Abstract
Glass transition temperature (Tg) is an important parameter for the physical quality control of hard candies. In order to understand the applicability of calcium maltobionate to hard candy, effect of calcium maltobionate addition on the Tg of model and hand-made hard candies was investigated. Freeze-dried calcium maltobionate-sugar (sucrose containing a small amount of glucose-fructose mixture) and calcium maltobionate-reduced isomaltulose mixtures were prepared as model candies, and their anhydrous Tg was evaluated using a differential scanning calorimetry. The anhydrous Tg increased linearly with the molar fraction of calcium maltobionate. From these results, it was expected that calcium maltobionate can improve the physical stability of normal and sugarless candies. For comparison, various commercial candies were employed, and their Tg was evaluated using a thermal rheological analysis. The Tg values were in the range of 28–49 °C. The Tg values were higher than 25 °C, which is significant with respect to the physical stability of the candies. Calcium maltobionate-sugar and calcium maltobionate-reduced isomaltulose candies were prepared as hand-made candies. The calcium maltobionate-reduced isomaltulose candies had higher Tg than the calcium maltobionate-sugar candies at each calcium maltobionate content, although reduced isomaltulose has a lower Tg than sugar. At a high calcium maltobionate content, calcium maltobionate-reduced isomaltulose candy had an equivalent Tg to the commercial sugarless candies, and thus practically acceptable stability was expected. In the case of calcium maltobionate-sugar candies, there was a possibility that the hydrolysis of sugar reduced their Tg. Vacuum-concentration will be useful to improve the Tg of the candies.
Keywords: calcium maltobionate, hard candy, glass transition temperature, differential scanning calorimetry, thermal rheological analysis
Abbreviations
MBCa, calcium maltobionate; DSC, differential scanning calorimetry; T g, glass transition temperature; RIM, reduced isomaltulose; RH, relative humidity; SG, sugar; TRA, thermal rheological analysis.
INTRODUCTION
Low-moisture food products are commonly amorphous, and thus the glass to rubber transition (glass transition) occurs at the glass transition temperature ( T g). The T g of hydrophilic amorphous materials decreases with an increase in the water content due to the water plasticizing effect, and thus the glassy (solid-like) product becomes a rubbery (liquid-like) product as a result of water sorption when T g becomes lower than room temperature. Therefore, it is practically important to understand the T g of low-moisture food products. 1) 2) 3) Hard candy is a typical glassy food product. However, hard candy shows softening, stickiness, and crystallization of sucrose when it is stored under high temperature and high relative humidity (RH) conditions. For example, Labuza et al. investigated the effect of storage conditions on the physical quality of hard-ball candy, and reported that the hard-ball candies change from free-flowing pieces to stuck pieces at a temperature higher than T g. 4) They also reported a similar result for cotton candy. 5)
The T g of candies mainly originates from sucrose and syrup. Since sucrose has a relatively low T g and tends to crystallize easily in a rubbery state, the physical stability of sucrose can be improved by the addition of high- T g materials. For example, the T g of sucrose could be elevated and the resistance to crystallization could be enhanced by the addition of high- T g materials. 6) 7) Sweeteners such as sugar alcohol can also be employed for the production of low-sugar or sugarless candies. 8) Since sugar alcohol commonly has a lower T g than sucrose, 9) it is important to improve the physical stability of low-sugar or sugarless candy by the addition of high- T g materials.
Calcium maltobionate (MBCa) is a potentially effective calcium-based material used for pharmaceutical and food products due to its high water solubility, high bioavailability, and mildly bitter taste. 10) In addition, MBCa has a high T g (anhydrous T g = 145 °C) and does not crystallize, even under high RH conditions. 11) In the MBCa-maltose mixture system, MBCa can elevate the T g of maltose and prevent the crystallization of maltose under high RH conditions. 12) Therefore, it is expected that MBCa is useful not only as an effective Ca-supplement, but also as a physical stabilizer of glassy food products.
