Abstract
Background
There have been insufficient data for African patients with COVID-19 who are critically ill. The African COVID-19 Critical Care Outcomes Study (ACCCOS) aimed to determine which resources, comorbidities, and critical care interventions are associated with mortality in this patient population.
Methods
The ACCCOS study was a multicentre, prospective, observational cohort study in adults (aged 18 years or older) with suspected or confirmed COVID-19 infection who were referred to intensive care or high-care units in 64 hospitals in ten African countries (ie, Egypt, Ethiopia, Ghana, Kenya, Libya, Malawi, Mozambique, Niger, Nigeria, and South Africa). The primary outcome was in-hospital mortality censored at 30 days. We studied the factors (ie, human and facility resources, patient comorbidities, and critical care interventions) that were associated with mortality in these adult patients. This study is registered on ClinicalTrials.gov, NCT04367207.
Findings
From May to December, 2020, 6779 patients were referred to critical care. Of these, 3752 (55·3%) patients were admitted and 3140 (83·7%) patients from 64 hospitals in ten countries participated (mean age 55·6 years; 1890 [60·6%] of 3118 participants were male). The hospitals had a median of two intensivists (IQR 1–4) and pulse oximetry was available to all patients in 49 (86%) of 57 sites. In-hospital mortality within 30 days of admission was 48·2% (95% CI 46·4–50·0; 1483 of 3077 patients). Factors that were independently associated with mortality were increasing age per year (odds ratio 1·03; 1·02–1·04); HIV/AIDS (1·91; 1·31–2·79); diabetes (1·25; 1·01–1·56); chronic liver disease (3·48; 1·48–8·18); chronic kidney disease (1·89; 1·28–2·78); delay in admission due to a shortage of resources (2·14; 1·42–3·22); quick sequential organ failure assessment score at admission (for one factor [1·44; 1·01–2·04], for two factors [2·0; 1·33–2·99], and for three factors [3·66, 2·12–6·33]); respiratory support (high flow oxygenation [2·72; 1·46–5·08]; continuous positive airway pressure [3·93; 2·13–7·26]; invasive mechanical ventilation [15·27; 8·51–27·37]); cardiorespiratory arrest within 24 h of admission (4·43; 2·25–8·73); and vasopressor requirements (3·67; 2·77–4·86). Steroid therapy was associated with survival (0·55; 0·37–0·81). There was no difference in outcome associated with female sex (0·86; 0·69–1·06).
Interpretation
Mortality in critically ill patients with COVID-19 is higher in African countries than reported from studies done in Asia, Europe, North America, and South America. Increased mortality was associated with insufficient critical care resources, as well as the comorbidities of HIV/AIDS, diabetes, chronic liver disease, and kidney disease, and severity of organ dysfunction at admission.
Funding
The ACCCOS was partially supported by a grant from the Critical Care Society of Southern Africa.
Introduction
SARS-CoV-2 has overwhelmed health-care systems by causing high rates of critical illness. The global case fatality rate of COVID-19 is approximately 3%,1 with older people (eg, people older than 62 years) who have comorbidities known to be more susceptible than younger people.2 Moreover, there is a concern of further mortality with subsequent waves across regions globally.
Our hypothesis was that critically ill patients with COVID-19 might have worse health outcomes in Africa than other continents because the ability to provide sufficient care is compromised by having a small workforce,3 having a low number of intensive care facilities, and the scarcity of critical care resources.4 We also hypothesised that unplanned admissions would further adversely affect critical care outcomes in Africa5 as the ability of health-care systems to respond to meet the clinical workload is limited. Finally, patient outcomes following critical care for COVID-19 were not sufficiently documented in this under-resourced environment,6 despite a call for prevention and response measures in low-income and middle-income countries.7
As there were little data for the management of critically ill patients with COVID-19,8 we designed the African COVID-19 Critical Care Outcomes Study (ACCCOS) to determine which resources, patient comorbidities, and critical care interventions were associated with mortality or survival in these patients. Wide dissemination of these findings could help to inform resource prioritisation necessary to manage patients who are critically ill with COVID-19 in Africa. This objective remains relevant as a recent meta-analysis6 reported no critical care outcomes data from Africa, or patient management data in resource-limited settings.
Research in context.
Evidence before this study
We searched MEDLINE, Embase, the Cochrane Library, Africa-Wide Information, and SciELO Citation Index between Jan 1 and Sept 23, 2020 (as well as an updated search from Sept 23 to Dec 6, 2020) using the search terms “(Betacoronavirus OR Betacoronaviruses)”, “(Corona Virus OR Corona Viruses OR Coronavirus OR Coronaviruses)”, “(COVID OR COVID19 OR COVID-19)”, “(CoV OR CoV2 OR HCoV-19 OR nCoV OR 2019nCoV)”, “(Severe Acute Respiratory Syndrome CoV OR severe acute respiratory syndrome coronavirus 2 OR SARS CoV 2 OR SARS-CoV-2 OR SARSCoV OR SARS-CoV OR SARS2)”, “(Intensive care OR intensive care unit* OR ICU*)”, “(ITU* or intensive treatment* OR intensive treatment unit*)”, “critical care”, “critical* ill*”, “1 or 2 or 3 or 4 or 5”, “6 or 7 or 8 or 9”, “10 and 11”, “Limit 12 to yr=“2020”. Studies that included patients with COVID-19 who were not in critical care, and critical care cohorts restricted to a specific patient subgroup were excluded. Studies published in all languages were considered in our search. There is little data to guide the management of critically ill patients with COVID-19 in under-resourced environments. Previously published systematic reviews confirmed that there were no published outcomes data from Africa, and little data for factors associated with mortality or survival in under-resourced environments.
Added value of this study
Mortality following critical care admission for patients with suspected COVID-19 infection and confirmed COVID-19 infection in this African cohort was 48·2% (95% CI 46·4–50·0). The meta-analysis reports a global mortality of 31·5% (27·5–35·5), with the African data reporting an excess mortality of 11 (in the best case scenario) to 23 (in the worst case scenario) deaths per 100 patients compared with the global average. The excess mortality could be explained by the shortage of critical care resources. In our study, only one in two patients referred for critical care were admitted. Patients were admitted to units with limited access to dialysis, proning, extracorporeal membrane oxygenation (ECMO), arterial blood gases, and pulse oximetry. Furthermore, at the patient level, access to interventions (eg, dialysis, proning, and ECMO) were estimated to be between seven-times and 14-times lower than what is needed to manage critically ill patients with COVID-19. Adjusted analyses suggest that critical care mortality is associated with increasing age, the patient comorbidities of HIV/AIDS, diabetes, chronic liver disease, and kidney disease, the severity of organ dysfunction on presentation to critical care, and the initial need for increasing respiratory and cardiovascular support. The quick sequential organ failure assessment score at admission was associated with patient mortality and could be a simple and feasible risk stratification tool to use in under-resourced environments.
Implications of all the available evidence
In our study, mortality was strongly associated with organ dysfunction and the level of organ support needed at critical admission. The use of the quick SOFA score could provide guidance for appropriate triage decision making at the time of referral to critical care in an under-resourced setting when managing critically ill patients with COVID-19. Strategies are needed to mitigate risk in patients with COVID-19 in Africa with coexisting HIV/AIDS, diabetes, chronic liver disease, and kidney disease. It is likely that patient outcomes will continue to be severely compromised until the problems surrounding critical care resource scarcity are addressed.
