Abstract
Alaskan wildfires have major ecological, social, and economic consequences, but associated health impacts remain unexplored. We estimated cardiorespiratory morbidity associated with wildfire smoke (WFS) fine particulate matter with a diameter less than 2.5 μm (PM2.5) in three major population centers (Anchorage, Fairbanks, and the Matanuska‐Susitna Valley) during the 2015–2019 wildfire seasons. To estimate WFS PM2.5, we utilized data from ground‐based monitors and satellite‐based smoke plume estimates. We implemented time‐stratified case‐crossover analyses with single and distributed lag models to estimate the effect of WFS PM2.5 on cardiorespiratory emergency department (ED) visits. On the day of exposure to WFS PM2.5, there was an increased odds of asthma‐related ED visits among 15–65 year olds (OR = 1.12, 95% CI = 1.08, 1.16), people >65 years (OR = 1.15, 95% CI = 1.01, 1.31), among Alaska Native people (OR = 1.16, 95% CI = 1.09, 1.23), and in Anchorage (OR = 1.10, 95% CI = 1.05, 1.15) and Fairbanks (OR = 1.12, 95% CI = 1.07, 1.17). There was an increased risk of heart failure related ED visits for Alaska Native people (Lag Day 5 OR = 1.13, 95% CI = 1.02, 1.25). We found evidence that rural populations may delay seeking care. As the frequency and magnitude of Alaskan wildfires continue to increase due to climate change, understanding the health impacts will be imperative. A nuanced understanding of the effects of WFS on specific demographic and geographic groups facilitates data‐driven public health interventions and fire management protocols that address these adverse health effects.
Keywords: Alaska, Alaska native, cardiorespiratory, epidemiology, particulate matter, smoke, wildfire
Key Points
The size and frequency of wildfires in Alaska are increasing, but there are no assessments of the direct health impacts of these events
In Alaska, wildfire smoke is positively associated with asthma emergency room visits, and this varies by age, sex, race, and geography
Alaska Native people had a higher risk of several cardiovascular outcomes during wildfire events
1. Introduction
Wildfires in Alaska have local, regional, and global implications for ecosystems and societies. Alaskan wildfires cause major landscape disturbances, which include degradation of permafrost and associated changes in vegetation cover, altered soil composition, and changes to water chemistry (Brown et al., 2016; Foster et al., 2019; Larouche et al., 2015; Mann et al., 2012; Sanford et al., 2015). The boreal‐, black spruce‐dominated forests covering much of the state are an important global carbon sink, but recent studies have shown that wildfires on these landscapes can be a significant source of carbon emissions, particularly as the average area burned each year grows (Turetsky et al., 2011; Veraverbeke et al., 2015; Walker et al., 2018). Wildfire suppression and management in Alaska are costly (Melvin et al., 2017), and the limited road network, vast forests, and dispersed rural populations add unique logistical challenges to fire management (Todd & Jewkes, 2006).
Recent assessments of trends in historical and future wildfire regimes in the western U.S. have not included Alaska (Brey et al., 2020; Dennison et al., 2014; Littell et al., 2018; Westerling, 2016), but almost one‐third of megafires (>100,000 acres) between 1997 and 2020 have occurred in the state (National Interagency Fire Center, 2020). The Alaskan wildfire season is getting longer; fires are starting earlier, and late season fires are becoming more common (Sanford et al., 2015). The frequency and extent of large fire years (in terms of acres burned) has increased over the past several decades (Kasischke et al., 2010; Markon et al., 2018; Pastick et al., 2017; Sanford et al., 2015; Thoman & Walsh, 2019). In parallel, the growing Alaskan population has coincided with increased fire occurrence at the wildland urban interface, frequently as a result of human‐caused ignitions (Calef et al., 2008, 2017). Additionally, wildland fires in remote areas outside human settlements are also becoming more extensive (Calef et al., 2008) and may represent a substantial source of smoke due to minimal suppression efforts (Melvin et al., 2017).
There have been a number of studies of health effects associated with wildfire smoke exposure in the western U.S. (Alman et al., 2016; Gan et al., 2017; Lassman et al., 2017; Phuleria et al., 2005; Reid, Brauer et al., 2016; Reid, Jerrett et al., 2016; Stowell et al., 2019; Wettstein et al., 2018), yet no epidemiological studies have been conducted in Alaska. Although the socio‐cultural context of perceptions of and adaptations to Alaskan wildfires have been broadly explored (Chapin et al., 2008; Huntington et al., 2006; Trainor et al., 2009), there have been no assessments of the direct impact of exposure to wildfire smoke (WFS) on human health. The intensifying wildfire regime, high frequency of megafires in the region, and the population distribution and demographics of Alaska provide a novel context for an epidemiologic assessment of WFS. Because the majority of the Alaskan population is concentrated in and around two urban areas (Anchorage and Fairbanks), the emergency department (ED) visits from these communities likely capture a significant portion of the acute cases of cardiorespiratory illness due to WFS in the state.
Although the composition of WFS depends on the fuel type, burn conditions, and atmospheric processing, current estimates suggest that 25% of primary fine particulate matter with a diameter less than 2.5 μm (PM2.5) in the U.S. are attributable to wildfires (US EPA, 2020). PM2.5 is a pollutant of primary public health concern given that these particles penetrate deeply into the lungs and interfere with gas exchange (US EPA, 2019). A significant challenge in epidemiologic studies of WFS is developing an accurate exposure assessment method that distinguishes WFS fine particulate matter from other sources of PM2.5. Previous studies have estimated WFS exposure from ground‐based measurements with geostatistical interpolation (Vora et al., 2011; Yao et al., 2016), satellite observations (Rappold et al., 2011; Wettstein et al., 2018), chemical transport model simulations (Alman et al., 2016; Haikerwal et al., 2015; Liu, Wilson, Mickley, Dominici, et al., 2017), or some combination of sources (Gan et al., 2017). Due to the clustering of a major portion of the Alaskan population, our study region does not necessitate extensive modeling approaches that are required to estimate WFS exposure in larger geographic domains. Empirical data from ground‐based monitors provides the most objective and reproducible exposure estimates of intraurban PM2.5 and have been widely implemented in small geographic areas (e.g., city and county) (Bell et al., 2004). However, distinguishing background PM2.5 from WFS requires additional data and methods to identify excess PM2.5 due to WFS.
In this study, we examined the effects of PM2.5 from recent wildfires on respiratory and cardiovascular ED visits in three major population centers in Alaska. The clustered populations allowed us to implement a novel exposure assessment approach for which a combination of measurements from in situ monitors and satellite observations were used to identify excess PM2.5 originating from WFS (O'Dell et al., 2019). Additionally, the large Alaska Native population provides an opportunity to explore potential differential effects of WFS exposure on this sub‐population.
2. Materials and Methods
2.1. Study Area
The study areas for this project were Anchorage, Fairbanks, and communities in the Matanuska‐Susitna Valley (Figure 1). The populations of these three boroughs (county equivalents) are ∼288, 97, and 108 thousand respectively, and Matanuska‐Susitna Valley is the fastest growing area of the state (U.S. Census Bureau, 2019). Together, these communities are home to over 65% of Alaskan residents. The three population centers included in our study are geographically and climatologically distinct. While the Matanuska‐Susitna valley is located only 35 miles north of Anchorage, the terrain opens up considerably into a series of river valleys. Fairbanks is located in the interior region of Alaska, over 350 miles north of Anchorage and the Matanuska‐Susitna Valley. Anchorage is characterized by a mild coastal climate with short, cool summers with average daytime temperatures ranging from ∼12.8°C to 25.6°C and average daytime winter temperatures between −15.0°C and −1.1°C (Koppen classification Dfc). The climate in Matanuska‐Susitna Valley is similar, with slightly more diurnal temperature variation because it is inland. Fairbanks has long cold winters and short warm summers with average monthly temperatures ranging between −22.2°C in January and 16.9°C in July (Koppen Dfc close to Dwc).
Figure 1.
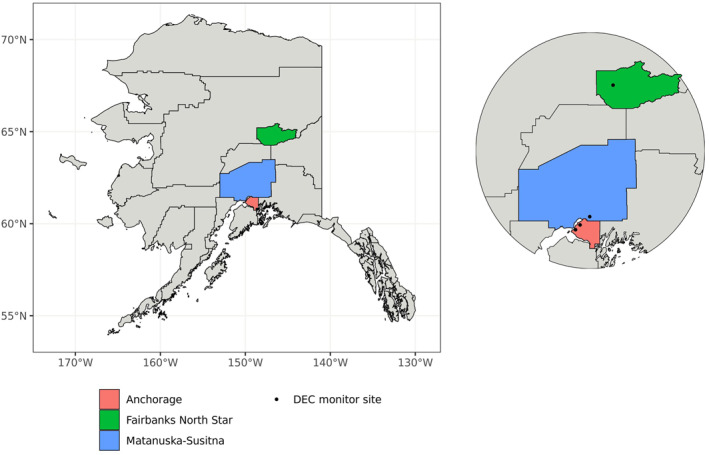
Map of Alaska, highlighting the major population centers in this study: Anchorage, Fairbanks, and communities in the Matanuska‐Susitna borough.