The purpose of this study was to understand the effect of MBCa addition on the T g of normal and low-sugar hard candies. For this purpose, sugar (sucrose containing a small amount of glucose-fructose mixture) and reduced isomaltulose were employed as the ingredients. First, freeze-dried MBCa-sugar and MBCa-reduced isomaltulose mixtures were prepared as hard candy models, and their T g values were evaluated using differential scanning calorimetry (DSC). Second, the T g of commercial hard candies were evaluated by thermal rheological analysis (TRA). TRA is a thermomechanical approach that is principally equivalent to thermal mechanical analysis, 13) thermal mechanical compression testing, 14) 15) 16) and phase transition analysis. 17) T g can be detected as a force-drop of baseline (force vs. temperature) by TRA. Although DSC is a widely used technique to evaluate the T g of amorphous materials, hard candy has to be powdered in order to place it into the DSC pan. However, the candies were too hard to obtain powder, and powdered candy is readily plasticized by water because of the increased surface area. In the case of TRA, there was an advantage in that the sample could be set directly in the equipment. 18) 19) 20) Finally, normal and low-sugar hard candies with added MBCa were prepared, and the T g values were compared to those of commercial products.
MATERIALS AND METHODS
Materials.
Analytical-grade calcium carbonate and maltose monohydrate were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Catalase and glucose oxidase were purchased from Amano Enzyme Inc. (Aichi, Japan). MBCa (70 % aqueous solution) was prepared according to our previous studies. 11) 12) The purity of the MBCa was confirmed to be greater than 99 % by high-performance liquid chromatography. Sugar and reduced isomaltulose (Mitsui Sugar Co., Ltd., Tokyo, Japan), and high-maltose corn syrup (San-ei Sucrochemical Co., Ltd., Aichi, Japan) were provided by the suppliers. The sugar was sucrose containing 1.2–1.6 % glucose-fructose mixture. The syrup was composed of 70 % (w/w) maltose, 25 % (w/w) water, and the other low-molecular weight carbohydrates given by hydrolyzed corn starch. Seven kinds of commercially available hard candy were purchased in a local market. Product information of the hard candies is given in Table 1. Based on the information, the candies were categorized into normal, sugarless, milk, and honey candies.
Table 1.
Table 1. T g and water content for commercial hard candies.
| Type | T g (ºC) | Water content (g/100g-solid) |
Composition (g/100g-product) | Product information | ||
|---|---|---|---|---|---|---|
| Protein | Fat | Carbohydrate | ||||
| Normal 1 | 48.3 ± 0.6 a | 2.3 ± 0.4 a | 0.0 | 0.0 | 97.2 | sugar, syrup, herbs extract, condensed chinese quince juice, malt extract, chinese quince extract, flavoring, caramel coloring, emulsifier, seasoning |
| Normal 2 | 47.7 ± 0.5 a, b | 1.4 ± 0.2 b | 0.0 | 1.6 | 95.5 | syrup, sugar, vegetable oil, condensed yogurt, condensed lemon extract, honey, acidulant, flavoring, emulsifier, gardenia pigment |
| Normal 3 | 49.1 ± 1.1 a | 1.4 ± 0.1 b | 0.0 | 0.0 | 97.5 | sugar, syrup, condensed blueberry extract, bilberry extract, acidulant, flavoring, marigold, vitamin A. biotin |
| Sugarless 1 | 44.6 ± 1.0 b, c | 0.6 ± 0.0 c | 0.0 | 7.9 | 90.2 | reduced sugar syrup, margarin, cream, coffee powder, salt, emulsifier, coloring, flavoring, sweetener |
| Sugarless 2 | 44.4 ± 0.5 c | 0.4 ± 0.1 c | 0.3 | 0.3 | 97.9 | reduced palatinose, reduced sugar syrup, herbs extract, chinese quince extract, vitamin C, flavoring, sweetener, coloring, vitamin B 2, vitamin B 1 |
| Milk | 31.8 ± 1.6 d | 2.7 ± 0.1 a | 2.6 | 10.2 | 81.8 | sugar, syrup, milk powder, cream, condensed milk, butter, salt, flavoring, emulsifier, acidulant |
| Honey | 27.9 ± 1.5 e | 0.9 ± 0.1 c | 0.0 | 0.0 | 99.3 | honey |
The T g and water content values are expressed as mean ± SD ( n = 3). The T gand water content values with the different letter are significantly different at p < 0.05.
Preparation of freeze-dried samples.
Freeze-dried MBCa-sugar and MBCa-reduced isomaltulose were prepared at various dry MBCa weight fractions as follows. A 10 % (w/w) MBCa aqueous solution was prepared by dissolving the reagents in distilled water. In glass vials, the MBCa solution was adjusted to various dry weight fractions by the addition of sugar or reduced isomaltulose, and they were then frozen at approximately –30 °C overnight in a freezer. The frozen preparations were placed in a pre-cooled freeze-drier, and evacuated to a pressure of less than 70 Pa as the temperature was increased from –35 °C to 10 °C over 2.5 days.