We aimed to determine which critical care resources, patient comorbidities, and hospital interventions were associated with in-hospital mortality in patients with suspected or confirmed COVID-19 who were referred to critical care in ten African countries.
Methods
Study design and participants
The ACCCOS study was a multicentre, prospective, observational cohort study in adults (aged 18 years or older) who were referred to intensive care or high-care units with suspected or confirmed COVID-19 in ten African countries (ie, Egypt, Ethiopia, Ghana, Kenya, Libya, Malawi, Mozambique, Niger, Nigeria, and South Africa). The study was open to all African countries, and these ten countries fulfilled the ethics and regulatory requirements to participate. A high-care unit was defined as a patient area that provides a level of care between that given in an intensive care unit and a general ward but that does not usually provide invasive ventilation. Eligible patients included all patients admitted to a high-care or intensive care unit with suspected or confirmed COVID-19. Patient follow-up was until hospital discharge, censored at 30 days if the patient was in hospital. The study recruited from May 7 to Dec 18, 2020. The primary ethics approval was from the Human Research Ethics Committee of the University of Cape Town (Cape Town, South Africa).
All patients received standard of care for patients with suspected or confirmed COVID-19 and who required critical care admission. We planned to recruit as many sites as possible in Africa. Sites were requested to include all eligible patients and to recruit for as long as possible with the understanding that they could stop recruiting at any point if they were overwhelmed by clinical commitments. Each site had to complete an eligible patients' screening log.
The primary ethics committee approved a so-called delayed consent process, as most patients would be unable to consent at the time of admission to critical care. The delayed consent process ensured consent by the patient (following stabilisation or recovery), a legal representative, or a proxy (in instances in which the patient was unable to provide consent). If there was no opportunity to acquire delayed consent before the study outcome was reached, then the ethics committee approved the inclusion of the patient's data in the study. The justification for this process was to minimise the risk of a non-consecutive patient enrolment, which would result in a biased sample. Some ethics committees waived consent. All sites fulfilled local ethics and regulatory requirements. The protocol and statistical analysis plan are posted at ClinicalTrials.gov, NCT04367207.
Procedures
Hospital specific data were collected by the hospital lead investigator before study registration and included: (1) the level of care, (2) the number of hospital and critical care beds, (3) reimbursement status, and (4) other factors affecting patient care (eg, nurse-to-patient ratio). The case record form and definitions document are in the appendix (pp 13–16).
To ensure a representative sample, we planned to include as many sites as possible with the requirement for inclusion of all consecutive patients using the delayed consent procedure.
Outcomes
The primary outcome of our study was in-hospital mortality within 30 days of admission. The secondary outcome was to determine the factors (ie, human and facility resources, patient comorbidities, and critical care interventions) that were associated with mortality in adult patients with suspected or confirmed COVID-19.
Statistical analysis
With 25–30 variables potentially associated with mortality in critically ill patients with COVID-19 (appendix pp 17–18) the minimum sample size required approximately 250–300 deaths to avoid violating the principle of approximately ten outcome events (ie, deaths) per variable in the regression.9 An interim analysis preprint10 was published in October, 2020, once that sample size was reached. The database was locked on Dec 18, 2020, with 1483 deaths in the cohort.
A statistical analysis plan was published on ClinicalTrials.gov before data evaluation for the interim analysis (appendix pp 17–18). Data were presented at an Africa wide level. Categorical variables were described as proportions and were compared using χ2 tests. Continuous variables were described as mean (SD) or median (IQR). Comparisons of continuous variables between groups were done using t-tests, one-way ANOVA, or equivalent non-parametric tests. The main model included patients with complete outcome data (ie, the main model excluded patients who were still in hospital and receiving therapy and who had not reached the outcome definition of death, discharge, or alive in hospital at 30 days). A three-level generalised linear mixed model (GLMM) was fitted using a logit link to identify independent risk factors for the primary outcome of mortality (with patients being at the first level, hospitals at the second level, and countries at the third level) to account for the expected correlation in outcomes within hospitals and countries. A fully conditional specification method was used to impute missing values for variables using an iterative Markov chain Monte Carlo method. We used a predictive mean matching method for scale variables. Five imputed datasets were constructed. All risk factors were considered for entry into the model provided there was no evidence of collinearity. The variables included subject variables, resource variables, and therapy variables. Subject variables included age, sex, body-mass index, coronary artery disease, congestive heart failure, hypertension, stroke or transient ischaemic attack, diabetes mellitus, cancer, whether the patient was a current smoker, chronic lung disease, active tuberculosis, chronic liver disease, HIV/AIDS, chronic or previous malaria, chronic kidney disease, cardiorespiratory arrest in the 24 h before referral to critical care, quick sequential organ failure assessment (SOFA) score,11 and full SOFA12 score on referral or admission. Resource variables included admission delayed due to the shortage of resources (eg, bed and staffing), nurse-to-patient ratio in critical care, ability to provide invasive ventilation, and physician availability on site 24 h per day, 7 days a week. Therapy variables included organ support at admission, respiratory support, proning, ventilatory support, intubation, inotropes or vasoconstrictors, dialysis, therapeutic anticoagulation, steroid therapy, repurposed or experimental COVID-19 drug therapy, and extracorporeal membrane oxygenation (ECMO). Collinearity was assessed using the variance inflation factor. Collinearity was associated with intubation, respiratory and ventilatory interventions, number of organs requiring support, and anticoagulation. Therefore, we created a single categorical variable for respiratory support (ie, none, oxygen, high flow nasal oxygen, continuous positive airway pressure, and invasive mechanical ventilation) and removed the dialysis and ECMO variables, which had collinearity with anticoagulation. The subsequent variance inflation factor showed collinearity between the subject variable “chronic malaria or malaria within 3 months” and the therapy variable “repurposed or experimental COVID-19 drug therapy”. We removed “repurposed or experimental COVID-19 drug therapy” as this therapy variable was a heterogeneous variable compared with “chronic malaria or malaria within 3 months”. No further collinearity was identified. A three-level random-intercept mixed effects logistic regression was done on each of the five imputed datasets using the glmer function in the lme4 package13 in R.14 Estimates were combined from the five repeated complete data analyses using the pool function from the mice package.15 The pool function implements the rules for combining the separate estimates and SEs from each of the imputed datasets to provide an overall estimate with SEs, CIs, and p values.16 A p value of less than 0·05 was considered significant. To allow for comparison with the imputed datasets, the complete case analysis was also presented.
The results of the GLMM are reported as adjusted odds ratios with 95% CIs. Sensitivity analyses defined a priori were: (1) confirmed SARS-CoV-2-positive patients only, (2) confirmed SARS-CoV-2 patients excluding patients who had life support withdrawn or therapy limited (ie, the decision not to provide additional therapy, such as ventilation, adrenaline, and dialysis, to the patient's current therapy because of the expected poor prognosis), and (3) only patients who died or who were discharged alive (excluding in-hospital patients). A post-hoc sensitivity analysis that was requested by the reviewers excluded “cardiorespiratory arrest within 24 h of admission” as a potential variable. All analyses were done by AH and BMB.
A post-hoc decision was taken to update the critical care meta-analyses of COVID-19 mortality by region,6, 8 done by EHT, KDMM, MElh, and JS. We have presented the case fatality rate for COVID-19 infections by region and did a meta-analysis of the mean age and SOFA score per region. The regional case fatality rate, critical care outcomes meta-analysis, and the meta-analysis of means for ages and SOFA scores provide context for rates of mortality in critical care in Africa.