2.2. Exposure Characterization
PM2.5 data were obtained from ground‐monitoring sensors maintained by the Alaska Department of Environmental Conservation (DEC) for Environmental Protection Agency (EPA) regulatory monitoring. These data are a measure of PM2.5 from all sources, including mobile sources, woodstoves, and industry. The data provided by DEC included daily average data (based on hourly samples) and data from continuous 24‐h monitors at four sites located in Anchorage, Fairbanks, and the Matanuska‐Susitna Valley. At each location, there were one, two, or three monitors that were DEC‐designated as primary, secondary, or tertiary. Hourly aggregate data obtained from primary monitors were selected preferentially. Daily data were excluded if fewer than 75% of hourly measurements were taken or if the recorded PM2.5 concentration was implausible (e.g., ≤0 µg/m3). During the study period, there were two active DEC monitoring sites in Anchorage. If both sites had eligible data available, the two daily concentrations, which were highly concordant, were averaged. When daily PM2.5 data were missing from a study site for two or fewer days, the missing data were imputed by taking the average of the leading and lagging days around the missing period.
To identify the excess PM2.5 originating from WFS, we combined satellite information with data from the ground‐level monitors. For a day within the data set to be considered a wildfire day (WFD), two criteria needed to be met, which were determined using both PM2.5 data and satellite information on smoke plume locations (Brey et al., 2018). First, the daily PM2.5 concentration needed to exceed one standard deviation above the long‐term monthly mean for daily PM2.5, as determined using 2008–2019 DEC monitoring data for Anchorage and Matanuska‐Susitna Valley and 2009–2019 data for Fairbanks. Second, the DEC monitor site had to be located within 50 km of a smoke plume on that day. This criterion was assessed using shapefiles of daily smoke plume locations that were obtained from the NOAA Hazard Mapping System. If a smoke plume was identified within 50 km of a monitor site on the same day that it had been flagged as having a daily PM2.5 that exceeded one standard deviation above the long‐term monthly mean, then that day was identified as a WFD for that study area. WFS PM2.5 (i.e., PM2.5 attributed to wildland fires, as opposed to anthropogenic sources), was estimated by subtracting the long‐term monthly mean PM2.5 from the daily PM2.5 measured on WFDs. Using this method, non‐WFDs were assigned a WFS PM2.5 concentration of zero.
Daily temperature and daily relative humidity were included in the analyses as time‐varying confounders. Meteorological data were compiled from the NOAA Automated Surface Observing System (ASOS) monitoring network. For each study area, temperature and relative humidity data were accessed from the nearest ASOS monitor to the DEC monitor from which daily PM2.5 data were obtained. For days during which a temperature or relative humidity measurement was not available from the nearest ASOS monitor, data from the second or third closest monitor were used. Like the daily PM2.5 data obtained from DEC monitors, these data were excluded if fewer than 75% of hourly measurements were collected. Daily temperature and relative humidity values were imputed if two or fewer consecutive days had missing data.
2.3. Health Outcomes
Hospitalization records were requested from the Alaska Health Facilities Data Reporting Program (HFDR) for years during which hospital reporting to the HFDR was mandatory (2015–2019) and for Quarters 2 and 3, when wildfires are most likely to occur (April‐September). The HFDR Program collects data from private, municipal, state, and federal hospitals, hospitals operated by Alaska Native organizations, psychiatric hospitals, residential psychiatric treatment centers, intermediate care facilities, and ambulatory surgical facilities. Records were requested based on International Classification of Diseases, 9th and 10th Revision, Clinical Modification codes (ICD‐9‐CM and ICD‐10‐CM) indicative of cardiorespiratory health outcomes. HFDR data were filtered to only include ED encounters for which the primary diagnosis was cardiorespiratory‐related. Other visits were excluded from the data set to eliminate planned medical care encounters and to isolate acute episodes that could have been precipitated by transient WFS exposures.
Separate analyses were conducted for each of the following health concerns indicated by the primary diagnosis ICD‐9‐CM or ICD‐10‐CM code: Asthma, chronic obstructive pulmonary disease (COPD), pneumonia, acute bronchitis, arrhythmia, cerebrovascular disease, heart failure, ischemic heart disease, and myocardial infarction. Diagnosis codes were also grouped to conduct analyses on all respiratory and all cardiovascular encounters. Age, sex, and race were also included for patient encounters in the HFDR data set.
2.4. Statistical Analyses
The associations between WFS PM2.5 exposure and cardiorespiratory ED visits in the study areas were evaluated using a time‐stratified case crossover study design (Janes et al., 2005a, 2005b). Each cardiorespiratory ED encounter that was recorded by a hospital located within one of the study areas and that occurred during the wildfire season in 2015–2019 was considered a case. All cases were assigned an exposure equal to the 24‐h WFS PM2.5 concentration that was derived from measurements taken by the DEC monitor (or monitors) located in the same study area. For each case, referent days were selected as the same day of the week for the duration of the wildfire season of the same year. Exposures were assigned to referent days using the same method as case days. Conditional logistic regression was used to estimate the associations between cardiorespiratory ED visits and a 10 μg/m3 increase in 24‐h WFS PM2.5.
Single‐day conditional logistic regression models were used to determine the effect of WFS PM2.5 on cardiorespiratory ED visits using same‐day exposures as well as lags of up to 5 days (six single‐day models: Lagged days 0–5). Distributed lag models (DLMs) were used to assess the effect of each lagged WFS PM2.5 day while accounting for WFS PM2.5 concentrations on the other five lagged days. These models were constrained using a natural spline with three degrees of freedom (Gasparrini et al., 2017). Time‐varying confounders (daily relative humidity and temperature) were lagged in the same way as daily WFS PM2.5 concentrations.
To understand how the associations between WFS PM2.5 and cardiorespiratory ED visits varied among the geographically and climatologically unique regions in our study, we conducted stratified analyses by borough. Stratified analyses were also conducted by age, race, and sex. Age was categorized into three groups: <15, 15–65, and >65 years of age. Race was stratified into two groups: Alaska Native and non‐Alaska Native.
2.5. Sensitivity Analyses
We conducted two sensitivity analyses. First, because we don't have a direct measure of WFS PM2.5, we tested two thresholds for defining a WFD. In addition to the definition presented above where a WFD occurred when the average daily PM2.5 concentration was at least one standard deviation above the long‐term monthly mean, we also tested a less sensitive (i.e., more strict) threshold of two standard deviations. All single day lag models were conducted using both thresholds, and a change in the effect estimate of more than 10% was considered evidence of a significant effect.
The second sensitivity analysis explored the potential time‐varying confounding from daily ozone concentrations. Ozone was considered a priori to be a potential time‐varying confounder; however, ozone data were only available from the Fairbanks monitor and therefore could not be included in all models. We performed a sub‐analysis using only Fairbanks data that included daily ozone concentration in the models. A difference in the effect estimate of at least 10% was considered to be indicative of confounding by ozone.
All analyses were conducted using R (version 3.6.1). The “tidyverse” package was primarily used for cleaning; “ggplot2” was used to create figures; “sf” supported spatial analyses; conditional logistic regression analyses were conducted with the “survival” package; and “riem” was used to obtain NOAA ASOS monitoring data. Human subjects approval was provided by the University of Alaska‐Anchorage Institutional Review Board (Protocol #1596177).
3. Results
3.1. Wildfire Smoke PM2.5 Concentrations
Between 2008 and 2019, average daily PM2.5 concentrations in Anchorage, Fairbanks, and Matanuska‐Susitna Valley tended to be higher in the winter months compared to rest of the year (Table 1). During this baseline period, these communities experienced 63, 44, and 47 WFDs, respectively, where the local PM2.5 concentrations were greater than one standard deviation above the long‐term mean and this could likely be attributed to WFS. The majority of the WFDs occurred between 2015‐2019 (43 WFDs in Anchorage, 26 WFDs in Fairbanks, and 35 WFDs in Matanuska‐Susitna) (Figure 2). In Anchorage and Matanuska‐Susitna, WFDs occurred between June and August, while in Fairbanks, these days were distributed between May and September. The average daily PM2.5 concentrations on WFDs were 3 to 17 times higher than the concentrations on non‐WFDs across the study regions (Table 1).
Table 1.