DSC measurement.
The T g values of the freeze-dried samples were evaluated using a DSC (DSC 120; Seiko Instruments Inc., Tokyo, Japan). Temperature and heat flow were calibrated using indium and distilled water, and alumina powder was used as a reference material. To evaluate anhydrous T g values of the freeze-dried samples, the samples (ca. 20 mg) were placed in the DSC pan and vacuum-dried at 80 °C for more than 16 h, and then hermetically sealed. 11) 12) Heat-scanning was performed in the temperature range between 0 and 180 °C at 5 °C/min. To reset the thermal history (i.e., enthalpy relaxation effect) of the glassy samples, a second scan was performed, and the T g values of the samples were determined from the onset of the endothermic shift observed in the second scan. 21) Measurements were performed in triplicate, and the results were averaged.
Preparation of hard candies.
Figure 1 shows the procedure for the preparation of the hard candies. For the preparation of conventional hard candy (control), sugar and syrup were mixed at dry weight fraction = 0.59 and 0.41, respectively, in an aluminum pot. For the preparation of the MBCa-sugar (MBCa-SG) and MBCa-reduced isomaltulose (MBCa-RIM) candies, MBCa was mixed with sugar or reduced isomaltulose at various dry weight fractions (0.1, 0.2, and 0.3 MBCa). Hereafter, the candies are described based on the dry weight fraction of MBCa; for example, MBCa-SG and MBCa-RIM candies containing MBCa at a dry weight fraction of 0.2 are described as 0.2 MBCa-SG and 0.2 MBCa-RIM, respectively. The samples were heat-condensed on an electric heater with brief stirring ( Fig. 1A). A temperature sensor was used as the stirring bar to confirm the sample temperature during the heating process. The weight loss of the sample was confirmed at various times during the processing ( Fig. 1B). Heat condensing was stopped at a water content of 3 g/100 g-dry matter (DM), which was evaluated from the weight loss, and the molten liquid was then poured into a 10 mm deep aluminum mold with a 20 mm diameter ( Fig. 1C). The sample was cooled in a desiccator with silica gel at room temperature ( Fig. 1D), and candies were then removed from the mold ( Fig. 1E). The candies (diameter = 20 mm, height = 8–10 mm) were individually placed into poly-packs and stored in a desiccator with silica gel.
Fig. 1. Procedure for the preparation of hand-made hard candy.

TRA measurement.
TRA measurements were conducted using a texture analyzer (CR-150; Sun Scientific Co., Ltd., Tokyo, Japan) with an attached heating stage (Nissin Seiki Co., Ltd., Hiroshima, Japan) and a temperature controller (TNX-400E; As One Co., Ltd., Osaka, Japan), according to a previously reported procedure with minor modifications. 18) 19) 20) 22) Hard candies were covered with a wrap film (Asahi Kasei Co., Tokyo, Japan) to prevent water sorption or evaporation during the measurement. The samples were placed on the sample stage and compressed at 80 N with a plate plunger (diameter = 5 mm). The force was a practical maximum force for the equipment, because force-drop induced by glass transition was expected to be enhanced. The initial compression was held for 1 min, and the sample was then heat-scanned at 3.5 °C/min in the temperature range between 10 and 80 °C. The temperature vs. time data correspond to mechanical force vs. time data, and a TRA curve (mechanical force vs. temperature) was then obtained. The mechanical T g was evaluated from the onset point of force-drop. It is known that mechanical T g does not always agree with calorimetric T g. 18) Thus, the T g values of freeze-dried maltose and sucrose with varying water content were preliminarily compared between DSC and TRA ( Fig. 2). On the basis of the linear relationship, the mechanical T g was calibrated to calorimetric T g. Measurements were performed in triplicate and the results were averaged.
Fig. 2. Relationship between mechanical T g and calorimetric T g for freeze-dried maltose (square) and sucrose (circle) with varying water content.

The values are expressed as mean ± SD ( n = 2–3).
Color properties.
The surface color of the hard candy was evaluated using a color-difference meter (NR-3000, Nippon Denshoku Ind., Co., Ltd., Tokyo Japan). The photometer was positioned at the center of the hard candy on a white paper, and the brightness ( L*), red to green ( a*), and yellow to blue ( b*) values were determined. Measurements were performed in triplicate and the results were averaged.
