Abstract
We reported a unique case with the coexistence of classic and cutaneous polyarteritis nodosa (PAN), and microscopic polyangiitis (MPA) in hepatitis virus-associated vasculitis. A 77-year-old Asian man presented with extremity weakness and weight loss found to have bilateral foot drop and rash on his hands and legs. Labs reveal positive for hepatitis B core antibody and perinuclear-antineutrophil cytoplasmic antibody (p-ANCA), decreased C3 and C4 levels. Skin biopsy of rash shows medium vessel vasculitis suggesting PAN. Interestingly, renal biopsy showed features of necrotising medium-sized arteritis consistent with PAN and focal crescentic glomerulonephritis consistent with MPA. The patient was treated with 1 g of solumedrol daily for 3 days, followed by oral steroids and cyclophosphamide treatment for vasculitis, and entecavir for chronic hepatitis B infection, resulting in resolution of symptoms. The patient has not had a relapse at 6 months.
Keywords: vasculitis, connective tissue disease
Background
Polyarteritis nodosa (PAN) is necrotising vasculitis targeting small to medium-sized arteries that can involve any organ of body but without glomerulonephritis (GN) and not associated with antineutrophil cytoplasmic antibodies (ANCAs).1 PAN may be triggered by viral infections, including particularly hepatitis B, but remain idiopathic in most case.2 Microscopic polyangiitis (MPA) is an antineutrophilic cytoplasmic antibody ANCA associated vasculitis that involve small and medium arteries. Rarely, MPA can occur in association with hepatitis B. Hepatitis B virus (HBV) infection has been reported to be associated with systemic vasculitis including PAN,3 mixed cryoglobulinaemia4 and leucocytoclastic vasculitis.5 Of note, classic HBV-associated PAN is ANCA (−), without GN. There has been no reported case of coexistence of the PAN and MPA, which is hepatitis B associated. Here we present a unique case with coexistence of classic and cutaneous PAN, and MPA in HBV-associated vasculitis.
Case presentation
A 77-year-old Asian man with a history of chronic obstructive pulmonary disease, hypertension, carotid stenosis status post bilateral carotid endarterectomy, coronary artery disease and significant L4 and L5 radiculopathy presented with a 3-month history of bilateral extremity weakness associated with numbness and weight loss. The patient denied recent gastrointestinal symptoms or infection.
Physical examination was notable for bilateral foot drop and a faint purple, lace-like discoloration on his hands and legs (figure 1). Laboratory testing (table 1) showed hepatitis B surface Ag (−), hepatitis B core IgG (+), hepatitis B surface Ab (−), HBV PCR (−), hepatitis C Ab (−), erythrocyte sedimentation rate 75 mm/hour, microscopic haematuria, serum creatinine 2.1 mg/dL, urine protein/creatinine ratio 0.3 g/g, p-ANCA (+) with ratio of 1:320, myeloperoxidase >100, decreased C3 and C4 complement levels and negative antinuclear antibodies. Skin biopsy of right lower leg shows medium vessel vasculitis, suggestive of PAN (figure 2). Electromyography (EMG) and nerve conduction studies showed the presence of moderately severe sensorimotor axonal neuropathy. Needle EMG findings are asymmetrical suggesting mononeuropathy multiplex. Interestingly, renal biopsy (figure 3A, B) showed focal crescentic GN consistent with MPA (figure 3A) and features of necrotising medium-sized arteritis consistent with PAN (figure 3B). Left vastus lateralis muscle biopsy showed an occluded endomysial blood vessel with inflammation indicating vasculitis (figure 4). Left sural nerve biopsy demonstrated a vasculopathy, with a single perineurial blood vessel with perivascular and transmural acid phosphatase staining and a thickened wall. There is also a mild increase in endoneurial acid phosphatase activity. There is no definite vasculitis on these sections.
Figure 1.
Skin rash of faint purple and lace-like discoloration on the right palm.
Table 1.