Monthly Mean (SD) of PM2.5 During the 2008–2019 Wildfire Seasons as Measured by Epa Regulatory Monitors Maintained by the Alaska Department of Environmental Conservation
| Month | Anchorage | Fairbanks north star | Matanuska‐susitna | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Long‐term | Non‐WFD during study period | WFD during study period | Long‐term | Non‐WFD during study period | WFD during study period | Long‐term | Non‐WFD during study period | WFD during study period | |
| January | 8.48 (5.54) | – | – | 23.21 (13.11) | – | – | 13.44 (12.31) | – | – |
| February | 6.68 (4.07) | – | – | 19.74 (11.57) | – | – | 10.10 (8.70) | – | – |
| March | 5.17 (2.38) | – | – | 10.90 (6.22) | – | – | 6.54 (6.04) | – | – |
| April | 4.69 (2.17) | 4.17 (1.86) | – | 5.18 (2.49) | 4.65 (2.51) | – | 3.70 (2.94) | 3.04 (2.73) | – |
| May | 4.26 (3.69) | 3.24 (1.45) | – | 4.22 (2.95) | 3.50 (1.83) | 10.99 (4.45) | 3.99 (4.15) | 2.88 (1.97) | – |
| June | 4.74 (3.99) | 3.73 (2.55) | 17.00 (8.07) | 8.36 (19.40) | 4.93 (6.52) | 84.13 (45.15) | 3.40 (3.54) | 2.74 (2.12) | 13.91 (5.05) |
| July | 5.39 (4.93) | 4.26 (2.08) | 18.74 (9.09) | 9.50 (24.17) | 8.73 (17.64) | 98.33 (61.97) | 3.88 (4.36) | 3.04 (2.14) | 13.04 (4.22) |
| August | 4.89 (7.51) | 4.24 (2.40) | 40.85 (21.21) | 4.28 (6.14) | 3.01 (2.03) | 17.80 (8.71) | 3.46 (4.61) | 2.32 (1.97) | 19.75 (7.43) |
| September | 3.48 (1.83) | 3.83 (2.06) | – | 4.33 (2.26) | 3.73 (1.75) | 13.37 (1.95) | 2.87 (3.07) | 2.56 (3.61) | – |
| October | 5.01 (2.79) | – | – | 9.55 (8.01) | – | – | 6.37 (5.36) | – | – |
| November | 7.90 (4.84) | – | – | 17.19 (12.86) | – | – | 13.11 (10.10) | – | – |
| December | 8.64 (5.66) | – | – | 20.04 (12.59) | – | – | 13.18 (10.97) | – | – |
Figure 2.
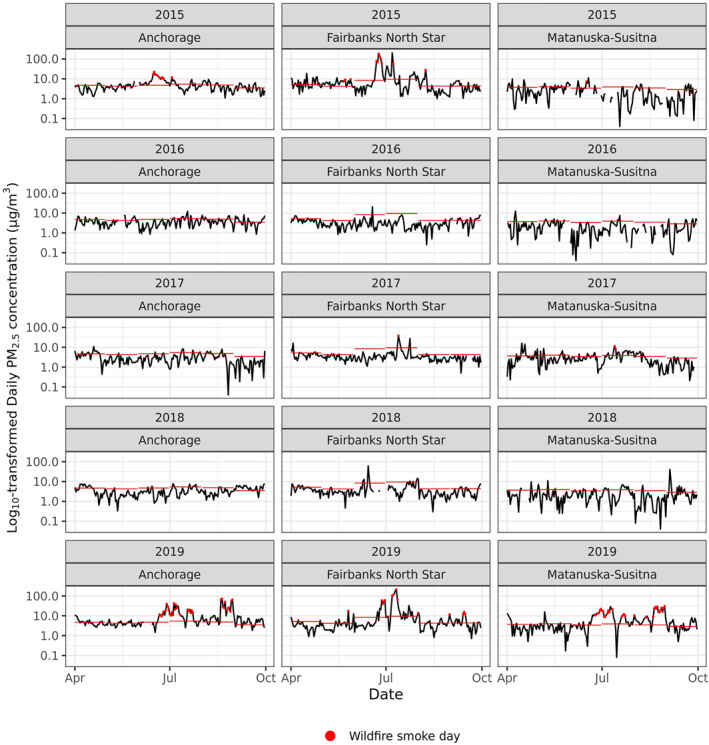
Log10‐transformed daily average PM2.5 concentration (μg/m3) between April 1 and September 30 in the Anchorage, Fairbanks, and Matanuska‐Susitna regions between 2015 and 2019. Lines mark the long‐term (2008–2019) monthly average in each community and dots mark wildfire smoke days, as defined for this study.
3.2. Cardiorespiratory Emergency Department Visits
Over 26,600 cardiorespiratory ED visits occurred during the study period, almost 80% of which were due to respiratory outcomes (n = 21,263) (Table 2). The majority of cardiovascular ED visits were male patients (58.0%), and slightly over half of the patients with respiratory concerns were female (54.2%). Almost half of the cardiovascular visits can be attributed to those older than 65 years old (44.4%). Very few patients less than 15 years old experienced ED visits for cardiovascular concerns (<1% of all cardiovascular ED visits); however, 16.1% of the respiratory visits were made by this age group, primarily for asthma and bronchitis. Almost one‐fifth of respiratory ED visits were made by people older than 65 years old, primarily for COPD and pneumonia. Of all ED visits due to respiratory outcomes, 29.2% were made by Alaska Native people; for cardiovascular outcomes, only 18.2% of patients were Alaska Native.
Table 2.
Summary of Cardiorespiratory Emergency Department (ED) Visits to Anchorage, Fairbanks, and Matanuska‐Susitna Valley Area Hospitals Between 2015‐2019
| Diagnosis | ICD‐9‐CM; ICD‐10‐CM codes | All (n) | Sex | Age | Race a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Female (%) | Male (%) | <15 years (%) | 15–65 years (%) | >65 years (%) | Alaskan Native (%) | Non‐Alaskan Native (%) | |||
| Respiratory | |||||||||
| Asthma | 493; J45 | 6422 | 55.8 | 44.2 | 20.5 | 73.5 | 6 | 31.8 | 65.1 |
| COPD | 490‐492, 494, 496; J40‐J44, J47 | 4868 | 53.9 | 46.1 | 0.8 | 62.2 | 36.9 | 22.7 | 74.7 |
| Pneumonia | 480‐486; J12‐J18 | 4889 | 50.1 | 49.9 | 19 | 59.2 | 21.8 | 31.8 | 64.7 |
| Bronchitis | 466; J20‐J22 | 5084 | 56.4 | 43.6 | 22.4 | 65.4 | 12.1 | 29.8 | 66.7 |
| All respiratory | 21263 | 54.2 | 45.8 | 16.1 | 65.7 | 18.2 | 29.2 | 67.6 | |
| Cardiovascular | |||||||||
| Arrhythmia | 427; I46‐I49 | 3256 | 42.3 | 57.7 | 1.4 | 57 | 41.6 | 15.2 | 80.4 |
| Cerebrovascular | 430‐438; I60‐I63, I65‐I69, G45, I23 | 868 | 47.7 | 52.3 | 0 | 48.3 | 51.7 | 15.9 | 78.8 |
| Ischemic | 410‐414; I20‐I22, I24‐I25 | 361 | 32.1 | 67.9 | 0.3 | 51.8 | 47.9 | 17.2 | 77.3 |
| Myocardial b | 410; I21‐I22 | 141 | 31.2 | 68.8 | 0 | 61 | 39 | 22 | 68.8 |
| Heart failure | 428; I50 | 871 | 39.5 | 60.5 | 0.1 | 53.6 | 46.3 | 31.9 | 65 |
| All cardiovascular | 5356 | 42 | 58 | 0.9 | 54.7 | 44.4 | 18.2 | 77.4 | |
| Total | 26619 | 51.7 | 48.3 | 13.1 | 63.5 | 23.5 | 27 | 69.6 | |
| Anchorage | Fairbanks North Star | Matanuska‐Susitna | |||||||
| 18279 | 4793 | 3547 | |||||||
Percentages may add to less than 100%; hospitalizations with “unknown” race was excluded.
Myocardial diagnoses are a subset of ischemic diagnoses; hence, myocardial primary diagnoses are not included in the total cardiovascular category.
3.3. Single Lag Models
In the single lag models, the strongest association between WFS PM2.5 and ED visits for cardiorespiratory outcomes was observed for asthma. The largest association was at Lag 0 (OR = 1.13, 95% CI = 1.10, 1.16) with elevated odds ratios for Lags 1–4 associated with a 10 μg/m3 increase in WFS PM2.5 (Lag 1 OR = 1.07, 95% CI = 1.04, 1.10; Lag 2 OR = 1.06, 95% CI = 1.03, 1.10; Lag 3 OR = 1.08, 95% CI = 1.05, 1.11; Lag 4 OR = 1.05, 95% CI = 1.02, 1.08) (Figure 3). We also found an increased odds of ED visits due to ischemic heart disease (Lag Day 2 OR = 1.18, 95% CI = 1.04, 1.33). When ED visits were grouped into “all respiratory” visits, we observed an association with same‐day WFS PM2.5 (OR = 1.04, 95% CI = 1.02, 1.06).
Figure 3.
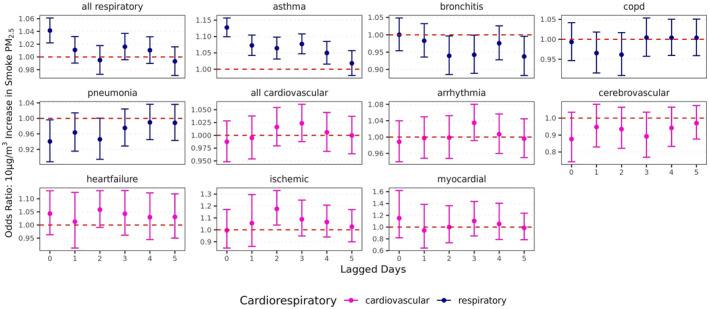
Unconstrained daily lag effects of a 10 µg/m3 increase in wildfire smoke PM2.5 on likelihood of respiratory and cardiovascular hospitalizations. Models adjusted for lagged temperature and relative humidity on the same day of lagged wildfire smoke exposure.