Water content.
Water content (g/100 g-DM) of the samples was evaluated gravimetrically by drying at 105 °C for 16 h. Measurements were performed in triplicate and the results were averaged.
Statistical analysis.
Analysis of variance (ANOVA) was performed with a Tukey HSD (honest significant difference) test ( p < 0.05) using Kaleida Graph (Version 3.6, Hulinks Inc., Tokyo, Japan).
RESULTS AND DISCUSSION
Anhydrous T g of freeze-dried samples.
Typical DSC thermograms (2nd scanning) for the anhydrous freeze-dried samples are shown in Figs. 3A (MBCa-sugar) and 3B (MBCa-reduced isomaltulose), respectively. The results indicated a clear endothermic shift due to glass transition, and the T g values were determined from the onset point. The anhydrous T g for sugar (66.4 ± 0.9 °C) agreed with a literature value (68 °C) for sucrose. 21) The anhydrous T g for reduced isomaltulose was determined to be 56.6 ± 0.8 °C. The anhydrous T g of sugar alcohol is slightly lower than that for typical sugars. 9) The anhydrous T g of MBCa was taken from our previous study. 11)
Fig. 3. Typical DSC thermograms for freeze-dried (A) MBCa-sugar and (B) MBCa- reduced isomaltulose.
Arrows indicate the T g.
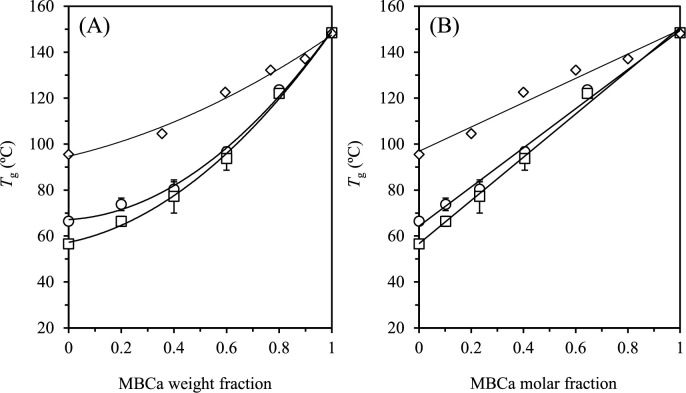
The effect of MBCa on the anhydrous T g of sugar and reduced isomaltulose is shown in Figs. 4A (as a function of the MBCa weight fraction) and 4B (as a function of the MBCa molar fraction), respectively. The T g values for the anhydrous MBCa-maltose system are also plotted for comparison. 12) The anhydrous T g increased quadratically and linearly with an increase in the MBCa weight and molar fractions, respectively. As mentioned above, it is important to elevate the T g of glassy foods because various undesirable physical changes occur in the rubbery state. Thus, it was expected that MBCa would improve the physical stability of normal and sugarless candies.
Fig. 4. Effect of (A) MBCa weight fraction and (B) MBCa molar fraction on the T g of freeze-dried MBCa-sugar (circles) and MBCa-reduced isomaltulose (squares).
The values are expressed as mean ± SD ( n = 3). MBCa-maltose (diamonds) was taken from a reference. 12) The solid lines were described by quadratic (A) and linear (B) equations.
T g of commercial hard candies.
The typical TRA curve of a commercial hard candy (normal 3 listed in Table 1) is shown in Fig. 5. A clear force-drop that reflects the glass transition was observed in the TRA curve, and the mechanical T g was determined from the onset point of the force-drop. As mentioned above, mechanical T g does not always agree with calorimetric T g. 18) Thus, the mechanical T g was calibrated to calorimetric T g based on the coefficients determined preliminarily ( Fig. 2).
Fig. 5. Typical TRA curve for a commercial candy (normal 3 listed in Table 1).

The inset shows a close up of the glass transition. The vertical dotted line indicates the T g.
