<0.05, GM decrease from baseline ≥33%). At week 24, IL-17A and IL-17F levels were not significantly different from HCs, suggesting normalisation of peripheral IL-23/Th17 axis effector cytokines postguselkumab treatment. Reductions in IL-17A/IL-17F levels were greater in guselkumab-treated versus ustekinumab-treated participants, whereas effects on CRP levels were similar.
Conclusion
Guselkumab treatment reduced serum protein levels of acute phase and Th17 effector cytokines and achieved comparable levels to those in HCs. In participants with PsA, reductions of IL-17A and IL-17F were of greater magnitude after treatment with guselkumab than with ustekinumab.
Keywords: Cytokines, Arthritis, Psoriatic, Biological Therapy
Key messages.
What is already known about this subject?
Psoriatic arthritis (PsA) is a heterogeneous chronic inflammatory disease that can be treated with biological therapies, however, a better understanding of the interleukin (IL)-23/T-helper cell 17 (Th17) pathway in response to therapies can help identify which therapies may be the most effective.
What does this study add?
Inhibition of IL-23 p19 with guselkumab led to a high reduction of Th17 effector cytokine levels to the range seen in healthy controls in PsA patients and greater than the reduction seen with IL-12/IL-23 p40 inhibition with ustekinumab.
While IL-17A and IL-17F levels correlated with disease activity and response to therapy in cutaneous psoriasis in PsA, these levels were not associated with disease activity or response to therapy in the joints in PsA, suggesting that local and/or other systemic factors also play a role in the musculoskeletal disease.
How might this impact on clinical practice or further developments?
The presence of residual joint disease in the face of apparent ‘normalisation’ of serum IL-17A and IL-17F levels with guselkumab treatment indicates that further work is required to better elucidate the kinetics and underlying biology of PsA joint disease to understand the additional factors that need to be addressed to improve outcomes in the musculoskeletal domain in PsA beyond those seen with current biological therapies.
Introduction
Psoriatic arthritis (PsA) is a heterogeneous chronic inflammatory rheumatic condition that affects 14%–23% of adults with psoriasis.1 There have been significant advances in the management of PsA with the development of biological drugs. Although the initial biological agents target tumour necrosis factor (TNF), the identification of the key role of the interleukin (IL)-23/T-helper cell 17 (Th17) pathway in psoriatic disease has led to the successful development of several efficacious agents targeting various proteins in this pathway. Clinical trials using targeted biological therapies offer an opportunity to identify potential biomarkers and to understand disease processes.2
Agents targeting the IL-23/Th17 pathway have led to high hurdle responses in psoriasis, in excess of those seen with TNF inhibitors,3 4 confirming the key pathogenetic role of IL-23/Th17 in the skin in psoriasis. By contrast, in PsA, there are limited head-to-head studies5 6 and effects of treatment on joints appear more heterogeneous than in the skin.7 Furthermore, in clinical practice, treatment remains largely empirical, with many patients with PsA exhibiting no or only partial response to available therapies, while others exhibit dichotomous responses in the skin and musculoskeletal system. Therefore, there remains a significant unmet need in the management of the musculoskeletal component of PsA and for a better understanding of the IL-23/Th17 pathway in response to therapies.
Currently available IL-23/Th17 therapies for psoriatic disease either target the effector cytokines IL-17A and/or IL-17F or the upstream cytokine IL-23. IL-23 is a heterodimer formed by p19 and p40 subunits, with the latter shared with IL-12. Targeting either of these subunits has demonstrated efficacy in both psoriasis and PsA. We have previously reported on IL-17A and IL-17F changes in PsA in response to the IL-12/IL-23 p40 inhibitor ustekinumab in the PSUMMIT phase III programme.2 Here, we extend this work to IL-23 p19 inhibition and a wider range of biomarkers, using data from the phase III clinical studies of guselkumab, a fully human immunoglobulin G1 lambda IL-23 p19 monoclonal antibody. The DISCOVER-1 and DISCOVER-2 multicenter, double-blind, placebo-controlled studies demonstrated the efficacy of guselkumab for the cutaneous and musculoskeletal domains of PsA.8 9
Serum samples were collected as an opportunity to identify the potential biomarkers to better understand the underlying inflammatory processes that drive PsA. The aim of these biomarker analyses is several-fold, namely to better understand the biology of PsA, to provide a biological assessment of the response of participants to treatment with guselkumab, to analyse differences between responders and non-responders, and to determine if these markers can be used to classify participants as potential responders prior to treatment. We also compared the immune modification of cytokine expression in response to IL-23 p19 inhibition with IL-12/IL-23 p40 inhibition to determine how these two strategies may differ in the protein changes they induce.
Patients and methods
Study design
No patients were involved in the design of this study. Detailed descriptions of the phase III DISCOVER-1 and DISCOVER-2 study designs, participant populations, and results have been reported elsewhere.8 9 Briefly, the study population included participants with active PsA who have had inadequate response to standard therapies. Approximately 30% of the DISCOVER-1 study population could have been previously exposed to up to two TNF inhibitors, whereas the DISCOVER-2 population was restricted to biological-naïve participants. Participants in both studies were randomly assigned 1:1:1 to treatment with subcutaneous guselkumab 100 mg every 4 weeks (q4w) from week 0 through week 48; guselkumab 100 mg at weeks 0 and 4, then every 8 weeks (q8w) through week 48; or placebo. The primary endpoint of both studies was the proportion of participants achieving an American College of Rheumatology 20% (ACR20) response at week 24. Participants who met one of the treatment failure criteria prior to week 24 were considered as ACR20 non-responders at week 24, regardless of the observed ACR20 response status.