Univariate analyses and the imputations were done using SPSS (version 26.0). The mixed effects logistic regressions were done using R.17 The meta-analyses were done using Stata 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). This study is registered on ClinicalTrials.gov, NCT04367207.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
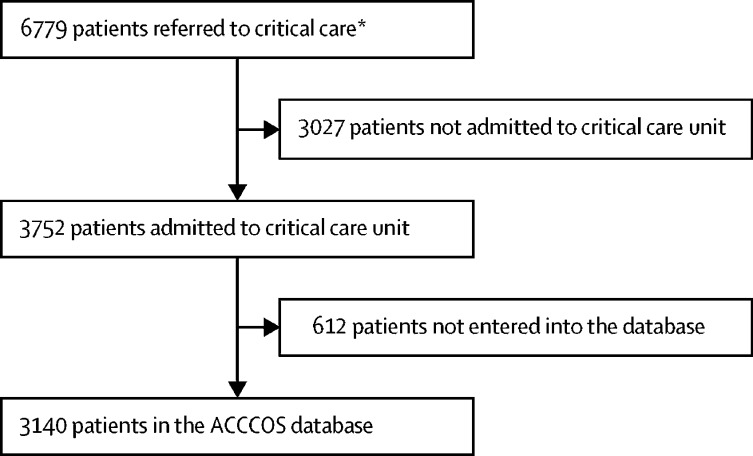
Of the 40 African countries that were invited to participate via the African Perioperative Research Group (with 26 country leaders accepting the invitation), ten countries (which included 64 hospitals) participated; Egypt (ten hospitals), Ethiopia (seven hospitals), Ghana (two hospitals), Kenya (three hospitals), Libya (14 hospitals), Malawi (three hospitals), Mozambique (two hospitals), Niger (two hospitals), Nigeria (eight hospitals), and South Africa (13 hospitals). 57 (89%) hospitals provided hospital level data (table 1 ). Most of the hospitals were university affiliated, government funded, and tertiary level hospitals. There was a median of two intensivists (IQR 1–4), with a median nurse-to-patient ratio of 1:2 (1:2–1:1) during the day. 27 (47%) of 57 hospitals had oxygen available from vacuum insulated evaporators, with a median surge ventilator capacity of five intensive care ventilators (3–10) and four anaesthesia ventilators (1–8). Only 49 (86%) sites could provide pulse oximetry to all patients in critical care, and 39 (68%) could provide renal replacement therapy. 3752 (55·3%) of 6779 patients who were referred to critical care were admitted (figure ). 3140 (83·7%) of 3752 patients who were eligible were included in our study, with a median of 25 patients (11–59) per hospital. The age range was 18 to 100 years, with men comprising 1890 (60·6%) of 3118 patients.
Table 1.
The characteristics of the hospitals included in the study
| Number of hospitals or median values | |
|---|---|
| Hospital level of care | |
| Primary | 4/57 (7%) |
| Secondary | 13/57 (23%) |
| Tertiary | 40/57 (70%) |
| Funding | |
| Government | 50/57 (88%) |
| Private | 3/57 (5%) |
| Dual funding | 4/57 (7%) |
| Hospital type | |
| University affiliated | 44/57 (77%) |
| Non-university affiliated | 13/57 (23%) |
| Population served | 2·0 million (0·2 million–5·0 million) |
| Hospital beds | 500 (250–775) |
| Critical care beds providing invasive ventilation | 12 (7–25) |
| Critical care beds unable to provide invasive ventilation | 11 (8–29) |
| Specialist intensivists | 2 (1–4) |
| Specialist doctors (not intensivists) | 4 (2–8) |
| Non-specialist doctors | 6 (2–11) |
| Nurse-to-patient ratio (day) | 1:2 (1:2–1:1) |
| Nurse-to-patient ratio (night) | 1:2 (1:3–1:1) |
| Doctor to patient ratio | 1:4 (1:6–1:3) |
| Doctor on site in critical care after hours | 49/57 (86%) |
| Surge capacity | |
| Number of extra intensive care ventilators | 5 (3–10) |
| Number of operating room ventilators available for critical care upgrade | 4 (1–8) |
| Haematology laboratory on hospital site | 56/57 (98%) |
| Ability to do arterial blood gases on hospital site | 45/55 (82%) |
| Critical care oxygen supply | |
| Vacuum insulated evaporator | 27/57 (47%) |
| Cylinder oxygen | 22/57 (39%) |
| Oxygen concentrator | 8/57 (14%) |
| Pulse oximetry | |
| All patients | 49/57 (86%) |
| Selected patients | 7/57 (12%) |
| No patients | 1/57 (2%) |
| Ability to provide prone ventilation | 40/57 (70%) |
| Ability to provide renal replacement therapy | 39/57 (68%) |
| Ability to provide extra-corporeal oxygenation | 9/57 (16%) |
Data are median (IQR) or n/N (%).
Figure.
The ACCCOS recruitment
ACCCOS=African COVID-19 Critical Care Outcomes Study. *Three of 64 hospitals did not submit a screening log. The three hospitals without screening logs contributed 18 patients (ten patients from one hospital, six patients from another hospital, and two patients from a third hospital)
From May 7 to Dec 18, 2020, 6779 patients were referred to critical care (figure). 2995 (95·4%) of 3140 patients in the cohort were confirmed positive for SARS-CoV-2 infection. Patients were referred from the emergency department (937 [30·2%] of 3103 patients), from another department within the same hospital (926 [29·8%] patients), or another hospital (1240 [40·0%] patients). Mortality differed between the sites of referral (p=0·002), with in-hospital mortality of 401 (43·5%) of 921 patients from emergency departments, 443 (48·5%) of 914 patients from in-hospital referrals, and 629 (51·2%) of 1228 patients from other hospitals. Admission to critical care was delayed in 248 (8·4%) of 2947 patients because of a shortage of resources at the time of admission. A quick SOFA score of three had a mortality of 203 (82%) of 247 patients. Patients who required mechanical ventilation had a mortality rate of 78·9% (918 of 1164 patients).
The patients enrolled in our study had a mean age of 56 years with few comorbidities, and 39·4% were women (table 2 ). The most common comorbidities were hypertension, diabetes, HIV/AIDS, coronary artery disease, and chronic kidney disease. The quick SOFA score was completed in 3069 (97·7%) of 3140 patients at admission.11 Most (2136 [68·0%] of 3140 patients) did not have a full SOFA score done at admission.
Table 2.