The T g and water content of the candy products are listed in Table 1. The T g values of the commercial candies were in the range of 28–49 °C. The T g of a hard candy evaluated previously by DSC (35 °C) failed in this range. 23) It is noted that their T g values were much higher than 25 °C. This is an important fact with respect to their storage stability because the structural change and recrystallization of sucrose may occur at a temperature above T g. 9) Labuza et al. characterized the physical deterioration of hard-ball candies by five stages: 1) free-flowing and separate, 2) stuck pieces break apart with gentle shaking, 3) very sticky pieces that pull apart by hand, 4) pieces stuck in one mass, and 5) pieces all flow into one mass. 4) The hard-ball candies could remain free-flowing at 20 °C lower than T g. At a higher temperature than T g, the deterioration level increased to a higher class at intervals of approximately 10 °C. According to these results, it is concluded that the commercial candies are sufficiently stable under typical storage conditions. However, it should be considered that the temperature may increase to as high as 60 °C in a closed car during the summer season. 4) In addition, water sorption reduces the T g of candies, so that lower RH should be maintained to prevent deterioration.
The T g values of the sugarless candies (44 °C) were slightly lower than the normal candies (47–49 °C), despite a lower water content. This is because sugar alcohols have a lower T g than sugars; therefore, the water content of sugarless candy must be reduced fully to elevate T g (and establish sufficient storability).
The milk candy did not contain sugar alcohol; however, the T g (32 °C) was much lower than those for the sugarless candies. This is due to the contribution of hydrophobic materials (milk, cream, and butter). Although hydrophobic materials cannot interact with the amorphous region constructed by hydrophilic carbohydrate materials, intrinsic surfactants facilitate molecular interaction. It is known that fats and surfactants act as plasticizers in amorphous materials. 19) 24) 25)
The honey candy had the lowest T g (28 °C) among the candies employed in this study. According to the product information, this candy was manufactured using only honey. Although the composition of honey is more or less dependent on the product components, the anhydrous T g values of honey are typically reported to be 15 °C 26) and 30 °C 27) . The major components of honey are fructose and glucose, the anhydrous T g values of which are 5 and 31 °C, respectively. 9) Thus, the honey candy will have a lower T g than the other candies. The honey candy has a higher T g than 25 °C; therefore, high storage stability is expected, at least under typical storage conditions. However, there is a possibility that the candy reveals sticky character when eaten; the candy may turn into a rubber state in the mouth (approximately 37 °C). Hard candy is a unique food in that the eating time is much longer than other foods.
T g of MBCa-added candies.
During hard candy preparation, the sample temperature was maximally increased up to 145 °C over a period at which the water content was reduced up to approximately 3 g/100 g-DM (data not shown). The T g, water content, and color parameters ( L *, a *, and b *) of the hand-made candies are listed in Table 2. As expected, the water content (1.9−2.6 g/100 g-DM) of the candies was lower than 3 g/100 g-DM. The T g of the control (sugar-syrup candy) was 47 °C, which was almost equivalent to commercial candies (47–49 °C); therefore, it was confirmed that an appropriate hard candy could be produced by this method. Since the MBCa-added candies had a higher T g than 25 °C, physical stability can be guaranteed under typical storage conditions without water sorption. The 0.1 MBCa-sugar was cloudy due to the crystallization of sucrose, and thus further experiments were not performed. The other MBCa-added candies were clear yellow color. A comparison of the color parameters between the control and the MBCa-added candies revealed no significant difference of L *. However, a * and b * of the MBCa-added candies were significantly higher than those of the control. The increased a * and b * values indicate the enhancement of red and yellow colors, respectively.
Table 2.
Table 2. T g, water content, and color parameters for hand-made hard candies.
| Sample | Mechnaical T g (ºC) | Water content (g/100g-DM) | Color parameters | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| Control | 46.8 ± 0.5 a | 1.9± 0.3 a | 53.0± 3.2 a | -2.9± 0.2 a | 4.9± 1.4 a |
| 0.2 MBCa-SG | 30.4 ± 0.6 b | 2.3± 0.2 a | 47.8± 1.8 a | 10.8± 0.8 b | 51.1± 1.7 b |
| 0.3 MBCa-SG | 33.6 ± 1.8 b, c | 2.6± 0.3 a | 48.7± 3.0 a | 8.5± 2.1 b | 48.2± 1.1 b |
| 0.1 MBCa-RIM | 35.7 ± 1.8 c, d | 2.6± 0.4 a | 50.2± 0.8 a | 10.6± 1.2 b | 51.0± 1.7 b |
| 0.2 MBCa-RIM | 38.6 ± 2.1 e | 2.5± 0.4 a | 50.8± 1.9 a | 9.4± 0.7 b | 49.7± 1.8 b |
| 0.3 MBCa-RIM | 44.6 ± 1.2 a | 2.1± 0.6 a | 49.4± 4.1 a | 10.5± 1.7 b | 51.6± 0.9 b |
The values are expressed as mean ± SD ( n = 3). The values with the different letter are significantly different at p < 0.05. 0.1 MBCa-SG crystalized immedeately after preparation. Control, sugar and high-maltose corn syrup mixture; MBCa, calcium maltobionate; SG, sugar; RIM, reduced isomaltulose.