Participants and biomarker analysis
In DISCOVER-1 and DISCOVER-2, approximately 100 participants per treatment arm (n=300 participants total) were randomly selected for the biomarker studies. Serum samples were collected for the analysis of potential biomarkers at weeks 0 (pretreatment), 4, and 24. Blood samples were collected in standard serum separation tubes as per standard protocol, prepared into aliquots, frozen at ≤−20°C, and then shipped frozen on dry ice to Covance Central Laboratory Services. Samples were later sent to Janssen R&D, Spring House, PA Biobank prior to bioanalysis.
Healthy control samples (n=34) were procured independently of the DISCOVER clinical studies (BioIVT, Westbury, NY; Biological Specialty, Colmar, Pennsylvania, USA). Subjects were selected to reflect the demographics of the clinical study participants (matched for age, sex, race/ethnicity). Subjects were considered ‘healthy’ based on the criteria described in the US Food and Drug Administration Code of Federal Regulations Title 21, Part 630.10 Briefly, healthy subjects had no signs of active infection based on physical assessment, medical history and current medication.
Twenty-one serum proteins were analysed: the acute phase reactants C reactive protein (CRP) and serum amyloid A (SAA) (Meso Scale Discovery (MSD) Platform, Rockville, Maryland, USA) and the following inflammatory cytokines/chemokines: IL-17A, IL-17F and IL-22 (Single Molecule Counting Erenna Immunoassay Platform, Millipore, Burlington MA); IL-1β, IL-2, IL-4, IL-6, CXCL-8 (IL-8), IL-10, IL-12p70, IL-13, interferon (IFN)-γ, CCL2 (MCP-1), CCL4 (MIP-1β), CCL22 (MDC), TNFα, soluble Intracellular Adhesion Molecule 1 (ICAM-1), and soluble Vascular Cell Adhesion Protein 1 (VCAM-1) (MSD Platform); and YKL-40 (Quantikine Immunoassay, R&D Systems, Minneapolis, Minnesota, USA). The following analytes were not analysed further because ≥75% of participants in each treatment arm and the overall population, including healthy controls, had detection levels below the lower limit of quantification for the assays: IL-1β, IL-2, IL-4, IL-10, IL-12p70 and IL-13.
Data from the PSUMMIT-1 and PSUMMIT-2 phase III programmes11 12 were used for indirect comparisons with ustekinumab. As previously reported,2 biomarkers were assayed at weeks 0, 4, and 24; IL-17A (n=784) and IL-17F (n=535) were assayed using Single Molecule Counting Human Immunoassay Kits (formerly Singulex), and CRP (n=925) was measured using CardioPhase high-sensitivity CRP assay.
Statistical analysis
The DISCOVER biomarker sample size was 300 participants, and no power calculations were performed as this was an exploratory analysis. Serum protein biomarker expression levels generally had log-normal distributions and were log2 transformed for statistical analysis. Combined data from DISCOVER-1 and DISCOVER-2 were analysed, unless otherwise noted. For clinical variables, last observation carried forward (LOCF) imputation for missing data was applied for continuous variables, and missing efficacy variables (ie, ACR20) were considered as non-response. Missing biomarker data were retained as missing, with no LOCF rules applied. Missing baseline values were retained as missing. Participants with missing baseline values were not included in this analysis.
Summary statistics for demographic variables and baseline major clinical parameters for samples included in the biomarker analyses were generated using Fisher’s exact test or analysis of variance, with significance defined as p<0.05. For comparison of disease to healthy and for change from baseline analyses, differences of at least 1.4-fold with p<0.05 were considered significant, with generalised linear model analyses performed on log2-transformed data. For change from baseline analyses, within-subject changes from baseline in log2-transformed levels were derived for testing with generalised linear models. Covariate of StudyID was used for DISCOVER-1 and DISCOVER-2 combined analyses. Correlations with serum biomarkers levels were tested using Spearman correlation of ranks tests, with absolute value of correlation coefficient (r)>0.25 and p<0.05 considered significant.