Results of laboratory test
| Laboratory tests | Normal range | Result |
| Serum creatinine (mg/dL) | 0.6–1.2 | 2.1 |
| Urine protein/creatinine ratio | <0.2 | 0.3 |
| Hepatitis B surface Ag | negative | negative |
| Hepatitis B surface Ab | negative | negative |
| Hepatitis B core IgG | negative | positive |
| Hepatitis B virus PCR | negative | negative |
| Hepatitis C antibody | negative | negative |
| P-ANCA | negative | positive with ratio of 1:320 |
| Anti-nuclear antibody | negative | negative |
| Myeloperoxidase Ab (IU/mL) | <3.5 | >100 |
| ESR (mm/hour) | <15 | 75 |
ANCA, antineutrophil cytoplasmic antibody; ESR, erythrocyte sedimentation rate.
Figure 2.
Skin biopsy from right lower leg showing medium vessel vasculitis, suggestive of polyarteritis nodosa.
Figure 3.
Renal biopsy shows focal crescentic glomerulonephritis (A) and necrotising medium-sized arteritis (B).
Figure 4.
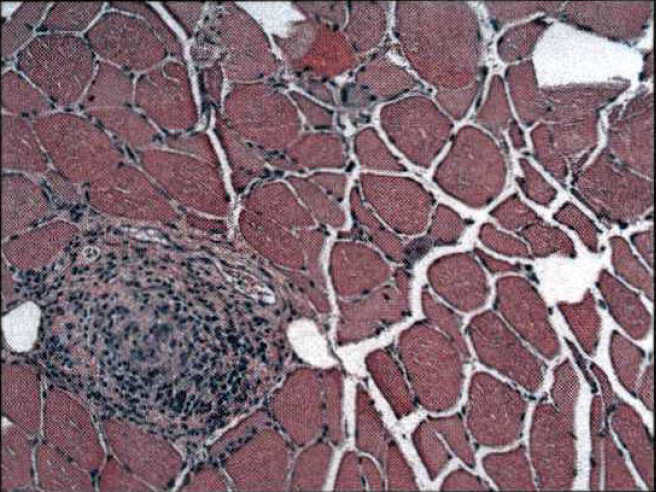
Left vastus lateralis muscle biopsy showed an occluded endomysial blood vessel with inflammation indicating vasculitis.
Treatment
The patient was treated with 1 g of solumedrol daily for 3 days, followed by oral steroids and cyclophosphamide treatment of vasculitis, and entecavir for chronic HBV infection, resulting resolution of symptoms including no foot drop, increased strength of lower extremities and resolution of skin rash without scar tissue. Patient has not had relapse at 6 months.
Discussion
Either acute or chronic HBV infection can lead to various extrahepatic manifestation which are considered to be mediated by immune complex.6 Among those, systemic vasculitis is one of the most serious but rare manifestation. In comparison with hepatitis C infection which is more commonly associated with small vessel vasculitis, HBV infection is predominantly associated with medium to large vessel disease.
HBV and HCV and other viruses such as HIV have been associated with systemic vasculitis including PAN. The form of systemic vasculitis most commonly seen is PAN, although cryoglubulinaemic and leucocytoclastic vasculitis were also reported. PAN usually presents with abdominal pain, rash, weight loss and polyarthritis which progresses to eventually involved the kidney, skin, nervous system and gastrointestinal tract. HBV-associated PAN has a higher incidence of gastrointestinal involvement than PAN without HBV.7
The pathogenic mechanism associated with HBV related PAN is assumed to be an immune complex related vascular injury. Immune complexes will result in severe form of PAN, but if the virus is eliminated then complete remission of the disease with no recurrence of relapse or late hepatic complication may occur. In our case, a patient with chronic HBV infection, PAN presented with no gastrointestinal syndrome however with involvement of skin, kidney and nervous system. Further, our unique patient presented with coexistence of p-ANCA and crescentic GN.
HBV infection has been associated with ANCA associated vasculitis in only three reports, but mainly as c-ANCA seropositivity8 9 (table 2). While the frequency of ANCA using immunofluorescence assays at low titers, has been reported in HBV cases, the majority were of c-ANCA but with no major clinical vasculitis features.10
Table 2.