Description of African COVID-19 critical care cohort
| All patients (n=3140) | Patients who died (n=1483) | Patients who survived (n=1594) | p value | |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 56 (16·11) | 58·74 (15·74) | 52·68 (15·88) | <0·0001 |
| Age quartiles, years | ||||
| 18–45 | 832/3072 (27·1%) | 302/1480 (20·4%) | 530/1592 (33·3%) | <0·0001 |
| 46–56 | 713/3072 (23·2%) | 317/1480 (21·4%) | 396/1592 (24·9%) | .. |
| 57–67 | 769/3072 (25·0%) | 409/1480 (27·6%) | 360/1592 (22·6%) | .. |
| >67 | 758/3072 (24·7%) | 452/1480 (19·2%) | 306/1592 (19·2%) | .. |
| Sex | ||||
| Male | 1890/3118 (60·6%) | 902/1481 (60·9%) | 966/1593 (60·6%) | 0·883 |
| Female | 1228/3118 (39·4%) | 579/1481 (39·1%) | 627/1593 (39·4%) | .. |
| Body-mass index categories | ||||
| <25 | 397/1596 (24·9%) | 169/715 (23·6%) | 221/851 (26·0%) | 0·030 |
| 25–29·9 | 548/1596 (34·3%) | 239/715 (33·4%) | 300/851 (35·3%) | .. |
| 30–34·9 | 353/1596 (22·1%) | 151/715 (21·1%) | 198/851 (23·3%) | .. |
| 35–39·9 | 154/1596 (9·6%) | 82/715 (11·5%) | 65/851 (7·6%) | .. |
| ≥40 | 144/1596 (9·0%) | 74/715 (10·3%) | 67/851 (7·9%) | .. |
| Missing | 1544 | 768/1511 (50·8%) | 743/1511 (49·2%) | .. |
| Comorbidities | ||||
| Coronary artery disease | 237/3093 (7·7%) | 128/1469 (8·7%) | 109/1586 (6·9%) | 0·058 |
| Congestive heart failure | 209/3087 (6·8%) | 100/1465 (6·8%) | 106/1584 (6·7%) | 0·885 |
| Hypertension | 1572/3104 (50·6%) | 794/1476 (53·8%) | 759/1588 (47·8%) | 0·001 |
| Stroke or transient ischaemic attack | 139/3088 (4·5%) | 77/1465 (5·3%) | 62/1584 (3·9%) | 0·082 |
| Diabetes | 1175/3090 (38·0%) | 623/1471 (42·4%) | 542/1582 (34·3%) | <0·0001 |
| Cancer | 96/3088 (3·1%) | 49/1466 (3·3%) | 45/1586 (2·8%) | 0·463 |
| Current smoker | 140/3078 (4·5%) | 56/1458 (3·8%) | 80/1581 (5·1%) | 0·114 |
| Chronic lung disease | 216/3088 (7·0%) | 105/1465 (7·2%) | 108/1586 (6·8%) | 0·722 |
| Active tuberculosis | 51/3084 (1·7%) | 30/1462 (2·1%) | 20/1583 (1·3%) | 0·116 |
| Chronic liver disease | 46/3086 (1·5%) | 29/1465 (2·0%) | 17/1585 (1·1%) | 0·052 |
| HIV/AIDS | 237/3084 (7·7%) | 149/1460 (10·2%) | 86/1587 (5·4%) | <0·0001 |
| Antiretroviral therapy | 180/273 (65·9%) | 115/175 (65·7%) | 64/96 (66·7%) | 0·894 |
| Chronic malaria or malaria within 3 months | 190/3089 (6·2%) | 60/1465 (4·1%) | 130/1586 (8·2%) | <0·0001 |
| Chronic kidney disease | 241/3085 (7·7%) | 149/1465 (10·2%) | 90/1585 (5·7%) | <0·0001 |
| Condition at admission | ||||
| Cardiorespiratory arrest in 24 h before critical care referral | 100/3086 (3·2%) | 77/1467 (5·2%) | 22/1583 (1·4%) | 0·001 |
| Organ support required at admission | ||||
| Respiratory support | 2743/3102 (11·6%) | 1375/1479 (93·0%) | 1332/1587 (83·9%) | <0·0001 |
| Cardiovascular support | 609/296 (19·4%) | 451/1396 (32·3%) | 155/1537 (10·1%) | <0·0001 |
| Renal support | 282/3072 (9·2%) | 185/1462 (12·7%) | 95/1581 (6·0%) | <0·0001 |
| Other support | 510/3024 (16·9%) | 217/1432 (15·2%) | 293/1568 (18·7%) | 0·011 |
| Number of organ systems requiring support | ||||
| No organ system | 271/3066 (8·8%) | 92/1479 (6·2%) | 179/1587 (11·3%) | <0·0001 |
| One organ system | 1816/3066 (59·2%) | 797/1479 (53·9%) | 1019/1587 (64·2%) | .. |
| Two organ systems | 705/3066 (23·0%) | 389/1479 (26·3%) | 316/1587 (19·9%) | .. |
| Three organ systems | 219/3066 (7·1%) | 151/1479 (10·2%) | 68/1587 (4·3%) | .. |
| Four organ systems | 55/3066 (1·8%) | 50/1479 (3·4%) | 5/1587 (0·3%) | .. |
| Quick SOFA score on presentation | ||||
| SBP ≤100 mm Hg | 471/3088 (15·3%) | 326/1472 (22·1%) | 141/1576 (8·9%) | <0·0001 |
| Respiratory rate ≥22 breaths per min | 2502/3085 (81·1%) | 1237/1468 (84·3%) | 1235/1578 (78·3%) | <0·0001 |
| Glasgow Coma Scale score ≤14 | 850/3072 (27·7%) | 588/1459 (40·3%) | 253/1574 (16·1%) | <0·0001 |
| Quick SOFA score | ||||
| No risk factors | 395/3069 (12·9%) | 116/1458 (8·0%) | 275/1573 (16·1%) | <0·0001 |
| One risk factor | 1786/3069 (58·2%) | 744/1458 (51·0%) | 1015/1573 (64·5%) | .. |
| Two risk factors | 641/3069 (20·9%) | 395/1458 (27·1%) | 240/1573 (15·3%) | .. |
| Three risk factors | 247/3069 (8·0%) | 203/1458 (13·9%) | 43/1573 (2·7%) | .. |
| Full SOFA score on admission | 4 (3–6) | 5 (4–8) | 4 (2–5) | <0·0001 |
| Full SOFA score missing data | 2136 | .. | .. | .. |
| Intensive care unit resources | ||||
| Admission delayed because of shortage of resources (eg, bed and staffing) | 248/2947 (8·4%) | 146/1382 (10·6%) | 100/1530 (6·5%) | 0·0001 |
| Nurse-to-patient ratio | 0·5 (0·25–1·00) | 0·5 (0·33–1·00) | 0·5 (0·25-0·67) | <0·0001 |
| Ability to provide invasive ventilation for patient if required | 2780/3091 (87·7%) | 1361/1470 (92·6%) | 1319/1585 (83·2%) | <0·0001 |
| Physician available on site 24 h/7 days a week | 2760/3084 (89·5%) | 1353/1463 (92·5%) | 1376/1583 (86·9%) | <0·0001 |
| Respiratory support (ie, highest level of support) | ||||
| None | 421/2995 (14·1%) | 237/1416 (16·7%) | 182/1546 (11·8%) | <0·0001 |
| Oxygen mask | 1352/2995 (43·1%) | 489/1416 (34·5%) | 860/1546 (55·6%) | .. |
| High-flow nasal oxygenation | 589/2995 (19·7%) | 293/1416 (20·7%) | 274/1546 (17·7%) | .. |
| CPAP | 633/2995 (21·1%) | 397/1416 (28·0%) | 230/1546 (14·9%) | .. |
| Prone ventilation | ||||
| None | 1516/2826 (53·6%) | 664/1305 (50·9%) | 846/1503 (56·3%) | <0·0001 |
| Not ventilated | 989/2826 (35·0%) | 396/1305 (30·3%) | 581/1503 (38·7%) | .. |
| Invasive mechanical ventilation | 321/2826 (11·4%) | 245/1305 (18·8%) | 76/1503 (5·1%) | .. |
| Ventilatory support | ||||
| None | 1358/2902 (46·8%) | 298/1384 (21·5%) | 1053/1494 (70·5%) | <0·0001 |
| Non-invasive ventilation | 380/2902 (13·1%) | 168/1384 (12·1%) | 206/1494 (13·8%) | .. |
| Invasive mechanical ventilation | 1164/2902 (40·1%) | 918/1384 (66·3%) | 235/1494 (15·7%) | .. |
| Intubation | ||||
| No | 1896/3062 (61·9%) | 532/1458 (36·5%) | 1346/1576 (85·4%) | <0·0001 |
| Yes (elective) | 385/3062 (12·6%) | 293/1458 (20·1%) | 90/1576 (5·7%) | .. |
| Yes (emergency) | 781/3062 (25·5%) | 633/1458 (43·4%) | 140/1576 (8·9%) | .. |
| Inotropes or vasoconstrictors | 931/3086 (30·2%) | 748/1472 (50·8%) | 179/1587 (11·3%) | <0·0001 |
| Dialysis | 330/3073 (10·7%) | 177/1467 (12·1%) | 150/1579 (9·5%) | 0·022 |
| Therapeutic anticoagulation | 2430/3084 (78·8) | 1218/1478 (82·4%) | 1188/1579 (75·2%) | <0·0001 |
| Steroid therapy | 2519/3011 (83·7%) | 1211/1430 (84·7%) | 1281/1552 (82·5%) | 0·125 |
| Repurposed or experimental COVID-19 drug therapy | 367/2937 (12·5%) | 122/1367 (8·9%) | 243/1549 (15·7%) | <0·0001 |
| Extracorporeal membrane oxygenation (if available) | 7/862 (0·8%) | 5/359 (1·4%) | 2/501 (0·4%) | 0·110 |
Data are mean (SD), n (%), n (proportion), or median (IQR). Odds ratios were constructed for in-hospital mortality with univariate binary logistic regression analysis. CPAP=continuous positive airway pressure. SBP=systolic blood pressure. SOFA=sequential organ failure assessment.