The anhydrous T g values of sugar and reduced isomaltulose were 66 and 57 °C, respectively. The T g of MBCa (148 °C) is much higher than that for sugar and reduced isomaltulose; therefore, it was expected that the T g of the hard candies would increase with the MBCa content. The anhydrous T g values of the freeze-dried MBCa-sugar and MBCa-reduced isomaltulose systems increased linearly with the MBCa molar fraction ( Fig. 4). Although the effect of water content on the T g of MBCa-sugar and MBCa-reduced isomaltulose was not investigated in this study, it can be predicted by an empirical approach that the effect of water content on the T g of hydrophilic amorphous materials can be described by the Gordon–Taylor (GT) equation:
where W 1 and W 2 are the respective weight fractions of the solute and water, and T g1 and T g2 are T g (K) for the anhydrous solute and water, respectively, and k is a constant. T g2 (136 K) can be obtained from previous reports. 28) 29) The T g1 of the MBCa-SG and MBCa-RIM systems can be predicted from the linear function of anhydrous T g as a function of the MBCa molar fraction ( Fig. 4). k is an unknown parameter; however, there is an empirical linear relationship between T g1 (°C) and k. 9)
This equation can be applied to water-soluble low-molecular-weight carbohydrates, although polysaccharides 30) and other complex systems 31) show a deviation from this equation. According to the T g-predictive approach, the T g values of the MBCa-SG and MBCa-RIM candies were calculated at each water content, and compared with experimentally determined T g values ( Fig. 6). To confirm the applicability of the T g-predictive approach, T g values for freeze-dried 0.2 MBCa-maltose with water contents of 3.03–8.33 g/100 g-DM were calculated and compared with experimental values taken from the literature. 12) The results indicated that freeze-dried 0.2 MBCa-maltose and MBCa-RIM candies exhibited good agreement between the calculated and experimentally determined T g. However, MBCa-SG showed a much lower experimentally determined T g than the calculated T g. The T g reduction of MBCa-SG cannot be simply related to the red and yellow coloring observed in the candies, because red and yellow coloring was also observed in the MBCa-RIM candies. This result suggests that sugar was thermally degraded during candy production. The hydrolysis of sucrose is known to be promoted at high temperature 32) and under low pH conditions. 33) Although MBCa is a mild acid (pH = 5.5) in aqueous solution (data not shown), the effect of maltobionate ion (acid) on the hydrolysis of sucrose will be emphasized under dehydrated conditions. 33) The hydrolysis of sucrose results in low-molecular-weight compounds such as glucose and fructose; therefore, T g will be reduced by their plasticizing effect.
Fig. 6. Relationship between experimentally determined T g and calculated T g for MBCa-SG candies (circles) and MBCa-RIM candies (squares).

The experimentally determined T g values are expressed as mean ± SD ( n = 3). Freeze-dried MBCa-maltose samples with various water contents (diamonds) were taken from a reference. 12) The color of the data points indicates MBCa weight fraction of the samples (gray = 0.1, white = 0.2, and black = 0.3).
The MBCa-RIM candies had higher T g than the MBCa-SG candies at each MBCa content, although reduced isomaltulose has a lower T g than sugar. Sugar alcohol is chemically stable; therefore, there will have been little or no hydrolysis during candy preparation. As expected, the T g values of the MBCa-RIM candies increased with the MBCa content. The 0.3 MBCa-RIM candy had an equivalent T g to the commercial sugarless candies (approximately 44 °C), and thus practically acceptable stability would be expected. It should be emphasized that the hand-made candies had higher water content than the commercial candies. According to the T g-predictive approach, when the water content of the MBCa-RIM candies was reduced (typically to 0.5 g/100 g-DM), which is similar to the commercial products (0.4–0.6 g/100 g-DM), the T g of the 0.1, 0.2, and 0.3 MBCa-RIM candies would be expected to be 55, 60, and 65 °C, respectively, which are sufficiently high T g values for hard candies ( Table 1).