Results
Baseline biomarker levels and baseline disease activity
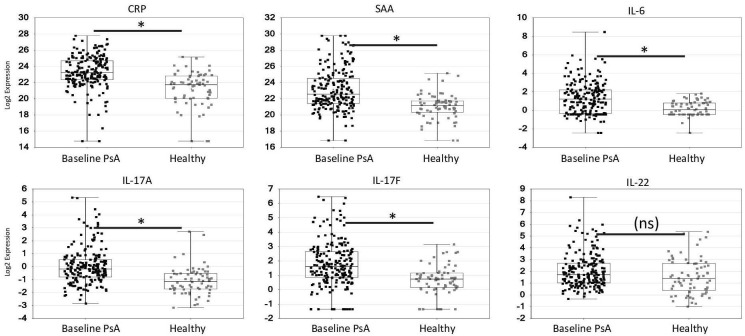
The demographics and treatment outcomes of the 300 randomly selected participants from the DISCOVER-1 and DISCOVER-2 studies used for the biomarker studies are shown, together with the demographics of the healthy controls, in table 1. Serum levels of the acute phase proteins CRP, SAA and IL-6 and the IL-23/Th17 effector cytokines IL-17A and IL-17F, but not IL-22 (figure 1), were significantly elevated at baseline in PsA participants in the DISCOVER studies compared with matched healthy controls (p<0.05 and geometric mean ≥40% higher) (figure 1). TNF levels had a significant (p<0.05) trend towards an increase in PsA participants versus healthy controls, with a mean increase over healthy of approximately 25%; however, this did not meet the predefined criteria for significance. By contrast, there was no significant difference in detectable serum soluble ICAM-1, soluble VCAM-1, CCL2 (MCP-1), CCL22 (MDC), CCL4 (MIP-1β), IFN-γ, IL-8 or YKL-40 in PsA participants compared with healthy controls (online supplemental table 1).
Table 1.
Demographics and treatment outcomes of the randomly selected participants from the DISCOVER studies and demographics of the healthy controls used for the biomarker studies
| Guselkumab 100 mg q4w | Guselkumab 100 mg q8w | Placebo | Healthy | ||
| n=101 | n=100 | n=99 | n=34 | ||
| Demographics | |||||
| Age, years | Mean (SD) | 47.4 (10.7) | 47.3 (11.8) | 47.7 (11.3) | 46.6 (11.9) |
| Sex, n (%) | Female | 51 (50.5) | 46 (46.0) | 49 (49.5) | 18 (52.9) |
| Male | 50 (49.5) | 54 (54.0) | 50 (50.5) | 16 (47.1) | |
| Race, n (%) | White | 97 (96.0) | 95 (95.0) | 94 (94.9) | 33 (97.1) |
| BMI | Mean (SD) | 29.5 (5.6) | 29.8 (6.4) | 29.7 (6.1) | NR |
| Baseline disease | |||||
| Psoriasis disease duration (years) | Mean (SD) | 16.9 (12.8) | 17.0 (12.6) | 17.0 (12.3) | |
| PsA disease duration (years) | Mean (SD) | 7.0 (6.9) | 5.1 (4.8) | 7.0 (5.8) | |
| Body surface area affected by psoriasis (%) | Mean (SD) | 14.9 (18.0) | 16.8 (21.5) | 13.3 (17.4) | |
| PASI | Mean (SD) | 10.1 (11.8) | 9.6 (11.2) | 7.8 (9.7) | |
| Swollen joint count (66) | Mean (SD) | 10.9 (7.4) | 11.8 (8.5) | 11.4 (6.4) | |
| Tender joint count (68) | Mean (SD) | 20.3 (13.5) | 20.9 (13.0) | 20.5 (14.7) | |
| Enthesitis | Number (%) | 68 (67.3) | 65 (65.0) | 60 (60.6) | |
| Dactylitis | Number (%) | 36 (35.6) | 40 (40.0) | 36 (36.4) | |
| Baseline medication usage | |||||
| Prior anti-TNF use | Number (%) | 16 (15.8) | 16 (16.0) | 16 (16.2) | |
| No of prior anti-TNF agents | 1 | 13 (12.9) | 13 (13.0) | 14 (14.1) | |
| 2 | 3 (3.0) | 3 (3.0) | 2 (2.0) | ||
| Current conventional synthetic DMARDs including MTX | Number (%) | 68 (67.3) | 71 (71.0) | 65 (65.7) | |
| No of prior DMARDs | 0 | 11 (10.9) | 11 (11.0) | 6 (6.1) | |
| 1 | 55 (54.5) | 63 (63.0) | 62 (62.6) | ||
| 2 | 29 (28.7) | 19 (19.0) | 23 (23.2) | ||
| 3+ | 6 (5.9) | 7 (7.0) | 8 (8.1) | ||
| Current MTX use | Number (%) | 59 (58.4) | 57 (57.0) | 52 (52.5) | |
| Current conventional synthetic DMARDs other than MTX | Number (%) | 9 (8.9) | 12 (12.0) | 13 (13.1) | |
| Current oral corticosteroid use for PsA | Number (%) | 19 (18.8) | 18 (18.0) | 22 (22.2) | |
| Current NSAID use for PsA | Number (%) | 58 (57.4) | 63 (63.0) | 65 (65.7) | |
| Clinical efficacy endpoints | Number (%) | ||||
| ACR20 response at week24 | Responder | 61 (60.4) | 60 (60.0) | 26 (26.3) | |
| Non-responder | 40 (39.6) | 40 (40.0) | 73 (73.7) | ||
| ACR50 response at week 24 | Responder | 36 (35.6) | 27 (27.0) | 12 (12.1) | |
| Non-responder | 65 (64.4) | 73 (73.0) | 87 (87.9) | ||
| IGA Response at week 24 | Responder | 49 (70.0) | 49 (69.0) | 10 (15.6) | |
| Non-responder | 21 (30.0) | 22 (31.0) | 54 (84.4) | ||
| PASI75 Response at week 24 | Responder | 86 (85.2) | 81 (81.0) | 20 (20.2) | |
| Non-responder | 15 (14.9) | 19 (19.0) | 79 (79.8) | ||
ACR, American College of Rheumatology response criteria; BMI, body mass index; DMARDs, disease modifying anti-rheumatic drugs; IGA, Investigator Global Assessment; MTX, methotrexate; NR, not recorded; NSAID, non-steroidal anti-inflammatory drug; PASI75, Psoriatic Area and Severity Index ≥75%; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; q4w, every 4 weeks; q8w, every 8 weeks; TNF, tumour necrosis factor.