The length of critical care stay was 7 days (IQR 4–12). The decision to limit therapy was made in 284 (9·2%) of 3086 patients, and therapy was withdrawn in 81 (2·6%) patients. 72 (88·9%) of the 81 patients in whom therapy was withdrawn had already had therapy limited.
In-hospital mortality in the 30 days after admission to a high-care or critical care unit occurred in 1483 (48·2%; 95% CI 46·4–50·0) of 3077 patients, with 261 (16·4%) of 1594 patients alive and in hospital at 30 days (35 of these 244 [data from 17 patients is missing with respect to whether they were in an intensive care unit or not] patients were still in intensive care), and 1333 (83·6%) patients had been discharged. The primary outcome was unknown for 63 patients.
For secondary outcome measures, the missingness of data for the GLMM is given in the appendix (p 19). The GLMM for the risk factors associated with mortality (ie, resources, comorbidities, and interventions) is shown in table 3 . Risk factors that were independently associated with mortality were increasing age and the comorbidities of HIV/AIDS, diabetes, chronic liver disease or kidney disease, an increasing quick SOFA score, high flow nasal oxygenation, continuous positive airway pressure, invasive mechanical ventilation, and cardiorespiratory arrest within 24 h before admission to critical care, and the need for inotropes. Steroid therapy was associated with increased survival. The sex of the patient was not associated with mortality. The sensitivity analyses supported these findings (appendix pp 20–25). No human resource factors (ie, physician available on site 24 h/7 days a week for patients and nurse-to-patient ratio) were associated with mortality.
Table 3.
Generalised linear mixed model (pooled results of the imputed datasets) for patients referred to critical care with suspected or confirmed COVID-19 infection with full dataset for mortality
| Odds ratio (97·5% CI) | p value | ||
|---|---|---|---|
| Patient characteristics | |||
| Intercept | 0·01 (0–0·04) | <0·0001 | |
| Age, years | 1·03 (1·02–1·04) | <0·0001 | |
| Female | 0·86 (0·69–1·06) | 0·15 | |
| Body-mass index | |||
| <25 | 1 (ref) | .. | |
| 25·0–29·9 | 1·12 (0·78–1·61) | 0·52 | |
| 30·0–34·9 | 1·06 (0·73–1·53) | 0·77 | |
| 35·0–39·9 | 1·16 (0·74–1·81) | 0·51 | |
| ≥40 | 1·11 (0·66–1·86) | 0·70 | |
| Comorbidities | |||
| Coronary artery disease | 0·80 (0·54–1·19) | 0·27 | |
| Congestive heart failure | 0·93 (0·63–1·38) | 0·71 | |
| Hypertension | 0·95 (0·76–1·19) | 0·65 | |
| Stroke or transient ischaemic attack | 1·04 (0·63–1·69) | 0·89 | |
| Diabetes | 1·25 (1·01–1·56) | 0·04 | |
| Cancer | 1·62 (0·90–2·91) | 0·11 | |
| Current smoker | 0·59 (0·35–1·01) | 0·06 | |
| Chronic lung disease | 0·96 (0·65–1·42) | 0·85 | |
| Active tuberculosis | 1·86 (0·85–4·06) | 0·12 | |
| Chronic liver disease | 3·48 (1·48–8·18) | 0·004 | |
| HIV/AIDS | 1·91 (1·31–2·79) | 0·0008 | |
| Chronic malaria or malaria within 3 months | 1·06 (0·52–2·15) | 0·88 | |
| Chronic kidney disease | 1·89 (1·28–2·78) | 0·001 | |
| Cardiorespiratory arrest in 24 h before critical care referral | 4·43 (2·25–8·73) | <0·0001 | |
| Quick SOFA score | |||
| No risk factors | 1 (ref) | .. | |
| One risk factor | 1·44 (1·01–2·04) | 0·04 | |
| Two risk factors | 2·00 (1·33–2·99) | 0·0008 | |
| Three risk factors | 3·66 (2·12–6·33) | <0·0001 | |
| Intensive care unit resources | |||
| Admission delayed because of shortage of resources (eg, bed and staffing) | 2·14 (1·42–3·22) | 0·0003 | |
| Physician available on site 24/7 for patient | 0·84 (0·50–1·42) | 0·51 | |
| Nurse-to-patient ratio (per unit increase) | 1·31 (0·98–1·74) | 0·07 | |
| Respiratory support | |||
| None | 1 (ref) | .. | |
| Oxygen mask | 1·28 (0·74–2·20) | 0·37 | |
| High flow nasal oxygenation | 2·72 (1·46–5·08) | 0·002 | |
| CPAP | 3·93 (2·13–7·26) | <0·0001 | |
| Invasive mechanical ventilation | 15·27 (8·51–27·37) | <0·0001 | |
| Organ systems requiring support at admission | |||
| No organ system | 1 (ref) | .. | |
| One organ system | 0·93 (0·56–1·52) | 0·76 | |
| Two organ systems | 0·93 (0·54–1·58) | 0·78 | |
| Three organ systems | 1·49 (0·76–2·93) | 0·25 | |
| Four organ systems | 2·99 (0·88–10·18) | 0·08 | |
| Prone ventilation | |||
| None | 1 (ref) | .. | |
| On spontaneous ventilation | 0·82 (0·61–1·09) | 0·17 | |
| On invasive mechanical ventilation | 1·42 (0·88–2·30) | 0·15 | |
| Inotropes or vasoconstrictors | 3·67 (2·77–4·86) | <0·0001 | |
| Therapeutic anticoagulation | 1·18 (0·85–1·63) | 0·33 | |
| Steroid therapy | 0·55 (0·37–0·81) | 0·003 | |
CPAP=continuous positive airway pressure. SOFA=sequential organ failure assessment. Controls are patients alive in hospital and alive and discharged at 30 days (n=3140).