Although the candies used in this study were prepared by heat-concentration under atmospheric pressure, commercial candy products have been commonly produced under vacuum conditions. Since product temperature can be reduced by the vacuum-concentration, it is expected that the red and yellow coloring of MBCa-added candies and the T g reduction of the MBCa-SG candies are diminished or prevented. For further study, it is necessary to prepare the candies as similar to industrial procedures.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
Mitsui Sugar Co., Ltd. (Tokyo, Japan) is acknowledged for providing the reduced isomaltulose.
REFERENCES
- 1).Bhandari B.R. and Howes T.: Implication of glass transition for the drying and stability of dried foods. J. Food Eng., 40, 71–79 (1999). [Google Scholar]
- 2).Le Meste M., Champion D., Roudaut G., Blond G., and Simatos D.: Glass transition and food technology: A critical appraisal. J. Food Sci., 67, 2444–2458 (2002). [Google Scholar]
- 3).Rahman M.S.: Food stability determination by macro–micro region concept in the state diagram and by defining a critical temperature. J. Food Eng., 99, 402–416 (2010). [Google Scholar]
- 4).Labuza T.P., Labuza T.J.,Labuza K.M., and Labuza P.S.: Soft condensed matter: a perspective on the physics of food states and stability. in Water Properties in Food, Health, Pharmaceutical and Biological Systems, D.S. Reid, T. Sajjaanantakul, P.J. Lillford, and S. Charoenrein, eds., Wiley-Blackwell, Singapore, pp. 87–114 (2010). [Google Scholar]
- 5).Labuza T.P., and Labuza P.S.: Influence of temperature and relative humidity on the physical sates of cotton candy. J. Food Process Pres., 28, 274–287 (2004). [Google Scholar]
- 6).Saleki-Gerhardt A. and Zografi G.: Non-isothermal and isothermal crystallization of sucrose from the amorphous state. Pharm. Res., 11, 1166–1173 (1994). [DOI] [PubMed] [Google Scholar]
- 7).Roe K.D., and Labuza T.P.: Glass transition and crystallization of amorphous trehalose-sucrose mixtures. Int. J. Food Prop., 8, 559–574 (2005). [Google Scholar]
- 8).Celeghin A.G. and Rubiolo A.C.: The water content effect on the glass transition temperature of low calory candy formulations. in Water Properties of Food, Health, Pharmaceutical, and Biological Materials, M.P. Buera, J. Welti-Chanes, P.J. Lillford, and H.R. Corti, eds., CRC Press, Boca Raton, pp. 703–708 (2006). [Google Scholar]
- 9).Roos Y.H.: Food components and polymers. in Phase Transition in Foods. Academic Press, San Diego, pp.109–156 (1995). [Google Scholar]
- 10).Suehiro D., Okada M., Fukami K., Ohtsuka M.,Nakagawa T., and Hayakawa T.: Effect of calcium maltobionate on enhancement of calcium absorption in rats. LuminacoidsRes., 21, 1–7 (2017). (in Japanese) [Google Scholar]
- 11).Fukami K., Kawai K., Takeuchi S., Harada Y., and Hagura Y.: Effect of water content on the glass transition temperature of calcium maltobionate and its application to the characterization of non-Arrhenius viscosity behavior. Food Biophys., 11, 410–416 (2016). [Google Scholar]
- 12).Fukami K., Takeuchi S., Fukujyu T., Hagura Y., and Kawai K.: Water sorption, glass transition, and freeze-concentrated glass-like transition properties of calcium maltobionate–maltose mixtures. J. Therm. Anal. Calorim., >135, 2775–2781 (2019). [Google Scholar]
- 13).Chang B.S., and Randall C.S.: Use of subambient thermal analysis to optimize protein lyophilization. Cryobiology, >29, 632–656 (1992). [Google Scholar]
- 14).Boonyai P., Howes T. and Bhandari B.: Instrumentation and testing of a thermal mechanical compression test for glass–rubber transition analysis of food powders. J. Food Eng., 78, 1333–1342 (2007). [Google Scholar]
- 15).K.Shrestha A., Ua-arak T., Adhikari B.P., Howes T., and Bhandari B.R.: Glass transition behavior of spray dried orange juice powder measured by differential scanning calorimetry (DSC) and thermal mechanical compression test (TMCT). Int. J. FoodProp., 10, 661–673 (2007). [Google Scholar]