Figure 1.
Baseline levels of acute phase reactants (A–C) and IL-23/Th17 effector cytokines (D–F) in patients with PsA in DISCOVER studies compared with matched healthy controls. Concentration (log2) of analyte (indicated at top of plots) for PsA patients (baseline) and healthy controls. Data presented as symbols representing individual participants and summarised by box plots. Each box represents the upper and lower IQR; lines inside the boxes represent the median; and whiskers represent the range. *Generalised linear model p<0.05 and absolute value of fold difference ≥1.4 vs healthy controls. CRP, C reactive protein; IL, interleukin; PsA, psoriatic arthritis; SAA, serum amyloid A; Th17, T-helper cell 17.
rmdopen-2021-001679supp001.pdf (228.2KB, pdf)
The baseline levels of IL-23/Th17 effector cytokines (IL-17A, IL-17F and IL-22) and the chemokine CCL22 were significantly correlated with baseline psoriasis disease activity, as assessed by both body surface area and Psoriatic Area and Severity Index (PASI) (Spearman signed rank p<0.05 and r>0.25), but not with baseline joint disease activity (table 2). By contrast, the acute phase proteins CRP, SAA and IL-6 and the inflammatory protein YKL-40 were significantly associated with baseline 28 joint disease activity score 28 (DAS28-CRP) (Spearman p<0.05 and r>0.25), but not with baseline swollen or tender joint counts or psoriasis skin severity in participants with PsA (table 2, with correlation between biomarkers shown in online supplemental table 2) (baseline for all participants) and online supplemental table 3) (week 24 change from baseline for guselkumab-treated participants)). The association of DAS28-CRP and CRP was not surprising as CRP is a component of this composite score. There was no significant difference in baseline serum biomarker levels between participants with or without current methotrexate usage (data not shown).
Table 2.
Correlation of baseline biomarker levels with baseline clinical activity measures
| Biomarker | PsA duration | Psoriasis duration | Time from psoriasis to PsA | Psoriasis BSA | Psoriasis PASI | Swollen joint count (66) | Tender joint count (68) | Das28-CRP | |
| CRP | r | 0.07 | 0.01 | −0.04 | 0.11 | 0.09 | 0.12 | 0.12 | 0.39 |
| p | 0.20 | 0.89 | 0.51 | 0.07 | 0.14 | 0.04 | 0.04 | <0.0001 | |
| SAA | r | 0.14 | −0.03 | −0.11 | 0.12 | 0.07 | 0.14 | 0.16 | 0.35 |
| p | 0.02 | 0.64 | 0.06 | 0.04 | 0.24 | 0.01 | 0.01 | <0.0001 | |
| IL-17A | r | 0.14 | 0.17 | 0.10 | 0.48 | 0.48 | 0.01 | 0.04 | 0.10 |
| p | 0.01 | 0.003 | 0.09 | <0.0001 | <0.0001 | 0.91 | 0.47 | 0.10 | |
| IL-17F | r | 0.12 | 0.17 | 0.12 | 0.49 | 0.53 | −0.003 | 0.05 | 0.05 |
| p | 0.03 | 0.004 | 0.04 | <0.0001 | <0.0001 | 0.96 | 0.37 | 0.38 | |
| IL-22 | r | −0.03 | 0.12 | 0.11 | 0.46 | 0.45 | 0.08 | 0.08 | 0.14 |
| p | 0.61 | 0.04 | 0.07 | <0.0001 | <0.0001 | 0.18 | 0.16 | 0.01 | |
| TNFα | r | 0.11 | 0.09 | 0.03 | 0.19 | 0.23 | 0.07 | 0.12 | 0.13 |
| p | 0.06 | 0.13 | 0.62 | 0.001 | <0.0001 | 0.24 | 0.04 | 0.02 | |
| IL-6 | r | 0.13 | 0.01 | −0.07 | 0.08 | 0.09 | 0.15 | 0.20 | 0.36 |
| p | 0.03 | 0.80 | 0.26 | 0.15 | 0.12 | 0.01 | 0.001 | <0.0001 | |
| IFN-γ | r | 0.04 | −0.03 | −0.04 | 0.09 | 0.09 | 0.06 | 0.06 | 0.08 |
| p | 0.53 | 0.57 | 0.47 | 0.12 | 0.13 | 0.32 | 0.31 | 0.14 | |
| sICAM-1 | r | 0.04 | 0.12 | 0.09 | 0.09 | 0.10 | 0.06 | 0.09 | 0.15 |
| p | 0.47 | 0.05 | 0.13 | 0.10 | 0.10 | 0.30 | 0.11 | 0.01 | |
| sVCAM-1 | r | 0.08 | 0.06 | 0.01 | 0.01 | 0.00004 | 0.004 | 0.04 | 0.08 |
| p | 0.15 | 0.28 | 0.81 | 0.88 | 1.0 | 0.94 | 0.47 | 0.19 | |
| CCL2 (MCP-1) | r | 0.09 | 0.13 | 0.06 | 0.17 | 0.13 | 0.19 | 0.17 | 0.18 |
| p | 0.12 | 0.02 | 0.28 | 0.003 | 0.03 | 0.001 | 0.003 | 0.002 | |
| CCL22 (MDC) | r | 0.01 | 0.06 | 0.08 | 0.37 | 0.37 | 0.08 | 0.04 | 0.07 |
| p | 0.90 | 0.30 | 0.19 | <0.0001 | <0.0001 | 0.17 | 0.46 | 0.22 | |
| CCL4 (MIP-1β) | r | 0.02 | −0.01 | −0.01 | −0.03 | −0.06 | −0.06 | −0.06 | −0.06 |
| p | 0.73 | 0.80 | 0.90 | 0.57 | 0.32 | 0.30 | 0.31 | 0.31 | |
| IL-8 | r | −0.02 | 0.04 | 0.07 | 0.16 | 0.2 | 0.12 | 0.09 | 0.13 |
| p | 0.67 | 0.44 | 0.26 | 0.004 | 0.001 | 0.04 | 0.13 | 0.02 | |
| YKL-40 | r | 0.14 | 0.11 | 0.04 | 0.14 | 0.13 | 0.12 | 0.16 | 0.31 |
| p | 0.02 | 0.06 | 0.52 | 0.02 | 0.02 | 0.03 | 0.007 | <0.0001 |
Significance is defined by p<0.05 and fold change ≥1.4 (shown in bold).