A post-hoc analysis exploring the frequency of interventions received by patients who required invasive mechanical ventilation showed that 148 (12·9%) of 1146 of these patients also received dialysis.
The updated meta-analyses are shown in the appendix (pp 26–40). The overall reported global mortality was 31·5% (95% CI 27·5–35·5) and the mortality in Africa was higher than the mortality in the other regions. The excess mortality in Africa was 11 excess deaths per 100 admissions in Africa (in the best case scenario) and 23 excess deaths per 100 admissions in Africa (in the worst case scenario), compared with the global rate. The age and SOFA scores of this cohort were significantly lower than the global cohort, and the point estimates of this cohort were lower than the other regional cohorts (appendix pp 30–31). The case fatality rate in Africa was approximately 0·1% higher than in Europe and the global average, and similar to North America's case fatality rate. In Africa, up until Dec 11, 2020, there were 57 (40·1% of the global average) cases of COVID-19 per 100 000 population, which was lower than the global average of 140 cases per 100 000 population (appendix p 41).18
Discussion
The principal finding of our study was that in-hospital mortality following critical care admission for COVID-19 infection in Africa occurred in 48·2% (95% CI 46·4–50·0) of 3077 patients in the 30 days after high-care or intensive care unit admission, with an excess mortality of 11–23 deaths per 100 patients compared with the global average. Mortality was associated with increasing age, HIV/AIDS, diabetes, chronic liver disease, kidney disease, a high severity of organ dysfunction on presentation, and increasing respiratory and cardiovascular support. A shortage of critical care resources could have contributed to increased mortality. The SOFA score could represent a simple, quick tool for risk stratification of patients with COVID-19 at the point of critical care admission.
Compared with other studies that have found men at higher risk of mortality with COVID-19, the finding of no sex differences in mortality in our study was unexpected.19 It is possible that women have an increased mortality risk generally because of the barriers to accessing care and limitations or biases in care when critically ill,20 which could have moderated the differences between men and women here. Previously, the clinical course of patients with HIV and COVID-19 infection was unknown.21 Our data suggests that HIV/AIDS is an important risk factor for COVID-19 mortality. Our study also supports the use of steroid therapy to decrease mortality from COVID-19 in this patient population.22
It is possible that overall mortality following SARS-CoV-2 infection is lower in Africa than in the other parts of the world (appendix p 41). However, the critical care in-hospital mortality was higher for African countries than for other, non-African countries. There are several possible reasons to explain this finding. First, the scarcity of critical care resources in African countries might contribute to the high critical care mortality. Second, there are inadequate critical care beds,4 with only one in two patients referred to critical care being admitted. Yet, the full SOFA scores suggest that the patients who were admitted could be relatively healthier than patients admitted in countries with more critical care resources (appendix p 31). Third, when considering the proportion of sites that could provide dialysis, proning, ECMO, arterial blood gases, and pulse oximetry, data from our study suggests that the African countries included in our study had very under-resourced critical care facilities. These data suggest that these countries had a low-volume critical care capacity, which could have adversely affected outcomes.23 Lastly, merely counting the available critical care resources necessary for intervention does not accurately reflect the proportion of patients who actually receive the interventions. We estimate that patient access to interventions was between seven-times lower (for dialysis and proning) and 14-times lower (for ECMO) than what is required. Dialysis was available in 39 (68%) of 57 sites and was offered to only 330 (10%) of 3073 patients. Yet, acute kidney injury could occur in over 90% of patients with COVID-19 admitted to intensive care units, with one in four patients who have been ventilated requiring renal replacement therapy.24
Proning is included in acute respiratory distress syndrome management strategies and has been provided in over 90% of patients.25 As 75% of patients with COVID-19 referred to critical care develop acute respiratory distress syndrome,26 at least six times more patients should have received proning in our cohort. Similarly, ECMO was only available in nine (15·8%) of 57 sites (table 1), but ECMO was offered to less than 1% of patients; yet large registry data supports its use in patients with COVID-19 with refractory respiratory failure.27 Lack of access to these interventions could partly explain the high mortality in Africa, and why one in eight patients had therapy withdrawn or limited.
The human resources available to these critical care units were somewhat good with respect to availability of a physician 24 h per day (7 days a week), and nurse-to-patient ratio. However, the inability to admit approximately half of the referred patients to the critical care unit could reflect intensive care units working at full capacity with the available resources, and therefore the effect of limited critical care human resources might have resulted in adverse outcomes outside the critical care unit, which we could not assess.
There are several limitations to our study. First, our study presents data from predominantly tertiary hospitals, yet pulse oximetry was not universally available. It is likely that lower level hospitals with less resourced critical care units might have had worse outcomes than those reported in this cohort. Furthermore, referral to higher level centres might have further increased mortality before patients were able to reach an appropriate critical care unit. We did not distinguish between cardiorespiratory arrests that occurred in hospital and those out of hospital, and it is likely that patients who had cardiorespiratory arrests outside of hospital might have had a higher mortality, and might be poorly represented in this cohort. It is therefore possible that the mortality for patients with COVID-19 who are critically ill might be higher than the mortality we report. The outcomes of the patients who were referred to critical care but not admitted are also unknown. It is unlikely that the findings of this study are generalisable to those patients, as their disease severity and resources available for therapy would differ from patients who are admitted to critical care.
Furthermore, we cannot report on the association between severity of comorbidities (eg, CD4 cell count in HIV/AIDS and mortality). Compared with other COVID-19 critical care cohorts (appendix p 30), our cohort was on average younger and it is therefore likely that the severity of comorbidities was less in this African cohort. Increasing age is associated with adverse outcomes in COVID-19 infections,28 and it is therefore likely that our estimate of excess mortality is an underestimate when matched for age and severity of comorbidities.
The inability to recruit all eligible patients reflects the difficulties of doing research while providing a critical care service in an under-resourced environment during a pandemic. We have little data to help us to understand how one in two patients died without receiving oxygen, and how one in three patients died without receiving inotropes. It is unclear whether these events occurred because of unavailability of resources, limitation of early therapy, or underuse of resources. Finally, this cohort represents ten African countries, despite 26 country leaders agreeing to participate. This result shows the difficulty in fulfilling ethics and regulatory requirements and other barriers associated with doing research in an under-resourced environment.29 It is therefore difficult to determine the generalisability of these results although, to the best of our knowledge, these data provide the largest cohort of critically ill patients with COVID-19 who are from under-resourced environments (appendix p 28).
This is a large, prospective, multicentre study from a previously unreported African setting and, to the best of our knowledge, the only study in this setting that has also included a large number of patients with HIV. The statistical analysis plan was published before data inspection and was adequately powered to adjust for the association between human resources, patient comorbidities, and critical care interventions and mortality. All prespecified sensitivity analyses confirm the main findings.
Hospital and critical care resources are scarce in Africa. Moreover, admission to critical care is restricted and access to critical care interventions are between seven-times and 14-times less than what is needed. Patient comorbidities of HIV/AIDS, diabetes, chronic liver disease and kidney disease, as well as increasing age, are associated with increased mortality from COVID-19 in Africa. Poor prognosis was associated with the degree of organ dysfunction at admission and the need for invasive mechanical ventilation or inotropic support. Although the full SOFA score has been shown to have superior performance to the quick SOFA score,30 most sites analysed in our study could not assess a full SOFA score because of the scarcity of resources. The quick SOFA score is a simple risk stratification or triage tool that is feasible in low resource environments.