Inverse effects for ED visits for two respiratory outcomes were also observed. There was a decreased odds of pneumonia ED visits associated with WFS PM2.5 at Lag 0 (OR = 0.94, 95% CI = 0.89, 0.996). Bronchitis ED visits were also observed to have an inverse association with WFS PM2.5 (Lag 2 OR = 0.94, 95% CI = 0.89, 0.997; Lag 5 OR = 0.94, 95% CI = 0.88, 0.996).
3.4. Distributed Lag Models
Similar to the single lag models, the distributed lag models suggest that the strongest relationship between WFS PM2.5 and cardiorespiratory outcomes exists for ED visits for asthma (Figure 4 and supporting information). There were significantly increased odds of asthma ED visits associated with a 10 μg/m3 increase in WFS PM2.5 when controlling for the WFS PM2.5 during the 5 days preceding a patient encounter (Lag 0 OR = 1.11, 95% CI = 1.07, 1.14; Lag 1 OR = 1.04, 95% CI = 1.03, 1.05). We also observed an increased odds of ED visits for “all respiratory” causes on the day of exposure to WFS PM2.5 (OR = 1.04, 95% CI = 1.02, 1.07).
Figure 4.
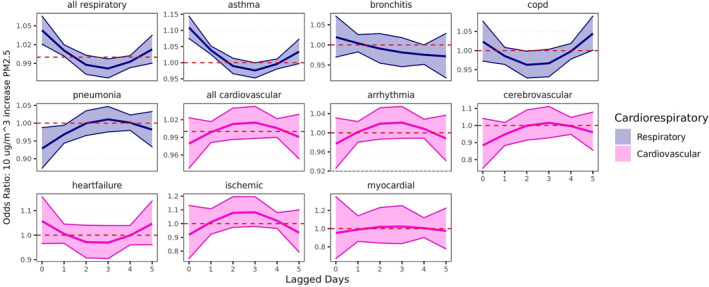
Constrained distributed lag effects of a 10 µg/m3 increase in wildfire smoke PM2.5 on likelihood of respiratory and cardiovascular hospitalizations. Models adjusted for daily temperature and relative humidity lagged on the same day as wildfire smoke exposure.
Weak associations were found for COPD visits that had not been observed in the single lag models. There was a decrease in ED visits for COPD at Lag 2 (OR = 0.96, 95% CI = 0.93, 0.998 and Lag 3 (OR = 0.97, 95% CI = 0.93, 1.00), although the 95% confidence interval for the estimate for Lag 3 contained the null. We also found an increased odds of visits for COPD 5 days after a WFD (OR = 1.04, 95% CI = 1.00, 1.09) when controlling for WFS PM2.5 during the 5 days preceding a patient encounter, but the 95% confidence interval for this estimate contained the null.
The inverse associations between WFS PM2.5 and bronchitis and pneumonia ED visits were consistent in the adjusted results. Significant inverse associations were observed for pneumonia ED visits (Lag 0 OR = 0.93, 95% CI = 0.87, 0.99; Lag 1 OR = 0.97, 95% CI = 0.94, 0.99) and for bronchitis ED visits (Lag 4 OR = 0.98, 95% CI = 0.95, 1.00), although the 95% confidence interval around the bronchitis risk estimate included the null.
Unlike the single day lag models, there were no significant associations between WFS PM2.5 and any of the cardiovascular outcomes in the distributed lag models.
3.5. Stratified Analyses
Stratified analyses revealed significant differences in the association between WFS PM2.5 and a number of cardiorespiratory outcomes by age, sex, race, and geographic location (supporting Tables).
3.5.1. Respiratory Outcomes
The strongest effects for asthma were observed on a WFD or the following day across all strata (Table S1). Particularly on the day of exposure to WFS PM2.5, there was an increased odds of asthma ED visits among the 15–65 year old age group (OR = 1.12, 95% CI = 1.08, 1.16) and among people older than 65 years of age (OR = 1.15, 95% CI = 1.01, 1.31). Asthma‐related ED visits were also more likely among Alaska Native people (OR = 1.15, 95% CI = 1.09, 1.23) compared to non‐Alaska Native people (OR = 1.09, 95% CI = 1.05, 1.13), although the risk of ED admission for asthma was elevated in both groups. Asthma ED visits were also more likely in Anchorage (OR = 1.10, 95% CI = 1.05, 1.15) and Fairbanks (OR = 1.12, 95% CI = 1.07, 1.17) compared to the Matanuska‐Susitna Valley where there was no association between WFS PM2.5 and asthma ED visits on the day of exposure (OR = 1.00, 95% CI = 0.72, 1.37). There was also an elevated risk of asthma ED visits among females (OR = 1.13, 95% CI = 1.08, 1.18) compared to males (OR = 1.08, 95% CI = 1.03, 1.14) on the day of exposure to elevated PM2.5 from WFS. Two to four days after a WFD, the odds of asthma ED visits decreased across most strata (Table S1). Results were similar when ED visits were grouped into “all respiratory” visits (Table S5).
Stratified results for COPD show a weak inverse association between WFS PM2.5 and ED visits among 15–65 year olds (OR = 0.95, 95% CI = 0.91, 1.00) and people living in Anchorage (OR = 0.95, 95% CI = 0.90, 0.998 2 days after exposure (Table S3), although the 95% confidence interval for the age strata included the null. However, we found an increased odds of COPD ED visits 5 days after a WFD among 15–65 year olds (OR = 1.06, 95% CI = 1.00, 1.12), people living in Anchorage (OR = 1.09, 95% CI = 1.02, 1.16), and Alaska Natives people (OR = 1.09, 95% CI = 1.00, 1.17), although the 95% confidence interval for the age and race strata included the null.
The strongest effect for bronchitis in the stratified analyses was an inverse association between WFS PM2.5 and ED visits among residents of Matanuska‐Susitna Valley 4 days after a WFD (OR = 0.65, 95% CI = 0.46, 0.92) (Table S2). Other inverse associations were observed among people less than 15 years of age (Lag 1 OR = 0.93, 95% CI = 0.86, 0.999), 15–65 year olds (Lag 4 OR = 0.96, 95% CI = 0.93, 0.99; Lag 5 OR = 0.92, 95% CI = 0.84, 1.00), and females (Lag 4 OR = 0.96, 95% CI = 0.93, 0.999), but the 95% confidence interval around the estimate for Lag 5 among 15–65 year olds included the null. Similarly, we found only weak inverse associations between WFS PM2.5 and ED visits for pneumonia (Table S4). These associations were most apparent among non‐Alaska Native people and people living in Fairbanks within a day of exposure to WFS.
3.5.2. Cardiovascular Outcomes
Relative to the respiratory outcomes described above, the associations between WFS PM2.5 and ED visits for individual cardiovascular outcomes were less apparent in the stratified analyses. There were no significant associations for cerebrovascular (Table S7), ischemia (Table S9), or myocardial infarction‐related (Table S10) ED visits in the stratified analyses. There were increased odds of arrhythmia‐related ED visits associated with a 10 μg/m3 increase in WFS PM2.5 among Alaska Native people (Lag 3 OR = 1.08, 95% CI = 1.00, 1.16; Lag 4 OR = 1.06, 95% CI = 1.02, 1.10), although the 95% confidence interval on Lag 3 included the null (Table S6). There was a stronger association for Alaska Native people (Lag 5 OR = 1.13, 95% CI = 1.02, 1.25) and males (Lag 0 OR = 1.12, 95% CI = 1.01, 1.24) for heart failure‐related ED visits (Table S8). When all cardiovascular ED visits were grouped, we again observed increased risk among Alaska Native people (Lag 4 OR: 1.04, 95% CI = 1.01, 1.07) (Table S11). Additionally, we found very strong associations among residents living in the Matanuska‐Susitna Valley that were not apparent in the unstratified models. On the day of exposure, there was a strong inverse association between WFS PM2.5 and ED visits for all cardiovascular causes (OR = 0.70, 95% CI = 0.50, 0.98). However, 2–3 days later, we observed an increase in the risk of cardiovascular ED visits within this population (Lag 2 OR = 1.29, 95% CI = 1.04, 1.60; Lag 3 OR = 1.29, 95% CI = 1.05, 1.60).
3.6. Sensitivity Analyses
When comparing the effect estimates from the single‐day lag models using the one and two standard deviation thresholds for defining WFDs, only one relationship (the association between WFS PM2.5 and myocardial infarction ED visits on Lag 1) was found to be significantly different by at least 10%. Using the two standard deviation cutoff, the association between WFS PM2.5 and myocardial infarction‐related ED visits was OR = 0.80 (95% CI = 0.46, 1.37). Using the one standard deviation cutoff, the OR = 0.94 (95% CI = 0.64, 1.38).