Case reports summary of ANCA associated vasculitis in HBV infection
| Case report author | Year | Clinical features | Vasculitis disorder | Treatment |
| Gil9 | 2011 | Left ear pain, cough, fevers, night sweats and weight loss over 6 weeks following hepatitis B vaccination | Granulomatosis with polyangiitis and PAN | Pulsed intravenous Methylprednisolone 500 mg daily for 3 days followed by oral corticosteroids (prednisolone) with concomitant bone protection and proton-pump inhibitor. Pulsed intravenous cyclphosphamide 500 mg fortnightly was initiated during admission and was continued for 3 months. Patient completed the course of cyclophosphamide infusions and is on azathioprine and low dose prednisolone maintenance therapy. |
| Joshi8 | 2017 | Acute-onset flaccid paraplegia in the background of a 10-day history of headache and puffiness of the face found to have acute transverse myelitis | Immune complex-medicated vasculitis (not specified) | Intravenous methylprednisolone 1 g daily for 3 days, followed by a short course of oral prednisolone for myelitis. Oral entecavir for hepatitis B infection. |
| Ijaz14 | 2018 | Initially presented with abdominal pain and scrotal swelling found to have PAN. Two years later, patient presented again with abdominal pain, orchitis, haemoptysis and acute kidney injury found to have MPA. | PAN and MPA | During first presentation, the patient was treated with pulse steroid and Cytoxan for treatment of PAN and Entecavir for HBV. During second presentation, patient was treated with intravenous pulsed dose of steroid for 3 days along with plasma exchange for 5 days; later transitioned to oral prednisone 60 mg daily along with rituximab. |
ANCA, antineutrophil cytoplasmic antibody; HBV, hepatitis B virus; PAN, polyarteritis nodos.
ANCAs are only occasionally detected in HBV-related PAN, unlike the classic PAN.11 MPA, an ANCA-associated vasculitis, rarely occurs in association with HBV infection. Interestingly, our case not only demonstrated features of PAN including mononeuritis multiplex and cutaneous (skin rash with biopsy of medium vessel vasculitis), also unusually MPA manifested as p-ANCA-related crescentic GN.
Our case is the first direct evidence of co-existent of PAN and MPA associated with HBV infection. Such as association has not heretofore been reported. Furthermore, this patient manifested an overlap of systemic vasculitis of medium, small and microscopic vessel disease but had no features that definitely fit into a distinct category of vasculitis, per Chapel Hill 2012 classification. Overlapping systemic vasculitis with ANCA associated vasculitis has been reported, to include eosinophilic granulomatosis with polyangiitis-PAN, MPA-PAN. The ANCA test has been an important differentiating feature, though some have been reported to manifest systemic vasculitis, but has features that do not put them into a specific vasculitis disorder.
The pathogenesis of systemic vasculitis of HBV associated infection is thought mostly due to immune complex or type III reaction. The vascular injury is caused by precipitation of immune complexes which trapped in the vessel walls. Interestingly, in our case, given positive p-ANCA and the finding of focal crescentic GN consistent with MPA, it suggests that ANCA may also contributes to the pathogenesis of HBV related systemic vasculitis. We suggest that HBV may trigger systemic vasculitis including an overlap of systemic vasculitis of ANCA-associated vasculitis and PAN as indicated in our case.
Management of HBV-related vasculitis established includes control the immune complex formation and reaction as well as antiviral agents to reduce the antigenic load resulting in reduction of inflammation.3 12 13 In patients of PAN which is associated with hepatitis B, main focus of therapy is placed on the treatment of underlying viral disorder with the use of antiviral agents. In our case, patient with coexistence PAN and MPA responded very well to the cotherapy of steroid and cyclophosphamide and obtain response in 2 weeks. Importantly, patient also started on antiviral treatment concurrently. There is no relapse for 6 months so far.
Our case demonstrates that given the different systemic vasculitis phenotypes, evaluation for hepatitis virus infection is needed on all cases with overlapping features, as remission can be achieved through targeted and more effective therapeutic options which include concomitant use of glucocorticoids, immunosuppression and an antiviral agent.
Learning points.
This case suggests the potential role of antineutrophil cytoplasmic antibody contributing to the development of vasculitis in the setting of chronic hepatitis B virus infection.