Mortality is associated with organ dysfunction and organ support needed at critical admission; yet there are insufficient resources to provide adequate support in this setting. Early warning systems, risk stratification, and early intervention are needed to avoid delays in instituting necessary organ support. Strategies are needed to mitigate risk in patients who are infected with SARS-CoV-2 in Africa with coexisting HIV/AIDS, diabetes, chronic liver disease, and kidney disease.
Correspondence to: Prof Bruce Biccard, Department of Anaesthesia and Perioperative Medicine, University of Cape Town, Cape Town 7925, South Africa; Department of Anaesthesia and Perioperative Medicine, Groote Schuur Hospital, Cape Town 7935, South Africa bruce.biccard@uct.ac.za
Data sharing
Data will be disclosed only upon request and approval of the proposed use of the data by the steering committee. Data are available to the journal for evaluation of reported analyses. Data requests from non-ACCCOS investigators will not be considered until 2 years after the close of the trial. Data will be de-identified for participant, hospital, and country, and will be available with a signed data access agreement.
Declaration of interests
MMe has received honoraria for services related to speakers bureau and advisory boards. These have related to purely educational talks that have been given in an objective fashion for educational purposes and with no vested interest or agenda other than for educational purposes. Companies that MMe gave talks to were Pfizer, Merck, Astellas, Sanofi-Aventis, Aspen, and Sun. IJ is the former president of the Critical Care Society of Southern Africa and is a current councillor and board member of the Critical Care Society of Southern Africa. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We would like to acknowledge Rema Ramakrishnan for their assistance with the meta-analysis forest plot and Dilshaad Brey for their assistance with the database search for the meta-analysis. The ACCCOS was supported by a grant from the Critical Care Society of Southern Africa.
Contributors
BMB contributed to the overall conception and design of the study, acquisition of data, local and national study leadership, statistical analysis, writing the first draft of the paper, critically revising the work for submission, and the final approval of the version of the study to be submitted. MMi, WLM, DT, AA, EA, GC, HTD, MElfi, MG, AGB, IJ, FK, H-LK, ZM, AM, WM, AN, ZN, AO, JLP, JS, YSA, DEAvS, and PDG contributed to the overall conception and design of the study, acquisition of data, local and national study leadership, critical revision of the paper for submission, and were involved in final approval of the study to be submitted. MSC contributed to the overall conception and design of the study, acquisition of data, local and national study leadership, patient recruitment, and data collection. MElh applied for nationwide and local hospital ethical approvals and recruited collaborators for data collection from the hospital, was involved in local and national study leadership, critically revised the study for submission, and was involved in the final approval of the version of the study to be submitted. MF contributed to the overall conception and design of the study, acquisition of data, local and national study leadership, critical revision the work for submission, final approval of the version of the study to be submitted, was a national hospital team leader, and was involved in patient recruitment in the study. DF was involved in designing the intensive care unit triage form, acquisition of data, critical revision of the work for submission, and final approval of the version of the study to be submitted. AH was involved in the statistical analyses, critical revision of the work for submission, and the final approval of the version of the study to be submitted. VM and CO were involved in the acquisition of data, local and national study leadership, critical revision of the work for submission, and final approval of the version of the study to be submitted. EHT was involved in the conception and design of the meta-analysis, the acquisition of data for meta-analysis, data analysis and writing, critical revision of the work for submission, and final approval of the version to be submitted. Mme contributed to the overall conception and design of the study, local and national study leadership, and the final approval of the version of the study to be submitted. MEl was involved in local and national leadership, critical revision of the work for submission, and final approval of the version of the study to be submitted. KDMM was involved in the acquisition of data for meta-analysis, data analysis and writing, critical revision of the work for submission, and final approval of the version to be submitted. FP was involved in designing the intensive care unit triage form, acquisition of data, critical revision of the work for submission, and final approval of the version of the study to be submitted. BMB and AH accessed and verified the data.
Contributor Information
The African COVID-19 Critical Care Outcomes Study (ACCCOS) Investigators:
Bruce M Biccard, Pragasan Dean Gopalan, Malcolm Miller, William Lance Michell, David Thomson, Adesoji Ademuyiwa, Ernest Aniteye, Greg Calligaro, Maman Sani Chaibou, Hailu Tamiru Dhufera, Mohamed Elfagieh, Mahmoud Elfiky, Muhammed Elhadi, Maher Fawzy, David Fredericks, Meseret Gebre, Abebe Genetu Bayih, Anneli Hardy, Ivan Joubert, Fitsum Kifle, Hyla-Louise Kluyts, Kieran Macleod, Zelalem Mekonnen, Mervyn Mer, Atilio Morais, Vanessa Msosa, Wakisa Mulwafu, Andrew Ndonga, Zipporah Ngumi, Akinyinka Omigbodun, Christian Owoo, Fathima Paruk, Jenna Louise Piercy, Yakob Seman, Elliott H Taylor, Dawid van Straaten, Mahmoud Elfiky, Maher Fawzy, Ahmed Awad, Hend Hussein, Mahmoud Shaban, Merihan Elbadawy, Ahmed O Elmehrath, Ahmed Cordie, Mohamed Elganainy, Mostafa El-Shazly, Mahmoud Essam, Omar A. Abdelwahab, Aboubakr Ali, Aliae Mohamed Hussein, Emad Zarief kamel, Fatma A. Monib, Islam Ahmed, Mahmoud M. Saad, Mohammed Ali Al-Quossi, Nashwa Rafaat, Islam Galal, Beshoui labib, Dalia Omran Omran, Maher Fawzy, Mahmoud Elfiky, Ahmed Azzam, Mohammed Azab, Sherief Ghozy, Ahmed Tawheed, Mahmoud Gamal, Mohamed El Kassas, Aml Azzam, Neama Ahmed, Yasmin NasrEldin, Ali Abdelsalam, Omar Abdewahab, Mohamed Elganainy, Omar Elmandouh, Hailu Tamiru Dhufera, Meseret Gebre, Abebe Genetu Bayih, Fitsum Kifle, Zelalem Mekonnen, Yakob Seman, Abebe Addisie, Akine Eshete, Fitsum Kifle, Kokeb Desita, Hiruy Araya, Yared Agidew, Addisu Desalegn Andabo, Emnet Tesfaye, Elias Ali Yesuf, Gelaw Hailemariam, Habtamu Sime, Dame Fikadu Keneni, Menbeu Sultan Mohammed, Yemane Gebremedhin, Yoseph Taye, Tamiru Assefa Mebrate, Tirunesh Busha Gemechu, Tigist Tesfaye Bedane, Elias Tewabe Abera, Ayele Teshome, Ernest Aniteye, Christian Owoo, Alfred Doku, Christian Owoo, Jane Sandra Afriyie-Mensah, Aba Lawson, Christian