BSA, body surface area; CRP, C reactive protein; DAS28, 28 joint Disease Activity Score; IFN γ, interferon γ; IL, interleukin; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; r, correlation coefficient; SAA, serum amyloid A; TNF, tumour necrosis factor.
Baseline biomarker levels and response to guselkumab treatment
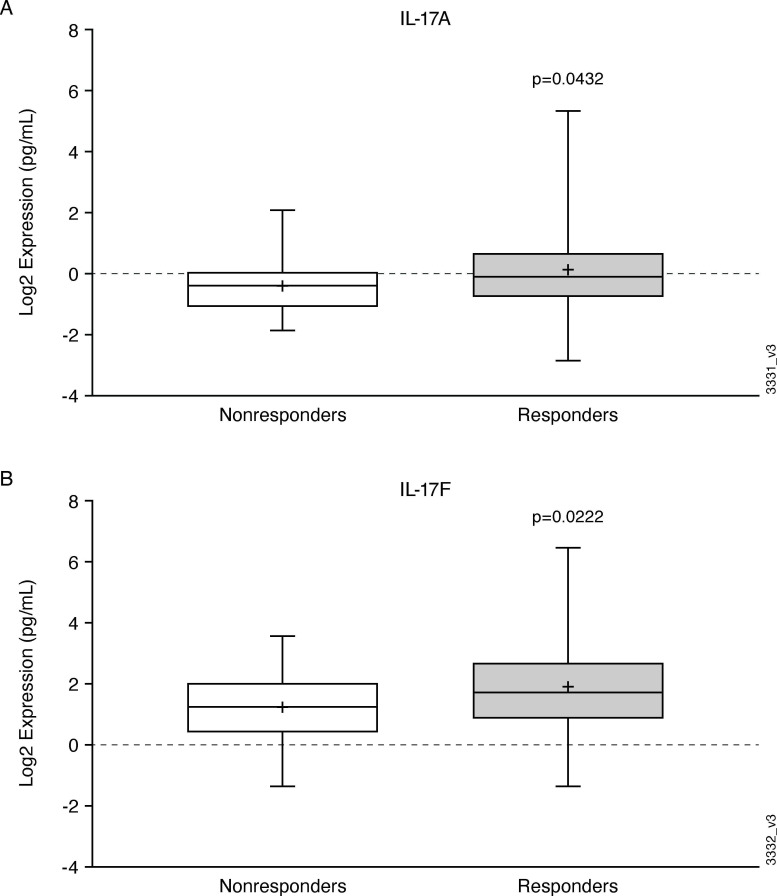
None of the baseline serum protein levels were significantly associated with ACR20 response to guselkumab treatment at week 24, nor correlated with week 24 change in DAS28-CRP from baseline (|r|<0.25; data not shown). For the cutaneous responses, PASI75 responders had significantly higher baseline levels of IL-17A and IL-17F compared with PASI75 non-responders (p=0.04 and p=0.02, respectively) (figure 2).
Figure 2.
Boxplots of Baseline IL-17A (A) (p=0.0432) and IL-17F (B) (p=0.0222) levels in PASI75 guselkumab non-responders (open circles) and guselkumab responders (grey) at week 24. Each box represents the upper and lower IQR; lines inside the boxes represent the median; and whiskers represent the range. IL interleukin; PASI, Psoriasis Area and severity index.