In our second sensitivity analysis to assess potential confounding by ozone, effect estimates and surrounding confidence intervals for daily ozone concentrations were extremely unstable. For this reason, we were not able to determine if daily ozone concentrations are an important confounder of the relationship between WFS PM2.5 and cardiorespiratory ED visits, and this limitation is discussed below.
4. Discussion
Over half of the Alaskan population was exposed to 26–43 days of poor air quality due to WFS between 2015 and 2019. These elevated levels of WFS PM2.5 were positively associated with ED visits for a number of cardiorespiratory health outcomes. In particular, a 10 μg/m3 increase in WFS PM2.5 was associated with up to a 13% increase in the odds of an asthma‐associated ED visit on the day of smoke exposure. This association was still apparent after adjusting for exposure to WFS PM2.5 on the 5 days preceding a patient encounter. The relationships between WFS PM2.5 and asthma varied by age, race, sex, and geography. Alaska Native people, people over 15 years of age, females, and people living in Anchorage and Fairbanks were more likely to seek emergency care for asthma during wildfire events. These ED visits were more likely to occur on the same day as the exposure to elevated WFS PM2.5 concentrations; the odds of asthma‐related ED visits decreased 2–3 days later across most of these population groups.
There were weaker and mixed associations between WFS PM2.5 and ED visits for other respiratory outcomes such as COPD, bronchitis, and pneumonia. The strongest effect among these was for bronchitis, where we observed a 35% decrease in the odds of ED visits in Matanuska‐Susitna Valley 4 days after exposure to elevated levels of WFS PM2.5.
Although there were fewer associations between WFS PM2.5 and cardiovascular outcomes, there were notable disparities in risk for Alaska Native people. There were increased odds of ED visits for arrhythmia, heart failure, and “all cardiovascular” causes among this population during wildfire events. There was also a unique pattern in ED visits for cardiovascular causes in the Matanuska‐Susitna Valley. While cardiovascular ED visits among this population initially decreased on days with elevated WFS PM2.5, we observed a 30% increase in the odds of cardiovascular ED visits 2–3 days later.
Our results are consistent with a growing literature on the respiratory health impacts of exposure to WFS, which demonstrates associations with exacerbations of asthma and acute all‐cause respiratory morbidity (Cascio, 2018; Kondo et al., 2019; Liu et al., 2015; Reid, Brauer, et al., 2016; Reid & Maestas, 2019). Similarly, the weak associations with respiratory tract infections are not unexpected based on similar studies in other regions. Previous studies of WFS exposure and respiratory infections have sometimes grouped bronchitis and pneumonia, while more recent studies have assessed these health outcomes independently (Reid & Maestas, 2019). Many of the combined analyses found positive associations between WFS exposures and respiratory infections (Delfino et al., 2009; Morgan et al., 2010; Rappold et al., 2011). However, only a handful of recent studies assessing pneumonia independently found significant associations with WFS (Hutchinson et al., 2018), and in one case, this finding was not consistent across exposure methods (Gan et al., 2017). Few studies have found significant associations between WFS and bronchitis (Reid et al., 2019), although Hutchinson et al. (2018) found an association for ED and outpatient visits but not for hospitalizations. Similarly, there is not a consensus in the literature on the associations between WFS exposure and COPD, nor is there a consistent pattern even among studies using similar exposure assessment methodologies and data sources for health endpoints (Reid & Maestas, 2019).
There is mixed evidence on the relationship between WFS exposure and cardiovascular morbidity (Cascio, 2018; Reid, Brauer, et al., 2016). Although some of this discrepancy may be due to the variety of exposure assessment methods and sources of health data, these inconsistencies are apparent even among studies that utilized emergency department visits to measure cardiovascular outcomes. In a comparison of counties exposed to a spike in PM2.5 from a peat bog wildfire to nearby counties that were unexposed, Rappold et al. (2011) found an increased risk for heart failure, but not for myocardial infarction or cardiac dysrhythmias. In a case‐crossover study of daily smoke exposures in Sydney, Australia over more than a decade, Johnston et al. (2014) found significant associations between a smoke day and emergency department visits for ischemic heart disease among people >65 years of age and for cardiac failure among 15–64 year olds on specific lag days, but no associations for cerebrovascular outcomes or grouped cardiovascular events. They also found an inverse association with arrhythmias but concluded that this was likely a spurious result. Haikerwal et al. (2015) focused on cardiovascular outcomes after a two‐month period of intense wildfires in Victoria, Australia. They found an increased risk of ED visits for ischemic heart disease, but no association for acute myocardial infarction or angina.
Only a small number of studies have explored how these cardiorespiratory health impacts change across demographic groups, and of those that have, results are inconsistent (Alman et al., 2016; Hanigan et al., 2008; Liu, Wilson, Mickley, Ebisu et al., 2017; Reid, Jerrett, et al., 2016; Wettstein et al., 2018). While some found the strongest associations between smoke and cardiovascular or respiratory outcomes among those 65 years and older (Alman et al., 2016; Wettstein et al., 2018), others have found that individuals aged 20–64 had a higher risk of COPD‐related morbidity, and still others found that youth were at highest risk of respiratory and other chest symptoms compared to other age groups (Tinling et al., 2016). Gan et al. (2017) found that the effect of age (and sex) depended on the WFS exposure estimation method. In comparisons of sex, some found stronger associations for asthma and hypertension (Reid, Jerrett, et al., 2016), respiratory illness (Liu, Wilson, Mickley, Ebisu et al., 2017), or pneumonia, acute bronchitis, and upper respiratory infections (Rappold et al., 2011) among women, while others found either no association (Tinling et al., 2016; Wettstein et al., 2018) or elevated risk of COPD‐related morbidity (Rappold et al., 2011) and reduced lung capacity (Kim et al., 2017) among men. The few studies that have assessed the impact of socioeconomic status or income on cardiorespiratory health outcomes during wildfires have found marginal to no effect (Liu, Wilson, Mickley, Ebisu et al., 2017; Rappold et al., 2012; Reid, Jerrett, et al., 2016). Race has not been rigorously examined in this context. There is some evidence of increased risk of hospitalization for respiratory illness associated with WFS among African‐Americans in the western U.S. (Liu, Wilson, Mickley, Ebisu et al., 2017), and among Indigenous people in Australia (Hanigan et al., 2008). A recent modeling study found that because of their geographic distribution in areas of high WFS concentration in Interior Alaska, African‐Americans and Alaskan Athabascans may be more likely to be exposed (Woo et al., 2020).
This is the first study to show an increased risk of adverse health outcomes associated with WFS among Alaska Native people. In particular, the associations with arrhythmia, heart failure, and “all cardiovascular” causes are likely due to underlying disparities in heart disease in this population. The heart disease mortality rate among Alaska Native people is 1.6 times the rate of the general Alaskan population (Alaska Native = 218.6/100,000; All Alaskans = 133.4/100,000) and 1.3 times the rate of the U.S. population (165/100,000) (AK BRFSS, 2017). The increased risk of asthma ED visits among Alaska Native people found in this study may be due to a similar disparity in underlying asthma prevalence (AK BRFSS, 2018). It may also be related to increased personal exposure due to differences in the amount of time spent outside or use of home modifications to prevent exposure to WFS indoors.
For three of the health effects assessed here (asthma, bronchitis, and all cardiovascular outcomes), we found either no association (asthma), a negative association (bronchitis), or evidence of a delayed association (all cardiovascular outcomes) during wildfire events in the Matanuska‐Susitna Valley. Of the three population centers, the Matanuska‐Susitna Valley is the most remote, and many people in this area live on rural properties off‐the‐grid, further from health care facilities. It is possible that residents in this rural region may avoid or delay seeking healthcare during wildfires due to the relative hassle or choice to “wait it out.” In contrast, our results show that people living in the more urban centers of Anchorage and Fairbanks are more likely to present at the ED with asthma exacerbations within 1–2 days of exposure to WFS PM2.5. Others have noted disparities in travel time as an important indicator of healthcare access among rural populations in the U.S. and Canada, particularly in the context of rapid care for acute cardiovascular morbidity (Pedigo & Odoi, 2010; Scott et al., 1998).
This study has a number of limitations. Ozone data were only available for one of the population centers in our study, and they were not sufficient to assess confounding. Although there is a large literature on the adverse health effects of exposure to ambient ozone (Chen et al., 2007; Mirowsky et al., 2017; Thurston & Ito, 2001), there are very few studies that have examined the health effects of ozone from wildfires (Azevedo et al., 2011; Reid et al., 2019; Tham et al., 2009). Reid et al. (2019) modeled daily 24‐h average exposures to PM2.5 and 8‐h maximum ozone concentrations during the 2008 Northern California wildfires and ran single and multipollutant models for a number of respiratory health outcomes. They found that ozone was not significantly associated with any respiratory hospitalizations but that asthma and “all respiratory” hospitalizations were associated with PM2.5 after adjusting for ozone. Although there is currently not clear evidence of an association between exposure to wildfire‐related ozone and adverse health effects, future studies should continue to incorporate this pollutant to help build our understanding of its potential role in the observed health effects during these events.