This patient manifested an overlap of systemic vasculitis of medium, small and microscopic vessel disease but had no features that definitely fit into a distinct category of vasculitis.
Our case demonstrates that given the different systemic vasculitis phenotypes, evaluation for hepatitis virus infection is needed on all cases with overlapping features, as remission can be achieved through targeted and more effective therapeutic options which include concomitant use of glucocorticoids, immunosuppression and an antiviral agent.
Acknowledgments
We would like to thank Dr Nicola Bravo from Bethesda Dermatopathology Laboratory to provide valuable skin biopsy imaging.
Footnotes
Contributors: Patient care involvement: WC and ZM. Renal biopsy pictures provided by LJA. Drafting the manuscript and revising the manuscript critically for important intellectual content: ZM and JM. Approval of the version of the manuscript to be published: ZM, WC, LJA and JM.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international chapel Hill consensus conference Nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11. 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 2.De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev 2016;15:564–70. 10.1016/j.autrev.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Sharma K. Hepatotropic viral infection associated systemic vasculitides-hepatitis B virus associated polyarteritis nodosa and hepatitis C virus associated cryoglobulinemic vasculitis. J Clin Exp Hepatol 2013;3:204–12. 10.1016/j.jceh.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terrier B, Marie I, Lacraz A, et al. Non HCV-related infectious cryoglobulinemia vasculitis: results from the French nationwide CryoVas survey and systematic review of the literature. J Autoimmun 2015;65:74–81. 10.1016/j.jaut.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 5.Bonkovsky HL, Liang TJ, Hasegawa K, et al. Chronic leukocytoclastic vasculitis complicating HBV infection. Possible role of mutant forms of HBV in pathogenesis and persistence of disease. J Clin Gastroenterol 1995;21:42–7. 10.1097/00004836-199507000-00011 [DOI] [PubMed] [Google Scholar]
- 6.Baig S, Alamgir M. The extrahepatic manifestations of hepatitis B virus. J Coll Physicians Surg Pak 2008;18:451–7. doi:07.2008/JCPSP.451457 [PubMed] [Google Scholar]
- 7.Ebert EC, Hagspiel KD, Nagar M, et al. Gastrointestinal involvement in polyarteritis nodosa. Clin Gastroenterol Hepatol 2008;6:960–6. 10.1016/j.cgh.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 8.Joshi U, Subedi R, Gajurel BP. Hepatitis B virus induced cytoplasmic antineutrophil cytoplasmic antibody-mediated vasculitis causing subarachnoid hemorrhage, acute transverse myelitis, and nephropathy: a case report. J Med Case Rep 2017;11:91. 10.1186/s13256-017-1255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil E, Lutalo P, D'Cruz D. Systemic vasculitis: a dual diagnosis? BMJ Case Rep 2011;2011:bcr1020114968. 10.1136/bcr.10.2011.4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calhan T, Sahin A, Kahraman R, et al. Antineutrophil cytoplasmic antibody frequency in chronic hepatitis B patients. Dis Markers 2014;2014:1–5. 10.1155/2014/982150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillevin L, Visser H, Noel LH, et al. Antineutrophil cytoplasm antibodies in systemic polyarteritis nodosa with and without hepatitis B virus infection and Churg-Strauss syndrome--62 patients. J Rheumatol 1993;20:1345–9. [PubMed] [Google Scholar]
- 12.Guillevin L, Raffray L, Nguyen Y. Treatment of ANCA-Associated Vasculitides. : Anti-Neutrophil cytoplasmic antibody (ANCA) associated vasculitis. Springer, Cham, 2020: 313–28. [Google Scholar]
- 13.Pagnoux C, Mendel A. Treatment of systemic necrotizing vasculitides: recent advances and important clinical considerations. Expert Rev Clin Immunol 2019;15:939–49. 10.1080/1744666X.2019.1656527 [DOI] [PubMed] [Google Scholar]
- 14.Ijaz SH, Taseem S, Usman K. Relapsing hepatitis B (HBV)- associated vasculitis with features of polyarteritis nodosa (PAN) and cANCA-associated vasculitis. JOJCS 2018;9. 10.19080/JOJCS.2018.09.555768 [DOI] [Google Scholar]