- 16).Thuc T.T., Fukai S., Truong V., and Bhandari B.: Measurement of glass-rubber transition temperature of rice by thermal mechanical compression test (TMCT). Int. J. Food Pro., 13, 176–183 (2010). [Google Scholar]
- 17).Avaltroni F., Bouquerand P.E., and Normand V.: Maltodextrin molecular weight distribution influence on the glass transition temperature and viscosity in aqueous solutions. Carbohydr. Polym., 58, 323–334 (2004). [Google Scholar]
- 18).Sogabe T., Kawai K., Kobayashi R., Jothi J.S., and Hagura Y.: Effects of porous structure and water plasticization on the mechanical glass transition temperature and textural properties of freeze-dried trehalose solid and cookie. J. Food Eng., 217, 101–107 (2018). [Google Scholar]
- 19).Jothi J.S., Ebara T., Hagura Y., and Kawai K.: Effect of water sorption on the glass transition temperature and texture of deep-fried models. J. Food Eng., 237, 1–8 (2018). [Google Scholar]
- 20).Ebara T.,Hagura Y., and Kawai K.: Effect of water content on the glass transition and textural properties of hazelnut. J. Therm. Anal. Calorim., 135, 2629–2634 (2019). [Google Scholar]
- 21).Kawai K., Hagiwara T., Takai R., and Suzuki T.: Comparative investigation by two type analytical approaches on enthalpy relaxation for glassy glucose, sucrose, maltose and trehalose. Pharm. Res., 22, 490–495 (2005). [DOI] [PubMed] [Google Scholar]
- 22).Kawai K., Toh M., and Hagura Y.: Effect of sugar composition on the water sorption and softening properties of cookie. Food Chem., 145, 772–776 (2014). [DOI] [PubMed] [Google Scholar]
- 23).Reinheimer M.A., Mussati S., Scenna N.J., and Pérez G.A.: Influence of the microstructure and composition on the thermal–physical properties of hard candy and cooling process. J. Mol. Struct., 980, 250–256 (2010). [Google Scholar]
- 24).Madrigal L., Sandoval A.J., and Müller A.J.: Effects of corn oil on glass transition temperatures of cassava starch. Carbohydr. Polym., 85, 875–884 (2011). [Google Scholar]
- 25).Pommet M., Redl A., Morel M.H., Guilbert S.: Study of wheat gluten plasticization with fatty acids. Polymer, 44, 115–122 (2003). [Google Scholar]
- 26).Lazaridou A., Biliaderis C.G., Bacandritsos N., and Sabatini A.G.: Composition, thermal and rheological behaviour of selected Greek honeys. J. Food Eng., 64, 9–21 (2004). [Google Scholar]
- 27).Venir E., Spaziani M., and Maltini E.: Crystallization in “Tarassaco” Italian honey studied by DSC. Food Chem., 122, 410–415 (2010). [Google Scholar]
- 28).Johari G.P., Hallbrucker A., and Mayer E.: The glass–liquid transition of hyperquenched water. Nature, 330, 552–553 (1987). [Google Scholar]
- 29).Sastry S.: Supercooled water: Going strong or falling apart? Nature, 398, 467–469 (1999). [Google Scholar]
- 30).Kawai K., Fukami K., Thanatuksorn P., Viriyarattanasak C., and Kajiwara K.: Effects of moisture content, molecular weight, and crystallinity on the glass transition temperature of inulin. Carbohydr. Polym., 83, 934–939 (2011). [Google Scholar]
- 31).Fongin S., Kawai K., Harnkarnsujarit N., and Hagura Y.: Effects of water and maltodextrin on the glass transition temperature of freeze-dried mango pulp and an empirical model to predict plasticizing effect of water on dried fruits. J. Food Eng., 210, 91–97 (2017). [Google Scholar]
- 32).Buera M.P., Chirife J., and Karel M.: A study of acid-catalyzed sucrose hydrolysis in an amorphous polymeric matrix at reduced moisture contents. Food Res. Int., 28, 359–365 (1995). [Google Scholar]
- 33).Shalaev E.Y., Lu Q., Shalaeva M., and Zografi G.: Acid-catalyzed inversion of sucrose in the amorphous state at very low levels of residual water. Pharm. Res., 17, 366–370 (2000). [DOI] [PubMed] [Google Scholar]