One of the primary challenges in WFS exposure assessments is distinguishing the PM2.5 originating from WFS from ambient PM2.5 originating from all other sources, including mobile sources, industry, or wood‐burning stoves (Kaulfus et al., 2017; O'Dell et al., 2019). Here we used data from ground‐based monitors, which likely provides the most realistic assessment of ground‐level exposures to PM2.5. Our methodology for distinguishing WFS PM2.5 from ambient PM2.5 relied on a subjective threshold of PM2.5 concentrations greater than one standard deviation above the long‐term mean concentration. Although we tested an additional threshold and found that the use of one threshold or the other did not substantially affect our results, it is possible that we are misclassifying some of the exposure to PM2.5 originating from WFS when it could be from other PM2.5 sources.
Although the adverse health impacts of chronic exposure to poor air quality are well‐documented (Eftim et al., 2008), little is known about the impact of seasonal exposures on increasing sensitivity to air pollution. Recent work in Montana has shown that PM2.5 exposure during the wildfire season is associated with increased influenza the following winter (Landguth et al., 2020). Fairbanks, Alaska has been designated as a nonattainment area based on the 24‐h PM2.5 National Ambient Air Quality Standard, in part due to a high prevalence of wood‐burning stoves and wintertime atmospheric inversions in the region (Ye & Wang, 2020). Future work could assess the impact of wintertime exposures on sensitivity to WFS PM2.5 the following summer.
This study focused on major population centers in Alaska where air quality data were available from ground‐based regulatory monitors. Future studies exploring the health impacts of WFS in rural Alaskan communities could use modeled or satellite‐based estimates of WFS exposure. Our results can be used to target public health interventions to prevent WFS‐related health issues. For example, public health efforts could include: 1) testing the EPA SmokeSense phone application (Rappold et al., 2019) among people with asthma in different age and cultural groups, 2) tracking the concentration of WFS PM2.5 in addition to total PM2.5, 3) strengthening public health recommendations about avoiding outdoor sports games or practice when WFS PM2.5 concentration is elevated, and 4) disseminating information to health care providers about discussing patient preparedness plans for dealing with WFS, particularly among patients with chronic cardiorespiratory diseases. Additional qualitative studies that include participants across a broad age range, cultural groups, underlying chronic medical conditions, and geographies can help elucidate care‐seeking behavior during wildfire events.
5. Conclusions
This is the first epidemiologic assessment of the association between WFS and cardiorespiratory health in Alaska. We found significant effects for asthma, particularly among Alaska Native people, people >15 years of age, females, and people living in Anchorage and Fairbanks on the day of exposure to elevated levels of WFS. There were weaker associations between WFS and COPD, bronchitis, and pneumonia. We observed a higher risk of adverse cardiovascular outcomes among Alaska Native people, compared to non‐Alaska Native people. People living in a more rural region (Matanuska‐Susitna Valley) had a lower odds of visiting the ED for both respiratory and cardiovascular issues compared to those living in the more urban population centers of Anchorage and Fairbanks. As the frequency and magnitude of wildfires continue to increase in Alaska due to climate change, understanding the health impacts of these events will be imperative. Our findings suggest that, even in relatively small communities compared to many western U.S. cities affected by recurring wildfires, smoke from long‐burning fires can cause substantial morbidity. This may have implications for decisions around prescribed burns or minimized suppression efforts to reduce fuel loads. A nuanced understanding of the effects of WFS exposure on specific demographic and geographic groups facilitates data‐driven public health interventions and fire management protocols that account for these adverse health effects.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
Table S9
Table S10
Table S11
Acknowledgments
Funding for this study was provided by the National Science Foundation under award #OIA‐1753748. S. Magzamen's contribution to the project was supported by the AJ Kauvar Foundation.
Hahn, M. B. , Kuiper, G. , O'Dell, K. , Fischer, E. V. , & Magzamen, S. (2021). Wildfire smoke is associated with an increased risk of cardiorespiratory emergency department visits in Alaska. GeoHealth, 5, e2020GH000349. 10.1029/2020GH000349
Data Availability Statement
Air quality data are available for download from the EPA Air Quality Program (https://www.epa.gov/outdoor-air-quality-data). Data from the Alaska Health Facilities Data Reporting Program are not publically available due to privacy concerns. Data can be requested from the Alaska Department of Health and Social Services with appropriate Institutional Review Board approvals (http://dhss.alaska.gov/dph/VitalStats/Pages/HFDR/default.aspx).
References
- AK BRFSS . (2017). Complete health indicator report of heart disease mortality rate. Retrieved from http://ibis.dhss.alaska.gov/indicator/complete_profile/HeaDisDth.html
- AK BRFSS . (2018). Health indicator report of asthma—Current asthma—adults (18+). Retrieved from http://ibis.dhss.alaska.gov/indicator/view/AsthAdltPrev.AK_US_time.html
- Alman, B. L. , Pfister, G. , Hao, H. , Stowell, J. , Hu, X. , Liu, Y. , & Strickland, M. J. (2016). The association of wildfire smoke with respiratory and cardiovascular emergency department visits in Colorado in 2012: A case crossover study. Environmental Health, 15(1), 64. 10.1186/s12940-016-0146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo, J. M. , Gonçalves, F. L. T. , & de Fátima Andrade, M. (2011). Long‐range ozone transport and its impact on respiratory and cardiovascular health in the north of Portugal. International Journal of Biometeorology, 55(2), 187–202. 10.1007/s00484-010-0324-2 [DOI] [PubMed] [Google Scholar]
- Bell, M. L. , Samet, J. M. , & Dominici, F. (2004). Ozone and mortality: A meta‐analysis of time‐series studies. Department of Biostatistics Working Papers, Working Paper 57. Johns Hopkins University. Retrieved from http://biostats.bepress.com/jhubiostat/paper57
- Brey, S. J. , Barnes, E. A. , Pierce, J. R. , Swann, A. L. S. , & Fischer, E. V. (2020). Past variance and future projections of the environmental conditions driving western U.S. summertime wildfire burn area. Earth's Future, 9(2), e2020EF001645. 10.1029/2020ef001645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey, S. J. , Ruminski, M. , Atwood, S. A. , & Fischer, E. V. (2018). Connecting smoke plumes to sources using hazard mapping system (HMS) smoke and fire location data over North America. Atmospheric Chemistry and Physics, 18, 1745–1761. 10.5194/acp-18-1745-2018 [DOI] [Google Scholar]
- Brown, D. R. N. , Jorgenson, M. T. , Kielland, K. , Verbyla, D. L. , Prakash, A. , & Koch, J. C. (2016). Landscape effects of wildfire on permafrost distribution in interior Alaska derived from remote sensing. Remote Sensing, 8(8), 654. 10.3390/rs8080654 [DOI] [Google Scholar]
- Calef, M. P. , McGuire, A. D. , & Chapin, F. S. (2008). Human influences on wildfire in Alaska from 1988 through 2005: An analysis of the spatial patterns of human impacts. Earth Interactions, 12(1), 1–17. 10.1175/2007EI220.1 [DOI] [Google Scholar]
- Calef, M. P. , Varvak, A. , & McGuire, A. D. (2017). Differences in human versus lightning fires between urban and rural areas of the boreal forest in interior Alaska. Forests, 8(11), 422. 10.3390/f8110422 [DOI] [Google Scholar]
- Cascio, W. E. (2018). Wildland fire smoke and human health. The Science of the Total Environment, 624, 586–595. 10.1016/j.scitotenv.2017.12.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin, F. S. , Trainor, S. F. , Huntington, O. , Lovecraft, A. L. , Zavaleta, E. , Natcher, D. C. , et al. (2008). Increasing wildfire in Alaska's boreal forest: Pathways to potential solutions of a wicked problem. BioScience, 58(6), 531–540. 10.1641/B580609 [DOI] [Google Scholar]
- Chen, C. , Arjomandi, M. , Balmes, J. , Tager, I. , & Holland, N. (2007). Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environmental Health Perspectives, 115(12), 1732–1737. 10.1289/ehp.10294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino, R. J. , Brummel, S. , Wu, J. , Stern, H. , Ostro, B. , Lipsett, M. , et al. (2009). The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occupational and Environmental Medicine, 66(3), 189–197. 10.1136/oem.2008.041376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison, P. E. , Brewer, S. C. , Arnold, J. D. , & Moritz, M. A. (2014). Large wildfire trends in the western United States, 1984–2011. Geophysical Research Letters, 41(8), 2928–2933. 10.1002/2014GL059576 [DOI] [Google Scholar]