Owoo, Daniel Akwanfo Sottie, Emma Addae, Ernest Ofosu-Appiah, William Obeng, Andrew Ndonga, Zipporah Ngumi, Andrew Ndonga, Anne Mugera, Caesar Bitta, Mohamed Elfagieh, Muhammed Elhadi, Mohammed Abdalraheem Huwaysh, Mohammed Mahdi Ali Yahya, Alsnosy Abdullah Khalefa Mohammed, Amrajaa Alsalihin Mohammed Majeed, Amkhatirah Emad Mousay Mohammed, Elsalhein Majeed, Abdurraouf A Abusalama, Ehab Altayr, Taha Abubaker, Akram Mohammed Alkaseek, Butaina Abdulhafith, Zainab Alziyituni, Marwa F Gamra, Mohamed Muftah Anaiba, Samer Khel, Mohammed Abdelkabir, Saedah Abdeewi, Safia Adam, Abdulmueti Alhadi, Ahmed Alsoufi, Muhannad Binnawara, Ahmed Msherghi, Ahmad Elmabri Mohammad Bouhuwaish, Ahmed SA Taher, Vanessa Msosa, Wakisa Mulwafu, Francis Masoo, Singatiya Stella Chikumbanje, Palesa Chisala, Delia Mabedi, Atilio Morais, Antonio Carlos, Atilio Morais, Cesaltina Lorenzoni, Jorge Mambo, Isabel Isabel Chissaque, Mouzinho Mouzinho Saide, Maman Sani Chaibou, Maikassoua Mamane, Foumakoye Amadou, Adesoji Ademuyiwa Adesoji Ademuyiwa, Akinyinka Omigbodun Akinyinka Omigbodun, Ademola Adeyeye, Akinola Akinmade, Yakubu Momohsani, John Bamigboye, Donald Orshio, Erdoo Suckie Isamade, Henry Embu, Samuel Nuhu, Samuel Ojiakor, Ahmed Nuhu, Salisu Kwayabura, Adeola Fowotade, Arinola Sanusi, Babatunde Osinaike, Olusola Idowu, Olukemi Adekanmbi, Abdullahi Oteikwu Amali, Sanusi Ibrahim, Adamu Abba Adamu, Ibrahim Kida, Job Otokwala, Mahmoud Essam, Olubusola Alagbe-Briggs, Sylvanus Ojum, Fathima Paruk, Juan Scribante, Ismail Sikander Kalla, Aurence Mdladla, Tebogo Mabotja, Ria Naidoo, Roel Matos-Puig, Arisha Ramkillawan, Michelle Smith, Christel Arnold-Day, David Thomson, Greg Calligaro, Ivan Joubert, Jagga Jagga, Jenna Piercy, Lance Michell, Liam Devenish, Malcolm Miller, Nicole Fernandes, Pragasan Dean Gopalan, Santosh Pershad, Nicola Grabowski, Mapule Rammego, Sabelo Zwane, Masikhanyise Elizabeth Dhlamini, Matthew Neuhoff, Tobisa Fodo, Anthony Usenbo, Busisiwe Mrara, Freddy Kabambi, Estie Cloete, Leonel De Caires, Roger Dickerson, Candice Louw, Alida Theron, Ryan Herselman, Jannes Badenhorst, Godfrey Moletsane, Helene Loots, Frans Christiaan Vorster, Fathima Paruk, Julian Chausse, Matthew Neuhoff, Melinda Sebastian, Nicola Grabowski, Paul Rheeder, Wesley van Hougenhouck-Tulleken, Carin Snyman, Durotolu Adeleke, Jovan Esterhuizen, Leoni de Man, Matema Mosola, Pieter van der Linde, Reinier Swart, Shaun Maasdorp, Tina Martins, and Veneshree Govender
Supplementary Material
References
- 1.The Johns Hopkins University School of Medicine Coronavirus resource center. https://coronavirus.jhu.edu/map.html
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biccard BM, Madiba TE, Kluyts HL, et al. Perioperative patient outcomes in the African Surgical Outcomes Study: a 7-day prospective observational cohort study. Lancet. 2018;391:1589–1598. doi: 10.1016/S0140-6736(18)30001-1. [DOI] [PubMed] [Google Scholar]
- 4.Ayebale AET, Kassebaum NJ, Roche AM, Biccard BM. Africa's critical care capacity before COVID-19. S Afr J Anaesthesiol Analg. 2020;26:162–164. [Google Scholar]
- 5.Skinner DL, De Vasconcellos K, Wise R, et al. Critical care admission of South African (SA) surgical patients: results of the SA Surgical Outcomes Study. S Afr Med J. 2017;107:411–419. doi: 10.7196/SAMJ.2017.v107i5.11455. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75:1340–1349. doi: 10.1111/anae.15201. [DOI] [PubMed] [Google Scholar]
- 7.Gupta M, Wahl B, Adhikari B, et al. The need for COVID-19 research in low- and middle-income countries. Glob Health Res Policy. 2020;5:33. doi: 10.1186/s41256-020-00159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor EH, Hofmeyr R, Torborg A, et al. Risk factors and interventions associated with mortality or survival in adult COVID-19 patients admitted to critical care: a systematic review and meta-analysis. S Afr J Anaesthesiol Analg. 2020;26:116–127. [Google Scholar]
- 9.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 10.Biccard BM, Miller MG, Michell WL, et al. An African, multi-centre evaluation of patient care and clinical outcomes for patients with COVID-19 infection admitted to high-care or intensive care units. SSRN. 2020 https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3707415 published online Oct 22. (preprint). [Google Scholar]
- 11.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 13.Bates D, Maechler M, Bolker B, Walker SA. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 14.RStudio Team . PBC; Boston, MA: 2020. RStudio. [Google Scholar]
- 15.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 16.Rubin DB. John Wiley & Sons; New York: 1987. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 17.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. A language and environment for statistical computing. [Google Scholar]
- 18.Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Mortality risk of COVID-19. 2020. https://ourworldindata.org/mortality-risk-covid [DOI] [PMC free article] [PubMed]
- 19.Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2021;64:36–47. doi: 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langer A, Meleis A, Knaul FM, et al. Women and health: the key for sustainable development. Lancet. 2015;386:1165–1210. doi: 10.1016/S0140-6736(15)60497-4. [DOI] [PubMed] [Google Scholar]
- 21.Costenaro P, Minotti C, Barbieri E, Giaquinto C, Donà D. SARS-CoV-2 infection in people living with HIV: a systematic review. Rev Med Virol. 2021;31:1–12. doi: 10.1002/rmv.2155. [DOI] [PubMed] [Google Scholar]
- 22.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen YL, Wallace DJ, Yordanov Y, et al. The volume-outcome relationship in critical care: a systematic review and meta-analysis. Chest. 2015;148:79–92. doi: 10.1378/chest.14-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 26.Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero Starke K, Petereit-Haack G, Schubert M, et al. The age-related risk of severe outcomes due to COVID-19 infection: a rapid review, meta-analysis, and meta-regression. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conradie A, Duys R, Forget P, Biccard BM. Barriers to clinical research in Africa: a quantitative and qualitative survey of clinical researchers in 27 African countries. Br J Anaesth. 2018;121:813–821. doi: 10.1016/j.bja.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Yao N, Qiu Y, He C. Predictive performance of SOFA and qSOFA for in-hospital mortality in severe novel coronavirus disease. Am J Emerg Med. 2020;38:2074–2080. doi: 10.1016/j.ajem.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be disclosed only upon request and approval of the proposed use of the data by the steering committee. Data are available to the journal for evaluation of reported analyses. Data requests from non-ACCCOS investigators will not be considered until 2 years after the close of the trial. Data will be de-identified for participant, hospital, and country, and will be available with a signed data access agreement.