Pharmacodynamics of biomarkers with guselkumab treatment
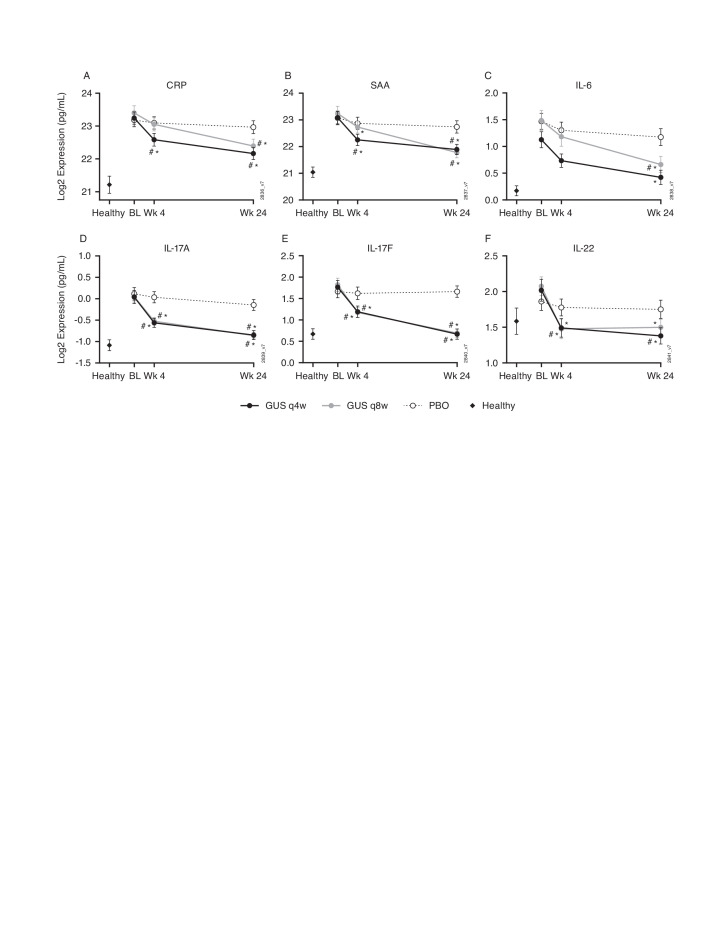
In the combined DISCOVER-1 and DISCOVER-2 biomarker cohort, a strong pharmacodynamic effect was observed in elevated protein biomarkers in response to both 100 mg q4w and 100 mg q8w dosing regimens of guselkumab. Guselkumab treatment resulted in statistically significant decreases in serum levels of CRP, SAA, IL-17A, IL-17F and IL-22 as early as week 4, while no significant change was observed in the placebo arm (figure 3). The expression of these proteins and IL-6 further decreased at week 24 in the guselkumab-treated participants on either dosing regimen and to a greater extent than observed in the placebo group. The week 24 IL-17A and IL-17F expression levels for the PsA participants treated with either dose of guselkumab were no longer significantly different from the healthy controls. No discernable differences in biomarker changes were observed between the two guselkumab dosing regimens (figure 3). There was no significant change from baseline in the other biomarkers (IL-1β, IL-2, IL-4, CXCL-8 (IL-8), IL-10, IL-12p70, IL-13, IFN-γ, CCL2 (MCP-1), CCL4 (MIP-1β), CCL22 (MDC), TNFα, soluble ICAM-1 and soluble VCAM-1) measured in this study.
Figure 3.
Change in serum CRP (A), SAA (B), IL-6 (C), IL-17A (D), IL-17F (E) and IL-22 (F) levels in PsA patients in response to treatment with guselkumab compared with placebo over 24 weeks. *P≤0.05 vs baseline, fold difference ≥1.4; #p≤0.05 vs PBO, fold difference ≥1.4; error bars represent two SEs of the mean (~95% CI). BL, baseline; CRP, C reactive protein; GUS, guselkumab; IL, interleukin; PBO, placebo; PsA, psoriatic arthritis; SAA, serum amyloid A; qw4, every 4 weeks; qw8, every 8 weeks; Wk, week.
Pharmacodynamics of biomarkers and clinical response to guselkumab
The reduction in serum proteins from baseline was not significantly associated with treatment-specific ACR20 clinical response at week 24, indicating that the change in these serum proteins with guselkumab treatment is a pharmacodynamic effect irrespective of the ACR20 response (data not shown).
Although CRP, SAA, IL-6, IL-17A and IL-17F levels decreased with guselkumab treatment, there was no significant difference between PASI75 responders and non-responders (data not shown).
Biomarker pharmacodynamics with guselkumab compared with ustekinumab
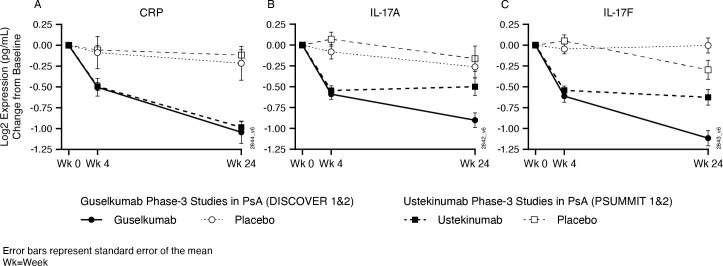
To assess the comparative pharmacodynamic effects of p19 IL-23 inhibition with guselkumab and p40 IL-12/–23 inhibition with ustekinumab, we used available biomarker data from the ustekinumab PSUMMIT-1 and PSUMMIT-2 programmes for comparison with the guselkumab results from the DISCOVER studies. Both ustekinumab and guselkumab significantly reduced expression of IL-17A, IL-17F and CRP at week 4 compared with placebo. Although CRP decreased further from week 4 to week 24 with both guselkumab and ustekinumab, IL-17A and IL-17F levels were further reduced by week 24 with guselkumab but not with ustekinumab treatment (figure 4).