- Eftim, S. E. , Samet, J. M. , Janes, H. , McDermott, A. , & Dominici, F. (2008). Fine particulate matter and mortality. Epidemiology, 19(2), 209–216. 10.1097/EDE.0b013e3181632c09 [DOI] [PubMed] [Google Scholar]
- Foster, A. C. , Armstrong, A. H. , Shuman, J. K. , Shugart, H. H. , Rogers, B. M. , Mack, M. C. , et al. (2019). Importance of tree‐ and species‐level interactions with wildfire, climate, and soils in interior Alaska: Implications for forest change under a warming climate. Ecological Modelling, 409, 108765. 10.1016/j.ecolmodel.2019.108765 [DOI] [Google Scholar]
- Gan, R. W. , Ford, B. , Lassman, W. , Pfister, G. , Vaidyanathan, A. , Fischer, E. , et al. (2017). Comparison of wildfire smoke estimation methods and associations with cardiopulmonary‐related hospital admissions. GeoHealth, 1(3), 122–136. 10.1002/2017GH000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini, A. , Scheipl, F. , Armstrong, B. , & Kenward, M. G. (2017). A penalized framework for distributed lag non‐linear models. Biometrics, 73(3), 938–948. 10.1111/biom.12645 [DOI] [PubMed] [Google Scholar]
- Haikerwal, A. , Akram, M. , Monaco, A. D. , Smith, K. , Sim, M. R. , Meyer, M. , et al. (2015). Impact of fine particulate matter (PM2.5) exposure during wildfires on cardiovascular health outcomes. Journal of the American Heart Association, 4(7), e001653. 10.1161/JAHA.114.001653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan, I. C. , Johnston, F. H. , & Morgan, G. G. (2008). Vegetation fire smoke, indigenous status and cardio‐respiratory hospital admissions in Darwin, Australia, 1996–2005: A time‐series study. Environmental Health, 7(1), 42. 10.1186/1476-069X-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington, H. P. , Trainor, S. F. , Natcher, D. C. , Huntington, O. H. , DeWilde, L. , & Chapin, F. S., III . (2006). The significance of context in community‐based research: Understanding discussions about wildfire in Huslia, Alaska. Ecology and Society, 11(1). 10.5751/ES-01723-110140 [DOI] [Google Scholar]
- Hutchinson, J. A. , Vargo, J. , Milet, M. , French, N. H. F. , Billmire, M. , Johnson, J. , & Hoshiko, S. (2018). The San Diego 2007 wildfires and Medi‐Cal emergency department presentations, inpatient hospitalizations, and outpatient visits: An observational study of smoke exposure periods and a bidirectional case‐crossover analysis. PLOS Medicine, 15(7), e1002601. 10.1371/journal.pmed.1002601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes, H. , Sheppard, L. , & Lumley, T. (2005a). Case‐crossover analyses of air pollution exposure data: Referent selection strategies and their implications for bias. Epidemiology, 16(6), 717–726. 10.1097/01.ede.0000181315.18836.9d [DOI] [PubMed] [Google Scholar]
- Janes, H. , Sheppard, L. , & Lumley, T. (2005b). Overlap bias in the case‐crossover design, with application to air pollution exposures. Statistics in Medicine, 24(2), 285–300. 10.1002/sim.1889 [DOI] [PubMed] [Google Scholar]
- Johnston, F. H. , Purdie, S. , Jalaludin, B. , Martin, K. L. , Henderson, S. B. , & Morgan, G. G. (2014). Air pollution events from forest fires and emergency department attendances in Sydney, Australia 1996–2007: A case‐crossover analysis. Environmental Health, 13(1), 105. 10.1186/1476-069X-13-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke, E. S. , Verbyla, D. L. , Rupp, T. S. , McGuire, A. D. , Murphy, K. A. , Jandt, R. , et al. (2010). Alaska's changing fire regime—implications for the vulnerability of its boreal forests. Canadian Journal of Forest Research, 40(7), 1313–1324. 10.1139/X10-098 [DOI] [Google Scholar]
- Kaulfus, A. S. , Nair, U. , Jaffe, D. , Christopher, S. A. , & Goodrick, S. (2017). Biomass burning smoke climatology of the United States: Implications for particulate matter air quality. Environmental Science & Technology, 51(20), 11731–11741. 10.1021/acs.est.7b03292 [DOI] [PubMed] [Google Scholar]
- Kim, Y. , Knowles, S. , Manley, J. , & Radoias, V. (2017). Long‐run health consequences of air pollution: Evidence from Indonesia's forest fires of 1997. Economics and Human Biology, 26, 186–198. 10.1016/j.ehb.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Kondo, M. C. , De Roos, A. J. , White, L. S. , Heilman, W. E. , Mockrin, M. H. , Gross‐Davis, C. A. , & Burstyn, I. (2019). Meta‐analysis of heterogeneity in the effects of wildfire smoke exposure on respiratory health in North America. International Journal of Environmental Research and Public Health, 16(6), 960. 10.3390/ijerph16060960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landguth, E. L. , Holden, Z. A. , Graham, J. , Stark, B. , Mokhtari, E. B. , Kaleczyc, E. , et al. (2020). The delayed effect of wildfire season particulate matter on subsequent influenza season in a Mountain West region of the USA. Environment International, 139, 105668. 10.1016/j.envint.2020.105668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larouche, J. R. , Abbott, B. W. , Bowden, W. B. , & Jones, J. B. (2015). The role of watershed characteristics, permafrost thaw, and wildfire on dissolved organic carbon biodegradability and water chemistry in Arctic headwater streams. Biogeosciences, 12, 4221–4233. 10.5194/bg-12-4221-2015 [DOI] [Google Scholar]
- Lassman, W. , Ford, B. , Gan, R. W. , Pfister, G. , Magzamen, S. , Fischer, E. V. , & Pierce, J. R. (2017). Spatial and temporal estimates of population exposure to wildfire smoke during the Washington state 2012 wildfire season using blended model, satellite, and in situ data. GeoHealth, 1(3), 106–121. 10.1002/2017GH000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell, J. S. , McKenzie, D. , Wan, H. Y. , & Cushman, S. A. (2018). Climate change and future wildfire in the western United States: An ecological approach to nonstationarity. Earth's Future, 6(8), 1097–1111. 10.1029/2018EF000878 [DOI] [Google Scholar]
- Liu, J. C. , Pereira, G. , Uhl, S. A. , Bravo, M. A. , & Bell, M. L. (2015). A systematic review of the physical health impacts from non‐occupational exposure to wildfire smoke. Environmental Research, 136(203), 120–132. 10.1016/j.envres.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. C. , Wilson, A. , Mickley, L. J. , Dominici, F. , Ebisu, K. , Wang, Y. , et al. (2017). Wildfire‐specific fine particulate matter and risk of hospital admissions in urban and rural counties. Epidemiology, 28(1), 77–85. 10.1097/EDE.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. C. , Wilson, A. , Mickley, L. J. , Ebisu, K. , Sulprizio, M. P. , Wang, Y. , et al. (2017). Who among the elderly is most vulnerable to exposure to and health risks of fine particulate matter from wildfire smoke? American Journal of Epidemiology, 186(6), 730–735. 10.1093/aje/kwx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, D. , Rupp, T. , Olson, M. , & Duffy, P. (2012). Is Alaska's boreal forest now crossing a major ecological threshold? Arctic Antarctic and Alpine Research, 44(3), 319–331. 10.1657/1938-4246-44.3.319 [DOI] [Google Scholar]
- Markon, C. , Gray, S. , Berman, M. , Eerkes‐Medrano, L. , Hennessy, T. , Huntington, H. P. , et al. (2018). Chapter 26: Alaska. Impacts, Risks, and Adaptation in the United States: The Fourth National Climate Assessment (Vol. II). Washington, DC. Retrieved from 10.7930/NCA4.2018.CH26 [DOI] [Google Scholar]
- Melvin, A. M. , Murray, J. , Boehlert, B. , Martinich, J. A. , Rennels, L. , & Rupp, T. S. (2017). Estimating wildfire response costs in Alaska's changing climate. Climatic Change, 141(4), 783–795. 10.1007/s10584-017-1923-2 [DOI] [Google Scholar]
- Mirowsky, J. E. , Carraway, M. S. , Dhingra, R. , Tong, H. , Neas, L. , Diaz‐Sanchez, D. , et al. (2017). Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environmental Health: A Global Access Science Source, 16(1), 126. 10.1186/s12940-017-0335-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, G. , Sheppeard, V. , Khalaj, B. , Ayyar, A. , Lincoln, D. , Jalaludin, B. , et al. (2010). Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology, 21(1), 47–55. 10.1097/EDE.0b013e3181c15d5a [DOI] [PubMed] [Google Scholar]
- National Interagency Fire Center . (2020). 100,000 acre fires. Retrieved from https://www.nifc.gov/fireInfo/fireInfo_stats_lgFires.html [Google Scholar]
- O'Dell, K. , Ford, B. , Fischer, E. V. , & Pierce, J. R. (2019). Contribution of wildland‐fire smoke to US PM 2.5 and its influence on recent trends. Environmental Science & Technology, 53(4), 1797–1804. 10.1021/acs.est.8b05430 [DOI] [PubMed] [Google Scholar]
- Pastick, N. J. , Duffy, P. , Genet, H. , Rupp, T. S. , Wylie, B. K. , Johnson, K. D. , et al. (2017). Historical and projected trends in landscape drivers affecting carbon dynamics in Alaska. Ecological Applications, 27(5), 1383–1402. 10.1002/eap.1538 [DOI] [PubMed] [Google Scholar]