Figure 4.
Comparison of changes in serum CRP (A), IL-17A (B) and IL-17F (C) levels with guselkumab (data from DISCOVER-1 and DISCOVER-2 phase 3 studies) and ustekinumab (data from PSUMMIT-1 and PSUMMIT-2 phase 3 studies) over 24 weeks. CRP, C reactive protein; IL, interleukin; PsA, psoriatic arthritis; Wk, week.
Discussion
The results presented here build on existing data regarding the relevance of the IL-23/Th17 pathway in PsA, with clinical and drug development implications. Baseline levels of acute phase proteins, IL-17A, and IL-17F, but not the other measured biomarkers, were significantly higher in people with active PsA than matched healthy controls. To be able to participate in these studies, participants had to have clinical evidence of active PsA with raised CRP and swollen and tender joint counts (≥0.3 mg/dL and ≥3 each, respectively, for DISCOVER-1 and ≥0.6 mg/dL and ≥5 each, respectively, for DISCOVER-2),8 9 so these biomarker results cannot be assumed to be the same for the wider PsA population and are unlikely to be of general diagnostic value in PsA.
Guselkumab treatment of participants with active PsA resulted in a significant reduction in the levels of these serum biomarkers at week 4, with further reduction and apparent ‘normalisation’ of IL-17A and IL-17F levels by week 24 down to levels within the range seen in healthy controls. Although caution should be applied when comparing data between different studies, it is notable that while treatment with ustekinumab also reduced CRP, IL-17A, and IL-17F at week 4, there was no further reduction in IL-17A or IL-17F at week 24, despite further reduction in CRP. This data suggests that in PsA, p19 inhibition with guselkumab results in deeper reduction in IL-17A and IL-17F levels, compared with p40 inhibition with ustekinumab at the doses used in these studies. While there are no head-to-head studies of guselkumab and ustekinumab in ustekinumab-naïve patients, comparison using individual participant-level data from randomised controlled trials in psoriasis demonstrated that guselkumab has a significantly higher probability of achieving and maintaining high levels of PASI response and complete remission than ustekinumab.13 It is possible that the stronger suppression of Th17 effector cytokines with guselkumab accounts for this difference in cutaneous psoriasis, although the clinical implications of this in musculoskeletal disease in PsA are currently not known. The mechanisms for this greater and longer reduction of circulating Th17 effector cytokines is unclear. One possibility is that by targeting the p19 subunit, guselkumab spares IL-12 inhibition, and that IL-12 may facilitate protection from autoimmune inflammation and T-cell exhaustion.14 15 p19 and p40 inhibitors may also have differential effects on tissue production of cytokines or on the regulatory effects of Th1/T-helper type 1 cells.16 It would also be interesting to compare these results with direct inhibition of IL-17, although monoclonal IL-17A antibodies may confound direct measurement of IL-17A levels.
Baseline Th17 cytokine levels correlated with baseline cutaneous psoriasis disease activity, and baseline serum IL-17A/IL-17F levels correlated with PASI75 response to guselkumab at 24 weeks. In contrast to the association observed with skin disease in our study, neither the baseline nor the change in serum levels of Th17 cytokines correlated with baseline joint disease activity or week 24 ACR20 response in PsA. The association of acute phase proteins with DAS28-CRP is to be expected as CRP forms part of this composite score. However, the baseline acute phase proteins CRP, SAA and IL-6 were only weakly correlated with baseline swollen joint counts and did not predict ACR20 responses at week 24. These findings are consistent with our previous observations in the PSUMMIT trials of ustekinumab2 and support the emerging picture of a closer association of IL-23/Th17 with cutaneous psoriasis disease activity compared with the more heterogeneous and complex situation in the joints in psoriatic disease.5–7 Therefore, these serum Th17 effector cytokine levels may be of limited clinical utility as markers of disease activity or predictors of response to therapy in joint disease in PsA. A recent study comparing IL-23 expression in skin and joint of PsA patients, found homogeneous high expression of IL23p40, IL-23p19 and IL-23R in psoriatic skin while expression in synovial tissue was heterogeneous.17 The presence of residual joint disease in the face of apparent ‘normalisation’ of serum IL-17A and IL-17F levels with guselkumab treatment indicates that further work is required to better elucidate the kinetics and underlying biology of PsA joint disease in order to understand the additional factors that need to be addressed to improve outcomes in the musculoskeletal domain in PsA beyond those seen with current biologic therapies.
A limitation of this study is that peripheral serum biomarkers may not accurately reflect the situation in the skin or musculoskeletal tissues. Although tissue assays would provide more detailed pathogenetic information, these procedures are invasive and would not be feasible for the numbers of participants reported here. Furthermore, in order for a biomarker to be of use in clinical practice, it needs to be easy to obtain on repeated occasions. An additional limitation is that the data reported here is from phase III studies, which required participants to have highly active PsA for inclusion, and thus may not reflect the wider PsA population, although patients receiving biologic therapies in most clinical settings have active disease.18 19 Data presented here are of relatively short follow-up but do have the advantage of a 24-week placebo-controlled period, which would not be available in observational studies. These data are from single intervention studies, and head-to-head and observational studies are required to evaluate these biomarkers with other agents targeting the IL-23/Th17 and other pathways to better understand their relevance.
In conclusion, inhibition of IL-23 p19 with guselkumab led to a high reduction of Th17 effector cytokine levels to the range seen in healthy controls and greater than the reduction seen with IL-12/IL-23 p40 inhibition with ustekinumab. While IL-17A and IL-17F levels correlated with disease activity and response to therapy in cutaneous psoriasis in PsA, these levels were not associated with disease activity or response to therapy in the joints in PsA, suggesting that local and/or other systemic factors also play a role in the musculoskeletal disease.
Acknowledgments
Writing/editing assistance was provided by Mia DeFino, MS, ELS of Synchrogenix on behalf of Janssen Research & Development, LLC. The data in this manuscript have been presented previously as an oral presentation at EULAR 2020 and encore presentations at PANLAR 2020, BSR 2020, APLAR 2020, and ACR 2020.
Footnotes
Contributors: KS, SS, IBM and MJL drafted the manuscript; KM, KL, VL, CF and PC generated the data and verified raw data output; KS and QS performed comparative analysis; all authors reviewed and revised the manuscript and gave final approval of the version to be published.
Funding: Study funded by Janssen Research and Development. ClinicalTrials.gov identifiers: NCT03162796 (DISCOVER-1), NCT03158285 (DISCOVER-2).
Competing interests: KS, QS, MJL, KM, KL, VL, CF and PC are employees of Janssen Research & Development, a wholly owned subsidiary of Johnson & Johnson and own company stock. SS, FRCP, PhD has received research grants, consulting fees and/or honoraria (all <US$10 000 per annum) from AbbVie, Amgen (previously Celgene), Biogen, Janssen, and Novartis. Iain B. McInnes, FRCP, PhD has received research grants, consulting fees, and/or honoraria (all US$<10 000 per annum) from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Gilead, GSK, Janssen, Novartis, Pfizer, Sanofi and UCB.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement
Data are available on reasonable request. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted in this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
References
- 1.Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019;80:251–65. 10.1016/j.jaad.2018.06.027 [DOI] [PubMed] [Google Scholar]
- 2.Siebert S, Sweet K, Dasgupta B, et al. Responsiveness of serum C-reactive protein, interleukin-17A, and Interleukin-17F levels to ustekinumab in psoriatic arthritis: lessons from two phase III, multicenter, double-blind, placebo-controlled trials. Arthritis Rheumatol 2019;71:1660–9. 10.1002/art.40921 [DOI] [PubMed] [Google Scholar]
- 3.Mahil SK, Ezejimofor MC, Exton LS, et al. Comparing the efficacy and tolerability of biologic therapies in psoriasis: an updated network meta-analysis. Br J Dermatol 2020;183:638–49. 10.1111/bjd.19325 [DOI] [PubMed] [Google Scholar]
- 4.Siebert S, Millar NL, McInnes IB. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann Rheum Dis 2019;78:1015–8. 10.1136/annrheumdis-2018-213654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 2020;79:123–31. 10.1136/annrheumdis-2019-215386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3B trial. Lancet 2020;395:1496–505. 10.1016/S0140-6736(20)30564-X [DOI] [PubMed] [Google Scholar]
- 7.Belasco J, Louie JS, Gulati N, et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol 2015;67:934–44. 10.1002/art.38995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1115–25. 10.1016/S0140-6736(20)30265-8 [DOI] [PubMed] [Google Scholar]
- 9.Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1126–36. 10.1016/S0140-6736(20)30263-4 [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration . Part 630—Requirements For Blood and Blood Components Intended For Transfusion Or For Further Manufacturing Use [Internet]. Electronic Code of Federal Regulations, 2020. [Google Scholar]
- 11.McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780–9. 10.1016/S0140-6736(13)60594-2 [DOI] [PubMed] [Google Scholar]
- 12.Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. 10.1136/annrheumdis-2013-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diels J, Thilakarathne P, Cameron C, et al. Adjusted treatment comparisons between guSelkumab and uStekinumab for treatment of moderate-to-severe plaque psoriasis: the COMPASS analysis. Br J Dermatol 2020;183:276–84. 10.1111/bjd.18634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 2003;198:1951–7. 10.1084/jem.20030896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schurich A, Raine C, Morris V, et al. The role of IL-12/23 in T cell-related chronic inflammation: implications of immunodeficiency and therapeutic blockade. Rheumatology 2018;57:246–54. 10.1093/rheumatology/kex186 [DOI] [PubMed] [Google Scholar]
- 16.Nussbaum L, Chen YL, Ogg GS. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br J Dermatol 2021;184:14–24. 10.1111/bjd.19380 [DOI] [PubMed] [Google Scholar]
- 17.Nerviani A, Boutet M-A, Tan WSG, et al. IL-23 skin and joint profiling in psoriatic arthritis: novel perspectives in understanding clinical responses to IL-23 inhibitors. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-218186. [Epub ahead of print: 26 Nov 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. 10.1002/art.40726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-001679supp001.pdf (228.2KB, pdf)
Data Availability Statement
Data are available on reasonable request. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted in this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.