- Pedigo, A. S. , & Odoi, A. (2010). Investigation of disparities in geographic accessibility to emergency stroke and myocardial infarction care in East Tennessee using geographic information systems and network analysis. Annals of Epidemiology, 20(12), 924–930. 10.1016/j.annepidem.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Phuleria, H. C. , Fine, P. M. , Zhu, Y. , & Sioutas, C. (2005). Air quality impacts of the October 2003 southern California wildfires. Journal of Geophysical Research Atmospheres, 110(7), 1–11. 10.1029/2004JD004626 [DOI] [Google Scholar]
- Rappold, A. G. , Cascio, W. E. , Kilaru, V. J. , Stone, S. L. , Neas, L. M. , Devlin, R. B. , & Diaz‐Sanchez, D. (2012). Cardio‐respiratory outcomes associated with exposure to wildfire smoke are modified by measures of community health. Environmental Health, 11(1), 71. 10.1186/1476-069X-11-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold, A. G. , Hano, M. C. , Prince, S. , Wei, L. , Huang, S. M. , Baghdikian, C. , et al. (2019). Smoke Sense initiative leverages citizen science to address the growing wildfire‐related public health problem. GeoHealth, 3(12), 443–457. 10.1029/2019GH000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold, A. G. , Stone, S. L. , Cascio, W. E. , Neas, L. M. , Kilaru, V. J. , Carraway, M. S. , et al. (2011). Peat bog wildfire smoke exposure in rural North Carolina is associated with cardiopulmonary emergency department visits assessed through syndromic surveillance. Environmental Health Perspectives, 119(10), 1415–1420. 10.1289/ehp.1003206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, C. E. , Brauer, M. , Johnston, F. H. , Jerrett, M. , Balmes, J. R. , & Elliott, C. T. (2016). Critical review of health impacts of wildfire smoke exposure. Environmental Health Perspectives, 124(9), 1334–1343. 10.1289/ehp.1409277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, C. E. , Considine, E. M. , Watson, G. L. , Telesca, D. , Pfister, G. G. , & Jerrett, M. (2019). Associations between respiratory health and ozone and fine particulate matter during a wildfire event. Environment International, 129, 291–298. 10.1016/j.envint.2019.04.033 [DOI] [PubMed] [Google Scholar]
- Reid, C. E. , Jerrett, M. , Tager, I. B. , Petersen, M. L. , Mann, J. K. , & Balmes, J. R. (2016). Differential respiratory health effects from the 2008 northern California wildfires: A spatiotemporal approach. Environmental Research, 150, 227–235. 10.1016/j.envres.2016.06.012 [DOI] [PubMed] [Google Scholar]
- Reid, C. E. , & Maestas, M. M. (2019). Wildfire smoke exposure under climate change. Current Opinion in Pulmonary Medicine, 25(2), 179–187. 10.1097/MCP.0000000000000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford, T. , Wang, R. , & Kenward, A. (2015). The age of Alaskan wildfires. Climate Central. Princeton, NJ: Princeton University. Retrieved from www.climatecentral.org [Google Scholar]
- Scott, P. A. , Temovsky, C. J. , Lawrence, K. , Gudaitis, E. , & Lowell, M. J. (1998). Analysis of Canadian population with potential geographic access to intravenous thrombolysis for acute ischemic stroke. Stroke, 29(11), 2304–2310. 10.1161/01.STR.29.11.2304 [DOI] [PubMed] [Google Scholar]
- Stowell, J. D. , Geng, G. , Saikawa, E. , Chang, H. H. , Fu, J. , Yang, C.‐E. , et al. (2019). Associations of wildfire smoke PM2.5 exposure with cardiorespiratory events in Colorado 2011–2014. Environment International, 133, 105151. 10.1016/j.envint.2019.105151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham, R. , Erbas, B. , Akram, M. , Dennekamp, M. , & Abramson, M. J. (2009). The impact of smoke on respiratory hospital outcomes during the 2002–2003 bushfire season, Victoria, Australia. Respirology, 14(1), 69–75. 10.1111/j.1440-1843.2008.01416.x [DOI] [PubMed] [Google Scholar]
- Thoman, R. , & Walsh, J. (2019). Alaska's changing environment: Documenting Alaska's physical and biological changes through observations. In McFarland H. (Ed.), International Arctic Research Center. University of Alaska‐Fairbanks. [Google Scholar]
- Thurston, G. D. , & Ito, K. (2001). Epidemiological studies of acute ozone exposures and mortality. Journal of Exposure Analysis and Environmental Epidemiology, 11(4), 286–294. 10.1038/sj.jea.7500169 [DOI] [PubMed] [Google Scholar]
- Tinling, M. A. , West, J. J. , Cascio, W. E. , Kilaru, V. , & Rappold, A. G. (2016). Repeating cardiopulmonary health effects in rural North Carolina population during a second large peat wildfire. Environmental Health, 15(1), 12. 10.1186/s12940-016-0093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, S. K. , & Jewkes, H. A. (2006). Wildland fire in Alaska: A history of organized fire suppression and management in the last frontier. School of agriculture and land Resources management, agricultural and forestry experiment station. Retrieved from http://hdl.handle.net/11122/1313 [Google Scholar]
- Trainor, S. F. , Calef, M. , Natcher, D. , Chapin, F. S. , Mcguire, A. D. , Huntington, O. , et al. (2009). Vulnerability and adaptation to climate‐related fire impacts in rural and urban interior Alaska. Polar Research, 28(1), 100–118. 10.1111/j.1751-8369.2009.00101.x [DOI] [Google Scholar]
- Turetsky, M. R. , Kane, E. S. , Harden, J. W. , Ottmar, R. D. , Manies, K. L. , Hoy, E. , & Kasischke, E. S. (2011). Recent acceleration of biomass burning and carbon losses in Alaskan forests and peatlands. Nature Geoscience, 4(1), 27–31. 10.1038/ngeo1027 [DOI] [Google Scholar]
- U.S. Census Bureau . (2019). Alaska population and housing unit estimates. Retrieved from https://www.census.gov/quickfacts/fact/table/matanuskasusitnaboroughalaska,fairbanksnorthstarboroughalaska,anchoragemunicipalityalaskacounty,AK/PST045219 [Google Scholar]
- US EPA . (2019). Integrated science assessment (ISA) for particulate matter (Final Report, 2019). Washington DC: U.S. Environmental Protection Agency. [PubMed]
- US EPA . (2020). National Emissions Inventory (NEI) Documentation. U.S. Environmental Protection Agency. Retrieved from https://www.epa.gov/air-emissions-inventories/2020-national-emissions-inventory-nei-documentation
- Veraverbeke, S. , Rogers, B. M. , & Randerson, J. T. (2015). Daily burned area and carbon emissions from boreal fires in Alaska. Biogeosciences, 12, 3579–3601. 10.5194/bg-12-3579-2015 [DOI] [Google Scholar]
- Vora, C. , Renvall, M. J. , Chao, P. , Ferguson, P. , & Ramsdell, J. W. (2011). 2007 San Diego wildfires and asthmatics. Journal of Asthma, 48(1), 75–78. 10.3109/02770903.2010.535885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, X. J. , Rogers, B. M. , Baltzer, J. L. , Cumming, S. G. , Day, N. J. , Goetz, S. J. , et al. (2018). Cross‐scale controls on carbon emissions from boreal forest megafires. Global Change Biology, 24(9), 4251–4265. 10.1111/gcb.14287 [DOI] [PubMed] [Google Scholar]
- Westerling, A. L. (2016). Increasing western US forest wildfire activity: Sensitivity to changes in the timing of spring. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1696), 20150178. 10.1098/rstb.2015.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein, Z. S. , Hoshiko, S. , Fahimi, J. , Harrison, R. J. , Cascio, W. E. , & Rappold, A. G. (2018). Cardiovascular and cerebrovascular emergency department visits associated with wildfire smoke exposure in California in 2015. Journal of the American Heart Association, 7(8). 10.1161/JAHA.117.007492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, S. H. L. , Liu, J. C. , Yue, X. , Mickley, L. J. , & Bell, M. L. (2020). Air pollution from wildfires and human health vulnerability in Alaskan communities under climate change. Environmental Research Letters, 15(9), 094019. 10.1088/1748-9326/ab9270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, J. , Eyamie, J. , & Henderson, S. B. (2016). Evaluation of a spatially resolved forest fire smoke model for population‐based epidemiologic exposure assessment. Journal of Exposure Science and Environmental Epidemiology, 26(3), 233–240. 10.1038/jes.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, L. , & Wang, Y. (2020). Long‐term air quality study in Fairbanks, Alaska: Air pollutant temporal variations, correlations, and PM2.5 source apportionment. Atmosphere, 11(11), 1203. 10.3390/atmos11111203 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
Table S9
Table S10
Table S11
Data Availability Statement
Air quality data are available for download from the EPA Air Quality Program (https://www.epa.gov/outdoor-air-quality-data). Data from the Alaska Health Facilities Data Reporting Program are not publically available due to privacy concerns. Data can be requested from the Alaska Department of Health and Social Services with appropriate Institutional Review Board approvals (http://dhss.alaska.gov/dph/VitalStats/Pages/HFDR/default.aspx).


